Significance
Reduced bioavailable nitric oxide generated within the vascular wall is a key pathogenic mechanism involved in the formation and rupture of an atheromatous plaque, the latter leading to myocardial infarction or stroke. We show that inorganic nitrate supplementation through delivery of nitric oxide reduces systemic leukocyte rolling and adherence, circulating neutrophil numbers, and monocyte activation through direct repression of neutrophil activation and up-regulation of interleukin-10–dependent antiinflammatory pathways in athero-prone mice. These antiinflammatory effects of inorganic nitrate result in reduced macrophage content of atherosclerotic plaques coupled with an elevation of smooth muscle content, resulting in a stable plaque phenotype. Our data suggest that inorganic nitrate supplementation has antiinflammatory effects, which may have clinical utility in the prophylaxis of atheroma development.
Keywords: atherosclerosis, inflammation, nitrate, nitric oxide, diet
Abstract
Reduced bioavailable nitric oxide (NO) plays a key role in the enhanced leukocyte recruitment reflective of systemic inflammation thought to precede and underlie atherosclerotic plaque formation and instability. Recent evidence demonstrates that inorganic nitrate (NO3−) through sequential chemical reduction in vivo provides a source of NO that exerts beneficial effects upon the cardiovascular system, including reductions in inflammatory responses. We tested whether the antiinflammatory effects of inorganic nitrate might prove useful in ameliorating atherosclerotic disease in Apolipoprotein (Apo)E knockout (KO) mice. We show that dietary nitrate treatment, although having no effect upon total plaque area, caused a reduction in macrophage accumulation and an elevation in smooth muscle accumulation within atherosclerotic plaques of ApoE KO mice, suggesting plaque stabilization. We also show that in nitrate-fed mice there is reduced systemic leukocyte rolling and adherence, circulating neutrophil numbers, neutrophil CD11b expression, and myeloperoxidase activity compared with wild-type littermates. Moreover, we show in both the ApoE KO mice and using an acute model of inflammation that this effect upon neutrophils results in consequent reductions in inflammatory monocyte expression that is associated with elevations of the antiinflammatory cytokine interleukin (IL)-10. In summary, we demonstrate that inorganic nitrate suppresses acute and chronic inflammation by targeting neutrophil recruitment and that this effect, at least in part, results in consequent reductions in the inflammatory status of atheromatous plaque, and suggest that this effect may have clinical utility in the prophylaxis of inflammatory atherosclerotic disease.
One of the early hallmarks of atherosclerosis is endothelial dysfunction, which is also synonymous with a lack of bioavailable endothelial nitric oxide synthase (eNOS)-derived nitric oxide (NO). Accordingly, atherosclerosis is associated with reduced levels of endogenous NO (1, 2). Tonic NO generation by the endothelium plays a pivotal role in sustaining cardiovascular health by promoting vasodilatation, exerting antithrombotic and, importantly, antiinflammatory effects. A reduction in bioavailable endothelium-derived NO has been implicated in several phenomena associated with atherogenesis, including the low-grade systemic inflammation evident in hypercholesterolemia and the enhanced inflammatory cell recruitment that triggers and perpetuates atheroma formation (3). Thus, restoration of bioavailable NO levels in atherosclerosis may offer a therapeutic approach for improvements in inflammation and therefore, potentially, disease prevention.
Attempts to restore NO levels using NO donors, particularly the organic nitrates, l-arginine (the substrate for conventional NO synthesis) or tetrahydrobiopterin (an essential cofactor for conventional NO synthesis), have yielded many positive outcomes in both preclinical and clinical assessments. All three have been associated with beneficial effects, including improvements in vascular function, systemic inflammatory profile, local inflammation at lesional sites, and an improved platelet profile (4–6). However, several clinical investigations, particularly long-term studies, have failed to translate these observations into clinical benefit (e.g., refs. 7–9). The reasons for these discordant findings are variously attributed to an uncoupling of NOS for l-arginine (10), tolerance and induction per se of vascular dysfunction with the organic nitrates (11), and oxidation of tetrahydrobiopterin (9). These issues suggest that the failure of translation may lie in the failure of effective delivery of NO rather than a lack of efficacy of NO. Thus, identification of alternative strategies to improve bioavailable NO levels are warranted.
A potential “alternative” route for NO delivery has been proposed in the form of inorganic nitrate. Orally ingested or endogenously produced nitrate is extracted from the circulation by the salivary glands and then secreted in the saliva into the oral cavity, where it is reduced to nitrite (NO2−) by bacteria-expressing nitrate reductase activity (12). This nitrite-rich saliva is then swallowed and enters the blood, where it can be further reduced to NO by vascular nitrite reductases, including xanthine oxidoreductase (XOR) (13, 14). Recent evidence suggests that dietary nitrate pretreatment or acute nitrite administration, by delivering NO to the vasculature, reduces leukocyte activation in response to an acute chemokine-induced inflammation (15) or following prolonged cholesterol treatment (16). Because recruitment and activation of leukocytes is a pivotal process in both plaque initiation and progression, we investigated whether dietary nitrate might reduce atherosclerosis through targeting of inflammatory mechanisms in athero-prone Apolipoprotein (Apo)E knockout (KO) mice.
Results
Dietary Nitrate Causes Dose-Dependent Elevation of Nitrite and Nitrate Levels Across all Compartments.
To confirm previous reports that atherosclerosis per se results in deficiencies in bioavailable NO, we measured the levels of nitrite and nitrate across blood and tissue compartments in 12-wk-old WT and ApoE KO littermate mice. Interestingly, at baseline lower levels of nitrate, but not nitrite, were evident in plasma and tissue of ApoE KO compared with WT mice (Table 1).
Table 1.
Comparison of baseline levels of nitrite and nitrate in blood and tissue homogenates
| Tissue | Nitrite | Nitrate | ||||
| WT | ApoE KO | P value | WT | ApoE KO | P value | |
| Plasma (µmol/L) | 0.6 ± 0.1 (11) | 0.5 ± 0.1 (12) | 0.18 | 37.7 ± 2.3 (11) | 20.0 ± 2.8 (12) | <0.0001 |
| RBC (nmoles/g) | 0.4 ± 0.1 (5) | 0.3 ± 0.1 (5) | 0.26 | 13.1 ± 1.4 (5) | 6.3 ± 1.3 (5) | <0.01 |
| Aorta (nmoles/g) | 102.5 ± 7.3 (10) | 112.9 ± 10.1 (13) | 0.44 | 1014.4 ± 142.5 (10) | 503.7 ± 51.6 (13) | <0.01 |
| Kidney (nmoles/g) | 4.9 ± 0.7 (13) | 5.2 ± 0.7 (10) | 0.80 | 352.6 ± 36.9 (13) | 105.1 ± 13.8 (10) | <0.0001 |
| Lung (nmoles/g) | 12.6 ± 1.4 (11) | 17.4 ± 1.8 (11) | 0.05 | 349.1 ± 33.3 (11) | 148.4 ± 31.9 (11) | <0.001 |
| Heart (nmoles/g) | 4.7 ± 0.7 (11) | 3.3 ± 0.6 (11) | 0.12 | 204.9 ± 13.3 (11) | 126.9 ± 12.1 (11) | <0.001 |
| Liver (nmoles/g) | 5.0 ± 0.7 (13) | 4.4 ± 0.7 (12) | 0.50 | 130.7 ± 12.8 (13) | 80.3 ± 14.7 (12) | <0.05 |
| Mesentery (nmoles/g) | 29.2 ± 1.9 (11) | 56.0 ± 5.2 (8) | <0.0001 | 287.1 ± 37.2 (11) | 426.1 ± 61.11(8) | 0.056 |
Statistical comparison using unpaired t test between WT and ApoE KO littermate mice at 12 wk of age. Data are shown as mean ± SEM of (n) mice. Statistical significance determined using unpaired t test.
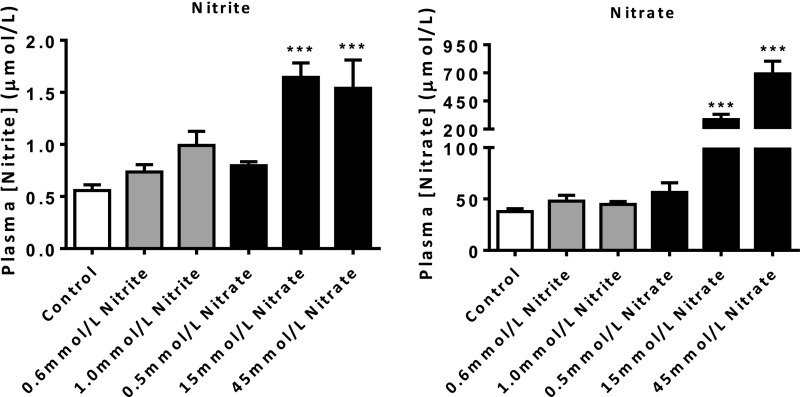
To establish a dose of KNO3 or KNO2 that reproducibly elevates levels of nitrite across most tissue compartments, we assessed the impact of various doses in blood and tissues of WT mice. Delivery of KNO3 in the drinking water for 2 wk provided consistent, dose-dependent, and statistically significant elevation of circulating nitrate and nitrite levels in WT mice, whereas KNO2 caused a rise in nitrite but not nitrate levels (Fig. S1). There were no differences in drinking water or food consumption between the groups (Table S1). Based upon these observations, 15 mmol/L KNO3 was used for all further dietary experiments.
Fig. S1.
Effect of dietary nitrite and nitrate on plasma NOx in WT mice. Dietary nitrate caused a dose-dependent elevation of circulating nitrate and nitrite levels, whereas dietary nitrite caused an elevation in nitrite but not nitrate levels. Data are shown as mean ± SEM of n = 8–12. Statistical significance was determined using a one-way ANOVA followed by Dunnett’s multiple posttest represented by ***P < 0.001 compared with control.
Table S1.
The amount of nitrite and nitrate consumed by WT mice during a 2-week treatment period with varying concentrations of KNO2 and KNO3 in the drinking water
| Treatment (n) | Amount nitrite consumed | Amount nitrate consumed | ||||
| Food consumed (g/d) | Water consumed (mL/d) | (mg⋅kg⋅d) | (mmol⋅kg⋅d) | (mg⋅kg⋅d) | (mmol⋅kg⋅d) | |
| Control (18) | 4.36 ± 0.50 | 6.41 ± 0.41 | <0.001 | <0.001 | <0.001 | <0.001 |
| 0.6 mmol/L KNO2 (12) | 3.85 ± 0.14 | 8.46 ± 0.80 | 7.00 ± 0.20 | 0.15 ± 0.01 | <0.001 | <0.001 |
| 1.0 mmol/L KNO2 (12) | 5.00 ± 0.54 | 8.85 ± 0.84 | 13.56 ± 0.43 | 0.29 ± 0.01 | <0.001 | <0.001 |
| 0.5 mmol/L KNO3 (12) | 4.18 ± 0.17 | 6.31 ± 0.59 | <0.001 | <0.001 | 7.59 ± 0.35 | 0.12 ± 0.01 |
| 15 mmol/L KNO3 (12) | 4.00 ± 0.24 | 7.11 ± 1.36 | <0.001 | <0.001 | 207.00 ± 11.25 | 3.34 ± 0.18 |
| 45 mmol/L KNO3 (12) | 5.12 ± 0.53 | 7.20 ± 0.55 | <0.001 | <0.001 | 698.90 ± 38.73 | 11.27 ± 0.62 |
Data are shown as mean ± SEM of (n) mice.
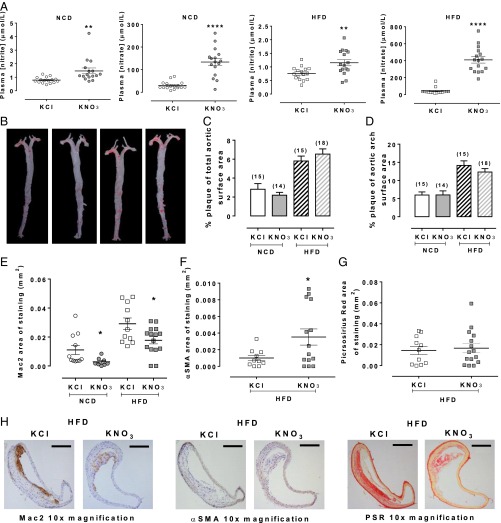
Treatment of mice with 15 mmol/L KNO3 for 2 wk caused 2- to 5-fold increases in nitrite and 4- to 14-fold increases in nitrate levels across tissues that were similar between the genotypes, with the exception of greater levels of both anions in the plasma and red blood cells (RBCs) of ApoE KO compared with WT mice (Table S2). Similarly, effects of this dose were evident in normal chow diet (NCD) and high-fat diet (HFD)-fed ApoE KO mice (Fig. 1A). In addition, KNO3 treatment also resulted in elevations of cGMP in WT (control 24.4 ± 2.5 nmol/L vs. nitrate 40.1 ± 12.3 nmol/L, n = 10, P = 0.23) and ApoE KO (control 18.1 ± 2.2 nmol/L vs. nitrate 26.9 ± 2.6 nmol/L, n = 10, P = 0.02). HFD feeding elevated cholesterol levels; however, there were no differences between the groups with respect to low-density lipoprotein (LDL), very LDL (VLDL), or high-density lipoprotein (HDL) levels (Table 2).
Table S2.
Comparison of nitrite and nitrate in blood and tissue homogenates following inorganic nitrate feeding
| Tissue | Nitrite | Nitrate | ||||
| WT (n) | ApoE KO (n) | P value | WT (n) | ApoE KO (n) | P value | |
| Plasma | 3.0 ± 0.2 (12) | 5.4 ± 0.6 (11) | <0.001 | 7.6 ± 1.2 (12) | 12.9 ± 1.7 (11) | <0.05 |
| RBC | 3.0 ± 0.2 (5) | 3.9 ± 0.2 (5) | <0.05 | 5.8 ± 1.2 (5) | 12.1 ± 1.4 (5) | <0.01 |
| Aorta | 3.0 ± 0.5(10) | 2.4 ± 0.25 (13) | 0.23 | 5.6 ± 1.5 (10) | 10.4 ± 2.5 (13) | 0.16 |
| Kidney | 2.7 ± 0.3 (10) | 3.3 ± 0.4 (10) | 0.29 | 3.4 ± 0.6 (10) | 14.2 ± 3.8 (10) | <0.05 |
| Lung | 3.3 ± 0.5 (11) | 2.4 ± 0.3 (11) | 0.13 | 4.3 ± 0.9 (11) | 8.6 ± 1.9 (11) | 0.06 |
| Heart | 3.4 ± 0.5 (10) | 5.6 ± 1.1 (11) | 0.09 | 4.5 ± 1.2 (10) | 4.1 ± 0.6 (11) | 0.73 |
| Liver | 2.9 ± 0.3 (13) | 2.3 ± 0.2 (12) | 0.09 | 3.6 ± 0.4 (13) | 5.0 ± 0.6 (12) | 0.06 |
| Mesentery | 3.3 ± 0.7 (11) | 2.7 ± 0.5 (8) | 0.49 | 6.9 ± 1.3 (11) | 7.3 ± 0.9 (8) | 0.82 |
Twelve-week-old WT and ApoE KO littermate mice were fed 15 mmol/L KNO3 in the drinking water for 2 wk. Data are shown as fold-increased expression to KCL control treated mice, mean ± SEM of (n) mice. Statistical comparisons were made between the relative fold-increases in the WT and ApoE KO littermate mice and determined using unpaired t test.
Fig. 1.
Dietary nitrate alters plaque composition in ApoE KO mice. Dietary nitrate elevates plasma nitrite and nitrate in NCD and HFD mice (A). A representative image of Oil red O staining in the aortic tree (B) and quantification showing total plaque area (C) and plaque area of the aortic arch (D), both of which were unchanged in response to dietary nitrate. Histological analyses demonstrated a reduction in macrophage accumulation (E), an increase in smooth muscle accumulation (F), and no change in collagen accumulation (G). Representative images of Mac-2, αSMA, and PSR (Picrosirius red) staining from HFD-fed mice (H). (Scale bars, 200 μm.) Data are shown as mean ± SEM of n = 16 for plasma NOx; n = 14–18 for plaque area; n = 9–15 for plaque composition. Statistical significance was determined using unpaired t test represented by *P < 0.05, **P < 0.01, ***P < 0.001, or ****P < 0.001 compared with control. (Mac-2, 1 data point in chow KNO3 and for αSMA, 1 data point in Western KNO3 excluded using Rout’s outlier test).
Table 2.
Dietary nitrate and lipid levels in NCD- or HFD-fed mice
| Diet | Treatment | HDL-C (mmol/L) (n) | LDL/VLDL-C (mmol/L) (n) |
| NCD | KCl | 1.1 ± 0.1 (10) | 10.5 ± 1.5 (10) |
| NCD | KNO3 | 0.9 ± 0.1 (9) | 8.4 ± 0.84 (9) |
| HFD | KCl | 2.5 ± 0.6 (12) | 20.3 ± 2.7 (12) |
| HFD | KNO3 | 1.6 ± 0.2 (11) | 27.6 ± 2.9 (11) |
ApoE KO mice were fed a NCD or HFD and either KNO3 or KCl (15 mmol/L) in the drinking water for 12 wk. Data are shown as mean ± SEM of (n) mice. Statistical significance was determined using unpaired t test comparing the KNO3-treated mice to KCl treatment in either NCD-fed mice or HFD-fed mice. There were no significant differences.
Inorganic Nitrate Alters Plaque Composition.
KNO3 treatment caused small but significant reductions in blood pressure in comparison with KCl controls, demonstrating efficacy of the dietary intervention (Table S3). In contrast, there was no difference in total plaque area between treatments in either NCD- or HFD-fed mice (Fig. 1 B and C) and no differences in percent plaque of aortic arch area (Fig. 1D). However, Mac2 staining within the areas of plaque was reduced in KNO3-fed mice compared with KCl controls: an effect evident in both NCD- and HFD-fed animals (Fig. 1 E and H). Although immunostaining exposed no evidence of α-smooth muscle actin (αSMA) in plaques of NCD-fed animals, with HFD feeding an increase in expression was evident in the KNO3-treated versus the KCl-treated mice (Fig. 1 F and H). There were no differences in collagen expression between any of the groups (Fig. 1 G and H).
Table S3.
Dietary nitrate reduces blood pressure in ApoE KO mice
| Diet | Treatment (n) | 24-h MAP (mmHg) | 24-h Mean SBP (mmHg) | 24-h Mean DBP (mmHg) | 24-h Mean pulse pressure (mmHg) | 24-h Mean heart rate (beats per minute) | 24-h Mean activity (a.u.) |
| NCD | KCl (6) | 106.4 ± 0.7 | 121.8 ± 0.7 | 90.3 ± 0.6 | 31.5 ± 0.3 | 561.1 ± 3.5 | 4.1 ± 0.2 |
| NCD | KNO3 (5) | 103.3 ± 0.7** | 120.2 ± 0.8 | 86.0 ± 0.6*** | 34.1 ± 0.3*** | 560.3 ± 3.2 | 4.3 ± 0.3 |
Dietary nitrate attenuates blood pressure in ApoE KO mice. Mean arterial (MAP), systolic (SBP), and diastolic (DBP) blood pressure, heart rate, and activity were recorded using radiotelemetry in ApoE KO mice (n = 5–6) fed a NCD with either KNO3 or KCl (15 mmol/L) in the drinking water for 12 wk. Data are shown as mean ± SEM of 15-min interval recordings over a full 24-h cycle. Statistical significance was determined using unpaired t test represented by **P < 0.01 or ***P < 0.001 compared with KCl treatment.
Nitrite Reduces Leukocyte Recruitment in ApoE KO Mice.
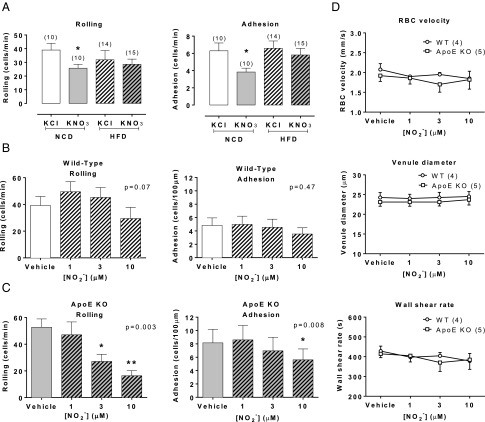
Because macrophage accumulation was reduced with nitrate-feeding, we used intravital microscopy to determine whether this might relate to a generalized suppression of systemic inflammation. Indeed, cremaster baseline cell rolling and adhesion were suppressed in KNO3 compared with KCl-fed mice on NCD (Fig. 2 A–C). In addition, nitrite superfusion caused concentration-dependent reductions in both leukocyte rolling and adhesion in ApoE KO (Fig. 2C) but not WT (Fig. 2B) mice. These effects were not a result of differences in blood flow because RBC velocity, wall shear rate, and venule diameter in response to nitrite were no different between the two genotypes (Fig. 2D).
Fig. 2.
Nitrite reduces leukocyte recruitment in ApoE KO mice. Dietary nitrate suppresses leukocyte rolling and adhesion in NCD-fed mice with no effect in HFD-fed mice (A). Nitrite had no effect on WT leukocyte rolling or adhesion (n = 7) (B), however caused a concentration-dependent reduction in ApoE KO leukocyte rolling and adhesion (n = 5) (C) with no effect on venule blood flow (n = 4/5) (D). All data are expressed as mean ± SEM of (n) mice. Statistical significance was determined using unpaired t test for comparison between KNO3 or KCl treated mice or one-way ANOVA followed by Dunnett’s posttest for comparison of nitrite-induced effects versus baseline and posttest significance represented by *P < 0.05 or **P < 0.01 compared with control.
Dietary Nitrate Suppresses Acute Inflammation in WT and ApoE KO Mice.
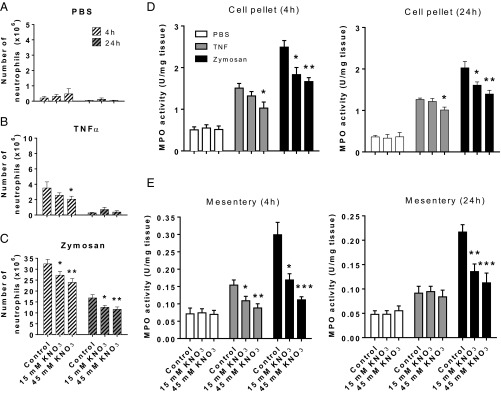
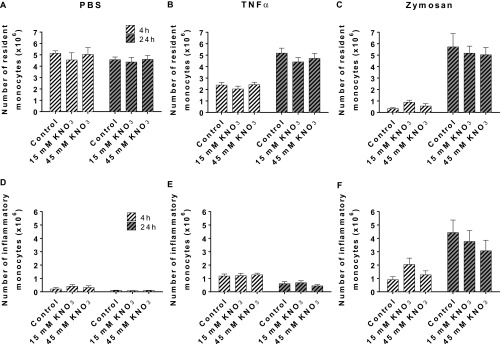
To explore the antiinflammatory activity of inorganic nitrate and to gain greater clarity upon the cell types targeted by this intervention, we assessed the impact of KNO3 treatment on tumor necrosis factor (TNF)-α and zymosan-induced peritonitis. We used this model as it allowed us to look specifically at the cell recruitment response but also to use TNF-α, a proinflammatory mediator implicated in atherogenesis. We assessed responses at 4 and 24 h following stimulus application because these time-points coincide with the early and later stages of the acute inflammatory response characterized by increased neutrophil and inflammatory monocyte presence, respectively. In WT mice both inflammogens caused increases in leukocyte number within the peritoneal wash that was inhibited by KNO3 in a dose-dependent manner, an effect particularly evident at the 4-h time-point (Table S4). Analysis of the leukocyte subsets within the lavage identified the cellular infiltrate at this time-point as predominantly neutrophilic (Fig. 3), the numbers of which were dose-dependently attenuated with inorganic nitrate (Fig. 3 and Fig. S2). In accordance with this observation, myeloperoxidase (MPO) activity levels were also reduced in both peritoneal cell pellet lysates and homogenized mesenteries (Fig. 3). Additionally, flow cytometric analysis demonstrated that the reduced neutrophil recruitment was associated with a concentration-dependent suppression of neutrophil CD11b but not CD162 or CD62L at both 4 and 24 h (Table S5).
Table S4.
Total peritoneal cell count at 4 and 24 h following intraperitoneal inflammogen administration in C57BL6 WT male mice
| Time point | Inflammogen | Treatment | Cell count (×106) | P value |
| 4 h | PBS | Control | 5.8 ± 0.29 (10) | 0.59 |
| 15 mmol/L KNO3 | 5.3 ± 0.54 (10) | |||
| 45 mmol/L KNO3 | 5.8 ± 0.44 (10) | |||
| TNF-α | Control | 7.1 ± 0.69 (10) | 0.04 | |
| 15 mmol/L KNO3 | 5.8 ± 0.34 (10) | |||
| 45 mmol/L KNO3 | 5.5 ± 0.23* (10) | |||
| Zymosan | Control | 33.7 ± 2.07 (17) | 0.02 | |
| 15 mmol/L KNO3 | 29.5 ± 1.77 (17) | |||
| 45 mmol/L KNO3 | 26.0 ± 1.83* (17) | |||
| 24 h | PBS | Control | 4.7 ± 0.23 (10) | 0.96 |
| 15 mmol/L KNO3 | 4.6 ± 0.46 (10) | |||
| 45 mmol/L KNO3 | 4.6 ± 0.44 (10) | |||
| TNF-α | Control | 6.3 ± 0.71 (8) | 0.8 | |
| 15 mmol/L KNO3 | 6.1 ± 0.51 (8) | |||
| 45 mmol/L KNO3 | 5.7 ± 0.67 (8) | |||
| Zymosan | Control | 22.9 ± 1.35 (16) | 0.07 | |
| 15 mmol/L KNO3 | 21.2 ± 1.30 (15) | |||
| 45 mmol/L KNO3 | 17.3 ± 2.39 (15) |
Data are shown as mean ± SEM of (n) mice. Statistical significance was determined using a one-way ANOVA followed by Dunnett's multiple posttest represented by *P < 0.05 compared with control.
Fig. 3.
Dietary nitrate reduces neutrophil recruitment in acute inflammation. Dietary nitrate did not alter baseline peritoneal cell numbers (n = 10) (A) but did reduce TNF-α (n = 10) (B) and zymosan (n = 17) (C) -induced neutrophil recruitment into the peritoneal cavity at 4 and 24 h. This effect was associated with reduced MPO activity in the (D) cell pellet (n = 6) and (E) mesentery (n = 6–10) at both time points. Data are shown as mean ± SEM and analyzed using one-way ANOVA followed by Dunnett’s multiple posttest represented by *P < 0.05, **P < 0.01, or ***P < 0.001 compared with control.
Fig. S2.
Effect of inorganic nitrate upon recruitment of specific leukocyte subsets in to the peritoneal cavity in WT mice. Resident (A–C) and inflammatory monocytes (D–F) in response to PBS (n = 10), TNF-α (n = 10), or zymosan (n = 17). Data are shown as mean ± SEM and analyzed using one-way ANOVA (no significant differences).
Table S5.
Inorganic nitrate specifically reduces CD11b expression on neutrophils
| Time point | Treatment | CD11b | P value | CD162 MFI | P value | CD62L MFI | P value |
| 4 h | Control | 4,845 ± 378 | 13,766 ± 1625 | 1,011 ± 151 | |||
| 15 mmol/L KNO3 | 4,491 ± 201 | 0.03 | 12,233 ± 1805 | 0.77 | 1,072 ± 182 | 0.96 | |
| 45 mmol/L KNO3 | 3,773 ± 198* | 14,121 ± 2306 | 1,058 ± 169 | ||||
| 24 h | Control | 1,641 ± 172 | 37,213 ± 3579 | 1,288 ± 205 | |||
| 15 mmol/L KNO3 | 1,255 ± 176 | 0.04 | 29,281 ± 2859 | 0.17 | 1,251 ± 187 | 0.91 | |
| 45 mmol/L KNO3 | 1,003 ± 153* | 27,200 ± 4674 | 1,170 ± 189 |
The effect of dietary KNO3 on CD11b, CD162, and CD62L cell surface expression, as indicated by median fluorescence intensity (MFI), on neutrophils at 4 and 24 h after intraperitoneal injection of zymosan (1 mg) in male C57BL6 WT mice. Data shown as mean ± SEM of n = 8 for each treatment and time-point. Statistical significance was determined using one-way ANOVA followed by Dunnett’s multiple posttest represented by *P < 0.05 compared with control.
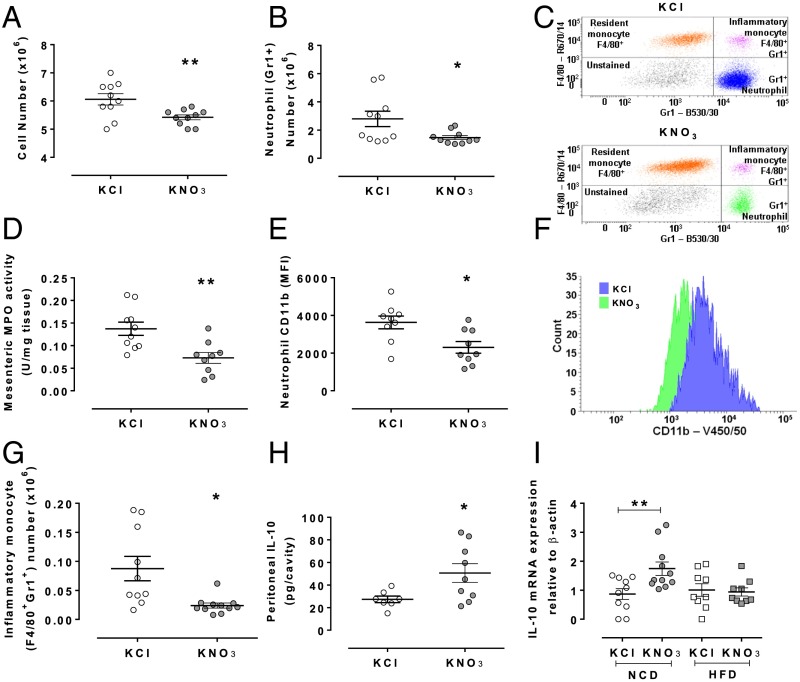
Based upon the above findings, we assessed the effects of KNO3 treatment on TNF-α–induced cell recruitment in ApoE KO mice. Similarly to WT mice, treatment with inorganic nitrate reduced peritoneal neutrophil recruitment, an effect associated with a reduction in MPO activity and CD11b expression (Fig. 4). Because within atherosclerotic plaques the key characteristic reflecting instability is the increased number of macrophages, in part a result of increased numbers of inflammatory monocytes, we assessed the possibility that the reduced neutrophil count in peritoneal lavages might also consequently lead to reductions in the number of inflammatory monocytes. Indeed, whereas at 4 h we saw no effect, at 24 h there was a near complete suppression of inflammatory monocyte numbers (Fig. 4G). A key endogenous mediator influencing inflammatory recruitment is the cytokine IL-10, released by the cellular infiltrate as a self-limiting pathway. Indeed, inorganic nitrate treatment was associated with elevated IL-10 in peritoneal lavage fluid (Fig. 4H).
Fig. 4.
Dietary nitrate suppresses TNF-α–induced neutrophil and inflammatory monocyte recruitment in ApoE KO mice. Dietary nitrate attenuated TNF-α–induced leukocyte recruitment (4 h) into the peritoneal cavity (A), and specifically a reduction in neutrophil numbers (B) with a representative scatter plot of neutrophil identification shown in C. This was associated with reduced MPO activity (D) and CD11b expression (E) with a representative histogram of neutrophil CD11b expression, blue and green representing control and nitrate-fed, respectively (F). Furthermore, dietary nitrate treatment caused a reduction of inflammatory monocyte recruitment into the peritoneal cavity at 24 h after TNF-α (G) and an elevation of IL-10 4 h after TNF-α in peritoneal lavage fluid (H). Finally, qRT-PCR for IL-10 mRNA of aortic arch from NCD or HFD ApoE KO mice demonstrates an increase of expression in NCD-fed mice (I). Data are shown as mean ± SEM of n = 8–10 mice. Statistical significance was determined using unpaired t test represented by *P < 0.05 or **P < 0.01 compared with control. (IL-10, 1 data point in KCl excluded using Routs outlier test).
Inorganic Nitrate Exerts a Modest Influence on Circulating Cell Numbers and Chemokine Levels in ApoE KO Mice.
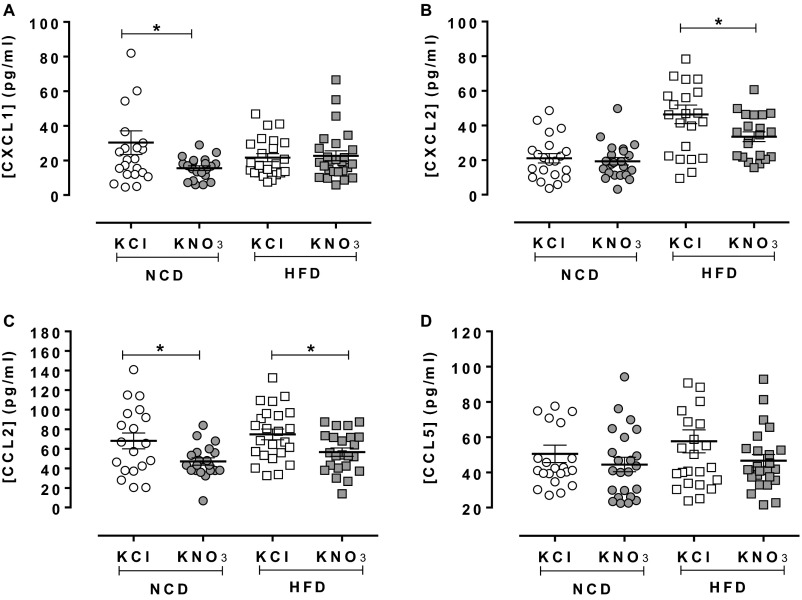
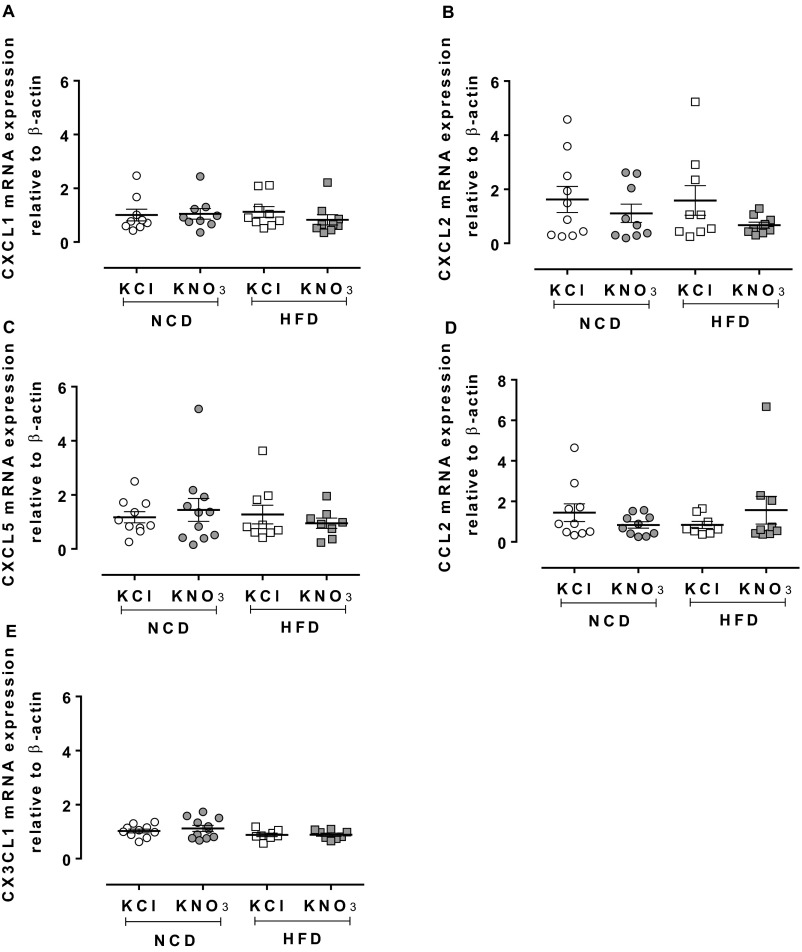
KNO3 treatment resulted in an overall decrease in circulating cell numbers in NCD-fed animals, with a trend for lower numbers in HFD-fed mice vs. KCl control (Table S6). In the NCD-fed animals this reduction in numbers appears to relate to a modest reduction in circulating neutrophils and a significant rise in resident monocytes. In addition, whereas CD11b expression of neutrophils was reduced with inorganic nitrate treatment, there were no differences in the expression of activation markers (CD11b, CD62L, and CD162) on any other cell type (Table S7). These differences were also associated with modest reductions in the circulating levels of the chemokines CXCL1 and CCL2, with a trend for reduction in CCL5 levels (Fig. S3). Interferon (IFN)-γ, IL-10, IL-1β, IL-4, and granulocyte macrophage colony-stimulating factor (GM-CSF) plasma levels were undetectable. However, assessment of tissue levels of the chemokines/cytokines within the plaque region (i.e., aortic arch segments) demonstrated no significant effects upon mRNA levels of CXCL1, CXCL2, or CCL2 (Fig. S4), but in contrast IL-10 mRNA levels were significantly enhanced in aortic arch by inorganic nitrate treatment in NCD-fed mice only (Fig. 4I).
Table S6.
Dietary nitrate (KNO3 15 mmol/L for 12 wk) exerts a modest effect on circulating leukocyte numbers in ApoE KO mice fed a NCD with no evidence of any effects with placebo KCl (15 mmol/L for 12 wk) or HFD for 12 wk
| Diet | Treatment (n) | Cell count (×106/mL) | Neutrophil | Resident monocyte | Inflammatory monocyte | CD4 T-cell | CD8 T cell | B cell |
| NCD | KCl (12) | 2.00 ± 0.24 | 12.8 ± 3.7 | 2.5 ± 0.4 | 2.3 ± 0.4 | 7.9 ± 0.8 | 3.7 ± 0.5 | 53.8 ± 5.1 |
| NCD | KNO3 (15) | 1.46 ± 0.13* | 10.3 ± 1.8 | 3.9 ± 0.5* | 2.4 ± 0.3 | 7.4 ± 0.8 | 2.9 ± 0.3 | 59.3 ± 3.5 |
| HFD | KCl (13) | 1.91 ± 0.31 | 9.4 ± 1.5 | 3.1 ± 0.4 | 2.7 ± 0.3 | 5.0 ± 0.4 | 2.0 ± 0.2 | 60.9 ± 2.7 |
| HFD | KNO3 (13) | 1.20 ± 0.20 | 14.1 ± 2.4 | 2.5 ± 0.4 | 2.3 ± 0.3 | 4.8 ± 0.6 | 2.2 ± 0.2 | 59.0 ± 3.4 |
Data shown as percentage of cells mean ± SEM of n = 12–15 for myeloid cells and n = 10–12 for lymphoid cells. Statistical significance was determined using unpaired t test represented by *P < 0.05 compared with KCl treatment in each diet.
Table S7.
The effect of dietary nitrate on leukocyte surface expression of CD11b, CD62L, and CD162
| CD11b, CD62L, and CD162 | Chow | Western | ||||
| KCL | KNO3 | P value | KCL | KNO3 | P value | |
| CD11b | ||||||
| Neutrophil; Gr1+ | 2,876 ± 357 | 2,076 ± 169* | 0.04 | 2,471 ± 137 | 2,973 ± 213 | 0.06 |
| Resident monocyte; F4/80+ | 7,821 ± 606 | 6,577 ± 423 | 0.10 | 6,038 ± 289 | 6,751 ± 534 | 0.24 |
| Inflammatory monocyte; F4/80+Gr1+ | 8,927 ± 872 | 7,735 ± 452 | 0.21 | 8,198 ± 357 | 9,658 ± 541* | 0.03 |
| T-cell CD4+ | 3,868 ± 1,672 | 3,476 ± 987 | 0.53 | 4,513 ± 1,430 | 3,963 ± 1,167 | 0.18 |
| T-cell CD8+ | 3,036 ± 1,611 | 2,980 ± 1,084 | 0.93 | 3,302 ± 1,210 | 3,339 ± 883 | 0.93 |
| B-cell CD19+ | 1,552 ± 651 | 1,558 ± 542 | 0.98 | 1285 ± 497 | 1,352 ± 393 | 0.72 |
| Percent neutrophil; Gr1+-expressing | 99.7 ± 0.1 | 99.6 ± 0.1 | 0.25 | 99.8 ± 0.1 | 99.9 ± 0.0 | 0.08 |
| Percent resident monocyte; F4/80+-expressing | 96.0 ± 0.8 | 95.2 ± 1.2 | 0.61 | 92.6 ± 1.7 | 91.2 ± 1.7 | 0.56 |
| Percent inflammatory monocyte; F4/80+Gr1+-expressing | 100 ± 0.0 | 99.9 ± 0.1 | 0.16 | 99.8 ± 0.1 | 99.7 ± 0.1 | 0.76 |
| Percent CD4+ cells-expressing | 12.6 ± 7.5 | 16.2 ± 3.1 | 0.31 | 16.7 ± 7.2 | 15.1 ± 4.6 | 0.53 |
| Percent CD8+ cells-expressing | 17.5 ± 9.2 | 25.6 ± 5.2* | 0.03 | 28.2 ± 10.4 | 26.6 ± 8.6 | 0.68 |
| Percent CD19+ cells-expressing | 4.4 ± 4.1 | 3.3 ± 1.5 | 0.4 | 3.9 ± 1.4 | 6.3 ± 7.2 | 0.28 |
| CD62L | ||||||
| Neutrophil; Gr1+ | 5,060 ± 351 | 5,155 ± 249 | 0.82 | 4,516 ± 267 | 4,581 ± 302 | 0.87 |
| Resident monocyte; F4/80+ | 2,315 ± 265 | 2,528 ± 295.7 | 0.60 | 2,459 ± 203 | 3,002 ± 501 | 0.32 |
| Inflammatory monocyte; F4/80+Gr1+ | 3,045 ± 244 | 3,224 ± 197 | 0.57 | 4,093 ± 501 | 4,120 ± 374 | 0.97 |
| T-cell CD4+ | 2,562 ± 416 | 2,655 ± 336 | 0.59 | 3,070 ± 194 | 3,138 ± 587 | 0.7 |
| T-cell CD8+ | 3,501 ± 607 | 3,660 ± 620 | 0.57 | 3,947 ± 443 | 3,947 ± 720 | 1 |
| B-cell CD19+ | 2,003 ± 380 | 1,945 ± 259 | 0.69 | 2,484 ± 497 | 2,434 ± 479 | 0.8 |
| Percent neutrophil; Gr1+-expressing | 99.6 ± 0.1 | 99.7 ± 0.1 | 0.54 | 99.4 ± 0.1 | 99.7 ± 0.1* | 0.02 |
| Percent resident monocyte; F4/80+-expressing | 19.0 ± 2.9 | 18.9 ± 7.8 | 0.97 | 31.8 ± 4.1 | 37.2 ± 4.7 | 0.40 |
| Percent inflammatory monocyte; F4/80+Gr1+-expressing | 89.2 ± 1.6 | 89.8 ± 1.7 | 0.8 | 91.7 ± 1.2 | 94.3 ± 1.1 | 0.12 |
| Percent CD4+ cells-expressing | 78.2 ± 7.6 | 76.5 ± 12.1 | 0.7 | 72.2 ± 6.6 | 74.5 ± 8.3 | 0.47 |
| Percent CD8+ cells-expressing | 92.4 ± 4.3 | 87.6 ± 8.4 | 0.13 | 88.6 ± 4.4 | 88.2 ± 5.8 | 0.83 |
| Percent CD19+ cells-expressing | 92.0 ± 4.4 | 92.2 ± 2.8 | 0.92 | 92.7 ± 3.5 | 94.2 ± 1.0 | 0.17 |
| CD162 | ||||||
| Neutrophil; Gr1+ | 22,631 ± 903 | 23,345 ± 1099 | 0.64 | 21,027 ± 712 | 20,414 ± 1,252 | 0.67 |
| Resident monocyte; F4/80+ | 14,147 ± 1,086 | 12,869 ± 379 | 0.23 | 14,867 ± 1,476 | 11,743 ± 557 | 0.07 |
| Inflammatory monocyte; F4/80+Gr1+ | 29,424 ± 1,932 | 30,559 ± 1,706 | 0.67 | 34,023 ± 1,265 | 32,211 ± 1,355 | 0.34 |
| T-cell CD4+ | 8,496 ± 642 | 8,708 ± 1,249 | 0.67 | 8,734 ± 1,047 | 8,425 ± 1,245 | 0.53 |
| T-cell CD8+ | 13,577 ± 2,013 | 15,926 ± 3,439 | 0.2 | 16,597 ± 4,052 | 16,318 ± 3,355 | 0.59 |
| B-cell CD19+ | 457 ± 146 | 494 ± 240 | 0.71 | 557 ± 241 | 685 ± 626 | 0.52 |
| Percent neutrophil; Gr1+-expressing | 100 ± 0 | 100 ± 0 | 0.34 | 100 ± 0 | 99.9 ± 0.1 | 0.27 |
| Percent resident monocyte; F4/80+-expressing | 97.3 ± 2.3 | 92.9 ± 5.8 | 0.54 | 90.9 ± 5.3 | 81.9 ± 7.2 | 0.32 |
| Percent inflammatory monocyte; F4/80+Gr1+-expressing | 99.6 ± 0.3 | 99.8 ± 0.1 | 0.5 | 99.9 ± 0.1 | 99.9 ± 0.1 | 0.86 |
| Percent CD4+ cells-expressing | 100 ± 0 | 100 ± 0 | 1 | 99.9 ± 0.1 | 100 ± 0.1 | 0.32 |
| PercentCD8+ cells-expressing | 100 ± 0 | 100 ± 0 | 1 | 100 ± 0 | 100 ± 0 | 1 |
| Percent CD19+ cells-expressing | 83.8 ± 4.1 | 82.9 ± 6.0 | 0.75 | 76.1 ± 15.8 | 79.9 ± 11.6 | 0.51 |
The effect of dietary nitrate on circulating neutrophils, resident monocytes, inflammatory monocytes, and T and B cells of CD11b, CD162, and CD62L expression, as measured by MFI, and the percentage of cells expressing these markers in ApoE KO mice. Data shown as mean ± SEM of n = 12–15 for myeloid cells and n = 10–12 for lymphoid cells. Statistical significance was determined using unpaired t test represented by *P < 0.05 compared with KCl treatment in each diet.
Fig. S3.
Inorganic nitrate causes modest attenuation of plasma chemokine levels. Inorganic nitrate reduced plasma CXCL1 in NCD-fed mice with no effect in mice fed HFD (A). Plasma CXCL2 was decreased in NCD- and HFD-fed mice, but significantly only in the latter (B). CCL2 was reduced in both diets (C). There was a trend toward reduction of CCL5 expression in both diets (D). Data are shown as mean ± SEM of n = 19–26. Statistical significance was determined using unpaired t test represented by *P < 0.05 compared with KCl in each treatment group. The unequal groups relate to technical failures during the assay.
Fig. S4.
Inorganic nitrate has no effect on mRNA expression of various chemokines. qRT-PCR of aortic arch from NCD or HFD ApoE KO mice showed no change in mRNA levels of CXCL1 (A), CXCL2 (B), CXCL5 (C), CCL2 (D), CX3CL1 (E) by inorganic nitrate. Data are shown as mean ± SEM of n = 8–11. Statistical significance was determined using unpaired t test; no significant differences were found.
Nitrite Reductase Activity Is Enhanced in ApoE KO Mice.
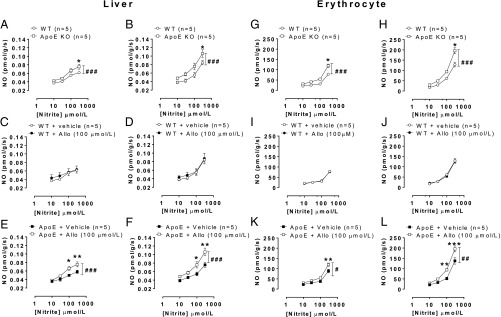
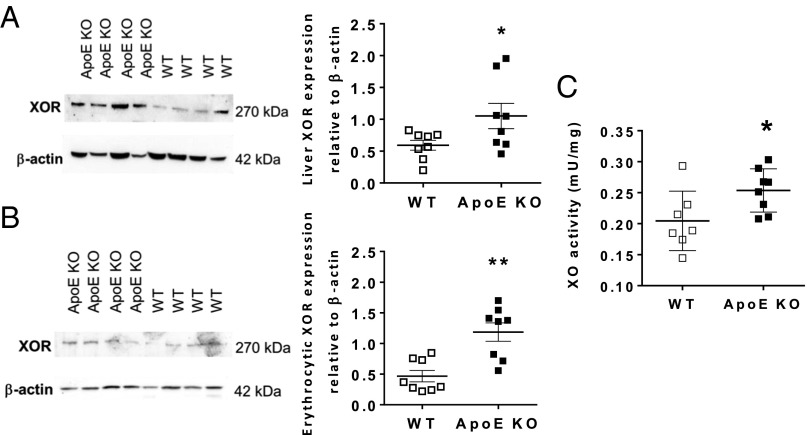
To determine the pathways for nitrite bioactivation in ApoE KO mice, we measured the nitrite reductase activity in the liver (17) and RBC (18, 19). These tissues were chosen because both sites represent significant sites for in vivo nitrite reductase activity but also as a surrogate for regions of plaque. Unfortunately, the sample size of plaque in mice provides insufficient amounts of tissue to measure this directly within the plaque region. Nitrite reduction was enhanced in tissues of ApoE KO vs. WT littermate mice in both tissues. Furthermore, treatment with the XOR inhibitor allopurinol attenuated this activity in homogenates of ApoE KO but not WT mice (Fig. 5). Western blotting of both tissue types demonstrated an approximate doubling of XOR expression in ApoE KO mice compared with WT littermate (Fig. 6 A and B). This increased expression was also associated with an increase in conventional XOR activity (i.e., purine catabolism) (Fig. 6C).
Fig. 5.
Liver and erythrocyte XOR nitrite reductase activity is enhanced in ApoE KO mice. Liver from ApoE KO mice exhibited greater nitrite reductase activity vs. WT at pH 7.4 (A) and pH 6.8 (B). The same pattern was observed in erythrocytes (G and H). Allopurinol had no effect on nitrite reductase activity from liver (C and D) or erythrocytes (I and J) from WT mice, in comparison ApoE KO mice exhibited reduced nitrite reductase activity in liver (E and F) and erythrocytes (K and L). Data are shown as mean ± SEM of n = 5. Statistical significance was determined using two-way ANOVA represented by #P < 0.05, ##P < 0.01, or ###P < 0.001, followed by Sidak’s multiple posttest represented by *P < 0.05, **P < 0.01, or ***P < 0.001, significantly different from WT or control.
Fig. 6.
Liver and erythrocyte XOR protein expression and activity is enhanced in ApoE KO mice. XOR expression in liver (A) and erythrocytes (B) and liver conventional XOR activity (C). Data are shown as mean ± SEM of n = 8. Statistical significance was determined using unpaired t test represented by *P < 0.05 or **P < 0.01 compared with WT.
Discussion
The enterosalivary circuit of inorganic nitrate is emerging as a “noncanonical” pathway of NO delivery in vivo that can be harnessed to exert beneficial cardiovascular effects, including blood pressure lowering, antiplatelet activity, and improvements in mitochondrial function. Herein, we demonstrate that delivery of inorganic nitrate in the diet also attenuates inflammation in atherosclerosis, resulting in changes within atherosclerotic plaque that support a stable plaque phenotype. We speculate that such an effect of inorganic nitrate might be useful in the therapeutics of atherosclerotic disease.
Endothelial dysfunction is a phenomenon occurring early in disease progression (20), particularly in atherosclerosis, with its presence reflected by reduced vascular flow responses (21, 22) and lower NOx levels in plasma (23, 24), although the evidence for the latter is scant. Our assessment of the distribution of both anions demonstrated a generalized reduction in the levels of nitrate but not nitrite in ApoE KO compared with WT mice across all of the compartments assessed, an observation also reported to occur in patients with coronary artery disease (23). The underlying mechanism of this effect is thought to be because of the endothelial dysfunction exhibited by these mice as a result of reduced eNOS activity (25). In contrast to our work, some studies suggest that reduced levels of plasma nitrite and not nitrate more accurately reflect disease severity and vascular flow responses (24, 26). However, it is accepted that the dominant pathway for NO metabolism occurs through its oxidation by hemoglobin directly to nitrate (27) rather than nitrite, and our data fit with this view. The difference between our observations and the above published reports may also reflect the difference between acute stimulation of endothelium-derived NO (as measured in the two studies assessing flow responses) and tonic NO generation (reflected from baseline NOx measures, as in our study). Nevertheless, treatment with KNO3 resulted in elevations of both nitrate and nitrite across all compartments, with no evidence of any particular organ selectivity, indicating that at least in ApoE KO mice the pathways for uptake of the two anions is universally present and that dietary nitrate effectively restores, and can elevate beyond, normal physiological levels. In support of the suggestion that dietary nitrate ultimately results in NO delivery in vivo are our observations demonstrating that nitrate feeding also results in elevations of cGMP, perhaps the most sensitive indicator of NO bioactivity (28).
Whereas a HFD regimen increased plaque burden compared with NCD in ApoE KO mice, dietary nitrate treatment for 12 wk with KNO3 (15 mmol/L)-enriched water did not alter the total plaque burden. This observation is in agreement with very recently published findings in LDL receptor KO mice, where a 14-wk NaNO3-treatment did not alter plaque size in HFD-fed mice (29). However, further interrogation of plaque composition in our study demonstrated that with KNO3 treatment there was a reduction in the macrophage load within the plaque region, whether the mice had consumed a diet high in fat or not. This change was associated with increased smooth muscle cell accumulation within the plaque; such a profile has been proposed to represent an indication of plaque stability (30), thus supporting the view that KNO3 facilitated a stable plaque presentation. This effect on smooth muscle may seem counterintuitive. It has been long known that NO inhibits smooth muscle proliferation with evidence of this effect both in vitro (31) and in vivo (32), and that nitrite also exerts inhibitory effects on proliferation in a vascular injury model (33). We propose that the accumulation of smooth muscle within the plaque is likely an indirect effect of reduced macrophage content. Indeed, it has been shown previously that smooth muscle cell proliferation is inhibited when in coculture with macrophages (34, 35), intimating that by lowering the macrophage content of the plaque this will result in a consequent removal of the inhibitory influence on smooth muscle cell proliferation. We acknowledge that our findings perhaps do not fit well with some previous data. Evidence has shown that genetic deficiency of eNOS, or inhibition of NOS activity using NOS inhibitors, in mice with a proatherogenic phenotype (i.e., ApoE KO or LDLr KO), substantially increase plaque size (36, 37). There is also a large body of evidence demonstrating improved endothelial function and levels of other surrogate markers in preclinical models and in patients with NO donors. In contrast however, there is little consensus regarding effects of NO donors upon plaque size, with several studies demonstrating no effect (36, 38). There is also some evidence suggesting that treatment with certain indirect NO donors, although not altering plaque size, do result in improvements in the inflammatory profile and stabilization of plaque, particularly in large-animal models (rabbit) (39). There is also some very recent data that might help further in understanding our observations. Studies using sGCα1 KO mice crossed with LDLr KO mice have shown surprisingly a decrease in plaque burden compared to LDLr KO alone. The authors suggest that PKG increases smooth muscle proliferation and that this underlies the apparent benefits of deletion of the gene by triggering a switch in smooth muscle cell phenotype (40). Interestingly the authors only consider that such an effect is detrimental; however, of course in the atherosclerotic scenario, a stimulus that is antiinflammatory but at the same time increases smooth muscle content of a plaque would be, in the long term, beneficial in terms of switching a plaque from an unstable to a stable phenotype. It is possible that such a phenomenon underlies the effects seen herein with dietary nitrate treatment.
It is noteworthy that in contrast to our findings, the study of Marsch et al. (29), using NaNO3, showed no differences in inflammatory markers in atherosclerotic lesions of athero-prone mice. An important difference between this study and ours is that in the Marsch study treatment with NaNO3 was not associated with elevations in circulating nitrite levels. It is likely that this absence of sufficient nitrite underlies the lack of bioactivity. It is also possible that the use of different nitrate salts may have resulted in differences in renal clearance of the anion. The mechanism by which nitrate is reabsorbed following glomerular filtration is unclear; however, it has been suggested that sodium and nitrate may be reabsorbed at the same site, intimating cotransport (41). Administration of NaNO3 in dogs resulted in reduced NOx reabsorption and greater NOx excretion (42). In these studies, no discrimination between nitrite and nitrate was made and further assessment of this would be of value. Such relationships between sodium and NOx anions have not to date been demonstrated for potassium, and thus it is possible that the difference in salt underlies the differences in circulating nitrite and nitrate levels evident between the study of Marsch et al. (29) and our study herein. Further investigations assessing this possibility would be of value.
Reduced macrophage numbers within plaque regions could be related to reduced monocyte recruitment to the vessel wall, a direct effect on the endothelial cell or the monocyte, or an indirect effect of reduced neutrophil accumulation. With respect to the latter, the recruitment of neutrophils in atherosclerosis is an event known to occur in the early stages of atherogenesis before monocyte recruitment (43, 44) and thought to play a role in instructing other key leukocytes, particularly monocytes, to accumulate at vascular sites with a predilection for atheroma formation (45). Our data suggest inorganic nitrate treatment results in a reduction in circulating leukocyte numbers driven by a reduction in neutrophil numbers. Assessment of activation state of these cells (CD11b, CD62L, and CD162) confirmed a neutrophil-specific effect of inorganic nitrate with neutrophil CD11b expression significantly attenuated, particularly in the NCD-fed mice, with no changes in expression of any of the other measured activation markers in neutrophils or any other cell type.
Interestingly, in accordance with a selective effect of nitrate on neutrophils, we also observed a generalized attenuation of neutrophil-targeting chemokines, CXCL1 and -2 and a trend for reduced CCL5. These chemokines have been implicated in neutrophil recruitment to athero-prone regions; in particular, evidence suggests that CXCL1 is a key chemokine involved in the mobilization of neutrophils from the bone marrow and platelet-derived CCL5 plays a critical role in neutrophil recruitment at athero-prone sites (46). The lack of any differences in any of the chemokines specifically within the affected plaque region supports the argument that the effects of inorganic nitrate upon neutrophil chemokines likely relate to an action upon the circulating cells. Interestingly, we did observe a near-doubling of the levels of IL-10 mRNA within the aortic arch. IL-10 is thought to play a prominent role in resolving an inflammatory response (47, 48), and importantly has been implicated in driving endogenous pathways limiting atherosclerosis (49). Interestingly, the effects of nitrate upon IL-10 were evident only in the NCD-fed animals and not the HFD-fed mice. The feeding of mice with HFD is an approach taken to accelerate the atherosclerotic process and this is aptly demonstrated in this study where plaque size with HFD is double that in NCD-fed animals and is associated with significant remodeling reflected by SMA. We speculate that the effect of dietary nitrate upon IL-10 in NCD-fed animals reflects a change triggered in the early stages of inflammation within the plaque and that in the HFD-fed mice additional experiments at earlier time-points are likely to expose similar changes.
To investigate whether the effects of inorganic nitrate relate to repression of leukocyte recruitment, we assessed baseline cell rolling and adherence in the cremaster muscle of mice. Although the cremaster circulation is not one prone to atheroma development, it does provide a window on the levels of systemic inflammation, as well as offers an opportunity to assess potential antiinflammatory agents. Using intravital microscopy, we have shown that nitrite caused concentration-dependent repression of both leukocyte rolling and adherence in ApoE KO mice that was not a result of changes in local blood flow, an effect not observed in WT littermates. This finding suggests that compared with controls, the bioactivity of nitrite is up-regulated in the setting of atherosclerosis, and fits with evidence demonstrating elevated expression and activity of vascular nitrite reductases in cardiovascular disease scenarios (12).
XOR as a nitrite reductase is of particular interest because its activity and expression is elevated in models of atherosclerosis (50) and in human disease within plaques (51), as well as the peripheral endothelium (52). The main source of XOR is the liver from where it is shed into the circulation during periods of stress (53) and then binds with high affinity to endothelial cells via glycosaminoglycans (54). More recently, we have demonstrated that XOR also binds to the erythrocyte (14), which is noteworthy because there is a substantial body of evidence implicating the erythrocyte as a key site for the chemical reduction of nitrite. It is likely that both the erythrocyte and the plaque itself are sites for nitrite reduction in atherosclerosis. Indeed, the hypoxic environment within the plaque (55) represents an ideal environment for nitrite reduction and is particularly an environment that potentiates XOR-dependent nitrite reduction. We show elevated XOR expression and activity in both the liver and erythrocyte of ApoE KO mice in comparison with WT littermates. In addition, we also show in both preparations that this elevated XOR expression/activity is associated with elevated nitrite reductase potential that is attenuated by XOR inhibition. These results suggest that in atherosclerosis nitrite, and hence nitrate, bioactivity is enhanced because of up-regulated XOR-dependent nitrite reductase activity. This profile of little or no contribution for XOR in health, but a more prominent role for the enzyme in nitrite reduction in disease, is very similar to the scenario demonstrated in hypertension (13). It is likely in the healthy environment, nitrite reduction is driven by other possible nitrite reductases, particularly deoxyhemoglobin (18).
Following chronic treatment with KNO3, a reduced leukocyte rolling and adherence was evident in ApoE KO mice, a finding in agreement with previous observations demonstrating reductions in leukocyte rolling in response to dietary nitrite in WT mice fed a HFD (16). Interestingly, however, the beneficial effects of inorganic nitrate were only evident in mice fed a NCD and not in HFD-fed animals. These results suggest perhaps that the mechanisms governing elevated cell activation in ApoE KO mice fed diets rich in cholesterol differ from those in mice fed a NCD, but may also relate to the relative differences in the stage of disease progression between NCD- and HFD-fed mice, as also reflected by the IL-10 measurements. It is also possible that inorganic nitrate at the dose we tested is simply insufficient to overcome the proinflammatory effects of the HFD. We suggest that perhaps the most clinically relevant data stems from the mice on a NCD because these mice had cholesterol and LDL levels commensurate with human hypercholesterolemia, whereas the HFD caused levels of LDL that far exceed those seen in human disease.
To interrogate more closely the possibility that inorganic nitrate specifically targets neutrophils, we explored the effects of nitrate treatment in peritoneal inflammation induced by two distinct inflammogens: TNF-α and zymosan. Zymosan activates the complement cascade to promote predominantly neutrophil accumulation by 4 h followed by monocyte influx that is still on-going at 24 h (56). TNF-α, on the other hand, causes a modest accumulation of neutrophils, evident at 4 h, with a very small increase in monocytes that is resolved at 24 h. Dietary nitrate pretreatment caused a dose-dependent reduction in neutrophil accumulation in response to either stimulus and concurs with previous findings demonstrating dietary nitrate induced reductions in CXCL2-induced leukocyte rolling and emigration (15). The reductions, we show, in MPO activity confirm that neutrophils were targeted by nitrate, as also does the demonstration of selective reductions in neutrophil CD11b expression.
The effects of nitrate are because of its chemical reduction to NO, and the above profile of activity against inflammation fits well with current understanding of NO bioactivity. NO itself has been shown, in models of atherosclerosis, to reduce leukocyte activity and adhesion (57) and repress expression of several key adhesion molecules, including vascular cell adhesion molecule-1, intercellular cell adhesion molecule-1 (58), E-selectin (59), P-selectin (60), and CD18 (61). In addition, there is evidence that NO influences neutrophilic CD11b (62) and, interestingly, the binding of CD11b with MPO plays an important role in activating signaling pathways in this cell type. It is possible that at least some of the reduction in CD11b expression with inorganic nitrate treatment relates to direct NO-induced repression of this MPO activity. Further analysis of this particular pathway is warranted.
Finally, we suggest that targeting of the neutrophil by inorganic nitrate plays a critical role in the consequent reductions in the inflammatory load within the plaque region, reflected by the decrease in macrophage number. In the early stages of atherosclerosis the neutrophil plays a key role, through the release of specific chemoattractant mediators, in the consequent recruitment of inflammatory monocytes to the athero-prone site (44, 63). Using an acute model of inflammation, we have shown that treatment of ApoE KO mice with inorganic nitrate reduces first neutrophil recruitment, followed by consequent reductions in the number of inflammatory monocytes. This effect is associated with an elevation of IL-10 levels. We also show that the reduced inflammatory load in atherosclerotic lesions of inorganic nitrate-fed ApoE KO mice is associated with elevated IL-10 mRNA levels. We suggest that the reduced inflammatory burden in the atherosclerotic plaques of inorganic nitrate-fed ApoE KO mice is caused, at least in part, by a nitrate-driven reduction in neutrophil-dependent recruitment of monocytes into the atherosclerotic plaque and an acceleration of the resolution of inflammation, driven by elevations in IL-10 expression and consequent activity (47, 48). Further investigations using mice doubly deficient in both ApoE and IL-10 (64) may offer an opportunity to interrogate more closely this relationship. Although our evidence supports an important role for the proposed pathway, we recognize that it is likely that other targets for NO also play a role, such as the repression of thrombospondin-1 (65).
In summary, we have demonstrated an antiinflammatory role for dietary inorganic nitrate in acute inflammation and in the chronic inflammation associated with atherosclerosis. We have shown that this effect of inorganic nitrate relates to XOR-dependent reduction of nitrite to NO that targets the neutrophil through, at least in part, a repression of both MPO and neutrophil CD11b activity and expression, together with an up-regulation of antiinflammatory IL-10. Taking these data together, we suggest that dietary nitrate as a method for elevating NO could have potential clinical utility in stabilizing the atheromatous plaque, and in this way might prove useful in the therapeutics of atherosclerotic disease.
Methods
Animal Studies.
All experiments were conducted according to the Animals (Scientific Procedures) Act 1986, United Kingdom, approved by the United Kingdom Home Office and reported according to the ARRIVE (Animals in Research: Reporting in Vivo Experiments) guidelines (66, 67). Male ApoE KO mice (on a C57BL6 background) and WT littermate mice at 6, 12, and 18 wk of age were bred in-house. In some experiments C57BL6 male mice (10–12 wk of age) were purchased from Charles River. Animals were randomly allocated to groups. Mice used at 6 or 12 wk of age were fed a NCD and tap water or water supplemented with KNO3 (0.5, 15, or 45 mmol/L) or KNO2 (0.5, 1 mmol/L) for 2 wk. Animals used at 18 wk of age were either fed a NCD or a “high fat” Western style diet (HFD) containing 21.4% crude fat (Western RD 829100, Special Diet Services) and received KCl (15 mmol/L) or KNO3 (15 mmol/L) in their drinking water from 6 wk of age. Up to six mice were housed according to their treatments in any single cage. For telemetry, mice were housed singly following treatment allocation.
Plaque Characterization.
Animals were perfusion-fixed and the entire aorta excised and cleaned of fat. The aorta was stained with Oil red O and imaged en face. Brachiocephalic arteries were paraffin wax-embedded, sectioned and stained with H&E, anti-αSMA, anti-Mac2 (for macrophage identification), and Picrosirius red. For full details, SI Methods.
Intravital Microscopy of the Cremaster Muscle.
ApoE KO or WT mice (6-, 12-, or 18-wk-old) were anesthetized using xylazine 7.5 mg/kg and ketamine 150 mg/kg, i.p., the cremaster muscle exposed and superfused with bicarbonate-buffered solution gassed with 5% (vol/vol) CO2 and 95% (vol/vol) N2 at 37 °C. Leukocyte rolling and adhesion were counted. In studies using 6- and 12-wk-old mice, KNO2 (1, 3, and 10 µmol/L) was superfused onto the preparation and leukocyte rolling and adhesion determined. For full details SI Methods.
Radiotelemtric Recording of Hemodynamics.
Radiotelemetric transmitters (TA11PA-C10, Data Sciences International) were implanted into 16-wk-old mice to record blood pressure. At 7–10 d following surgery, blood pressure was recorded for 24 h. During this period the mice were left undisturbed and maintained on a 12-h light/dark cycle. For full details, SI Methods.
Measurement of Nitrate, Nitrite, and cGMP Levels.
Plasma and tissue nitrite and nitrate levels (collectively termed NOx) were measured by ozone-based chemiluminescence (68) and cGMP using a commercially available assay. For full details, SI Methods.
Measurement of Liver and Erythrocytic Nitrite Reductase Activity.
The nitrite reductase activity of tissue supernatants was determined using gas-phase chemiluminesence (69) at pH 7.4 (representing physiological conditions) or pH 6.8 (severe acidosis but also conditions that favor nitrite reductase pathways). Involvement of XOR was ascertained by pretreatment with allopurinol (100 μmol/L) or vehicle for 30 min.
Peritoneal Inflammation.
WT or ApoE KO mice were injected intraperitoneally with TNF-α) (300 ng), zymosan (1 mg), or sterile PBS. After 4 or 24 h, the peritoneal cavity was washed with PBS and lavage fluid collected and a cell count performed using a hemocytometer. The lavage pellet was split and part used for flow cytometry and the remaining for a MPO assay (for full details, SI Methods). The mesentery from the same animals was collected, placed in liquid N2, and stored at −80 °C for subsequent MPO analysis. These stimuli were chosen because both complement and TNF-α have been implicated in the systemic and local inflammation that characterizes preclinical models (70, 71) of atherosclerosis and in human disease (45).
Flow Cytometry of Peritoneal Lavage Fluid and Blood.
Blood (50 μL) or peritoneal lavage fluid (5 × 105 cells) were incubated with various fluorochrome-conjugated antibodies following which erythrocytes were lysed using RBC lysis buffer (eBioscience). Viability solution (7-ADD) was added before acquisition on a LSR II Fortessa (BD Biosciences). All flow cytometric data were analyzed using FACS Diva software (BD Biosciences). For full details, SI Methods.
Western Blotting.
Equal amounts of protein homogenates of liver or erythrocytes were subjected to Western blotting to determine XOR expression levels. For full details, SI Methods.
Cytokine Assessment.
Following cardiac puncture, blood was centrifuged at 13,000 × g for 5 min at 4 °C and plasma collected and stored at −80 °C for later analysis. Plasma CXCL1, CCL2, CCL5, IFN-γ, IL-10, IL-1β, IL-4, and GM-CSF levels were determined using the BD Cytokine Bead Array according to manufacturer’s guidelines. Plasma (CXCL2) was determined using CXCL2 Quantikine ELISA (R&D systems). IL-10 was assessed in peritoneal lavage fluid using IL-10 DuoSet ELISA (R&D Systems).
Plasma Lipid Levels.
Plasma cholesterol was determined using a commercially available kit, according to the manufacturer’s guidelines (HDL and LDL/VLDL Cholesterol assay kit, Abcam).
Quantitative RT-PCR of Mouse Aorta.
Samples were homogenized and RNA extracted for quantitative RT-PCR (qRT-PCR) analysis with SYBR green, using specifically designed primers. For full details, SI Methods.
Liver XOR Activity.
Liver XO activity was determined using a commercially available kit, according to manufacturer guidelines (xanthine oxidase assay kit, Abcam).
Data and Statistical Analysis.
Power calculations for assessment of total aortic plaque indicated an n = 14 were required to expose statistical differences. Because of in-house breeding and a desire to allocate at least one animal to each intervention from any litter, there were variations in group size for this experiment of 14–18. For assessment of aortic mRNA levels, power calculations indicated an n = 10 necessary to expose statistical differences. In both cases an additional n value was added to account for technical difficulties. For 18-wk intravital microscopy, NCD n = 10 and HFD n = 15 animals were used. For assessment of the impact of nitrite on leukocyte recruitment, an n = 7 animals were allocated and n = 10 for all other inflammatory experiments in WT or ApoE KO mice, with the exception of n = 17 for experiments with zymosan. All data are shown as mean ± SEM. For statistical comparisons, either unpaired Students t test or one-way or two-way ANOVA followed by Dunnett’s or Sidaks posttest used for multiple comparisons as appropriate. Where ANOVA was applied, posttests have only been conducted where F achieved P < 0.05 and there was no significant variance in homogeneity. Any P value less than 0.05 was taken to infer statistical significance. Variation of n values relate to technical failure or from identification of outliers using the ROUT (72) exclusion test. Where any exclusions have occurred, this has been stated explicitly in the figure legends. All analysis was conducted using Graphpad Prism 6.0.
SI Methods
Plaque Characterization.
Animals were perfused with saline via the left ventricle, followed by 10% formal saline at 100 mmHg (physiological pressure) for 5 min. The entire aorta was excised and the surrounding fat removed. The brachiocephalic artery was isolated and stored in sterile saline containing 0.1% sodium azide at 4 °C for subsequent analysis. The aorta was opened longitudinally from the aortic root to the iliac bifurcation and atherosclerotic lesions were stained with 0.3% Oil red O [in 60% (vol/vol) propanol containing 0.6% dextrin] for 20 min. Images of en face staining were captured using a digital camera (Sony W570) and the total area covered by plaque quantified using ImageJ (NIH). Brachiocephalic arteries were paraffin wax-embedded, sectioned and stained with H&E, anti-αSMA (1:1,250; Abcam), anti-Mac2 (1:400; Cedarlane), and Picrosirius red.
Intravital Microscopy of the Cremaster Muscle.
Six- and 12-wk-old WT and ApoE KO mice and 18-wk-old mice were anesthetized with xylazine (7.5 mg/kg, s.c.) and ketamine (150 mg/kg, s.c.). The testicle was exteriorized, the cremaster cut longitudinally and pinned out and allowed to stabilize for 15 min before any recordings. The cremaster muscle was superfused with bicarbonate-buffered solution [132 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L MgSO, 17.9 mmol/L NaHCO3, and 2.0 mmol/L CaCl2 (pH 7.4), gassed with 5% (vol/vol) CO2 and 95% (vol/vol) N2] at 37 °C at a rate of 2 mL/min and animals maintained at 37 °C. Leukocyte rolling and adhesion was counted five times in three vessels in each animal and these values averaged to represent a single n value. A suitable vessel was identified as being 20–40 µm in diameter and 100 µm in length. Leukocyte rolling was counted as the number of leukocytes rolling past a fixed point in 1 min and adhesion was identified as a leukocyte remaining stationary for >30 s in a 1-min period. In studies using 6- and 12-wk-old mice, KNO2 (1, 3, and 10 µmol/L) was superfused onto the preparation and leukocyte rolling and adhesion counted in one vessel. Venular blood flow was calculated from the product of mean RBC velocity (Vmean = centerline velocity/1.6) and microvascular cross-sectional area, assuming a cylindrical geometry. Wall shear rate was calculated by the Newtonian definition: shear rate = 8,000 × (Vmean/diameter). In these latter experiments, because of the lengthened duration of the experiment only those animals where flow rate was maintained throughout the duration of the study were included.
Radiotelemtric Recording of Hemodynamics.
Blood pressure was recorded in conscious freely moving mice using radiotelemetric transmitters (TA11PA-C10, Data Sciences International) implanted into the aortic arch of 16-wk-old animals. Mice were anesthetized with 2% (vol/vol) isoflurane delivered with oxygen (0.4 L/min) and administered preoperative analgesia with buprenorphine 0.3 mg subcutaneously. After 10-d recovery, the blood pressure was recorded for 24 h. During this period the mice were left undisturbed and maintained on a 12-h light/dark cycle (7:00 AM to 7:00 PM/7:00 PM to 7:00 AM). Data were acquired for 2 min, every 15 min, and the average values for mean arterial blood pressure calculated for every time point (Dataquest Art Acquisition System).
Measurement of Plasma and Tissue Nitrate and Nitrite Levels.
Blood samples were collected from anesthetized animals by cardiac puncture into 1-mL syringes containing 0.08 mL 3.8% (wt/vol) sodium citrate and centrifuged at 4 °C, 13,000 × g for 5 min and the plasma collected. Tissue and RBC samples were collected, snap-frozen, and stored at −80 °C. Samples were homogenized in the presence of a protease inhibitor mixture containing 4-(2-Aminoethyl)benzenesulfonyl fluoride (1 mg/mL), antipain, aprotinin, benzamidine, leupeptin, and pepstatin A, all at a concentration of 10 μg/mL, using a Precellys homogenizer at 4 °C and the homogenate centrifuged at 10,000 × g, 5 min, 4 °C, and the supernatant collected. Plasma and tissue supernatant were filtered using Sartorius Vivaspin 500 3,000 molecular weight cut-off PES (Sartorius Stedim Biotech) at 4 °C, 14,000 × g for 60 min (plasma) or 90 min (tissue). Before use, filters were washed twice with low NOx containing 18 MΩ dH2O. For RBC determination, the compacted pellet was resuspended 1:4 in “nitrite preserving solution” containing potassium ferriccyanide (0.8 mol/L; Sigma) and N‐ethylmaleimide (0.1 mol/L; Sigma) and then deproteinated using ice‐cold methanol. Briefly, to determine total NOx concentration, samples were added to 0.1 mol/L vanadium (III) chloride in 1 mol/L hydrochloric acid refluxing at 95 °C under N2. Nitrite concentration was determined by addition of samples to 0.09 mol/L potassium iodide in glacial acetic acid under nitrogen at room temperature. Nitrate concentration was calculated by subtraction of the nitrite concentration from the total NOx.
Flow Cytometry of Peritoneal Lavage Fluid and Blood.
All antibodies were purchased from eBioscience unless otherwise stated. Lavage fluid was centrifuged at 800 × g for 5 min at 4 °C and resuspended in 0.2% BSA in PBS. Next, 5 × 105 cells were seeded in each flow tube and preincubated with 1:100 Fc receptor block (anti-mouse CD16/32) for 15 min. The cells were then stained with anti-mouse Ly-6G (Gr-1) FITC (1:500), anti-mouse F4/80 (1:16), anti-mouse CD11b eFlour450 (1:60), anti-mouse CD62L APC-efluor 780 (1:60), and anti-mouse CD162 PE (1:8; BD Biosciences), or the appropriate isotype control for 30 min at 4 °C in the dark. The 7-AAD viability solution was added before acquisition on a LSR II Fortessa (BD Biosciences).
Blood was collected by cardiac puncture into 5,000 U/mL heparin and stored on ice. Two flow cytometry panels were used, the first being anti-mouse Ly-6G (Gr-1) FITC (1:500), anti-mouse CD115 APC (1:33), anti-mouse CD11b eFlour450 (1:60), anti-mouse CD62L APC-efluor 780 (1:60), and anti-mouse CD162 PE (1:8; BD Biosciences). The second panel used anti-mouse CD3 PE-Cy7 (1:8), anti-mouse CD4 APC (1:33), anti-mouse CD8a FITC (1:20), anti-mouse CD19 Brilliant violet 605 (1:10; Biolegend), and anti-mouse CD11b, CD62L, and CD162 described above. Next, 50 μL blood was incubated with the above antibodies or the appropriate isotype controls for 30 min at 4 °C in the dark following which erythrocytes were lysed using RBC lysis buffer (eBioscience). The 7-AAD viability solution was added before acquisition on a LSR II Fortessa (BD Biosciences). All flow cytometric data were analyzed using FACS Diva software (BD Biosciences).
MPO Assay.
Mesentery samples from PBS, zymosan, or TNF-α–treated mice were homogenized in 1 mL of 0.5% hexadecyltrimethylammonium bromide using a precellys homogenizer (Bertin Technologies) and CK14-beaded tubes (Stretton Scientific). Samples were homogenized twice at 5,000 rpm for 30 s with 15 s between homogenizations. Following homogenization, samples were then centrifuged at 10,000 × g for 5 min at 4 °C to generate a supernatant, which was then collected and the pellet discarded. A standard curve was generated using purified MPO in PBS (2 U/mL) by serial dilution at 0.03125–1 U/mL). Standard and sample (20 μL) were loaded in triplicate on a 96-well plate and 160 μL of reagent (tetramethylbenzidine 4 mg/mL in DMSO then diluted 1:8 in PBS just before use) was added to each of the wells along with 20 μL of H2O2 (0.1 mmol/L diluted 1:30 in PBS just before use). The plate was protected from light using tin foil and left to incubate for 5 min. Light absorbance was measured using a spectrophotometric plate reader (MRX-TC Revelation, Dynex Technologies) at 620 nm and sample MPO concentration determined from the standard curve. A bicinchoninic acid protein assay (Thermo Scientific) was conducted, according to manufacturer’s guidelines, to determine the protein concentration of each supernatant sample, allowing normalization of MPO activity.
qRT-PCR of Mouse Aorta.
Samples were homogenized and RNA extracted for qRT-PCR analysis with SYBR green, using specifically designed primers for CXCL1: 5′-TGAGCTGCGCTGTCAGTGCCT-3′ and 5′-AGAAGCCAGCGTTCACCAGA-3′; CXCL2: 5′-GAGCTTGAGTGTGACGCCCCCAGG-3′ and 5′-GTTAGCCTTGCCTTTGTTCAGTATC-3′; CXCL5: 5′-GCATTTCTGTTGCTGTTCACGCTG-3′ and 5′-CCTCCTTCTGTTTTTCAGTTTAGC-3′; CCL2: 5′-TTAAAAACCTGGATCGGAACCAA-3′ and 5′-GCATTAGCTTCAGATTTACGGGT-3′; CX3CL1: 5′-TCCTGGAGACGACACAGCA-3′ and 5′-TGCCACCATTTTTAGTGAGGG-3′; IL-10: 5′-TGGCCCAGAAATCAAGGAGC-3′ and 5′-CAGCAGACTCAATACACACT-3′. qRT-PCR was performed using an ABI Prism 7900 sequence detection system. Expression of each gene was normalized to β-actin, 5′-GAAATCGTGCGTGACATCAAAG -3′ and 5′-TGTAGTTTCATGGATGCCACAG-3′, and expressed as a relative value using the comparative threshold cycle (Ct) method (2−ΔΔCt).
Western Blotting.
Equal amounts of protein homogenates of liver or erythrocytes were subjected to Western blotting to determine XOR expression levels. Tissue homogenates and RBCs were incubated in stock solution of tissue lysis buffer (10 mmol/L Tris⋅HCl, 50 mmol/L NaCL, 30 mmol/L NaPPi, and 2 mmol/L EDTA) and 0.5 mol/L NaF, 1% Triton X-100, 0.2 mol/L Na3VO4, as well as 1 µg/mL each of the protease inhibitors benzamidine, aprotinin, antipain, leupeptin, pepstatin A, and AEBSF. Equal amounts of protein (20 μg) were subjected to 10% (wt/vol) SDS gel electrophoresis under reducing conditions. Separated proteins were then electrotransferred onto 0.2-μm nitrocellulose membrane (GE Healthcare) using semidry electrophoretic transfer cell. Blots were blocked with 5% (wt/vol) milk and incubated overnight at 4 °C with primary antibody XOR; rabbit polyclonal anti-XOR antibody (XOR 1:2,000; Abcam), overnight. Membranes were then incubated with goat polyclonal anti-rabbit secondary antibody (1:5,000; Dako) for 1 h and visualized using the ECL Western blotting detection system (FluorChem E Protein Simple). The levels of protein were expressed relative to β‐actin expression (1:10,000; Millipore).
Plasma cGMP Determination.
Blood was collected via cardiac puncture into 5,000 U/mL heparin and 3-isobutyl-1-methylxanthine (final concentration 100 μmol/L). Blood was then immediately centrifuged at 4 °C, 13,000 × g for 5 min, and the plasma collected, snap-frozen, and stored at −80 °C until the day of assay. cGMP was determined using cGMP Enzymeimmunoassay Biotrak kit (GE Healthcare) according to the manufacturer’s guidelines.
Acknowledgments
This work was funded by the British Heart Foundation.
Footnotes
Conflict of interest statement: A.A. is a Director of HeartBeet Ltd.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613063114/-/DCSupplemental.
References
- 1.Böger RH, et al. Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation. 1997;95(8):2068–2074. doi: 10.1161/01.cir.95.8.2068. [DOI] [PubMed] [Google Scholar]
- 2.Böger RH, et al. Hypercholesterolemia impairs basal nitric oxide synthase turnover rate: A study investigating the conversion of L-[guanidino-(15)N(2)]-arginine to (15)N-labeled nitrate by gas chromatography--mass spectrometry. Nitric Oxide. 2004;11(1):1–8. doi: 10.1016/j.niox.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 4.Clarkson P, et al. Oral L-arginine improves endothelium-dependent dilation in hypercholesterolemic young adults. J Clin Invest. 1996;97(8):1989–1994. doi: 10.1172/JCI118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf A, et al. Dietary L-arginine supplementation normalizes platelet aggregation in hypercholesterolemic humans. J Am Coll Cardiol. 1997;29(3):479–485. doi: 10.1016/s0735-1097(97)00523-8. [DOI] [PubMed] [Google Scholar]
- 6.Theilmeier G, et al. Adhesiveness of mononuclear cells in hypercholesterolemic humans is normalized by dietary L-arginine. Arterioscler Thromb Vasc Biol. 1997;17(12):3557–3564. doi: 10.1161/01.atv.17.12.3557. [DOI] [PubMed] [Google Scholar]
- 7.Blum A, et al. Oral L-arginine in patients with coronary artery disease on medical management. Circulation. 2000;101(18):2160–2164. doi: 10.1161/01.cir.101.18.2160. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura Y, Moss AJ, Brown MW, Kinoshita M, Kawai C. Multicenter Myocardial Ischemia Research Group Long-term nitrate use may be deleterious in ischemic heart disease: A study using the databases from two large-scale postinfarction studies. Am Heart J. 1999;138(3 Pt 1):577–585. doi: 10.1016/s0002-8703(99)70163-8. [DOI] [PubMed] [Google Scholar]
- 9.Cunnington C, et al. Systemic and vascular oxidation limits the efficacy of oral tetrahydrobiopterin treatment in patients with coronary artery disease. Circulation. 2012;125(11):1356–1366. doi: 10.1161/CIRCULATIONAHA.111.038919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Förstermann U, Sessa WC. Nitric oxide synthases: Regulation and function. Eur Heart J. 2012;33(7):829–837, 837a–837d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Münzel T, Daiber A, Gori T. More answers to the still unresolved question of nitrate tolerance. Eur Heart J. 2013;34(34):2666–2673. doi: 10.1093/eurheartj/eht249. [DOI] [PubMed] [Google Scholar]
- 12.Lundberg JO, et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5(12):865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh SM, et al. Enhanced vasodilator activity of nitrite in hypertension: Critical role for erythrocytic xanthine oxidoreductase and translational potential. Hypertension. 2013;61(5):1091–1102. doi: 10.1161/HYPERTENSIONAHA.111.00933. [DOI] [PubMed] [Google Scholar]
- 14.Webb AJ, et al. Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia: Role for xanthine oxidoreductase and endothelial nitric oxide synthase. Circ Res. 2008;103(9):957–964. doi: 10.1161/CIRCRESAHA.108.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jädert C, et al. Decreased leukocyte recruitment by inorganic nitrate and nitrite in microvascular inflammation and NSAID-induced intestinal injury. Free Radic Biol Med. 2012;52(3):683–692. doi: 10.1016/j.freeradbiomed.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Stokes KY, et al. Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2009;296(5):H1281–H1288. doi: 10.1152/ajpheart.01291.2008. [DOI] [PubMed] [Google Scholar]
- 17.Bryan NS, et al. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol. 2005;1(5):290–297. doi: 10.1038/nchembio734. [DOI] [PubMed] [Google Scholar]
- 18.Cosby K, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 19.Gladwin MT, Kim-Shapiro DB. The functional nitrite reductase activity of the heme-globins. Blood. 2008;112(7):2636–2647. doi: 10.1182/blood-2008-01-115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celermajer DS, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 21.Neunteufl T, et al. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis. 1997;129(1):111–118. doi: 10.1016/s0021-9150(96)06018-2. [DOI] [PubMed] [Google Scholar]
- 22.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23(2):168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 23.Ishibashi T, et al. Negative NO3- difference in human coronary circulation with severe atherosclerotic stenosis. Life Sci. 2000;66(2):173–184. doi: 10.1016/s0024-3205(99)00575-5. [DOI] [PubMed] [Google Scholar]
- 24.Kleinbongard P, et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35(7):790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 25.d’Uscio LV, et al. Mechanism of endothelial dysfunction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21(6):1017–1022. doi: 10.1161/01.atv.21.6.1017. [DOI] [PubMed] [Google Scholar]
- 26.Rassaf T, et al. Vascular formation of nitrite after exercise is abolished in patients with cardiovascular risk factors and coronary artery disease. J Am Coll Cardiol. 2010;55(14):1502–1503. doi: 10.1016/j.jacc.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 27.Fukuto JM, Cho JY, Switzer CH. The chemical proerties of nitric oxide and related nitrogen oxides. In: Ignarro LJ, editor. Nitric Oxide: Biology and Pathobiology. Academic; San Diego: 2000. pp. 23–40. [Google Scholar]
- 28.Batchelor AM, et al. Exquisite sensitivity to subsecond, picomolar nitric oxide transients conferred on cells by guanylyl cyclase-coupled receptors. Proc Natl Acad Sci USA. 2010;107(51):22060–22065. doi: 10.1073/pnas.1013147107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsch E, et al. The effect of prolonged dietary nitrate supplementation on atherosclerosis development. Atherosclerosis. 2016;245:212–221. doi: 10.1016/j.atherosclerosis.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 30.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30(7):1282–1292. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 31.Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989-1777;83(5):1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNamara DB, et al. L-arginine inhibits balloon catheter-induced intimal hyperplasia. Biochem Biophys Res Commun. 1993;193(1):291–296. doi: 10.1006/bbrc.1993.1622. [DOI] [PubMed] [Google Scholar]
- 33.Alef MJ, et al. Nitrite-generated NO circumvents dysregulated arginine/NOS signaling to protect against intimal hyperplasia in Sprague-Dawley rats. J Clin Invest. 2011;121(4):1646–1656. doi: 10.1172/JCI44079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunigal S, et al. Monocyte-expressed urokinase inhibits vascular smooth muscle cell growth by activating Stat1. Blood. 2003;102(13):4377–4383. doi: 10.1182/blood-2002-12-3872. [DOI] [PubMed] [Google Scholar]
- 35.Proudfoot D, Fitzsimmons C, Torzewski J, Bowyer DE. Inhibition of human arterial smooth muscle cell growth by human monocyte/macrophages: A co-culture study. Atherosclerosis. 1999;145(1):157–165. doi: 10.1016/s0021-9150(99)00028-3. [DOI] [PubMed] [Google Scholar]
- 36.Kauser K, da Cunha V, Fitch R, Mallari C, Rubanyi GM. Role of endogenous nitric oxide in progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Physiol Heart Circ Physiol. 2000;278(5):H1679–H1685. doi: 10.1152/ajpheart.2000.278.5.H1679. [DOI] [PubMed] [Google Scholar]
- 37.Kuhlencordt PJ, et al. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104(4):448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, et al. Effects of chronic treatment with L-arginine on atherosclerosis in apoE knockout and apoE/inducible NO synthase double-knockout mice. Arterioscler Thromb Vasc Biol. 2003;23(1):97–103. doi: 10.1161/01.atv.0000040223.74255.5a. [DOI] [PubMed] [Google Scholar]
- 39.De Meyer GRY, et al. Nitric oxide donor molsidomine favors features of atherosclerotic plaque stability during cholesterol lowering in rabbits. J Cardiovasc Pharmacol. 2003;41(6):970–978. doi: 10.1097/00005344-200306000-00021. [DOI] [PubMed] [Google Scholar]
- 40.Segura-Puimedon M, et al. Proatherosclerotic effect of the α1-subunit of soluble guanylyl cyclase by promoting smooth muscle phenotypic switching. Am J Pathol. 2016;186(8):2220–2231. doi: 10.1016/j.ajpath.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Rahma M, et al. Effects of furosemide on the tubular reabsorption of nitrates in anesthetized dogs. Eur J Pharmacol. 2001;428(1):113–119. doi: 10.1016/s0014-2999(01)01357-7. [DOI] [PubMed] [Google Scholar]
- 42.Greene I, Hiatt EP. Renal excretion of nitrate and its effect on excretion of sodium and chloride. Am J Physiol. 1955;180(1):179–182. doi: 10.1152/ajplegacy.1954.180.1.179. [DOI] [PubMed] [Google Scholar]
- 43.van Leeuwen M, et al. Accumulation of myeloperoxidase-positive neutrophils in atherosclerotic lesions in LDLR-/- mice. Arterioscler Thromb Vasc Biol. 2008;28(1):84–89. doi: 10.1161/ATVBAHA.107.154807. [DOI] [PubMed] [Google Scholar]
- 44.Zernecke A, et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res. 2008;102(2):209–217. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- 45.Döring Y, Drechsler M, Soehnlein O, Weber C. Neutrophils in atherosclerosis: From mice to man. Arterioscler Thromb Vasc Biol. 2015;35(2):288–295. doi: 10.1161/ATVBAHA.114.303564. [DOI] [PubMed] [Google Scholar]
- 46.Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122(18):1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 47.Couper KN, Blount DG, Riley EM. IL-10: The master regulator of immunity to infection. J Immunol. 2008;180(9):5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 48.Mosser DM, Zhang X. Interleukin-10: New perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mallat Z, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85(8):e17–e24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 50.Schröder K, et al. Xanthine oxidase inhibitor tungsten prevents the development of atherosclerosis in ApoE knockout mice fed a Western-type diet. Free Radic Biol Med. 2006;41(9):1353–1360. doi: 10.1016/j.freeradbiomed.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 51.Patetsios P, et al. Identification of uric acid and xanthine oxidase in atherosclerotic plaque. Am J Cardiol. 2001;88(2):188–191, A6. doi: 10.1016/s0002-9149(01)01621-6. [DOI] [PubMed] [Google Scholar]
- 52.Spiekermann S, et al. Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: Relation to endothelium-dependent vasodilation. Circulation. 2003;107(10):1383–1389. doi: 10.1161/01.cir.0000056762.69302.46. [DOI] [PubMed] [Google Scholar]
- 53.Yokoyama Y, et al. Circulating xanthine oxidase: Potential mediator of ischemic injury. Am J Physiol. 1990;258(4 Pt 1):G564–G570. doi: 10.1152/ajpgi.1990.258.4.G564. [DOI] [PubMed] [Google Scholar]
- 54.Adachi T, Fukushima T, Usami Y, Hirano K. Binding of human xanthine oxidase to sulphated glycosaminoglycans on the endothelial-cell surface. Biochem J. 1993;289(Pt 2):523–527. doi: 10.1042/bj2890523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parathath S, et al. Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circ Res. 2011;109(10):1141–1152. doi: 10.1161/CIRCRESAHA.111.246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Getting SJ, Flower RJ, Perretti M. Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br J Pharmacol. 1997;120(6):1075–1082. doi: 10.1038/sj.bjp.0701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kubes P, Suzuki M, Granger DN. Nitric oxide: An endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spiecker M, Darius H, Kaboth K, Hübner F, Liao JK. Differential regulation of endothelial cell adhesion molecule expression by nitric oxide donors and antioxidants. J Leukoc Biol. 1998;63(6):732–739. [PubMed] [Google Scholar]
- 59.Kosonen O, Kankaanranta H, Uotila J, Moilanen E. Inhibition by nitric oxide-releasing compounds of E-selectin expression in and neutrophil adhesion to human endothelial cells. Eur J Pharmacol. 2000;394(1):149–156. doi: 10.1016/s0014-2999(00)00141-2. [DOI] [PubMed] [Google Scholar]
- 60.Davenpeck KL, Gauthier TW, Lefer AM. Inhibition of endothelial-derived nitric oxide promotes P-selectin expression and actions in the rat microcirculation. Gastroenterology. 1994;107(4):1050–1058. doi: 10.1016/0016-5085(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 61.Banick PD, Chen Q, Xu YA, Thom SR. Nitric oxide inhibits neutrophil β 2 integrin function by inhibiting membrane-associated cyclic GMP synthesis. J Cell Physiol. 1997;172(1):12–24. doi: 10.1002/(SICI)1097-4652(199707)172:1<12::AID-JCP2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 62.Klink M, Bednarska K, Jastrzembska K, Banasik M, Sulowska Z. Signal transduction pathways affected by nitric oxide donors during neutrophil functional response in vitro. Inflamm Res. 2007;56(7):282–290. doi: 10.1007/s00011-007-6205-4. [DOI] [PubMed] [Google Scholar]
- 63.Soehnlein O, et al. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112(4):1461–1471. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caligiuri G, et al. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9(1-2):10–17. [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Z, et al. Thrombospondin-1 (TSP1) contributes to the development of vascular inflammation by regulating monocytic cell motility in mouse models of abdominal aortic aneurysm. Circ Res. 2015;117(2):129–141. doi: 10.1161/CIRCRESAHA.117.305262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McGrath JC, Lilley E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br J Pharmacol. 2015;172(13):3189–3193. doi: 10.1111/bph.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buga GM, Griscavage JM, Rogers NE, Ignarro LJ. Negative feedback regulation of endothelial cell function by nitric oxide. Circ Res. 1993;73(5):808–812. doi: 10.1161/01.res.73.5.808. [DOI] [PubMed] [Google Scholar]
- 69.Webb A, et al. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci USA. 2004;101(37):13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohta H, et al. Disruption of tumor necrosis factor-α gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis. 2005;180(1):11–17. doi: 10.1016/j.atherosclerosis.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 71.Manthey HD, et al. Complement C5a inhibition reduces atherosclerosis in ApoE-/- mice. FASEB J. 2011;25(7):2447–2455. doi: 10.1096/fj.10-174284. [DOI] [PubMed] [Google Scholar]
- 72.Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression—A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 2006;7:123. doi: 10.1186/1471-2105-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]