Abstract
Context:
Surgical procedures carry significant morbidity and mortality depending on the type of surgery and patients. There is a dearth of evidence from India on the outcome of surgical patients admitted to an Intensive Care Unit (ICU).
Aims:
We aimed to describe the incidence and risk factors of postoperative complications and mortality in noncardiac surgical patients admitted to the ICU.
Settings and Design:
This was a prospective observational study on all perioperative patients admitted to a multidisciplinary ICU for 18 months.
Subjects and Methods:
Data on demography, admission Acute Physiology and Chronic Health Evaluation II (APACHE-II), Sequential Organ Failure Assessment (SOFA) scores, perioperative course, type and duration of surgery, reason for ICU admission, ICU interventions, and perioperative complications were recorded. The primary outcomes analyzed were perioperative complications and mortality.
Results:
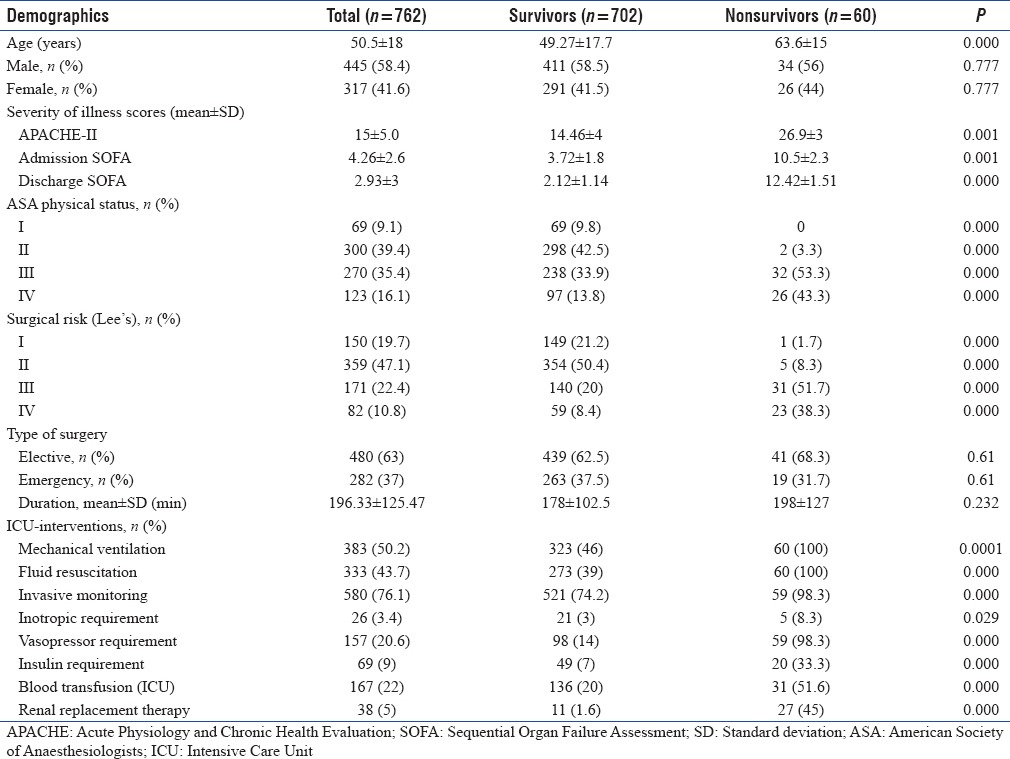
The study included 762 patients with a mean age of (mean ± standard deviation [SD]) 50.5 ± 18 years and a male (58.4%) preponderance. The mean (±SD) admission APACHE-II and SOFA scores were 15 (±5.0) and 4.26 (±2.6), respectively. The most common reason for ICU admission was elective mechanical ventilation 50%, followed by prolonged surgery 26.2% and hemodynamic instability 21.2%. Most (51.1%) patients belonged to American Society of Anaesthesiologists physical Status III or IV and Lee's surgical risk Category I and II (66.8%). The most common surgical procedures performed were gastro-intestinal (28.5%) followed by interventional Neuro-radiology (14.0%) and orthopedic (13.9%). Overall perioperative complications were observed in 51.4% (n = 392). Common complications observed were hemodynamic instability 24%, hypothermia 17.2%, sepsis 17.3%, poor glycemic control 11.2%, perioperative myocardial infarction 7.1%, cardiac arrest 0.13%, and acute kidney injury (AKI) 10.1%. The overall hospital mortality was 7.9%. Multivariate logistic regression analysis showed that admission APACHE-II score, sepsis, AKI, and ICU length of stay were independent predictors for mortality.
Conclusions:
High risk perioperative patients after noncardiac surgery have significant mortality and morbidity.
Keywords: Critically ill, noncardiac surgical patients, perioperative patients
Introduction
Advances in medical care have resulted in increased use of safe surgery in many disease conditions including high risk patient populations like elderly, those with multiple comorbid conditions and those undergoing major surgeries.[1,2,3]
Approximately 234 million major surgeries are performed annually with a mortality of 0.4–4% which may be even higher in high risk patients (12.3%–25%).[4] Studies have shown that reduced functional and organ reserve along with comorbid conditions impact perioperative mortality and morbidity.[3,5,6]
There is a dearth of evidence on the outcome of noncardiac surgical patients admitted to Intensive Care Unit (ICU) in India. Hence, in the present study, we primarily aimed to describe the incidence of postoperative complications and mortality in noncardiac surgical patients and secondarily to identify risk factors for incidence of complications.
Subjects and Methods
This was a prospective, observational study on consecutive perioperative patients admitted to ICU during a period of 18 months (April 2014–October 2015). Institutional Ethical Committee approval with a consent waiver was obtained due to the observational nature of the study. Patients <18 years, surgical duration <30 min and surgeries done under monitored anesthesia care/local anesthesia were excluded from the study. Data on demographics, severity of illness scores such as Acute Physiology and Chronic Health Evaluation II (APACHE-II) and Sequential Organ Failure Assessment (SOFA), type of surgery/anesthesia, duration of surgery, reason for ICU admission, interventions during ICU stay and perioperative complications as defined [Figure 1] were recorded. The primary outcomes analyzed were perioperative complications and hospital mortality. The secondary outcomes analyzed were duration of ICU stay, ventilator free days and ICU free days.
Figure 1.
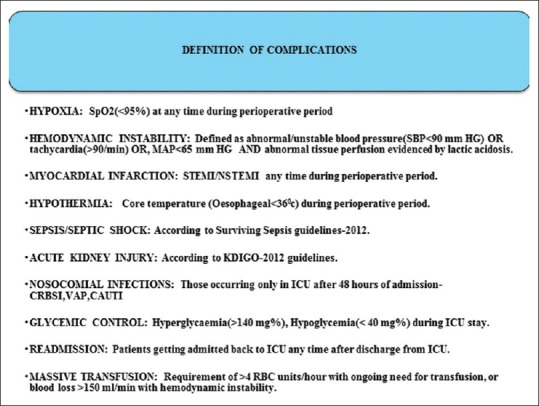
Definition of complications.
Results were expressed as mean ± standard deviation (SD) for quantitative data and frequencies for qualitative variables. Statistical analysis included Fisher's exact test, t-test and the Mann–Whitney U-test with P < 0.05 considered statistically significant. Multiple logistic regression was used to identify the independent risk factors for mortality SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc. was used.
Results
We recorded the data on 762 patients [Figure 2] were included and found their mean age to be 50.5 ± 18 years and were of male predominance 58.4% (n = 445). The mean APACHE-II score was 15 ± 5.0, SOFA score (admission) was 4.26 ± 2.6 and SOFA (discharge) was 2.93 ± 3 [Table 1].
Figure 2.
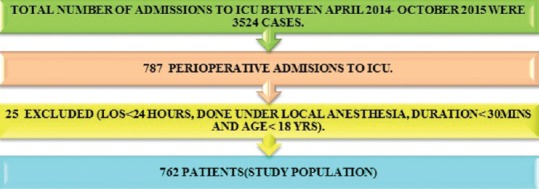
Admission data.
Table 1.
Profile and characteristics of survivors and nonsurvivors
The reasons for admission to ICU were for elective mechanical ventilation and observation in view of co-existing medical conditions 50% (n = 383), followed by prolonged duration of surgery 26.2% (n = 200) and hemodynamic instability 21.2% (n = 163).
Most (51.1%) of the patients belonged to American Society of Anesthesiologists (ASA) physical Status III or IV (ASA III – 35% and IV – 16.1%). Increasing ASA physical status has been associated with higher mortality (observed mortality 0%, 0.6%, 11.5%, and 21.16% in ASA I, II, III, and IV, respectively) [Table 1].
In the present study, most patients belonged to the Lee's surgical risk category of I and II (66.8%) (Lee's I – 19.7%, Lee's II – 47.1%), whereas 10.8% belonged to the highest surgical risk Category IV. The observed mortality increased with higher surgical risk (Lees I – 0.67%, II – 1.4%, III – 18% and IV – 29%) [Table 1].
The most common surgical procedures performed were gastro-intestinal (28.5%, n = 217), interventional neuro-radiological (14.0%, n = 107) and orthopedic (13.9%, n = 106) [Figure 3].
Figure 3.
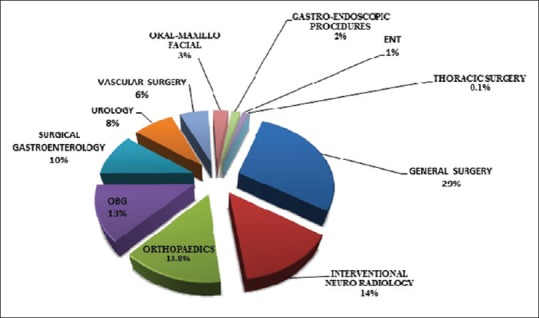
Case-mix of postoperative patients.
In our study, 379 patients (49.7%) received blood-product transfusion and most of it was used in the operating room (298 [39.1%]). The median perioperative blood loss in our study was 200 ml (interquartile range [IQR] 100–900). In patients who required blood-product transfusion this was 700 ml (IQR 280–1200). Out of the 379 patients who received blood-product transfusion only 20 patients (2.62%) received massive transfusion. The median massive transfusion volume was 5550 ml (5100–6000 ml). Eight of these twenty patients died accounting for an observed mortality of 40%, but the overall mortality in patients who received transfusion was 15.8% [Table 2].
Table 2.
Perioperative blood usage

Fifty percent (n = 383) of patients in our study received mechanical ventilation in ICU. Other ICU interventions observed in our study included invasive monitoring (76.1%, n = 580), fluid resuscitation (43.7%, n = 333), inotropic/vasopressor requirement (24%, n = 183), and renal replacement therapy (5%, n = 38), all these were more frequently used in nonsurvivors [Table 1].
The overall perioperative complications observed in the present study was 51.4% (n = 392) [Table 3]. The surgical complications included 5.5% (n = 42) and the nonsurgical complications was 45.9% (n = 350). Nonsurgical complications observed were hemodynamic instability 24% (n = 183), hypothermia 17.2% (n = 131), sepsis 17.3% (n = 132), poor glycemic control 11.2% (n = 90), perioperative myocardial infarction (MI) 7.1% (n = 54), cardiac arrest 0.13% (n = 14), and acute kidney injury (AKI) 10.1% (n = 77) [Table 3].
Table 3.
Complications and patients outcomes
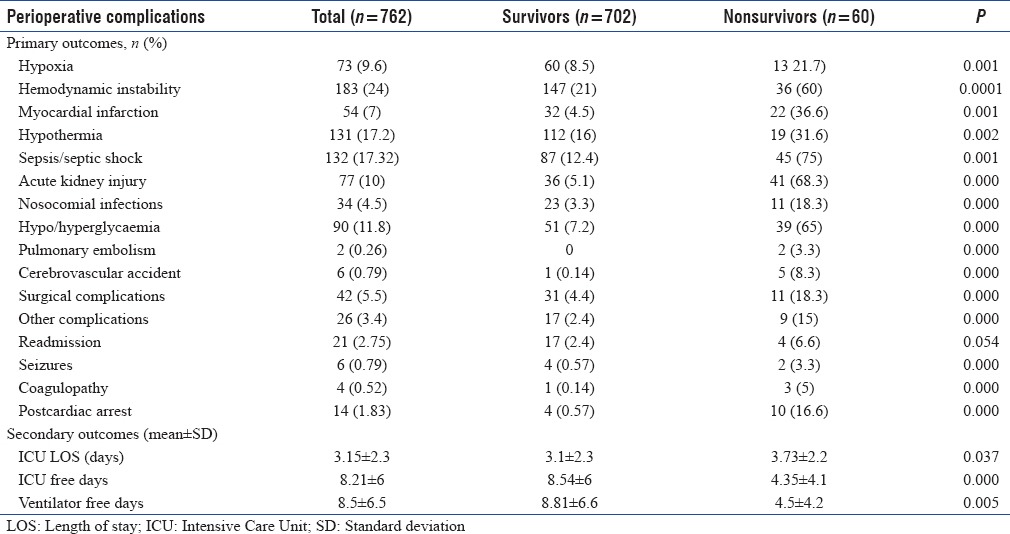
The overall hospital mortality in our study was 7.9% (n = 60).
Secondary outcome measures were ICU length of stay (LOS) (mean ± SD) 3.15 ± 2.3 days, ICU free days 8.21 ± 5.9 and ventilator free days 8.47 ± 6.5. Multivariate logistic regression analysis showed that admission APACHE-II score (odds ratio [OR] - 2.07, 95% confidence interval [CI] - 1.6-2.7; P = 0.000), ICU LOS (OR - 1.36, 95% CI - 1.43-1.78; P = 0.023), sepsis (OR - 0.088, 95% CI - 0.18-0.439; P = 0.003) and AKI (OR - 0.086, 95% CI - 0.016-0.46; P = 0.004) were independent risk factors for mortality.
Discussion
Identification of high risk surgical patients and development of strategies aimed at reducing perioperative morbidity and mortality is a major challenge for anesthesiologists and surgeons.[5,6] The objective of this study was to examine the characteristics and outcome of noncardiac surgical patients admitted to an ICU, which represents a heterogenous high risk patient population.
Our study group was predominantly male (58.4%) with a mean age of 50.5 ± 18 years. Studies described by Lobo et al. and Abelha et al.[7,8] had an elderlypopulation (62.4 ± 17 years and 64.11 ± 14 years respectively). We found that an increasing age correlated with a higher mortality (survivors [49.3 ± 17.8] vs. nonsurvivors [64 ± 15]; P = 0.000).
Our overall hospital mortality when compared to a study by Abelha et al.,[8] was lower (11.2%). This may be explained by the fact that their study group was older (64 vs. 50.5) and were of higher risk as categorized by the ASA physical status (ASA III/IV - 57% vs. 51%) than ours. In a study by Sakr et al.[9] the mortality rate was 9%. These patients had higher APACHE II score (22 ± 8.3 vs. 15.4 ± 5.1 current study) and were mainly cardiac surgical patients (26.4%) when compared to our study. Another study by Hashmi et al.[10] observed an overall mortality of 33% which was very high when compared to our study. This may be attributed to a higher incidence of emergency surgeries (62% vs. 37%) compared to ours. The INDICAPS[11] study described a mortality of (18.8%) in surgical ICU patients.
Several retrospective studies have demonstrated a correlation between ASA classification and perioperative mortality and have suggested its usefulness as a predictor of patient outcome.[8,12,13] Similar to our study [Table 1], Wolter et al.[14] in their study also demonstrated an increasing mortality with worse ASA physical status (ASA I – 0.1%, II – 0.7%, III – 3% and 5%, IV – 18.3%). Lee et al.[15] also found increasing mortality with patients who had higher surgical risk (Lee Class I – 0.4% vs. Lee Class IV – 11%). We found that patients in ASA III/IV had an APACHE in the range of 17–18 (predicted mortality – 26.2%) while those belonging to Lee's Class III/IV had an APACHE in the range of 18–19 (predicted mortality – 29.1%) [Table 1]. This may be the reason for the high observed mortality in our study compared to that observed by Wolter et al.,[14] and Lee et al.[15] The importance of the type of surgery has been emphasized in several studies[8,16] and poor outcome has been attributed to emergency surgery. However, our study did not find any association between emergency surgery and mortality (OR - 1.293, 95% CI - 0.735–2.275; P = 0.372) [Table 1].
Major hemorrhage that is life threatening and likely to result in the need for massive transfusion is not uncommon in the perioperative period.[16,17] This is associated with a high risk for respiratory and infectious complications and for mortality. In a study described by Turan et al.[16] the mortality rate was 21.5%. This was low compared to our study (40%) although their incidence of massive transfusions itself was lower (0.77% vs. 2.62%).
Studies that have examined perioperative sepsis are limited. They are mostly retrospective in nature or have looked at elective surgeries only.[18,19,20] The overall incidence of severe sepsis/septic shock among a mixed general ICU population in INDICAPS[11] study was higher than our study (28.3%, 17.3%). The predominant sources of perioperative sepsis in our study were abdominal (n = 52), soft tissue (n = 47), urological (n = 23), respiratory (n = 10). The National Surgical Quality Improvement Program (USA) data[20] evaluating sepsis in general surgical patients reported 34% mortality in patients with severe sepsis/septic shock which was similar to our study (34.01%) (OR - 21.2, 95% CI - 11.4–39.5; P < 0.0001). In addition 34 (4.5%) patients developed nosocomial infections. In a study by Custovic et al.[21] incidence of nosocomial infections in ICU was 11.25%. Pneumonia was the predominant infection in this study. Similiarly we too found pneumonia to be the most common nosocomial infection. Ventilator-associated pneumonia (n = 20, 60% of all nosocomial infections). The other nosocomial infections included catheter-associated urinary tract infection (n = 9, 25%), and catheter-related blood stream infection (n = 5, 15%). The incidence of nosocomial infections in INDICAPS[11] study was 12.2% and the mortality rate in such patients was 28.4%. The mortality rate in patients who developed nosocomial infections in our study was 32.3%. When compared to survivors nosocomial infections were more common in nonsurvivors (3.3% vs. 18.3%) (OR - 6.6, 95% CI - 3–14.3; P = 0.0001) [Table 3].
In various studies described earlier mortality after perioperative MI varied widely between 0.3%–3.5% in low risk patients and 25%–33% in high risk patients.[22,23] 7.1% (n = 54) of patients in our study had perioperative MI out of which almost one-third (29.6%) were new-onset and developed postoperatively. The mortality due to perioperative MI was high (41%) and this may be explained by the fact that 6 of 16 patients presented to our ICU in postcardiac arrest status. Nonsurvivors (36.6%) had a significantly higher incidence of MI than survivors (4.5%) (OR - 12, 95% CI - 6.43–22.8; P < 0.0001) [Table 3].
Many modern ICU treatment goals are aimed to prevent AKI. In an ICU setting, beginning and ending supportive therapy kidney investigators[24] showed major surgery as the second leading cause of AKI (34%) with overall mortality of 60%. AKI is a serious complication with even small rises in serum creatinine associated with both increased morbidity and mortality. In our study, although the incidence of AKI was lower, (10.1% [n = 77]). Thirty-eight patients (49.4%) required renal replacement. The observed mortality in patients with AKI was 53.2% and was consistent with earlier studies. AKI was more common in nonsurvivors than survivors in our study (68% vs. 5.1%) (OR - 19, 95% CI - 11.7–31.3; P = 0.000) [Table 3].
Limitations
The main limitation of the study was relatively small study population (n = 762). This number may be inadequate to identify independent risk factors for common perioperative complications. We also did not have posthospitalisation follow-up data and hence more relevant end points based on long-term outcomes could not be assessed.
Conclusions
High risk noncardiac surgical patients encounter significant morbidity and mortality. Higher admission APACHE-II scores, longer ICU LOS, sepsis and AKI were independent predictors for perioperative mortality in this study. The present study represents only a small sample size of 762 patients which in our opinion may not be adequate to draw major conclusions. A larger study is required to identify the risk factors for perioperative complications in this important high risk group of noncardiac surgical patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pearse RM. Another inconvenient truth: Meeting the challenge of preventing poor surgical outcomes. Curr Opin Crit Care. 2010;16:337–8. doi: 10.1097/MCC.0b013e32833c5cb7. [DOI] [PubMed] [Google Scholar]
- 2.Jhanji S, Pearse RM. The use of early intervention to prevent postoperative complications. Curr Opin Crit Care. 2009;15:349–54. doi: 10.1097/MCC.0b013e32832c4a7e. [DOI] [PubMed] [Google Scholar]
- 3.Moonesinghe SR, Mythen MG, Grocott MP. High-risk surgery: Epidemiology and outcomes. Anesth Analg. 2011;112:891–901. doi: 10.1213/ANE.0b013e3181e1655b. [DOI] [PubMed] [Google Scholar]
- 4.Catto JW, Alexander DJ. Pancreatic debridement in a district general hospital – Viable or vulnerable? Ann R Coll Surg Engl. 2002;84:309–13. doi: 10.1308/003588402760452394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ. Participants in the VA National Surgical Quality Improvement Program. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–41. doi: 10.1097/01.sla.0000179621.33268.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C, et al. Mortality after surgery in Europe: A 7 day cohort study. Lancet. 2012;380:1059–65. doi: 10.1016/S0140-6736(12)61148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lobo SM, Rezende E, Knibel MF, Silva NB, Páramo JA, Nácul FE, et al. Early determinants of death due to multiple organ failure after noncardiac surgery in high-risk patients. Anesth Analg. 2011;112:877–83. doi: 10.1213/ANE.0b013e3181e2bf8e. [DOI] [PubMed] [Google Scholar]
- 8.Abelha F, Maia P, Landeiro N, Neves A, Barros H. Determinants of outcome in patients admitted to a surgical Intensive Care Unit. Arq Med. 2007;21:135–43. [Google Scholar]
- 9.Sakr Y, Krauss C, Amaral AC, Réa-Neto A, Specht M, Reinhart K, et al. Comparison of the performance of SAPS II, SAPS 3, APACHE II, and their customized prognostic models in a surgical intensive care unit. Br J Anaesth. 2008;101:798–803. doi: 10.1093/bja/aen291. [DOI] [PubMed] [Google Scholar]
- 10.Hashmi M, Asghar A, Rashid S, Khan FH. APACHE II analysis of a surgical intensive care unit population in a tertiary care hospital in Karachi (Pakistan) Anaesth Pain & Intensive Care. 2014;18:338–44. [Google Scholar]
- 11.Divatia JV, Amin PR, Ramakrishnan N, Kapadia FN, Todi S, Sahu S, et al. Intensive Care in India: The Indian Intensive Care Case Mix and Practice Patterns Study. Indian J Crit Care Med. 2016;20:216–25. doi: 10.4103/0972-5229.180042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesthesia & Analgesia. 2005;100:4–10. doi: 10.1213/01.ANE.0000147519.82841.5E. [DOI] [PubMed] [Google Scholar]
- 13.Pittet D, Thiévent B, Wenzel RP, Li N, Gurman G, Suter PM. Importance of pre-existing co-morbidities for prognosis of septicemia in critically ill patients. Intensive care medicine. 1993;19:265–72. doi: 10.1007/BF01690546. [DOI] [PubMed] [Google Scholar]
- 14.Wolters U, Wolf T, Stützer H, Schröder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77:217–22. doi: 10.1093/bja/77.2.217. [DOI] [PubMed] [Google Scholar]
- 15.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–9. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 16.Turan A, Yang D, Bonilla A, Shiba A, Sessler DI, Saager L, et al. Morbidity and mortality after massive transfusion in patients undergoing non-cardiac surgery. Can J Anesth. 2013;60:761–70. doi: 10.1007/s12630-013-9937-3. [DOI] [PubMed] [Google Scholar]
- 17.Glance LG, Dick AW, Mukamel DB, Fleming FJ, Zollo RA, Wissler R, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114:283–92. doi: 10.1097/ALN.0b013e3182054d06. [DOI] [PubMed] [Google Scholar]
- 18.Leaper DJ, Van Goor H, Reilly J, Petrosillo N, Geiss HK, Torres AJ, et al. Surgical site infection–a European perspective of incidence and economic burden. International wound journal. 2004;1:247–73. doi: 10.1111/j.1742-4801.2004.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore LJ, McKinley BA, Turner KL, Todd SR, Sucher JF, Valdivia A, et al. The epidemiology of sepsis in general surgery patients. J Trauma Acute Care Surg. 2011;70:672–80. doi: 10.1097/TA.0b013e31820e7803. [DOI] [PubMed] [Google Scholar]
- 20.Moore LJ, Moore FA, Todd SR, Jones SL, Turner KL, Bass BL. Sepsis in general surgery: The 2005-2007 national surgical quality improvement program perspective. Arch Surg. 2010;145:695–700. doi: 10.1001/archsurg.2010.107. [DOI] [PubMed] [Google Scholar]
- 21.Custovic A, Smajlovic J, Hadzic S, Ahmetagic S, Tihic N, Hadzagic H. Epidemiological surveillance of bacterial nosocomial infections in the surgical intensive care unit. Materia socio-Medica. 2014;26:7–11. doi: 10.5455/msm.2014.26.7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gualandro DM, Calderaro D, Yu PC, Caramelli B. Acute myocardial infarction after noncardiac surgery. Arq Bras Cardiol. 2012;99:1060–7. doi: 10.1590/s0066-782x2012005000098. [DOI] [PubMed] [Google Scholar]
- 23.Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation. 2009;119:2936–44. doi: 10.1161/CIRCULATIONAHA.108.828228. [DOI] [PubMed] [Google Scholar]
- 24.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–8. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]


