Abstract
In this study, we determined the risk factors for acute kidney injury (AKI) following orthotopic liver transplantation (OLT) in China. We collected 5074 donation after cardiac death (DCD) OLT recipients who underwent surgery between January 1, 2010, and December 31, 2015, in 86 academic hospitals or transplant centers in China. Univariate and multivariate analyses were used to investigate the criticality of donor, graft, or recipient variables in the development of post-OLT AKI. In all, 4482 patients were included (median age, 49.31 years). Post-OLT AKI occurred in 3.97% patients, and 73.6% of all OLT patients were male. The 1- and 5-year cumulative survival rates (CSRs) of the AKI group were 33.95% and 25.24%, respectively, compared with 86.34% and 70.05%, respectively, of the non-AKI group (P < 0.001). The independent risk factors for post-OLT AKI were blood loss, cold ischemia time, warm ischemia time, preoperative serum creatinine, the treatment period with dopamine, overexposure to calcineurin inhibitor, and combined mycophenolate mofetil use (P < 0.05). These had a high prediction accuracy for post-OLT AKI (area under the curve [AUC] = 0.740).
Acute kidney injury (AKI) is a common complication of orthotopic liver transplantation (OLT) and is a major cause of mortality and morbidity1,2,3,4,5. The incidence rate of AKI after OLT ranks from 5 to 94%1,3,6 and approximately 8–17% need renal replacement therapy (RRT)3,7. The Risk, Injury, Failure, Loss, and End-stage Renal Dysfunction (RIFLE) classification was set up for stratifying the severity of early AKI by the Acute Dialysis Quality Initiative (ADQI) workgroup8.
Various factors are associated with the occurrence of AKI, and some depend on the preoperative recipient’s condition, and others stem from intraoperative vascular and metabolic dynamics and postoperative complications3,7,9,10,11. Recipients may have had intrinsic renal disease, induced by obstructive uropathy hypotension11, cerebrovascular diseases4, diabetes mellitus11, or hepatorenal syndrome (HS)12 before undergoing liver transplantation. Acute tubular necrosis (ATN) and glomerulonephritis mainly develop in patients with nephrotoxic drug use, cirrhosis, and IgA nephropathy, all of them thought as signs of AKI13. Besides, graft size and donor age14, low prothrombin activity, and high pre-transplantation serum creatinine (SCr) level15 were also important identifiable risk factors of AKI after OLT. In addition to low cryoprecipitate transfusion16 and low intraoperative volume of blood transfusion17, immunosuppressive therapy of low nephrotoxic potential, and careful operative technique18 were recommended to diminish the risk for AKI after OLT. Meanwhile, some investigations revealed that postoperative infection19, RRT induction14 were independent risk factors of AKI complicated to OLT. However, some risk factors, which may cause AKI after OLT, are still controversial or even contradicting in different investigations6,11,16,17,20,21,22,23,24,25.
China is a big country for organ transplantation, which began its first clinical liver transplantation (LT) in 1977, and the cumulative number of LT is at least 30,00026,27. However, no national laws were framed for the oversight of China’s transplantation system until 200728, and more than 90% of transplanted organs were obtained from executed prisoners29, an approach that violated medical ethics and was illegal. Thus, international scholars and journals boycotted to accept and publish the results of organ transplantation from China30. To date, limited data are available on patients with AKI who underwent OLT in China.
We attempted to explore in our study the independent risk factors of AKI after OLT with analysis of legal multicenter and large samples, and provide reliable data to recognize, prevent, and treat AKI after OLT in China.
Results
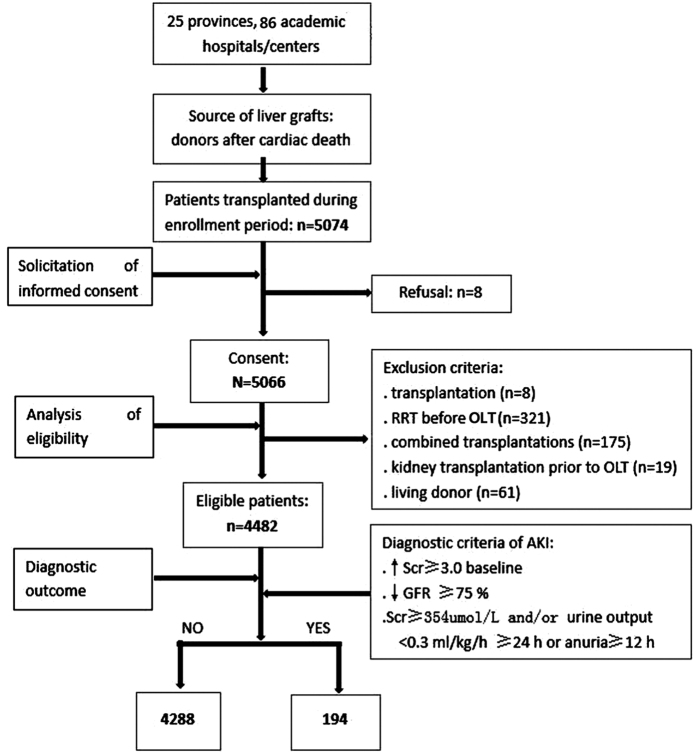
As shown in Fig. 1, 5074 adult recipients underwent OLT during our enrollment period, 584 patients were excluded (8, 321, 175, 19, and 61 cases for re-transplantation, RRT before OLT, combined transplantations, kidney transplantation before OLT, and living donor, respectively), and 8 patients refused to sign the informed consent. Finally, 4482 patients were included in our investigation, and their clinical and biochemical data were analyzed.
Figure 1. Study profile.
RRT = renal dialysis treatment; OLT = orthotopic liver transplantation.
Donor characteristics based on AKI and non-AKI after OLT
Some reports14,31 have confirmed that certain factors derived from donors influence the OLT prognosis. Herein, we attempted to explore the association between donor factors and occurrence of post-OLT AKI. The mean age in the non-AKI and AKI groups was 37.92 years (22.63, 47.63) and 39 years (24, 47.17), respectively (Table 1). Male patients accounted for 73.23% (3140/4288) and 79.90% (155/194) in the non-AKI and AKI groups, respectively. No significant differences were found in age, sex, donor blood type, and past medical history, but the China classification of DCD and cause of death showed a remarkable difference between groups (P = 0.005 and P = 0.007, respectively). Moreover, we analyzed biochemical markers of donor blood. No significant difference was found between groups regarding K+, ALP, total bilirubin, GGT, and ALT. However, the AKI group showed a higher concentration of Na+ (153.15 vs 144 mmol/L, P = 0.008), BUN (9.78 vs 6.83 mmol/L, P = 0.001), blood sugar (8.5 vs 7.65 mmol/L, P = 0.039), SCr (103.5 vs 79 μmol/L, P < 0.001), and AST (61 vs 50.4 U/L, P = 0.011), and a lower albumin level (30.6 vs 33.38 g/L, P = 0.003) than the non-AKI group.
Table 1. Donor characteristic with or without post-OLT AKI.
| Parameters (n = 4482) | Non-AKI | AKI | P value | Missing value |
|---|---|---|---|---|
| Age (years) | 37.92 (22.63, 47.63) | 39 (24, 47.17) | 0.597 | 679 |
| Sex (male) | 3140 (81.16) | 155 (81.58) | 0.885 | 423 |
| Blood type | 0.928 | 388 | ||
| O | 1535 (39.32) | 79 (41.58) | ||
| A | 1099 (28.15) | 52 (27.37) | ||
| B | 994 (25.46) | 47 (24.74) | ||
| AB | 276 (7.07) | 12 (6.32) | ||
| China classification of DCD | 0.005 | 60 | ||
| Grade I | 536 (12.67) | 19 (9.95) | ||
| Grade II | 1822 (43.06) | 105 (54.97) | ||
| Grade III | 1873 (44.27) | 67 (35.08) | ||
| Cause of donor death | 0.007 | 1765 | ||
| Trauma | 1452 (56.61) | 86 (56.58) | ||
| Cerebrovascular accident | 1452 (56.61) | 86 (56.58) | ||
| Brain tumor | 134 (5.22) | 16 (10.53) | ||
| Hypoxic brain injury | 73 (2.85) | 5 (3.29) | ||
| Others | 255 (9.94) | 4 (2.63) | ||
| Toxicosis | 7 (0.27) | 0 (0) | ||
| Preoperative data of donor | ||||
| Past medical history | ||||
| Diabetes | 8 (0.19) | 0 (0) | — | |
| Hypertension | 121 (2.82) | 8 (4.12) | 0.289 | |
| Respiratory diseases | 4 (0.09) | 1 (0.52) | 0.199 | |
| Cardiovascular diseases | 11 (0.26) | 0 (0) | — | |
| Others | 69 (1.61) | 9 (4.64) | 0.002 | |
| None | 1709 (39.86) | 91 (46.91) | 0.050 | |
| Donor blood examination | ||||
| Na+ | 144 (138, 155) | 153.15 (138.9, 164) | 0.008 | 2810 |
| K+ | 3.99 (3.57, 4.5) | 4 (3.5, 4.6) | 0.465 | 3040 |
| BUN | 6.83 (4.68, 10.83) | 9.78 (5.41, 15.14) | <0.001 | 2655 |
| Albumin | 33.38 (28, 39) | 30.6 (27.3, 36.6) | 0.003 | 2652 |
| ALP | 87 (58.5, 122) | 85 (61.5, 116.9) | 0.825 | 3122 |
| Blood sugar | 7.65 (5.5, 10.9) | 8.5 (6.8, 11.69) | 0.039 | 3428 |
| Total bilirubin (μmol/L) | 14.5 (9.3, 23) | 14.65 (9.6, 22.9) | 0.782 | 2601 |
| sCr (μmol/L) | 79 (53.9, 121.95) | 103.5 (64.85, 206.5) | <0.001 | 2598 |
| GGT | 37 (19, 79) | 42 (14, 87) | 0.607 | 3005 |
| AST (U/L) | 50.4 (30, 91.2) | 61 (37, 113) | 0.011 | 2641 |
| ALT (U/L) | 36 (21, 76) | 41.25 (23.5, 75) | 0.330 | 2624 |
Demographic and clinic data of recipients with or without AKI after OLT
The mean age of the 4482 enrolled patients was 49.31 years, and male patients accounted for 73.56% (3591/4882). AKI after OLT occurred in 3.97% (194/4882) recipients. The age in the AKI group was slightly increased than that in the non-AKI group (50.5 vs 48.4 years, P = 0.056). No significant difference was found regarding sex and BMI between these two groups (Table 2).
Table 2. Demographic and preoperative clinical data of recipients with or without post-OLT AKI.
| Characteristics | Non-AKI (n = 4288) | AKI (n = 194) | P value |
|---|---|---|---|
| Age, years (mean) | 48.4 (40.4, 55.8) | 50.5 (41.9, 58.7) | 0.056 |
| Male sex, n (%) | 3444 (80.32) | 147 (75.77) | 0.121 |
| BMI, (mean, SD) | 22.77 (20.76, 24.69) | 22.33 (20.76, 24.49) | 0.072 |
| Preoperative data, n (%) | |||
| Blood type compatibility, n (%) | 3757 (87.62) | 175 (90.21) | 0.004 |
| Hepatocarcinoma-related disease, n (%) | <0.001 | ||
| HCC | 1630 (38.01) | 54 (27.84) | |
| Benign disease | 1630 (38.01) | 68 (35.05) | |
| Others | 1028 (23.97) | 72 (37.11) | |
| Hepatitis B, n (%) | 2723 (63.5) | 130 (67.01) | 0.321 |
| Cirrhosis, n (%) | 3411 (79.55) | 138 (71.13) | 0.005 |
| Hepatic failure, n (%) | 311 (7.25) | 35 (18.04) | <0.001 |
| Preoperative dialysis, n (%) | 312 (7.28) | 20 (10.31) | 0.115 |
| Total bilirubin (μmol/L), (mean L b) | 102 (27.8, 356) | 143 (29, 450) | 0.461 |
| Serum creatinine (μmol/L), (mean L c) | 74.6 (56.2, 110) | 85 (64, 136) | 0.003 |
| INR, (mean score) | 1.58 (1.2, 2.62) | 1.63 (1.25, 2.5) | 0.485 |
| MELD score, (mean score) | 20 (12, 33) | 23 (13, 35) | 0.109 |
| Child-Pugh grade, (mean C-P) | 10 (8, 12) | 10 (8, 12) | 0.204 |
| Albumin (g/dL), (mean min) | 3.34 (2.91, 3.8) | 3.38 (3, 3.89) | 0.571 |
Tacrolimus and cyclosporine with or without MMF were administered to recipients on day 0 after surgery. Induction therapy using basiliximab, rabbit antithymocyte globulin (rATG) or anti-Tac mAb, sometimes combined with steroid drugs, was administered to 2151 (48%) patients. A steroid-based therapy was administered to 3675 (82%) patients (data not shown).
No difference was observed in the preoperative data analysis (Table 2) between the two groups regarding hepatitis B, preoperative dialysis, total bilirubin, INR, MELD score, Child–Pugh grade, and albumin, which were paradoxical with the findings in Naik et al.11 and Yuan et al.’s19 studies. Furthermore, we found that a remarkable association was found between the AKI and non-AKI groups regarding blood type compatibility (90.21% vs 87.62%, P = 0.004), hepatocarcinoma-related disease (P < 0.001), cirrhosis (71.13% vs 79.55%, P = 0.005), hepatic failure (18.04% vs 7.25%, P < 0.001), and SCr (85 vs 74.6 μmol/L, P = 0.003).
Wenger et al.25 have confirmed that many intraoperative data like duration of surgery and blood transfusion volume were independent factors contributing to post-OLT AKI. Here, we also observed a significant difference between groups in cold ischemia time (7 vs 6.17 h, P = 0.043), warm ischemia time (5 vs 5 min, P < 0.001), intraoperative blood loss (2500 vs 1600 mL, P < 0.001), red blood cells (2000 vs 1600 mL, P < 0.001), autologous blood cell transfusion (1225 vs 800 mL, P = 0.015), fresh frozen plasma (1850 vs 1450 mL, P < 0.001), and total intravenous infusion (6611.5 vs 5500 mL, P < 0.001). Furthermore, patients with post-OLT AKI had a greater need for noradrenaline (P < 0.001) and dobutamine (P < 0.001). However, no difference was found between groups regarding duration of surgery, whole blood, cell saver, and platelet (Table 3).
Table 3. Intraoperative clinic data (mean range) of recipients with or without post-OLT AKI.
| Parameters | Non-AKI (n = 4288) | AKI (n = 194) | P value |
|---|---|---|---|
| Duration of surgery (h) | 7.25 (6.07, 8.53) | 7.28 (5.67, 8.92) | 0.916 |
| Cold ischemia time (h) | 6.17 (4.5, 8.63) | 7 (5, 10.53) | 0.043 |
| Warm ischemia time (min) | 5 (2, 8) | 5 (3.25, 14) | <0.001 |
| Intraoperative blood loss (mL) | 1600 (800, 3000) | 2500 (1500, 6000) | <0.001 |
| Transfusion of blood products | |||
| Whole blood (mL) | 1200 (800, 2400) | 1260 (800, 2000) | 0.627 |
| Red blood cells (mL) | 1600 (800, 2400) | 2000 (1100, 4000) | <0.001 |
| Cell saver (mL) | 2800 (1600, 4000) | 4600 (800, 6400) | 0.701 |
| Autologous blood cell transfusion (mL) | 800 (500, 1500) | 1225 (600, 2950) | 0.015 |
| Fresh frozen plasma (mL) | 1450 (800, 2060) | 1850 (1080, 2810) | <0.001 |
| Platelet (units) | 2 (1, 10) | 2 (1, 9) | 0.764 |
| Total volume of intravenous infusion (mL) | 5500 (3500, 7652) | 6611.5 (4975, 9990) | <0.001 |
| Noradrenaline (%) | 1458 (34) | 107 (55.2) | <0.001 |
| Dobutamine (%) | 772 (18) | 72 (37.1) | <0.001 |
We attempted to postoperatively analyze the association between post-OLT complications and occurrence of AKI. Interestingly, a significant difference was found between the AKI and non-AKI groups regarding intraperitoneal hemorrhage (32.99% vs 4.52%, P < 0.001), vascular complications (8.76% vs 3.54%, P < 0.001), abnormal graft function (28.35% vs 1.66%, P < 0.001), postoperative infection (44.85% vs 15.79%, P < 0.001), diabetes (21.65% vs 12.52%, P < 0.001), hypertension (6.19% vs 3.36%, P = 0.036), new-onset diabetes (16.49% vs 10.03%, P = 0.004), pleural effusion (48.97% vs 23.06%, P < 0.001), pneumonedema (10.31% vs 0.93%, P < 0.001), and intra-abdominal abscess (32.99% vs 11.22%, P < 0.001). In addition, hospital stay length and follow-up duration of the AKI group were remarkably shorter compared with those in the non-AKI group (16 vs 25 days, P < 0.001; 0.72 vs 3.82 months, P < 0.001, respectively) (Table 4).
Table 4. Postoperative clinical data of recipients with or without post-OLT AKI.
| Factors | Non-AKI (n = 4288) (%) | AKI (n = 194) (%) | P value |
|---|---|---|---|
| Postoperative complications | |||
| Intraperitoneal hemorrhage | 194 (4.52) | 64 (32.99) | <0.001 |
| Biliary complications | 198 (4.62) | 10 (5.15) | 0.728 |
| Vascular complications | 152 (3.54) | 17 (8.76) | <0.001 |
| Abnormal graft function | 71 (1.66) | 55 (28.35) | <0.001 |
| Postoperative infection | 677 (15.79) | 87 (44.85) | <.001 |
| Diabetes | 537 (12.52) | 42 (21.65) | <0.001 |
| Hypertension | 144 (3.36) | 12 (6.19) | 0.036 |
| New-onset diabetes | 430 (10.03) | 32 (16.49) | 0.004 |
| New-onset hypertension | 94 (2.19) | 6 (3.09) | 0.406 |
| Cytomegalovirus infection | 51 (1.19) | 2 (1.03) | 0.847 |
| Pleural effusion | 989 (23.06) | 95 (48.97) | <0.001 |
| Pneumonedema | 40 (0.93) | 20 (10.31) | <0.001 |
| Intra-abdominal abscess | 481 (11.22) | 64 (32.99) | <0.001 |
| Initial induction of CNI | |||
| Tacrolimus | 2680 (62.51) | 146 (75.02) | 0.015 |
| Cyclosporine | 391 (9.11) | 25 (13.04) | 0.027 |
| Average CNI trough level (ng/ml) | |||
| Tacrolimus | 9.2 (8.8, 9.6) | 10.3 (9.8, 10.7) | 0.008 |
| Cyclosporine | 178.5 (172, 185) | 174.1 (162, 181) | 0.324 |
| Overexposure to CNI | 527 (12.30) | 93 (48.14) | <0.001 |
| MMF use | 3044 (71.08) | 81 (42.10) | <0.001 |
| Dobutamine (days) | 1.2 (0.0, 2.4) | 3.5 (0.8, 6.2) | <0.001 |
| Dopamine (days) | 3.2 (1.1, 5.3) | 5.4 (3.5, 7.3) | <0.001 |
| Length of hospital stay (days) | 25 (18,35) | 16 (6,34.5) | <0.001 |
| Follow-up duration (month) | 3.82 (0.92, 10.66) | 0.72 (0.2, 3.88) | <0.001 |
Besides, some previous studies24,32 showed that immunosuppressive therapy can result in post-OLT AKI. Here, we also attempted to analyze the association between post-OLT AKI and immunosuppressive agent use. The results show that a significant difference was found between the AKI and non-AKI groups regarding initial induction of CNI with tacrolimus (75.02% vs. 62.51%, P = 0.015), induction of CNI with cyclosporine (13.04% vs. 9.11%, P = 0.015), and mean CNI trough level for tacrolimus (10.3 vs. 9.2 ng/mL, P = 0.008). The overexposure rate to CNI was significantly higher in the AKI group (P < 0.001). With regard to MMF use, a remarkable difference was found between both groups (71.08% vs. 42.10%, P < 0.001).
Cumulative survival rates of recipients and grafts with or without post-OLT AKI
During long-term follow-up, we found that the 1- and 5-year cumulative survival rates (CSR) were 83.34% and 68.14%, respectively, in all eligible recipients. However, the CSR of the AKI group was significantly lower than that of the non-AKI group. The 1- and 5-year CSRs were 33.95% and 25.24% in the AKI group compared with 86.34% and 70.05% in the non-AKI group, respectively (P < 0.001) (Fig. 2A). Moreover, the 1- and 5-year CSR between the AKI and non-AKI groups were 31.69% vs 84.34% and 23.68% vs 68.12% (P < 0.001), respectively with respect to the grafts (Fig. 2B). In fact, approximately two thirds of recipients or grafts died within the first month after OLT in the AKI group. With regard to the recipients, the first-month CSR was 38% 95% in the AKI and non-AKI groups, respectively.
Figure 2.
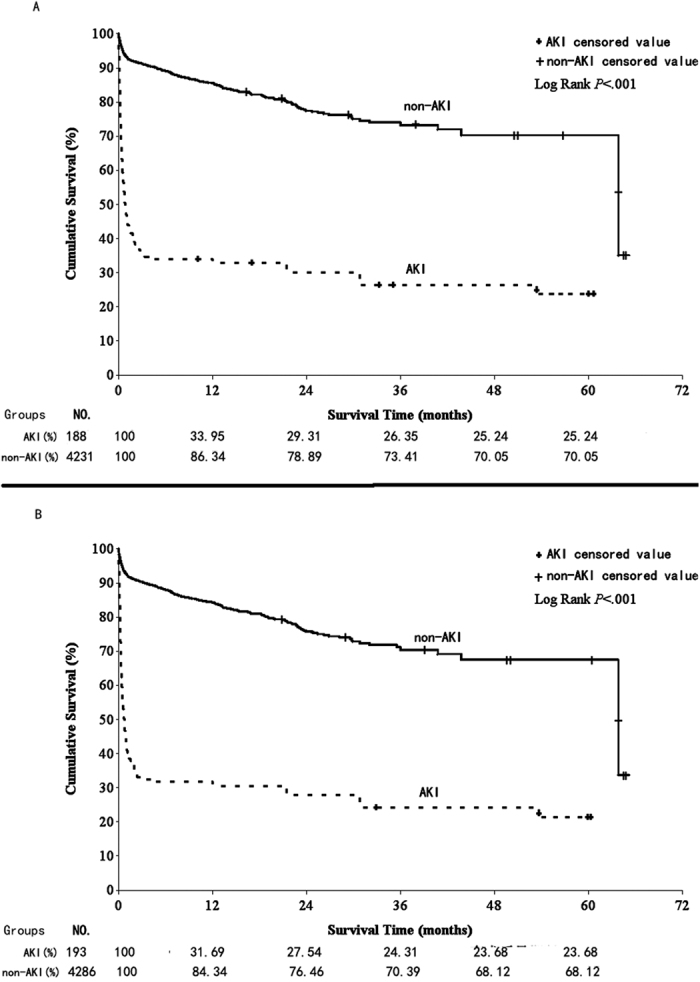
Kaplan-Meier Curves for (A) the recipients and (B) the grafts.
Multivariate analysis of risk factors associated with post-OLT AKI
A stepwise logistic regression model was constructed based on significant univariate analysis data to find the independent risk factors for post-OLT AKI. After multivariable risk adjustment for potential confounding factors (Table 5), cold ischemia time (OR, 1.061; 95% CI, 1.032–1.090; P < 0.001), warm ischemia time (OR, 1.028; 95% CI, 1.011–1.046; P = 0.001), blood loss (OR, 230; 95% CI, 1.001–1.451; P < 0.001), SCr (OR, 1.352; 95% CI, 1.181–1.763; P < 0.001), treatment period with dopamine (OR, 1.854; 95% CI, 1.425–2.281; P < 0.001), overexposure to CNI (OR, 2.841; 95% CI, 1.762–5.360; P < 0.001), and combined MMF use were still positively correlated with post-OLT AKI (P = 0.023).
Table 5. Multivariate analysis of the risk factors of post-OLT AKI.
| Factors | OR | 95% CI | P value | ||
|---|---|---|---|---|---|
| Cold ischemia time | <7 h | 1 | — | — | |
| >7 h | 1.061 | 1.032 | 1.09 | <0.001 | |
| Warm ischemia time | <10 min | 1 | — | — | |
| >10 min | 1.028 | 1.011 | 1.046 | 0.001 | |
| Blood loss | <2500 mL | 1 | — | — | |
| >2500 mL | 1.23 | 1.001 | 1.451 | <0.001 | |
| SCr | <354 μmol/L | 1 | — | — | |
| >354 μmol/L | 1.352 | 1.181 | 1.763 | <0.001 | |
| Treatment with dopamine | <6 days | 1 | — | — | |
| >6 days | 1.854 | 1.425 | 2.281 | <0.001 | |
| Overexposure to CNI | No | 1 | — | — | |
| Yes | 2.841 | 1.762 | 5.360 | <0.001 | |
| Combined use of MMF | Yes | 1 | — | — | |
| No | 2.184 | 1.338 | 6.89 | 0.023 | |
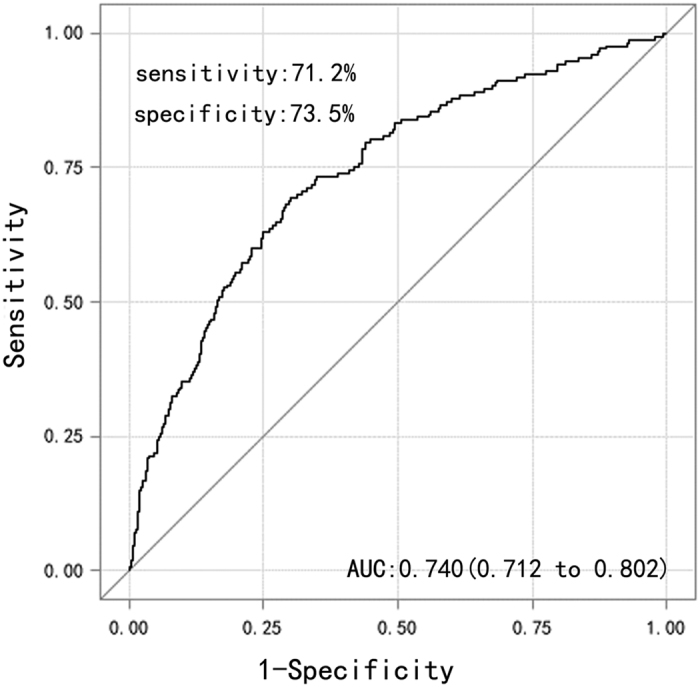
Moreover, the predictive ability of these seven risk factors for post-OLT AKI was evaluated by using ROC analysis. The AUC for this model was 0.740 (95% CI, 0.712–0.802; sensitivity = 71.2%; specificity = 73.5%; Fig. 3). Furthermore, the H–L test was conducted for logistic regression, and no evidence showed lack of fit (P = 0.142; data no shown). In summary, all these results indicated that the seven factors were independent risk factors the occurrence of post-OLT AKI.
Figure 3. Receiver operating characteristic (AUC) cure of the risk factors for the occurrence post-OLT AKI.
Discussion
AKI is a common and severe complication after OLT and seriously affects the prognosis of OLT patients2,33,34. Because the major proportion of transplanted organs in China were obtained from illegal and unethical approaches, such as convicted prisoners and human organ traders, before 2015, limited information regarding organ transplantation, including post-OLT complications, was reported in public or international journals29,30. Herein, we reported the incidence rate of post-OLT AKI and analyzed its possible risk factors in China. In addition, this study reported this complication with analysis of multicenter and large samples in China.
In the study, we discovered that the incidence rate of post-OLT AKI was 3.97%, which was less than the 11.1–90% reported in previous studies1,3,6,35,36,37, and similar to the finding of Kirnap et al.’s study (5%)20. This relatively low occurrence rate benefited from the development of transplantation surgery and application of precautionary measures to prevent complications after OLT17,18,22,38. Compared with the occurrence rate approximately 10 years prior10,34,39,40, it decreased gradually in recent years20,35, and our result is consistent with this tendency. In addition, using the difference diagnosis criteria of AKI also could be a reason for the reporting of different rates. One study have reported a 51.5% occurrence rate using the definition of SCr > 1.5 mg/dL41, and 17% was reported by using SCr > 2 mg/dL in another study11. In the present study, we defined AKI based on the RIFLE classification, such as that used in Liu et al.’s study16. This comprehensive definition may account for the relatively low incidence rate of AKI observed.
The 1- and 5-year survival rates were speculated as important marks for OLT prognosis. Previous studies have demonstrated that post-OLT AKI was associated with an increasing eight-fold mortality risk42, prolonged stay in the intensive care unit, and augmented hospital costs13. In our investigation, the 1- and 5-year CSRs were 83.34% and 68.14%, respectively, in all eligible recipients, but had a significant difference in subgroups (AKI group, 33.95% and 25.24%; non-AKI group, 86.34% and 70.05%; P < 0.05). This result was similar to that of Nonthasoot et al.’s study in Thailand (1- and 5-year CSRs: 85% and 69%)36, but remarkably differed from that of Kirnap et al.’s study in Turkey (1-year CSR in the AKI and non-AKI groups was 82% and 89%, respectively)20 and O’Riordan et al.’s study in Denmark (1-year CSR in the AKI group was 47.5%)35. The reason for these differences may be related to economic level and health care quality (Denmark and Turkey are developed countries, whereas China and Thailand are low-income countries). Studies have demonstrated that patients with AKI could have a recovery rate of 97% and result in a good prognosis with appropriate treatments13,43. In addition, with regard to the recipients, the first-month CSR was 38% in AKI group, which was lower than that in the non-AKI group. The exact reasons were unclear, and it may be related to poor management of AKI recipients44.
Donor factors were considered important in organ transplantation and directly affected survival rates of recipients14,31,45,46,47. Here, our results indicated that China classification of DCD, donor cause of death, and blood constituent have shown a remarkable difference between the AKI and non-AKI groups. However, no evidence supports in proving that the donor factors included in our study were independent risk factors associated with post-OLT AKI. Unfortunately, other donor factors, such as BMI47, graft weight and size24, and pre-arrest SCr31 confirmed to be related to AKI after OLT in previous studies, were not registered in our database, and feasible comparison cannot be conducted.
Furthermore, we analyzed the relationship between the features of recipient and occurrence of post-OLT AKI. The result indicated that males account for 73.6% of all recipients, which may be attributed to a higher occurrence rate of hepatocarcinoma (male:female = 3:1) in males48.
Besides, in preoperative factor analysis, we found that blood type compatibility, hepatocarcinoma-related diseases, cirrhosis, hepatic failure, and SC were significantly different between the AKI and non-AKI groups, which were correlated with previous studies11,16,19,35,49,50. However, in contrast with Utsumi et al.’s24 and Yuan et al.’s19 studies, no evidence supports that higher MELD score and Child–Pugh grade were related to post-OLT AKI in our investigation and Faenza et al.’s49. The reasons may be related to (1) the inclusion of patients in our study with relatively low SCr level (we excluded patients who are on RRT as they are not “at risk” for post-operative renal injury); (2) the MELD score lacking systematic observed indicators, such as ascites, blood loss, hepatic encephalopathy, etc., but not just SCr, INR, total bilirubin; hence, estimating the prognosis of patients who have no preoperative AKI was not suitable51; and (3) the Sequential Organ Failure Assessment score having the best discriminatory power for prediction of short-term mortality after OLT as indicated by Elsayed et al.52. In addition, hypoalbuminemia in Cabezuelo et al.’s study3 showed a significant difference between two groups, but it was contradictory to our results. The exact reasons were still unclear, it might be probably due to the following: (1) the small sample size in Cabezuelo et al.’s study (184 patients); (2) exclusion of the preoperative RRT population in our study, but not in their study; and (3) the development of parenteral alimentation, which can regulate serum protein level in recent years.
In the present study, we found an obvious difference between the AKI and non-AKI groups, regarding intraoperative blood loss, transfusion of blood products, cold or warm ischemia time, and total volume of intravenous infusion. These findings were consistent with previous studies6,16,18,19,24,25,49. However, regarding the duration of surgery, no significant difference was found between groups in our results, but some studies17,32,53 inferred that this can be attributed to post-OLT AKI. In fact, the duration of surgery is influenced by many factors. Only those factors, such as prolonged blood loss, long-standing hypoxemia, and so on, can result in the instability of systemic hemodynamics and kidney injury after surgery. A good explanation why venovenous bypass was not related to post-OLT AKI was because it can regulate the stability of systemic hemodynamics.
In addition, the changes of hemodynamic factors during surgery have an effect on the development of post-OLT AKI. Recipients requiring vasoactive medications usually had lower MAP and systolic arterial pressure during the anhepatic phase, and lower cardiac index during the post-anhepatic phase. Renal blood flow reduction could affect postoperative renal function through different mechanisms. Dealing with the changes of OLT hemodynamics was difficult. Bilbao et al.9 confirmed that patients with more vasoactive drugs use have a higher probability of requiring post-OLT RRT. Our results that post-OLT AKI group had a greater need for adrenergic agonist drugs and blood products than non-AKI group during and after OLT were in line with these studies3,54,55. Noradrenaline, dobutamine, and dopamine all showed a strong correlation with the occurrence of post-OLT AKI, and the treatment period with dopamine (>6 days) was an independent factor for post-OLT AKI. Thus, we believe that patients with greater hemodynamic instability are more susceptible to developing AKI3.
Moreover, we also analyzed the association between post-OLT AKI and other postoperative complications, and results indicated significant difference between the two groups regarding postoperative infection, diabetes, and hypertension, which were similar to previous studies6,19,24,35. The possible reason was these complications could increase the burden of renal function through different mechanisms in early post-OLT24. Furthermore, we compared the hospital stay length and follow-up duration in the AKI and non-AKI groups. Surprisingly, compared with those without AKI, the mean length of hospital stay was significantly lower for those with AKI. The reasons may be that (1) more patients with AKI died within the first 2 weeks post-transplantation; (2) patients with AKI were transferred to nephrology department or other kidney disease centers for RRT, because some organ transplant centers, in China, had not equipment for RRT; (3) patients with AKI could not afford for the cost of RRT and left hospitals reluctantly within two weeks post-transplantation, as the health insurance, in China, did not cover the cost of organ transplantation and RRT. In addition, follow-up duration may be related to the survival rate after surgery, and patients with post-OLT AKI have lower survival rate than those without AKI.
Overexposure to CNI has been established as an independent factor for postoperative AKI9,24,56,57. The direct toxic effects of CNI use were the formation of thrombotic microangiopathy, such as hemolytic uremic syndrome or thrombotic thrombocytopenic purpura24,57,58. The severity of renal dysfunction is affected along with the change of CNI concentration. De Simone et al.57,58 found that everolimus with reduced tacrolimus can improve renal function after liver transplantation. In our analysis, the initial induction of CNI with tacrolimus or cyclosporine was introduced in the AKI group than the non-AKI group. A significant higher average trough level of tacrolimus was found in the AKI group than the non-AKI group. Reduced CNI levels combined with MMF use can maintain adequate immunosuppression and mitigate the incidence of post-OLT AKI. However, CNI use without combined MMF was an independent risk factor of post-OLT AKI after matching, and this result was in agreement with previous reports24,32. In addition, overexposure to CNI may also be considered as an independent factor for postoperative AKI after matching. This may be related to MMF use with reducing CNI dose in patients with high CNI trough levels, or MMF use as an initial immunosuppressive protocol. Thus, we speculate that reducing CNI dosage with MMF use can contribute to the prevention of severe AKI and should be taken into consideration in the use of nephrotoxic immunosuppressive regimens in liver transplantation24,54.
Finally, multivariate analysis revealed that the independent risk factors for the occurrence of post-OLT AKI included intraoperative blood loss, pre-SCr, cold ischemia time, warm ischemia time, treatment period with dopamine, overexposure to CNI, and combined MMF use after adjusting for other variables. Some studies18,19,24,59 identified intraoperative blood loss (>2500 mL) as an independent risk factor for the occurrence of AKI. Our results support the previous findings that blood loss could result in hypotension, unstable systemic hemodynamics, and negative influence on post-transplant renal function. However, although we define SCr > 354 μmol/L as one of the diagnostic markers of AKI (failure stage), some slight injury to the kidney (risk and injury stages) might already have existed in patients before transplantation. These slight reversible injuries progress into severe AKI (failure stage) under the influence of the instability of intraoperative systemic hemodynamics. Besides, we also confirmed that cold (>6 h) and warm ischemia times (>10 min) are independent risk factors of post-OLT AKI, which are similar to the results of Leithead et al.55, Kubal et al.60, and other studies54,61,62. Although grafts are separated from donors and cut down the bloodstream, cell metabolism continues, and the metabolic waste of aerobic and anaerobic metabolism cannot be eliminated, leading to cell apoptosis. Ischemia–reperfusion injury can activate cell signals linked to invasion and migration by disrupting the hepatic microcirculation. A cellular cascade can be activated by ischemia, leading to large cellular proliferation, growth, and angiogenesis61,62. As a result, the “abnormal graft” increases the burden of the kidney, resulting in AKI. Besides, ROC analysis (AUC = 0.740) and H–L test (P = 0.142) also provided evidence for the prediction of post-OLT AKI with this seven-factor model.
In summary, to mitigate postoperative AKI incidence, the following terms should at least be performed: (i) strictly monitoring the dosage of CNI and combine it with MMF duly, (ii) reducing those risk factors influenced the hemodynamic stability of patients as much as possible during and after surgery, and (iii) reducing the ischemia time of grafts.
This study has some limitations, including a not comprehensive retrospective design and the difference of diagnosis and treatment level of different centers and hospitals, which contribute to missing or absent important data. Further studies should focus on the economic level, donor factors, induction therapy, and postoperative complications to better understand the pathophysiology of post-OLT AKI and to investigate factors associated with worse outcome. Early recognition, prevention, and treatment of AKI after OLT may be useful in determining its prognosis.
In conclusion, AKI is a common complication of OLT. Blood loss, cold ischemia time, warm ischemia time, preoperative SCr level, treatment period with dopamine, overexposure to CNI, and combined MMF use are the independent risk factors of post-OLT AKI, and not MELD score, Child–Pugh grade, preoperative hypoalbuminemia, cirrhosis, hepatic failure, and duration of surgery.
Methods
On January 6, 2016, we collected 5074 donation after cardiac death (DCD) OLT recipients who underwent surgery between January 1, 2010, and December 31, 2015. These data were obtained from 86 academic hospitals or transplant centers in mainland China and registered in a legal organ donation system, known as the China Liver Transplant Registry (CLTR)63, which was set up in 2005 and regulated by the Chinese government. The study was approved by the ethics committees of China Medical University and carried out in accordance with the principles of the Declaration of Helsinki as revised in 2008. All participants were supplied with oral and written information and gave written consent prior to inclusion and informed consent was obtained from all the study participants.
Inclusion and exclusion criteria
We included all patients (>18 years) who underwent OLT between January 1, 2010, and December 31, 2015. Exclusion criteria were the following: (1) re-transplantation (n = 8); (2) RRT before OLT (n = 321); (3) combined transplantations (e.g., liver and kidney combined transplantations) (n = 175); (4) kidney transplantation before OLT (n = 19); or (5) living donor (n = 61).
Definition of AKI
Based on the RIFLE classification8, AKI after OLT was classified into three groups (risk, injury, and failure) based on relative changes of SCr or urine output. We defined AKI as renal function reaching the level of failure (an increase in SCr ≥ 3.0 baseline, decrease in GFR ≥ 75%, or absolute SCr ≥ 354 μmol/L, with an acute increase of at least 44 μmol/L and/or urine output < 0.3 mL/kg/h ≥ 24 h or anuria ≥ 12 h) within their hospital stay period.
Chinese criteria for human organ donation
Classification I of Chinese criteria (C-I): donation after brain death64.
Classification II of Chinese criteria (C-II): DCD, which includes classifications I–V of the Maastricht criteria65.
Classification III of Chinese criteria (C-III): donation after brain death awaiting cardiac death (DBCD), which is similar to classification IV of the Maastricht criteria. The warm ischemia time of donor organ is controllable.
In China, no law exists regarding identifying brain death and allowing organ donation from patients or individuals who are brain dead. Second, the family members cannot accept that organs are donated from donors whose heart is still beating. Thus, we mostly used the DBCD criteria in China. These criteria are in line with Chinese circumstances.
Liver donor data
The following data were analyzed: age, sex, blood type, China classification of DCD64, cause of donor death, past medical history (including diabetes, hypertension, respiratory disease, etc.), blood components (including Na+, K+, blood urea nitrogen [BUN], albumin, alkaline phosphatase [ALP], blood sugar, total bilirubin, SCr, gamma-glutamyl transferase [GGT], aspartate aminotransferase [AST], and alanine aminotransferase [ALT]).
Pretransplantation recipient data
The following baseline characteristics were analyzed: age, sex, body mass index (BMI), and blood type. Furthermore, we analyzed underlying liver disease (including hepatocarcinoma related, Hepatitis B, cirrhosis, hepatic failure), dialysis, total bilirubin, SCr, international normalized ratio (INR), MELD score66, Child–Pugh grade67, and albumin.
Intraoperative data
All patients were transplanted without venovenous bypass68, we analyzed operative time, cold ischemia time, warm ischemia time, intraoperative blood loss, blood product transfusion (including whole blood, red blood cells, cell saver, autologous blood cell transfusion, fresh frozen plasma, and platelet), and total intravenous infusion. In addition, noradrenaline was used when the cardiac preload and systolic function were normal, but systemic vascular resistance index was low, and dobutamine was used when systolic dysfunction existed to maintain the essential hemodynamic objective (mean arterial pressure [MAP] ≥ 70 mmHg) and ensure sufficient cardiac function3.
Postoperative data
We collected the following data: postoperative complications (including intraperitoneal hemorrhage, biliary complications, vascular complications, abnormal graft function, postoperative infection, diabetes, new-onset diabetes, hypertension, new-onset hypertension, cytomegalovirus infection, pleural effusion, pneumonedema, and intra-abdominal abscess), hospital day, and follow-up duration. The conditions of the use of immunosuppression, induction therapy, steroid drugs, CNIs were analyzed. The CNIs were introduced on day 0 after surgery. Outpatient follow-up was performed once a week for the first 3 months and every 2 weeks within the first year after the surgery. The causes of death for each patient were recorded. All follow-up data were collected until December 31, 2015.
Statistical analysis
We first compared the data distribution of each covariate between the exposed and the non-exposed groups, using the Student t test (normal distribution) or Mann–Whitney U test (non-normal distribution) for continuous variables and X2 tests or Fisher’s exact test for categorical data, as appropriate. The Kaplan–Meier curves were constructed to determine survival and compared using the log rank test. A series of exact logistic regression models were implemented to obtain bivariate estimates and confidence intervals (CIs) with a small sample size. Significant predictors (P < 0.05) and only the variable that was more biologically plausible were included in subsequent multivariate model building with adjustments for covariates. Multivariate logistic regression models were used to identify whether these covariates had an independent effect on AKI after OLT. A receiver operating characteristic (ROC) curve and the goodness-of-fit test of Hosmer–Lemeshow (H–L) were used to assess the predictive power of the logistic regression model. We calculated the area under curve (AUC) and decided on a cutoff value by using the Youden method. All results were expressed as means and standard errors of the mean (SEMs). All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, United States). A P-value of <0.05 was considered significant.
Additional Information
How to cite this article: Zongyi, Y. et al. Risk factors of acute kidney injury after orthotopic liver transplantation in China. Sci. Rep. 7, 41555; doi: 10.1038/srep41555 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
We would like to thank the China Liver Transplant Registry (CLTR) and all organ transplant centers in China, for their help on the collection and analysis of the data. This study was supported by the National Natural Science Foundation of China (81370882), and the Natural Science Foundation of Liaoning Province (2013021058).
Footnotes
The authors declare no competing financial interests.
Author Contributions All authors contributed to the intellectual development of this paper. Li Baifeng had the original idea for the study. Yin Zongyi supervised data collection. Li Baifeng and Yin Zongyi designed the study and supervised data analysis. Yin Zongyi performed the analysis and wrote the first draft. Li Baifeng, Yin Zongyi, Zou Funian, Li Hao and Wang Xin provided critical corrections to the manuscript and approved the final manuscript.
References
- McCauley J., Van Thiel D. H., Starzl T. E. & Puschett J. B. Acute and chronic renal failure in liver transplantation. Nephron 55, 121–128 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. et al. Acute renal failure in orthotopic liver transplantation. Ir J Med Sci 165, 271–273 (1996). [DOI] [PubMed] [Google Scholar]
- Cabezuelo J. B. et al. Risk factors of acute renal failure after liver transplantation. Kidney Int 69, 1073–1080, doi: 10.1038/sj.ki.5000216 (2006). [DOI] [PubMed] [Google Scholar]
- Chen H. P., Tsai Y. F., Lin J. R., Liu F. C. & Yu H. P. Incidence and Outcomes of Acute Renal Failure Following Liver Transplantation: A Population-Based Cohort Study. Medicine (Baltimore) 94, e2320, doi: 10.1097/MD.0000000000002320 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMattina J. C. et al. Intraoperative Continuous Veno-Venous Hemofiltration Facilitates Surgery in Liver Transplant Patients With Acute Renal Failure. Transplant Proc 47, 1901–1904, doi: 10.1016/j.transproceed.2015.05.005 (2015). [DOI] [PubMed] [Google Scholar]
- Sirivatanauksorn Y. et al. Renal dysfunction after orthotopic liver transplantation. Transplant Proc 46, 818–821, doi: 10.1016/j.transproceed.2013.11.124 (2014). [DOI] [PubMed] [Google Scholar]
- Lekerika N. et al. Predicting fluid responsiveness in patients undergoing orthotopic liver transplantation: effects on intraoperative blood transfusion and postoperative complications. Transplant Proc 46, 3087–3091, doi: 10.1016/j.transproceed.2014.10.005 (2014). [DOI] [PubMed] [Google Scholar]
- Bellomo R. et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8, R204–212, doi: 10.1186/cc2872 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbao I. et al. Risk factors for acute renal failure requiring dialysis after liver transplantation. Clin Transplant 12, 123–129 (1998). [PubMed] [Google Scholar]
- Pham P. T., Pham P. C. & Wilkinson A. H. The kidney in liver transplantation. Clin Liver Dis 4, 567–590 (2000). [DOI] [PubMed] [Google Scholar]
- Naik P., Premsagar B. & Mallikarjuna M. Acute renal failure in liver transplant patients: Indian study. Indian J Clin Biochem 30, 94–98, doi: 10.1007/s12291-013-0410-4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Lago A. M. et al. Evolution of hepatorenal syndrome after orthotopic liver transplantation: comparative analysis with patients who developed acute renal failure in the early postoperative period of liver transplantation. Transplant Proc 39, 2318–2319, doi: 10.1016/j.transproceed.2007.07.070 (2007). [DOI] [PubMed] [Google Scholar]
- Yang L. et al. Acute kidney injury in China: a cross-sectional survey. Lancet 386, 1465–1471 (2015). [DOI] [PubMed] [Google Scholar]
- Iwata H. et al. Negative prognostic impact of renal replacement therapy in adult living-donor liver transplant recipients: preoperative recipient condition and donor factors. Transplant Proc 46, 716–720, doi: 10.1016/j.transproceed.2013.11.113 (2014). [DOI] [PubMed] [Google Scholar]
- Zhu F. X. et al. [Risk factors of renal failure in the early post-liver transplantation period]. Zhonghua Gan Zang Bing Za Zhi 13, 168–170 (2005). [PubMed] [Google Scholar]
- Liu S. et al. The effects of intraoperative cryoprecipitate transfusion on acute renal failure following orthotropic liver transplantation. Hepatol Int 7, 901–909, doi: 10.1007/s12072-013-9457-9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y. et al. Factors related to post-liver transplantation acute renal failure. Transplant Proc 38, 2982–2984, doi: 10.1016/j.transproceed.2006.08.156 (2006). [DOI] [PubMed] [Google Scholar]
- Smoter P. et al. Risk factors of acute renal failure after orthotopic liver transplantation: single-center experience. Transplant Proc 46, 2786–2789, doi: 10.1016/j.transproceed.2014.09.044 (2014). [DOI] [PubMed] [Google Scholar]
- Yuan C. H. et al. [The influential factors and clinical significance of acute renal failure complicated to orthotopic liver transplantation]. Zhonghua Wai Ke Za Zhi 49, 1003–1006 (2011). [PubMed] [Google Scholar]
- Kirnap M. et al. Acute renal injury in liver transplant patients and its effect on patient survival. Exp Clin Transplant 12 Suppl 1, 156–158 (2014). [PubMed] [Google Scholar]
- Komurcu O. et al. Postoperative effects of intraoperative hyperglycemia in liver transplant patients. Exp Clin Transplant 13 Suppl 1, 335–339 (2015). [DOI] [PubMed] [Google Scholar]
- Lee J. P. et al. Risk factors for consequent kidney impairment and differential impact of liver transplantation on renal function. Nephrol Dial Transplant 25, 2772–2785, doi: 10.1093/ndt/gfq093 (2010). [DOI] [PubMed] [Google Scholar]
- Tinti F. et al. Acute renal failure in liver transplant recipients: role of pretransplantation renal function and 1-year follow-up. Transplant Proc 43, 1136–1138, doi: 10.1016/j.transproceed.2011.02.042 (2011). [DOI] [PubMed] [Google Scholar]
- Utsumi M. et al. Risk factors for acute renal injury in living donor liver transplantation: evaluation of the RIFLE criteria, http://www.ncbi.nlm.nih.gov/pubmed/23855657 (2013). [DOI] [PubMed]
- Wenger U. et al. The relationship between preoperative creatinine clearance and outcomes for patients undergoing liver transplantation: a retrospective observational study. BMC Nephrol 14, 37, doi: 10.1186/1471-2369-14-37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Shusen Y. J. & Zhang Wu. current development of liver transplantation in China. J Clin Hepatol 30 (2014). [Google Scholar]
- Shusen Z. Chinese organ transplantation into the world. http://www.cma.org.cn/bainian/ (Accessed in may 16, 2016, in Chinese) (2015).
- Huang J., Millis J. M., Mao Y., Millis M. A., Sang X. & Zhong S. A pilot programme of organ donation after cardiac death in China. Lancet 397, 862–865, doi: 10.1016/S0140-6736(11)61086-6 (2012). [DOI] [PubMed] [Google Scholar]
- Huang J. Ethical and legislative perspectives on liver transplantation in the People’s Republic of China. Liver Transpl 13, 193–196, doi: 10.1002/lt.21081 (2007). [DOI] [PubMed] [Google Scholar]
- Caplan A. L., Danovitch G., Shapiro M., Lavee J. & Epstein M. Time for a boycott of Chinese science and medicine pertaining to organ transplantation. The Lancet 378, 1218, doi: 10.1016/s0140-6736(11)61536-5 (2011). [DOI] [PubMed] [Google Scholar]
- Sohrabi S. et al. Donation after cardiac death kidneys with low severity pre-arrest acute renal failure. Am J Transplant 7, 571–575, doi: 10.1111/j.1600-6143.2006.01639.x (2007). [DOI] [PubMed] [Google Scholar]
- Park M. H. et al. Clinical Risk Scoring Models for Prediction of Acute Kidney Injury after Living Donor Liver Transplantation: A Retrospective Observational Study. PLoS One 10, e0136230, doi: 10.1371/journal.pone.0136230 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan Menon K. V., Nyberg S. L., Harmsen W. S. et al. MELD and other factors associated with survival after liver transplantation. Am J Transplant 4, 819 (2004). [DOI] [PubMed] [Google Scholar]
- Cedidi C. et al. Urodilatin: a new approach for the treatment of therapy-resistant acute renal failure after liver transplantation. Eur J Clin Invest 24, 632–639 (1994). [DOI] [PubMed] [Google Scholar]
- O’Riordan A. et al. Acute renal disease, as defined by the RIFLE criteria, post-liver transplantation. Am J Transplant 7, 168–176, doi: 10.1111/j.1600-6143.2006.01602.x (2007). [DOI] [PubMed] [Google Scholar]
- Nonthasoot B. et al. Orthotopic liver transplantation at King Chulalongkorn Memorial Hospital: a report. J Med Assoc Thai 98 Suppl 1, S127–130 (2015). [PubMed] [Google Scholar]
- Biagioni E. et al. Acute renal failure and renal replacement therapy in the postoperative period of orthotopic liver transplant patients versus nonelective abdominal surgery patients. Transplant Proc 43, 1145–1147, doi: 10.1016/j.transproceed.2011.02.051 (2011). [DOI] [PubMed] [Google Scholar]
- Hovaguimian F., Schlapfer M. & Beck-Schimmer B. Organ protection in allograft recipients: anesthetic strategies to reduce postoperative morbidity and mortality. Curr Opin Organ Transplant 19, 121–130, doi: 10.1097/MOT.0000000000000062 (2014). [DOI] [PubMed] [Google Scholar]
- Navarro A. P. et al. Renal transplants from category III non-heart-beating donors with evidence of pre-arrest acute renal failure. Transplant Proc 38, 2635–2636, doi: 10.1016/j.transproceed.2006.08.007 (2006). [DOI] [PubMed] [Google Scholar]
- Kuse E. R. et al. [Urodilatin (INN: ularitide). A new peptide in the treatment of acute kidney failure following liver transplantation]. Anaesthesist 45, 351–358 (1996). [DOI] [PubMed] [Google Scholar]
- Bilbao I., Charco R., Balsells J. et al. Risk factors for acute renal failure requiring dialysis after liver transplantation. Clin Transplant 12, 123 (1998). [PubMed] [Google Scholar]
- Yalavarthy, R., Edelstein C. L. & Teitelbaum I. Acute renal failure and chronic kidney disease following liver transplantation. Hemodial Int 11 (Suppl 3), S7 (2007). [DOI] [PubMed] [Google Scholar]
- Cabezuelo J. B., Ramirez P., Rios A. et al. Risk factors of acute renal failure after liver transplantation. Kidney Int 69 (2006). [DOI] [PubMed] [Google Scholar]
- Khera N. Beyond Biology: Impact of Center- and Country-specific Economic Factors on Outcomes After Hematopoietic Cell Transplantation. EBioMedicine 2, 1869–1870, doi: 10.1016/j.ebiom.2015.12.005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Laudo M. et al. “Combined liver and dual kidney transplant: role in expanded donors”. Liver Transpl doi: 10.1002/lt.24472 (2016). [DOI] [PubMed] [Google Scholar]
- Zhang L. et al. Preliminary analysis of factors influencing organ donation rates in China. Transplant Proc 43, 1421–1424, doi: 10.1016/j.transproceed.2011.01.166 (2011). [DOI] [PubMed] [Google Scholar]
- Andert A. et al. Liver Transplantation and Donor Body Mass Index >30: Use or Refuse? Ann Transplant 21, 185–193 (2016). [DOI] [PubMed] [Google Scholar]
- Chen W. et al. Cancer statistics in China, 2015. CA Cancer J Clin 66, 115–132, doi: 10.3322/caac.21338 (2016). [DOI] [PubMed] [Google Scholar]
- Faenza S. et al. Acute renal failure after liver transplantation in MELD era. Transplant Proc 39, 1945–1946, doi: 10.1016/j.transproceed.2007.05.050 (2007). [DOI] [PubMed] [Google Scholar]
- Chuang F. R. et al. Acute renal failure after cadaveric related liver transplantation. Transplant Proc 36, 2328–2330, doi: 10.1016/j.transproceed.2004.07.002 (2004). [DOI] [PubMed] [Google Scholar]
- Feng Z. Z. et al. Renal insufficiency after liver transplantation in the MELD era compared to the pre-MELD era. Clin Transplant 23, 637–642, doi: 10.1111/j.1399-0012.2009.01020.x (2009). [DOI] [PubMed] [Google Scholar]
- Elsayed F. G., Sholkamy A. A., Elshazli M., Elshafie M. & Naguib M. Comparison of different scoring systems in predicting short-term mortality after liver transplantation. Transplant Proc 47, 1207–1210, doi: 10.1016/j.transproceed.2014.11.067 (2015). [DOI] [PubMed] [Google Scholar]
- Hilmi I. A. et al. Acute kidney injury following orthotopic liver transplantation: incidence, risk factors, and effects on patient and graft outcomes. Br J Anaesth 114, 919–926, doi: 10.1093/bja/aeu556 (2015). [DOI] [PubMed] [Google Scholar]
- Barreto A. G. et al. Risk factors for acute kidney injury and 30-day mortality after liver transplantation. Ann Hepatol 14, 688–694 (2015). [PubMed] [Google Scholar]
- Leithead J. A., Rajoriya N., Gunson B. K., Muiesan P. & Ferguson J. W. The evolving use of higher risk grafts is associated with an increased incidence of acute kidney injury after liver transplantation. J Hepatol 60, 1180–1186, doi: 10.1016/j.jhep.2014.02.019 (2014). [DOI] [PubMed] [Google Scholar]
- Boudjema K. et al. Reduced-dose tacrolimus with mycophenolate mofetil vs. standard-dose tacrolimus in liver transplantation: a randomized study. Am J Transplant 11, 965–976, doi: 10.1111/j.1600-6143.2011.03486.x (2011). [DOI] [PubMed] [Google Scholar]
- Rodriguez-Peralvarez M. et al. Tacrolimus trough levels, rejection and renal impairment in liver transplantation: a systematic review and meta-analysis. Am J Transplant 12, 2797–2814, doi: 10.1111/j.1600-6143.2012.04140.x (2012). [DOI] [PubMed] [Google Scholar]
- De Simone P. et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant 12, 3008–3020, doi: 10.1111/j.1600-6143.2012.04212.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey C. K. et al. Intraoperative blood loss in orthotopic liver transplantation: The predictive factors. World J Gastrointest Surg 7, 86–93, doi: 10.4240/wjgs.v7.i6.86 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubal C. et al. Optimization of Perioperative Conditions to Prevent Ischemic Cholangiopathy in Donation After Circulatory Death Donor Liver Transplantation. Transplantation Apr 29, doi: 10.1097/TP.0000000000001204 (2016). [DOI] [PubMed] [Google Scholar]
- Kornberg A., Witt U., Kornberg J., Friess H. & Thrum K. Extended Ischemia Times Promote Risk of HCC Recurrence in Liver Transplant Patients. Dig Dis Sci 60, 2832–2839, doi: 10.1007/s10620-015-3541-z (2015). [DOI] [PubMed] [Google Scholar]
- Nagai S. et al. Ischemia time impacts recurrence of hepatocellular carcinoma after liver transplantation. Hepatology 61, 895–904, doi: 10.1002/hep.27358 (2015). [DOI] [PubMed] [Google Scholar]
- China Liver Transplant Registry (CLTR). http://www.cltr.org/pages/index.jsp (accessed in January 6, 2016, in Chinses).
- Liu Y. F. Guidelines for the work of donation after cardiac death of China. China J Transplant (Electronic Edition), August 2012, Vol 6. No.3 (2012).
- Evrard P. & Belgian Working Group on, D. C. D. N. P. Belgian modified classification of Maastricht for donors after circulatory death. Transplant Proc 46, 3138–3142, doi: 10.1016/j.transproceed.2014.09.169 (2014). [DOI] [PubMed] [Google Scholar]
- Martin A. P., Bartels M., Hauss J. & Fangmann J. Overview of the MELD score and the UNOS adult liver allocation system. Transplant Proc 39, 3169–3174, doi: 10.1016/j.transproceed.2007.04.025 (2007). [DOI] [PubMed] [Google Scholar]
- Kamath P. S., Wiesner R. H., Malinchoc M., Kremers W., Therneau T. M., Kosberg C. L. et al. A model to predict survival in patients with end-stage liver disease Hepatology 33, 464–470 (2001). [DOI] [PubMed] [Google Scholar]
- McCormack L., S. M. & Clavien P.-A. The transplant operation. In Medical Care of Liver Transplantation. Edited by Killenberg P., Clavien P.-A.Oxford, England, Blackwell Publishing, 229–241 (2006). [Google Scholar]