Abstract
Context:
Regional citrate anticoagulation for continuous renal replacement therapy is associated with a longer filter-life, less bleeding events and improved mortality. Problems associated with using Prismocitrate 10/2 solution in continuous renal replacement therapy, include hypomagnesemia, hypophosphatemia and the need for additional bicarbonate infusion.
Aims:
This study uses the new Prismocitrate 18/0 solution for improved buffer balance and Phoxilium solution for a more favourable electrolyte profile.
Settings and Design:
A retrospective analysis of patients who underwent continuous venovenous hemofiltration (CVVH) using Prismocitrate 18/0 and Phoxilium in our 21-bed ICU was conducted from March to July 2014.
Methods and Material:
Continuous venovenous hemofiltration (CVVH) was performed at fixed rate by using Prismocitrate 18/0 predilution at 1250 ml/hour, a blood flow rate of 110 ml/min and post-replacement with Phoxilium at 1250 ml/hr. CVVH was run for 72 h or until filter clotting, transportation, or achievement of the clinical target.
Statistical Analysis Used:
The results were displayed as the median with the interquartile range (IQR). The trend in pH, electrolytes, and base excess are shown using a standard box plot. All analyses were performed by the Statistical Package for Social Science for Windows, version 17 (SPSS, Chicago, IL, USA).
Results:
Forty-five CVVH episodes were analysed. The median circuit lifetime was 44 h (interquartile range, IQR 29-55). Metabolic alkalosis, hypophosphatemia and hypomagnesemia occurred in 8.3%, 3.5% and 40.2% of the blood samples, respectively. No patient developed hypokalemia or citrate toxicity.
Conclusions:
This new CVVH regime is safe and easy to administer for critically ill patients.
Keywords: Acute renal failure, anticoagulation, citrate, continuous renal replacement therapy
Introduction
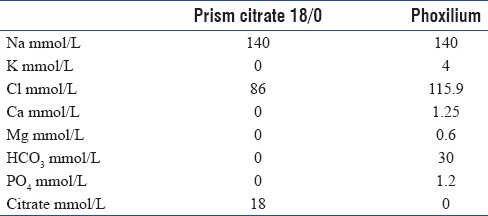
Continuous renal replacement therapy (CRRT) is an important treatment modality in intensive care of acute kidney injury, which is common in critically ill patients. Clotting of the circuit is a major factor contributing to treatment interruption.[1] Regional citrate anticoagulation (RCA) for CRRT is associated with a longer filter-life, less bleeding events, and improved mortality.[2,3] We previously reported the use of a commercially prepared citrate solution, Prismocitrate 10/2 (Baxter Gambro, Deerfield, IL, USA) with good metabolic control and a median circuit lifetime ranging from 26 to 50 h.[4,5] However, hypomagnesemia (41.6%) and hypophosphatemia (17.6%) were commonly encountered during previously reported citrate CRRT with Prismocitrate 10/2.[4] Moreover, to provide adequate buffer balance, a separate sodium bicarbonate infusion had to be used. In an attempt to circumvent these problems, we used another proprietary solution with higher citrate content (Prismocitrate 18/0, Baxter Gambro, Deerfield, IL, USA) and a phosphate containing hemofiltration replacement solution (Phoxilium, Baxter Gambro, Deerfield, IL, USA). The compositions of the solutions are shown in Table 1. The combined use of these two solutions has been reported in a number of studies.[6,7,8] The higher citrate concentration consequentially requires the titration of the replacement fluid flow rate or the blood flow rate to avoid overt metabolic alkalosis. Here, we optimize the use of these solutions in combination to provide a safe and effective alternative for critically ill patients who require CRRT.
Table 1.
Composition of the solutions
Subjects and Methods
A retrospective analysis of patients who underwent continuous venovenous hemofiltration (CVVH) using Prismocitrate 18/0 and Phoxilium in our 21-bed Intensive Care Unit (ICU) was conducted from March to July 2014. A treatment episode was defined as the lifespan of a single hemofilter. Indications for starting CVVH included fluid overload unresponsive to diuretic treatment, severe acid-base or electrolyte imbalance, and severe sepsis. Those who were contraindicated for RCA suffered from terminal malignancy or were pregnant were excluded from the study. Major contraindication for RCA included those patients with acute liver failure or shock with muscle hypoperfusion, which would increase the risk of citrate accumulation.[9]
A 12-Fr double lumen hemodialysis catheter was inserted into either the internal jugular or femoral vein for vascular access by the attending physicians. Citrate CVVH was performed using either a Prismaflex machine with either the HF 1400 or ST 100 hemofilter (Baxter Gambro, Deerfield, IL, USA) or MultiFiltrate machine with the Ultraflux AV 600S hemofilter (Fresenius Medical Care, Bad Homburg, Germany). Predilution CVVH without the use of dialysis was prescribed to all the patients. The schematic of the treatment regimen is shown in Figure 1. When using the Prismaflex machine, we did not use the Flexitrate program so that we could uncouple the linkage between blood flow and citrate flow. The blood flow rate was fixed at 110 ml/min. Replacement solution with Prismocitrate 18/0 (predilution) and Phoxilium (postdilution) was both given at the fixed rate of 1250 ml/h. The calculated citrate dose was 3.4 mmol/L of blood withdrawn. Ten percent calcium chloride was infused through a separate central venous catheter and was titrated to achieve a systemic ionized calcium (iCa) level of 1–1.2 mmol/L. The fluid withdrawal rate was adjusted to achieve the desired fluid balance. The circuit was allowed to run up to 72 h unless there was filter clotting, transportation for intervention or imaging, or achievement of the predefined clinical target.
Figure 1.
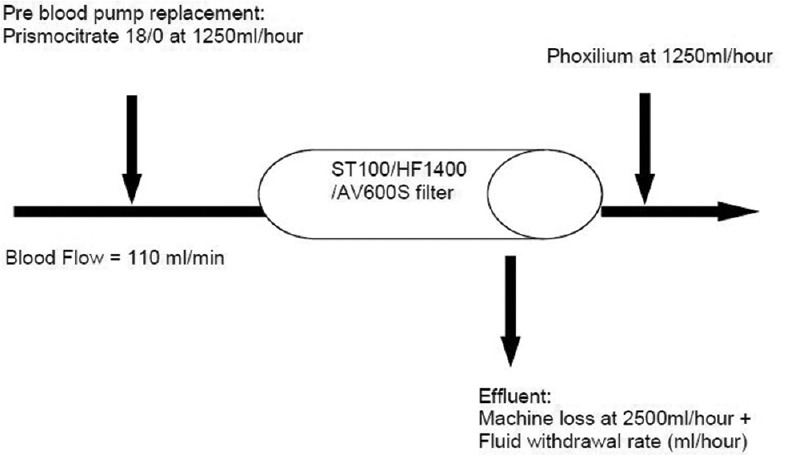
Schematic of predilutional continuous venovenous hemofiltration using Prismocitrate 18/0 and Phoxilium.
Serum samples for urea, creatinine, sodium, potassium, arterial blood gas, total calcium, iCa, prefilter iCa, and postfilter iCa were taken at 0, 2, and 4 h, then at 4 h intervals for 24 h and then at 8 h intervals up to 72 h. Serum samples for total calcium, magnesium, phosphate, and bilirubin were taken daily. Additional blood tests were performed as indicated. Demographic data were collected, including age, gender, predicted body weight, and APACHE II and IV scores. The primary outcome was circuit filter life. Filter clotting was defined as an overt sign of circuit clotting or a 100% increase in filter drop pressure (difference between pre- and post-filter pressure). ICU and hospital mortality rates and lengths of stay were also recorded. Potential side effects such as hypernatremia (defined as a sodium level >150 mmol/L), hypophosphatemia (defined as phosphate level <0.8 mmol/L), hypomagnesemia (defined as magnesium level <0.66 mmol/L), metabolic alkalosis (defined as pH ≥ 7.5), and citrate toxicity (defined as increasing metabolic acidosis with total calcium level to iCa ratio >2.5) were assessed.[10]
Statistical analysis
The results were displayed as the median with the interquartile range (IQR). The trend in pH, electrolytes, and base excess are shown using a standard box plot. All analyses were performed by the Statistical Package for Social Science for Windows, version 17 (SPSS Inc., Chicago, IL, USA). This trial was registered with the Australian and New Zealand Clinical Trials Registry, number ACTRN12614001000695.
Results
During the study, 36 patients receiving 50 sessions of citrate CVVH were evaluated. Five sessions were excluded, leaving 45 for analysis. Three exclusions were due to cardiac arrest unrelated to citrate accumulation, one due to a cannula problem, and one due to misinterpretation of the laboratory data.
Baseline patient characteristics are shown in Table 2. Four patients had preexisting end-stage renal failure. Indications for starting CVVH were fluid overload (39.5%), sepsis (30.2%), and pH or electrolyte disturbance (30.2%). The median ICU length of stay was 9.8 days (IQR 5.7–26.8). The ICU mortality rate was 24%, and the hospital mortality rate was 47.6%.
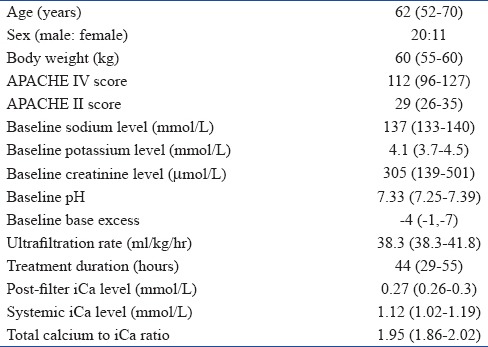
Table 2.
Patient characteristics data are presented as median (interquartile range)
The median circuit lifetime was 44 h (IQR 29–55). In 19 treatment episodes (42%), the filter lifetime was more than 48 h, and 9 episodes (20%) reached 72 h. CVVH was most commonly terminated for the achievement of the predefined clinical target (24 sessions, 53.3%), and then for filter clotting (16 sessions, 35.6%). Poor metabolic control (2 sessions, 4.4%) and need for transportation (3 sessions, 6.7%) were less common. The postfilter iCa was <0.3 mmol/L in 90.0% of the blood samples.
The time taken to correct metabolic acidosis as defined by a base excess of zero was 12–16 h [Figure 2]. Metabolic alkalosis was uncommon, with a pH value of ≥7.5 found in 34 out of 412 blood samples (8.3%). Hypernatremia, as defined by Na >150 mmol/L, occurred in 3 out of 393 blood samples (0.8%). These three samples originated from a single treatment episode with baseline hypernatremia, which was corrected later. No patient developed hypokalemia (K <3 mmol/L) [Figure 3]. Hypophosphatemia (PO4 <0.8 mmol/L) [Figure 4] occurred in 3 out of 86 samples (3.5%). Hypomagnesemia (Mg <0.66 mmol/L) [Figure 5] was common, found in 33 out of 82 samples (40.2%). The median daily magnesium supplement was 10 mmol (IQR 0–10). Urea and creatinine gradually decreased during treatment. The trend of acid-base parameters, serum electrolytes, and creatinine were displayed in Figures 2-8. The total/iCa ratio, as the surrogate marker for citrate accumulation, was consistently below the threshold value of 2.5 in all blood samples, including the two patients with CVVH terminated for poor metabolic control [Figure 8].
Figure 2.
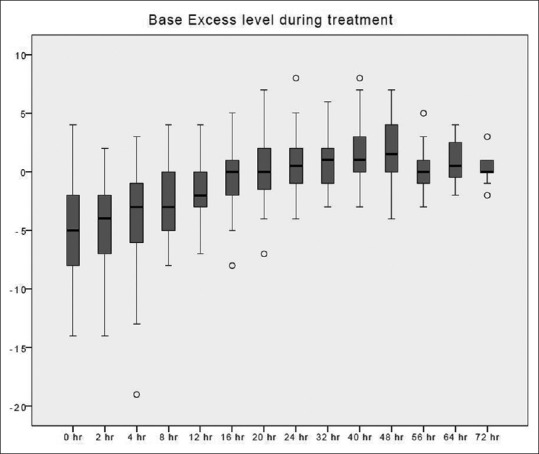
Base excess changes over time during citrate continuous renal replacement therapy. Standard box plot, in which the horizontal line represents the median, the thick line represents the interquartile range, and the thin line represents the maximum and minimum values. The circular dots represent the outliers.
Figure 3.
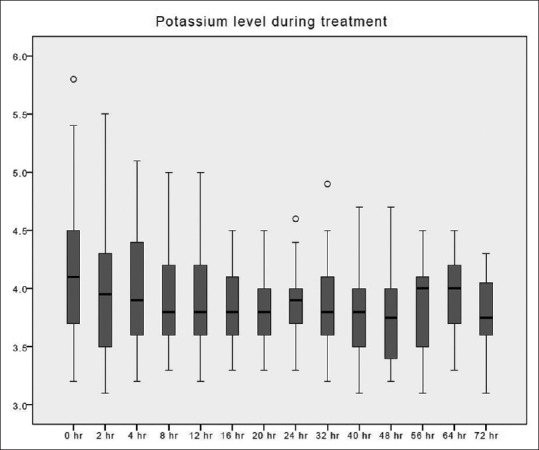
Potassium changes over time during citrate continuous renal replacement therapy. Standard box plot, in which the horizontal line represents the median, the thick line represents the interquartile range, and the thin line represents the maximum and minimum values. The circular dots represent the outliers.
Figure 4.
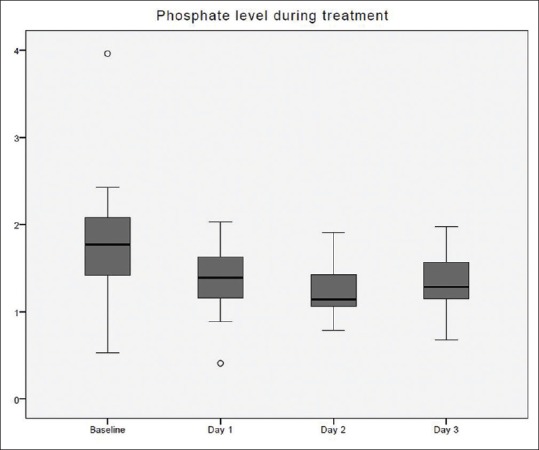
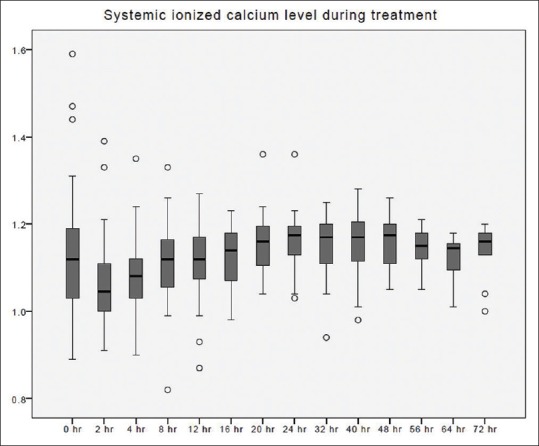
Systemic ionized calcium changes over time during citrate continuous renal replacement therapy. Standard box plot, in which the horizontal line represents the median, the thick line represents the interquartile range, and the thin line represents the maximum and minimum values. The circular dots represent the outliers.
Figure 5.
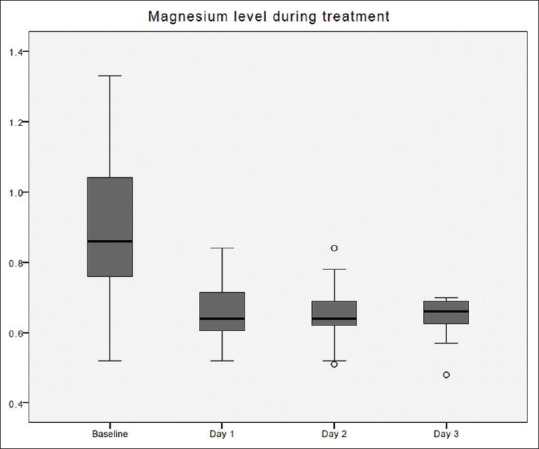
Magnesium changes over time during citrate continuous renal replacement therapy. Standard box plot, in which the horizontal line represents the median, the thick line represents the interquartile range, and the thin line represents the maximum and minimum values. The circular dots represent the outliers.
Figure 8.
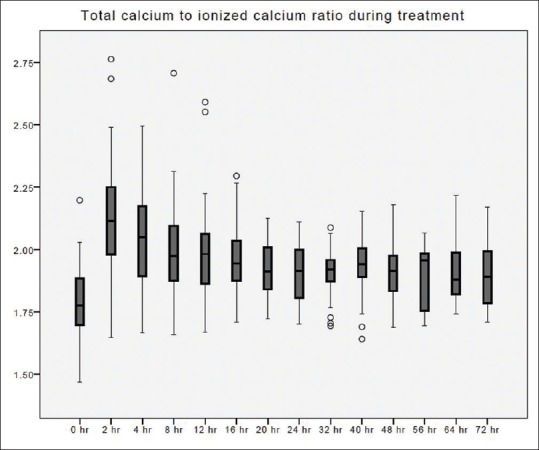
Total calcium to ionized calcium ratio changes over time during citrate continuous renal replacement therapy. Standard box plot, in which the horizontal line represents the median, the thick line represents the interquartile range, and the thin line represents the maximum and minimum values. The circular dots represent the outliers.
Figure 6.
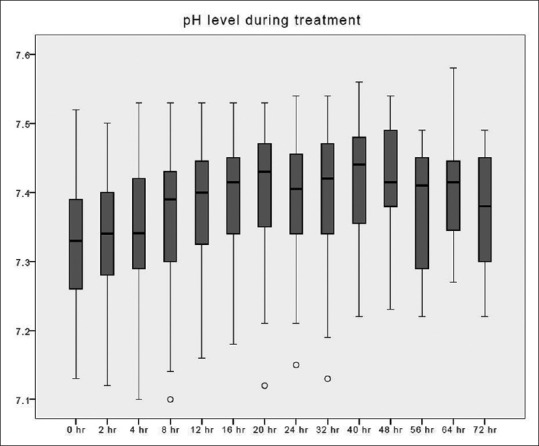
pH changes over time during citrate continuous renal replacement therapy. Standard box plot in which the horizontal line represents the median, the thick line represents the interquartile range, and the thin line represents the maximum and minimum values. The circular dots represent the outliers.
Figure 7.
Phosphate changes over time during citrate continuous renal replacement therapy. Standard box plot, in which the horizontal line represents the median, the thick line represents the interquartile range, and the thin line represents the maximum and minimum values. The circular dots represent the outliers.
Discussion
In the recent Kidney Disease Improving Global Outcomes Clinical Practice Guideline for acute kidney injury, RCA has been recommended in patients receiving CRRT with no contraindication for citrate.[9] Two meta-analyses compared RCA and systemic anticoagulation with heparin,[11,12] where RCA was shown to have a comparable or better circuit lifetime, induced less bleeding, and required fewer transfusions. The adoption of commercially prepared citrate solution has greatly simplified the use of citrate CRRT in recent years.
Circuit efficacy
Compared with our previously reported protocol,[4] the increase in buffer supply with 18 mmol/L citrate solution eliminated the need for a separate bicarbonate infusion. The citrate concentration of this new protocol was 3.4 mmol/L, and the median circuit lifetime was 44 h. Circuits that lasted for more than 48 h were up to 42%. The major advantage of this protocol was its simplicity and relative infrequency of metabolic disorders. The demographic, the circuit set up, the circuit efficiency, the safety of the regime, and the clinical outcome of this study, and the previously reported study was tabulated for comparison.
Tolwani et al. previously introduced the use of an 18 mmol/L citrate solution with a predilution CVVHDF set up.[13] The solution flow rate was 1000–2000 ml/h titrated to maintain a postfilter iCa of 0.25–0.5 mmol/L while providing a circuit citrate concentration of 2–6 mmol/L and reported an 80% circuit survival at 72 h. In an observational study by Morabito et al.,[8] a software program was used to titrate the citrate and blood flow to achieve circuit iCa of ≤0.4 mmol/L. The initial blood flow rate was 140 ml/min, the 18 mmol/L predilution citrate solution was started at rate of 1 L/h, while the Phoxilium solution was given as both dialysate and postreplacement solution at rate of 0.5 L/h and 0.6 L/h, respectively. They reported the median filter lifetime of 47.5 h (IQR 24–78.5). Another CVVHDF trial reported a median circuit lifetime of 42.1 h using the same citrate solution with machine integrated software algorithms for citrate dose adjustment to maintain the prefilter iCa between 0.3 and 0.44 mmol/L.[14]
In our current protocol, more than half of the treatment episodes were terminated due to the achievement of the predefined clinical criteria and only 36% developed filter/circuit clotting. We would expect the filter lifetime be longer if it was allowed to continue until the filter clotted. This was supported by the fact that over 90% of the postfilter iCa blood samples were <0.3 mmol/L.
Complications related to the use of citrate
Citrate toxicity and metabolic derangement are two areas of concern when using RCA.
There was no reported case of citrate accumulation or toxicity in this study, and the total to iCa ratios was below the threshold value of 2.5. In two treatment sessions where CVVH was stopped for poor metabolic control, both were attributed to bowel ischemia that led to worsening of metabolic acidosis.
Despite the increase in the citrate content with this new preparation, metabolic alkalosis could be avoided by reducing the replacement solution and blood flow rates. The incidence of an arterial pH ≥7.5 was lower than our prior findings, which included additional bicarbonate infusion (8.3% vs. 13.3%).
In this sample of patients, the serum sodium was either within the normal range or being corrected toward the normal range. There was no reported hypokalemia and only half of the treatment episodes required additional potassium supplement. With the use of a commercial phosphate and potassium containing replacement solution, we report a 5-fold reduction in the incidence of hypophosphatemia compared to our previous findings (3.5% vs. 17.6%). A similar reduction of hypophosphatemia had been reported when compare the use of Phoxilium with Hemosol B0 (Baxter Gambro, Deerfield, IL, USA) as dialysate and/or replacement during CVVH.[6,7] Landmark studies of renal replacement treatments had reported a high incidence of hypokalemia and hypophosphatemia.[15,16] In the renal study, the reported incidence of hypokalemia was 23.4% and up to 65.1% of patients in the high-intensity treatment group suffered from hypophosphatemia.[15] As hypophosphatemia may lead to respiratory muscle failure, arrhythmia, heart failure, rhabdomyolysis, hemolysis, and white cell dysfunction, this new change may provide a safety buffer from these harmful effects. Hypomagnesemia remained the most common electrolyte disturbance, and exogenous intravenous magnesium replacement was required. The relative high incidence of hypomagnesemia was related to the 79% increase in the circuit lifetime. The frequency of monitoring of Magnesium was reduced during this present study; given the presence of magnesium in the Phoxilum. Yet, the loss of magnesium through the circuit far exceeded the 0.6 mmol/L magnesium content of the replacement solution. Hence, this high incidence of hypomagnesemia could be avoided by frequent laboratory monitoring and replacement. The other option might be to increase the magnesium content of this phosphate containing replacement solution.
There are several limitations of this study. This was a small retrospective study; thus, bias may have occurred during data collection and review. In addition, the adoption of a CRRT regime with fixed blood and predilution solution flows, though simple to administer, did not allow for titration of CRRT dose according to body weight variations. However, the median CRRT dose of 38.3 ml/kg/h was still comparable to current recommendation.[17] Besides there was no titration of the citrate flow against the postfilter, iCa which could help to prolong the circuit lifetime. In the future, the use of integrated software algorithms regulating the citrate concentration according to blood flow and replacement fluid rate, and the coupling of calcium supplementation accordingly will further enhance the automation and safety of patients receiving CRRT.[18]
Conclusion
The combined use of Prismocitrate 18/0 and Phoxilium preformulated solutions in this fixed CVVH regime is safe and easy to administer in clinical practice. This CVVH regime has an acceptable circuit lifetime without major electrolyte disturbances apart from hypomagnesemia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Monti G, Herrera M, Kindgen-Milles D, Marinho A, Cruz D, Mariano F, et al. The DOse REsponse Multicentre International Collaborative Initiative (DO-RE-MI) Contrib Nephrol. 2007;156:434–43. doi: 10.1159/000102137. [DOI] [PubMed] [Google Scholar]
- 2.Bihorac A, Ross EA. Continuous venovenous hemofiltration with citrate-based replacement fluid: Efficacy, safety, and impact on nutrition. Am J Kidney Dis. 2005;46:908–18. doi: 10.1053/j.ajkd.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Oudemans-van Straaten HM, Bosman RJ, Koopmans M, van der Voort PH, Wester JP, van der Spoel JI, et al. Citrate anticoagulation for continuous venovenous hemofiltration. Crit Care Med. 2009;37:545–52. doi: 10.1097/CCM.0b013e3181953c5e. [DOI] [PubMed] [Google Scholar]
- 4.Leung AK, Shum HP, Chan KC, Chan SC, Lai KY, Yan WW. A retrospective review of the use of regional citrate anticoagulation in continuous venovenous hemofiltration for critically ill patients. Crit Care Res Pract 2013. 2013 doi: 10.1155/2013/349512. 349512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shum HP, Chan KC, Yan WW. Regional citrate anticoagulation in predilution continuous venovenous hemofiltration using Prismocitrate 10/2 solution. Ther Apher Dial. 2012;16:81–6. doi: 10.1111/j.1744-9987.2011.01001.x. [DOI] [PubMed] [Google Scholar]
- 6.Broman M, Carlsson O, Friberg H, Wieslander A, Godaly G. Phosphate-containing dialysis solution prevents hypophosphatemia during continuous renal replacement therapy. Acta Anaesthesiol Scand. 2011;55:39–45. doi: 10.1111/j.1399-6576.2010.02338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chua HR, Schneider AG, Baldwin I, Collins A, Ho L, Bellomo R. Phoxilium vs. Hemosol-B0 for continuous renal replacement therapy in acute kidney injury. J Crit Care. 2013;28:884.e7–14. doi: 10.1016/j.jcrc.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Morabito S, Pistolesi V, Tritapepe L, Vitaliano E, Zeppilli L, Polistena F, et al. Continuous venovenous hemodiafiltration with a low citrate dose regional anticoagulation protocol and a phosphate-containing solution: Effects on acid-base status and phosphate supplementation needs. BMC Nephrol. 2013;14:232. doi: 10.1186/1471-2369-14-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–84. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 10.Meier-Kriesche HU, Gitomer J, Finkel K, DuBose T. Increased total to ionized calcium ratio during continuous venovenous hemodialysis with regional citrate anticoagulation. Crit Care Med. 2001;29:748–52. doi: 10.1097/00003246-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Wu MY, Hsu YH, Bai CH, Lin YF, Wu CH, Tam KW. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy: A meta-analysis of randomized controlled trials. Am J Kidney Dis. 2012;59:810–8. doi: 10.1053/j.ajkd.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Hongying N. Efficacy and safety of regional citrate anticoagulation in critically ill patients undergoing continuous renal replacement therapy. Intensive Care Med. 2012;38:20–8. doi: 10.1007/s00134-011-2438-3. [DOI] [PubMed] [Google Scholar]
- 13.Tolwani AJ, Prendergast MB, Speer RR, Stofan BS, Wille KM. A practical citrate anticoagulation continuous venovenous hemodiafiltration protocol for metabolic control and high solute clearance. Clin J Am Soc Nephrol. 2006;1:79–87. doi: 10.2215/CJN.00040505. [DOI] [PubMed] [Google Scholar]
- 14.Brain MJ, Roodenburg OS, Adams N, McCracken P, Hockings L, Musgrave S, et al. Randomised trial of software algorithm driven regional citrate anticoagulation versus heparin in continuous renal replacement therapy: The filter life in renal replacement therapy pilot trial. Crit Care Resusc. 2014;16:131–7. [PubMed] [Google Scholar]
- 15.Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, et al. RENAL Replacement Therapy Study Investigators. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–38. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 16.Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, et al. VA/NIH Acute Renal Failure Trial Network. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jun M, Heerspink HJ, Ninomiya T, Gallagher M, Bellomo R, Myburgh J, et al. Intensities of renal replacement therapy in acute kidney injury: A systematic review and meta-analysis. Clin J Am Soc Nephrol. 2010;5:956–63. doi: 10.2215/CJN.09111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandl M, Strobl K, Hartmann J, Kellner K, Posnicek T, Falkenhagen D. A target-orientated algorithm for regional citrate-calcium anticoagulation in extracorporeal therapies. Blood Purif. 2012;33:7–20. doi: 10.1159/000332394. [DOI] [PubMed] [Google Scholar]


