ABSTRACT
Social bonds are necessary for many mammals to survive and reproduce successfully. These bonds (i.e. pair-bonds, friendships, filial bonds) are characterized by different periods of development, longevity and strength. Socially monogamous species display certain behaviors not seen in many other mammals, such as adult pair-bonding and male parenting. In our studies of prairie voles (Microtus ochrogaster) and titi monkeys (Callicebus cupreus), we have examined the neurohormonal basis of these bonds. Here, we discuss the evidence from voles that aspects of adolescent and adult social behavior are shaped by early experience, including changes to sensory systems and connections, neuropeptide systems such as oxytocin and vasopressin, and alterations in stress responses. We will compare this with what is known about these processes during development and adulthood in other mammalian species, both monogamous and non-monogamous, and how our current knowledge in voles can be used to understand the development of and variation in social bonds. Humans are endlessly fascinated by the variety of social relationships and family types displayed by animal species, including our own. Social relationships can be characterized by directionality (either uni- or bi-directional), longevity, developmental epoch (infant, juvenile or adult) and strength. Research on the neurobiology of social bonds in animals has focused primarily on ‘socially monogamous’ species, because of their long-term, strong adult affiliative bonds. In this Review, we attempt to understand how the ability and propensity to form these bonds (or lack thereof), as well as the display of social behaviors more generally, are transmitted both genomically and non-genomically via variation in parenting in monogamous and non-monogamous species.
KEY WORDS: Monogamy, Parental care, Vole, Oxytocin, Vasopressin
Summary: In this paper, we review the ways in which parents shape social behavior in offspring, in both monogamous and non-monogamous mammals.
What is monogamy?
Monogamy has been variously defined as a mating system, a genetic system or a social system, of which the basic unit is a pair (in most species, a male/female pair) and their offspring (Kleiman, 1977; Tecot et al., 2016). That is, a species can be sexually or genetically monogamous, with all reproduction occurring within the mated pair (although in reality, this rarely or never happens; with the possible exception of California mice, Peromyscus californicus) (Ribble, 1991). In contrast, many more species have been labeled as socially monogamous. This definition encompasses a suite of traits that include defense of a shared territory, mate-guarding, often coordinated movement or duetting, and also sometimes biparental care of offspring. The traditional assessment is that 5% of mammalian species are socially monogamous (Kleiman, 1977).
Monogamy has also sometimes been defined by the existence of a pair-bond, which is an attachment relationship displayed by two adults (Hazan and Shaver, 1987; Mason and Mendoza, 1998). Like attachments of infants to their mothers (Ainsworth et al., 1978; Bowlby, 1969, 1973, 1982), adult attachments are characterized by a preference for the partner over an unfamiliar animal, distress upon separation and the ability of the partner to buffer a stressful situation (Hazan and Shaver, 1987; Mason and Mendoza, 1998). Because of the strictness of this definition, very few species have even been sufficiently studied to demonstrate whether the adults display a pair-bond (Fuentes, 1999). Titi monkeys and prairie voles are prominent exceptions, with experimental evidence for partner preference (Carp et al., 2015; Getz et al., 1981; Thomas and Birney, 1979; Williams et al., 1992), separation distress (Mendoza et al., 2000; Mendoza and Mason, 1986; Sun et al., 2014) and stress buffering (Hennessy et al., 1995; Smith and Wang, 2014). Although how we refer to social structures is important, we would like to suggest that other important considerations might be the selectivity and the strength of their social bonds, their mode of transmission intergenerationally, their underlying mechanisms, and how all of these characteristics might affect the social structure of these species as well as our understanding of the evolution of social bonds.
In this Review, we focus primarily on prairie vole social bonds. Prairie voles meet the classic definition of social monogamy, in that they breed as pairs in the wild, living on small territories, with males that display parenting. Variation in social structure (females living alone, or two females in a group) is usually the result of the loss of a mate. Some males adopt a wandering strategy, but it is not clear whether this is an alternate reproductive strategy or indicates that they were just unable to find a pair-mate. Prairie voles demonstrate significant alloparenting by offspring (70–75% of offspring never disperse), which also makes them cooperative breeders (Carter and Getz, 1993; Getz and Hofmann, 1986; Getz et al., 1993). They display a strong social preference for a familiar partner (DeVries and Carter, 1999; Williams et al., 1992), with mate-guarding (Gobrogge et al., 2007; Williams et al., 1992; Winslow et al., 1993) and stress buffering (Burkett et al., 2016; Smith and Wang, 2014). They also demonstrate distress upon separation (Sun et al., 2014), although this has not been fully tested in this species, with isolation from all conspecifics always confounded with separation from the pair-mate.
The neurobiology and intergenerational transmission of social bonds
Our knowledge on the neurobiology of social bonds is limited to a few species, and primarily limited to prairie voles and titi monkeys for in-depth information (Gobrogge and Wang, 2015; Young et al., 2011). In brief, the formation of selective social bonds appears to be subserved by oxytocin (OT) and arginine vasopressin (AVP) receptors (OTR and V1aR, respectively) that, in prairie voles, are located in the same areas as dopamine (D1 and D2) receptors (Aragona et al., 2003; Gingrich et al., 2000; Liu and Wang, 2003). In other words, OT and AVP (‘social memory’) receptors are co-localized with D1 and D2 (‘reward’) receptors and work in concert for the facilitation of pair-bonding. The AVP system (Jarcho et al., 2011), and many of the same neural areas of social memory and reward, also subserve the pair-bond in titi monkeys (Bales et al., 2007b). The hedonic experience of mating and pair-bonding is mediated by mu opioid receptors (Ragen et al., 2013; Resendez et al., 2013), while the dysphoric effects of separation are mediated by kappa opioid receptors (Ragen et al., 2015a,b; Resendez and Aragona, 2013; Resendez et al., 2012).
List of abbreviations.
- ACTH
adrenocorticotropin hormone
- AVP
arginine vasopressin
- AVP-ir
arginine vasopressin immunoreactivity
- BNST
bed nucleus of the stria terminalis
- CORT
corticosterone
- CRH
corticotrophin-releasing hormone
- DA
dopamine
- D1
dopamine D1-type receptor
- D2
dopamine D2-type receptor
- E
estradiol
- ERα
estrogen receptor alpha
- GC
glucocorticoid
- GnRH-ir
gonadotropin-releasing hormone immunoreactivity
- GR
glucocorticoid receptors
- HPA
hypothalamic pituitary adrenal
- LS
lateral septum
- MPOA
medial preoptic area of the hypothalamus
- NAcc
nucleus accumbens
- OT
oxytocin
- OTR
oxytocin receptor
- PVN
paraventricular nucleus of the hypothalamus
- S1
primary somatosensory cortex
- V1aR
arginine vasopressin 1a receptor
- VTA
ventral tegmental area
But how is the propensity to form a bond passed from one generation to the next? While some of the variation in this behavior may be biological, such as inheritance of specific receptor patterns (Perkeybile et al., 2015), it has long been suggested that the intervening environmental variable may be the parental care received by the offspring (Smotherman and Bell, 1980; Smotherman et al., 1977). Variation in parenting behavior, and consequences for the offspring, has been best characterized in a non-monogamous species, the laboratory rat. However, data from monogamous species may be most useful in asking specific questions about the transmission of selective social bonds, and the neural substrates underlying them. Thus far, we know little about how early environmental variation impacts the formation and strength of pair bonds in this species. Here, we review findings in rats and other rodent models of natural variation in maternal behavior as well as our recent work on biparental care in the monogamous prairie vole in an effort to understand how aspects of social behavior and the neurobiology that regulates it are transmitted from one generation to the next. We then discuss how findings from these models can be used as a starting point for understanding the transmission of selective social bonds across generations.
Natural variation in parenting in non-monogamous species
Decades of research on the effects of early experience on offspring have used a rat model of early maternal separation and have demonstrated that short-term separation, or handling, results in offspring who display decreased anxiety-like behavior and hypothalamic-pituitary-adrenal (HPA) axis activation in response to stressors (Denenberg, 1964; Ladd et al., 1996; Levine, 1957; Levine et al., 1967; Ogawa et al., 1994; Plotsky and Meaney, 1993). It was proposed that differences in offspring outcomes after maternal handling or separation were due not to the manipulation itself but rather to a change in pup-directed maternal behavior upon reunion of the dam and pups following the manipulation. This is, in fact, the case – dams which experience a short separation from pups show a period of increased pup-directed care, including grooming and nursing, when they are returned to the nest compared with dams which are separated for a prolonged period of time or are not handled (Boccia and Pedersen, 2001; Smotherman and Bell, 1980; Smotherman et al., 1977).
Variation in maternal pup-directed care is also naturally occurring and has similar consequences for offspring. Rat mothers display variation in licking/grooming behavior across the first week postpartum and this variation is normally distributed across dams. The behavior appears to be a trait-like quality of the dam – the amount of licking/grooming care given by an individual dam is consistent across multiple litters and is not impacted by individual litter characteristics (litter weight, sex ratio, litter size) (Champagne et al., 2003a). Investigation of the two extremes in the distribution of care results in groups of dams that engage in high amounts of pup licking/grooming (1 standard deviation above the cohort mean) and dams that show low amounts of pup licking/grooming (1 standard deviation below the cohort mean) (Caldji et al., 1998; Champagne et al., 2003a; Liu et al., 1997). Several outcomes, including HPA function and anxiety-like and social behavior, show remarkable similarities between previous handling studies and natural variation in maternal licking/grooming, providing support for the hypothesis that early handling has its effects on offspring via alterations on maternal pup-directed care.
Variation in maternal behavior appears to be driven in large part by actions of OT, estradiol (E) and dopamine (DA). Variation in both ERα and OTR are associated with variation in maternal care. High licking/grooming dams have increased OTR binding density in several brain regions involved in the display of maternal behavior (Champagne et al., 2001) and have an increased number of OT-immunopositive cells in both the medial preoptic area (MPOA) and paraventricular nucleus of the hypothalamus (PVN) (Shahrokh et al., 2010) in comparison to low licking/grooming dams. OT action at these receptor sites contributes to the variation in offspring care, as an OT antagonist administered on postnatal day 3 decreases licking/grooming behavior in high licking/grooming dams to the level of low licking/grooming dams, effectively eliminating differences between groups. This is dependent, however, on OT's interaction with E in a highly site-specific manner, in particular the MPOA and the lateral septum (LS) (Champagne et al., 2001). Along with differences in OTR, high and low licking/grooming dams also show variation in ERα in the MPOA (Champagne et al., 2003a). This variation in ERα, where high licking/grooming dams have increased expression, likely moderates the increase in OT synthesis and OTR seen, producing an increase in the display of maternal behavior toward offspring.
The MPOA and PVN both contain OT projections to the ventral tegmental area (VTA), which contains DA neurons and projects to the nucleus accumbens (NAcc). This circuit, and the action of OT within these regions, is also involved in the control of maternal behavior. Increased DA activity in the NAcc during licking/grooming bouts is reported in high licking/grooming dams compared with low licking/grooming dams (Shahrokh et al., 2010). This DA activity during maternal behavior is very likely regulated in part by OT in the VTA, where OT infused directly into the VTA increases DA signaling in the NAcc while an OT antagonist infusion into the same region blocked the rise of DA activity during licking/grooming bouts in high but not low licking/grooming dams (Champagne et al., 2004; Shahrokh et al., 2010). High licking/grooming dams also show increased D1 receptor gene expression in the olfactory bulb (de Moura et al., 2015), a region implicated in the expression of maternal behavior (Lévy and Keller, 2009; Lévy et al., 2004). That the mesolimbic DA system is activated to a greater extent in high compared with low licking/grooming dams during licking/grooming bouts suggests there is variation in motivational aspects of maternal interaction with pups and that this may be tied to differences in OT signaling.
Social behaviors
As mentioned previously, effects in offspring are often similar following early handling and following naturally varying maternal care. Indeed, differential outcomes are found in offspring reared by high versus low licking/grooming dams in a number of social behaviors, including maternal care of their own offspring and the neurophysiology that regulates maternal behavior, social play and dominance behavior, and reproductive behavior. Variation in early maternal care, then, sets the stage for a rich diversity of social behavior across the species that is likely propagated from mother to offspring via non-genomic routes.
Variation in maternal licking/grooming behavior is transmitted from mother to offspring very reliably. Female offspring of high licking/grooming dams are likely to become high licking/grooming dams themselves, while female offspring of low licking/grooming dams are likely to become low licking/grooming dams (Champagne et al., 2003a; Francis et al., 1999). This variation is present even in virgin offspring, with high licking/grooming female offspring having a shorter latency to displaying a full suite of maternal behaviors toward pups (Champagne et al., 2001). Cross-fostering high licking/grooming pups to low licking/grooming dams results in offspring who become low licking/grooming dams, while low licking/grooming pups reared by high licking/grooming dams become high licking/grooming dams toward their own offspring, suggesting that transmission of behavior occurs via non-genomic mechanisms (Francis et al., 1999). This transmission appears to be regulating the same endocrine components in female offspring that mediate the display of maternal behavior in their mothers.
As mentioned previously, high licking/grooming dams have increased ERα, D1 and OTR binding in regions implicated in regulation of maternal behavior. The same is seen in high licking/grooming female offspring (Champagne et al., 2006; Francis et al., 2000, 2002; Peña et al., 2014), although not in all cases (Starr-Phillips and Beery, 2014). In some regions, differences in OTR between high and low licking/grooming offspring are dependent on lactation state (Francis et al., 2000), suggesting that early experience impacts the upregulation of OTR following parturition in dams and contributes to variation in pup care as discussed previously (Champagne et al., 2001, 2003b).
In addition to increases in maternal behavior, high licking/grooming offspring, both male and female, engage in greater amounts of social contact and social play behavior (Starr-Phillips and Beery, 2014; van Hasselt et al., 2012), although they show a decreased preference for social interactions with their siblings (Peña et al., 2014). Meanwhile, low licking/grooming offspring participate in more play fighting (Parent and Meaney, 2008) and may display increased dominance in adulthood compared with high licking/grooming offspring (Parent et al., 2013).
Although higher licking/grooming during rearing increases several social behaviors during adolescence and adulthood, the same is not true for measures of sexual behavior and reproduction. Female offspring reared by high licking/grooming dams display a later onset of puberty and decreased levels of sexual receptivity (Cameron et al., 2008a,b; Uriarte et al., 2007) compared with low licking/grooming female offspring. Given a choice, males are also less likely to choose a high licking/grooming female to mate with compared with a low licking/grooming female (Sakhai et al., 2011). High licking/grooming offspring have lower levels of progesterone and luteinizing hormone during proestrus, decreased ERα expression in the PVN, and a decreased number of gonadotropin-releasing hormone immunoreactive (GnRH-ir) cells in the MPOA due to a decreased sensitivity to positive feedback of E (Cameron et al., 2008a; Sakhai et al., 2011). Perhaps not surprisingly, these high licking/grooming females also have decreased fecundity compared with low licking/grooming females (Cameron et al., 2008b; Parent et al., 2013). Engaging in high amounts of offspring care may come at a cost to reproduction so that females develop a strategy of either greater investment in offspring with fewer total offspring produced or decreased investment in many offspring (Stearns, 1976). This model of variation in maternal care suggests that there are trade-offs to engaging in high amounts of offspring care, such that these dams may reach sexual maturity at a slower rate (Cameron et al., 2008a) and produce a lower number of offspring throughout their lifetime, yet invest a greater amount of time into each offspring to promote its survival (Champagne, 2011).
Stress responsiveness
Increased early maternal care by high licking/grooming dams is associated with decreases in fearfulness and anxiety-like behavior in offspring across several measures, both social (Menard and Hakvoort, 2007) and non-social (Caldji et al., 1998; Francis et al., 2000; Hancock et al., 2005; Menard et al., 2004; Menard and Hakvoort, 2007; Starr-Phillips and Beery, 2014). In reaction to a stressor, offspring of low licking/grooming dams mount what is potentially a less adaptive response, including increased adrenocorticotropic hormone (ACTH) and plasma corticosterone (CORT) as well as impaired glucocorticoid (GC) sensitivity, resulting in a delayed GC feedback on the hypothalamic-pituitary-adrenal axis and, therefore, an extended rise in CORT (Liu et al., 1997). These low licking/grooming offspring also have increased corticotropin-releasing hormone (CRH) mRNA in the PVN as well as decreased glucocorticoid receptor (GR) mRNA in the hippocampus, which has been linked to regulation of GC feedback (Jacobson and Sapolsky, 1991; Sapolsky et al., 1984). Behavioral responses to fear and anxiety-provoking stimuli, as well as the response of the HPA axis and associated circuitry to stressors, are very similar between high licking/grooming offspring and adult offspring who experience early maternal handling (Meaney and Aitken, 1985; Meaney et al., 1989, 1996; Viau et al., 1993). Early care also impacts the neural circuitry regulating fearfulness and outcomes show remarkable similarities to the effects of early handling (Caldji et al., 2003, 1998, 2000; Menard et al., 2004). The interactions between mother and offspring in early life, then, serve to program the neural functioning of offspring that mediate several behavioral and physiological responses to the environment and potentially prepare the offspring to be better suited for the environment they are likely to be a part of.
Epigenetic regulation
It is clear that variation in maternal care during the first week of life has profound consequences for a number of outcomes in offspring. One potential mechanism for these effects is differences in DNA methylation that, in turn, are associated with altered gene expression in high compared with low licking/grooming offspring. Thus far, effects of early licking/grooming on methylation patterns have been documented with regard to systems regulating maternal behavior and stress responsiveness. As discussed previously, ERα expression levels in the MPOA are a key component of the inheritance of maternal behavior patterns from mother to daughter. A source of these individual differences in ERα expression appears to be varying levels of DNA methylation of the ERα1b promoter. Adult female offspring of low licking/grooming dams have elevated levels of methylation across the ERα1b promoter compared with offspring of high licking/grooming dams. Increased DNA methylation generally results in decreased gene expression and in this case would lead to decreased levels of ERα gene expression in low licking/grooming offspring and, therefore, decreases in behavior mediated by ERα, such as maternal behavior. This is, in fact, the case. These differences are likely the direct result of varying early care, as cross-fostering indicates that ERα expression in the MPOA is a result of rearing experience rather than inheritance from the biological mother (Champagne et al., 2006).
In a similar fashion, maternal care impacts levels of DNA methylation of the GR promoter in the hippocampus, which is implicated in the variation in stress responsiveness between high and low licking/grooming offspring reviewed previously. Low licking/grooming offspring have elevated levels of methylation of the GR promoter compared with high licking/grooming offspring. These differences emerge by postnatal day 6 and persist into adulthood and, once again, are reversed by cross-fostering (Weaver et al., 2004). The differences in DNA methylation levels in regions involved in mediating behaviors that are impacted by varying early care, and the ability to reverse patterns of both behavior and methylation between groups via cross-fostering, suggests that variation in early maternal behavior acts to program individual difference in offspring through epigenetic regulation of associated gene expression and highlights a mechanism by which behavioral patterns of one generation can be transmitted to the next.
Variation in other rodents
The study of naturally occurring variation and its consequences for offspring is certainly not unique to rats. Variation in several aspects of maternal behavior has been documented between strains of mice (Champagne et al., 2007). Strain-specific variation in several behaviors (Francis et al., 2003; Franks et al., 2015) as well as neural circuits regulating fear response (Caldji et al., 2004) are likely influenced by this variation in early care, very similarly to rats. Both OTR and V1aR binding densities are also increased in the LS after increased maternal care (Curley et al., 2012). Interestingly, effects of maternal behavior owing to strain-specific variation follow patterns of non-genomic inheritance and are evident in a third generation (Curley et al., 2008), providing evidence for long-lasting alterations in maternal and anxiety-like behavior persisting over several generations.
The impact of paternal behavior on offspring has been explored in the California mouse (Peromyscus californicus), a monogamous rodent where both females and males engage in extensive parental care of offspring (Gubernick and Alberts, 1987) and the presence of the father in the nest is critical for offspring survival in the wild (Gubernick and Teferi, 2000). Decreased levels of early paternal care are associated with offspring displaying decreased exploratory and pup-directed behavior (Gleason and Marler, 2013), increases in AVP-ir in the PVN and increased basal CORT (Frazier et al., 2006), and decreased cognitive abilities (Bredy et al., 2004). A key behavior of fathers in this species is pup retrieval, which increases as offspring age toward weaning (Bester-Meredith et al., 1999). Following paternal retrieval, male offspring experience a transient increase in testosterone, hypothesized to be linked to later increases in aggression and AVP (Becker et al., 2010). Indeed, aggression in a resident–intruder test increases in male offspring following a greater number of early paternal retrievals (Bester-Meredith and Marler, 2003a; Frazier et al., 2006), as does retrieval of the male's own offspring (Bester-Meredith and Marler, 2003b). Both of these behaviors may be mediated by AVP-ir in the bed nucleus of the stria terminalis (BNST), which is increased following receipt of early retrievals (Frazier et al., 2006) and is associated with retrievals of one's own offspring as well as the display of several additional pup-directed behaviors (Bester-Meredith and Marler, 2003b). Increased AVP-ir in the BNST is also associated with greater amounts of maternal behavior in this species (Bester-Meredith and Marler, 2012), indicating similar means of regulating parental behavior in male and female California mice.
Natural variation in parenting in a monogamous species, the prairie vole
The prairie vole has been used extensively to understand the neurobiology of social bonds. These socially monogamous rodents form selective adult pair bonds and engage in biparental care of offspring, behaviors that are very rare in other mammals. The presence of both mother and father as early caregivers allows us to study the impacts of naturally occurring variation in early pup care on various developmental trajectories in offspring, including behaviors unique to socially monogamous mammals.
Parental care in the prairie vole is a trait-like quality. Levels of care are consistent within a breeder pair from one litter to the next, but vary greatly between pairs. In this model, breeding pairs who together engage in high amounts of offspring-directed parental care (the top quartile of a cohort) are used as high contact breeders while those that engage in low amounts of total pup-directed care (the bottom quartile of a cohort) are low contact breeders. This variation results in offspring which experience differences not only in the amount but also in the type of parental care. Distribution of offspring care between the mother and father differs between high contact and low contact pairs, where fathers of low contact pairs spend a greater amount of time caring for offspring than do fathers of high contact pairs, even while total combined care time from both the mother and father is still decreased in low contact pairs (Perkeybile et al., 2013). Our lab has been working to understand the consequences these natural differences in parental care have on a number of offspring outcomes such as social behavior, stress responsiveness, neuropeptide systems and sensory system organization.
Social behavior
Several species-typical behaviors in the prairie vole are highly sensitive to early life manipulations (Ahern and Young, 2009; Bales and Carter, 2003a,b; Bales et al., 2007a,c). In many regards, variation in early parenting has very similar consequences for offspring behavior as does early handling or early neuropeptide exposure, and these outcomes are often sex-dependent. High contact male offspring engage in a greater amount of alloparental care of infants (Perkeybile et al., 2013; Arias del Razo and Bales, 2016). Cross-fostering indicates that alloparenting in voles is transmitted non-genomically, with the parental behavior experienced by the offspring predicting the alloparental behavior shown (Perkeybile et al., 2015). There are additional group differences in other social behaviors, including an increase in affiliative behavior toward a novel juvenile in high contact offspring during the early adolescent period (Perkeybile et al., 2013). While alloparenting of unrelated offspring is sensitive to the early rearing environment, it is not yet known how parental care of own offspring is impacted. Previous data from meadow voles indicate that this early care likely affects later parental behavior. Meadow voles cross-fostered to prairie voles were found to cohabitate with their breeding partner following the birth of a litter – a behavior not seen in pairs that were in-fostered – and displayed increased maternal and paternal care of offspring compared with in-fostered pairs (McGuire, 1988). That species-typical social behavior (biparental care, postpartum cohabitation) of prairie voles is acquired at least to some extent by the meadow vole, which typically displays neither of these behaviors, suggests a complex inheritance of these behaviors from parent to offspring that may rely heavily on environment, at least in regards to social behavior, and corroborates findings in alloparenting in prairie voles.
Prairie voles show significant amounts of variation in several social behaviors in laboratory and field studies, including alloparenting (Lonstein and De Vries, 2000, 2001; Roberts et al., 1998), mating strategies (Getz and McGuire, 1993; Ophir et al., 2008) and parental care (Perkeybile et al., 2013; Wang and Novak, 1992). In the field, dispersal from the natal nest is also variable, with up to 70–75% of offspring remaining in the natal nest and serving as non-reproductive alloparents to future offspring of the breeding pair (Getz and McGuire, 1997; McGuire et al., 1993). As with other social behaviors discussed previously, dispersal rates may be influenced by early experience. Dispersal from the natal nest was tested at many time points from standard weaning age (postnatal day 20) to adulthood. At weaning, high contact offspring were less likely to ‘disperse’ (follow a long tube which simulated the size of a prairie vole territory) away from the home cage compared with low contact offspring (Arias del Razo and Bales, 2016). The decreased dispersal in the high contact offspring is likely not due to an unwillingness to explore new environments away from the home cage – high contact offspring display increases in prosocial interactions with infants and conspecifics as well as decreased anxiety-like behavior compared with low contact offspring. As offspring age closer to adulthood, the likelihood that both high contact and low contact offspring would disperse was predicted by the behavior of the parents, where parents that explored away from the home cage had offspring that also explored away from the home cage. This relationship between parental dispersal and offspring dispersal suggests that parents greatly influence the exploratory behavior of offspring as they approach reproductive age. This may occur either directly, where offspring actively follow a parent away from the nest and are more likely to explore in this manner in the presence of a social partner, or indirectly, by inheritance of a physiology that promotes exploratory behavior, perhaps via differences in systems regulating stress responsiveness or fearfulness and anxiety-like behavior.
Selective social bonds
At this point, very little is known about the effects of naturally occurring variation in the early environment and later development of social bonds in the prairie vole. Rearing by a single mother compared with having both the mother and father present delays formation of a partner preference in both male and female offspring (Ahern and Young, 2009). These same single-mother offspring receive decreased care in early life. Female offspring of low contact parents spend less time in contact with an opposite sex partner than do offspring of high contact parents (Arias del Razo and Bales, 2016). This suggests that decreased care in early life leads to disrupted abilities to form social bonds in adulthood. The mechanisms for why this might be, as well as whether this is specific to the formation of a selective partner preference or generalizes to a wider array of social bonds, is not yet known and is an interesting avenue for further exploration.
Stress responsiveness
In studying the effects of maternal licking/grooming behavior on HPA function in offspring in the rat, stressors used are almost exclusively acute and non-social. Perhaps missing, though, is investigation of social stressors as well as a comparison of acute and chronic stress exposure. Responses to social and non-social stressors do vary (Branchi et al., 2013), and social stressors, in particular social isolation housing, have been extensively studied in the prairie vole (Grippo et al., 2007b,c, 2009; Pan et al., 2009; Ruscio et al., 2007) as well as the rat (Malkesman et al., 2006; Parker and Morinan, 1986; Weiss et al., 2004; Yorgason et al., 2013). Similarly, HPA function varies between acute and chronic stress exposure (see Lightman, 2008 for review).
Stress responsiveness in low licking/grooming rat offspring is impaired in part because of a dysfunctional negative feedback on the HPA response, partially a result of increased methylation of GR in the hippocampus (Jacobson and Sapolsky, 1991; Liu et al., 1997; Sapolsky et al., 1984; Weaver et al., 2004). This results in a prolonged elevation in CORT following a stressor. This does not appear to be true for the prairie vole after either acute social or non-social stressors (Perkeybile and Bales, 2015a,b). CORT is increased in high contact female but not male offspring after chronic social isolation (Perkeybile and Bales, 2015b). It is not surprising that this is seen only in females, as sex-dependent responses to social isolation have been well documented in this species (Grippo et al., 2007a,b). However, that this elevation in CORT occurs only after high contact rearing suggests that high contact offspring, previously found to engage in more social behaviors in a number of contexts, appear to be more sensitive to the detrimental effects of long-term lack of social interaction. This may cause them to actively maintain social contact with conspecifics, an idea that is supported by their decreased rates of dispersal from the natal nest discussed previously. These high contact offspring display decreased anxiety-like behavior in an elevated plus maze as adolescents (Perkeybile et al., 2013; Arias del Razo and Bales, 2016), and adult male offspring also initiate aggression with a novel animal less often than their low contact counterparts (Perkeybile and Bales, 2015b). This again indicates that increased early parental care programs the offspring to engage in more pro-social behavior later in life.
Neuropeptide and sensory systems
Several of the species-typical behaviors that make the prairie vole a unique and excellent model for understanding the neurobiology of social behavior are regulated in part by the neuropeptides OT and AVP, including alloparenting (Bales et al., 2004; Olazábal and Young, 2006a,b) and pair bonding (Cho et al., 1999; Insel and Hulihan, 1995; Winslow et al., 1993). Previous research has demonstrated that these neuropeptide systems are sensitive to early life experiences (Bales et al., 2011; Carter et al., 2009; Yamamoto et al., 2004). It appears that early parental care also works to shape these systems. Levels of parental care are negatively associated with V1aR binding density in the central amygdala and BNST in male but not female offspring (Perkeybile et al., 2015). Density of OTR is increased in low contact compared with high contact males in the BNST, with a trend for the same pattern in female offspring (A.M.P. and K.L.B., unpublished data). For both OTR and V1aR, cross-fostering suggests that binding density is inherited genetically in a sex-specific pattern (Perkeybile et al., 2015). Given the role of the BNST in regulating anxiety-like behavior (Duvarci et al., 2009; Goodson, 2005), the increase in both OTR and V1aR binding densities in low contact male offspring after decreased amounts of early care may help to explain the increase in anxiety-like behavior these animals show.
Organization of sensory cortex and its connections are known to be determined prenatally through genetic mechanisms (reviewed in O'Leary and Sahara, 2008) and sensitive to experimental manipulations very early in development (Hubel et al., 1977; Killackey and Dawson, 1989; Simons and Land, 1987). Naturally occurring changes in sensory stimuli also have the potential to reorganize cortical maps. For example, organization of the somatosensory cortex is altered in the mother following nursing of offspring, in which representation of the ventrum is enlarged following nursing of pups (Xerri et al., 1994). Natural variation in early parental care may also have the potential to alter cortical organization and connectivity, in particular in the somatosensory cortical areas. Interaction with parents and siblings is a primary source of sensory experience in early life and much of this involves tactile stimulation such as licking, grooming and nursing.
Offspring of high contact parents receive greater amounts of tactile stimulation as infants compared with low contact offspring, and this subtle variation in early somatosensory input has lasting consequences for connectivity and general organization of sensory cortices. Neuroanatomical tracer injections into the primary somatosensory cortex (S1) show greater amounts of connectivity in S1 both contra- and ipsilateral from the injection site in high contact offspring, while low contact offspring have a greater number of connections in neighboring regions including the motor cortex and secondary somatosensory area/parietal ventral area, and nearly three times the total number of labeled cells contralaterally compared with high contact offspring (Seelke et al., 2016). These differences in somatosensory connectivity produced by relatively subtle variation in early care point toward a different approach to tactile information processing in high contact and low contact offspring. The diffuse patterns of connections of low contact offspring may indicate an enhanced ability to integrate multimodal sensory information (Seelke et al., 2016). These results show that variation in parental behavior is associated with individual differences in cortical connectivity and may provide yet another pathway, in addition to differences in neuropeptide systems, for parental care to modulate later behavior in offspring via central mechanisms.
Conclusions and future directions
It is clear that monogamous and non-monogamous species share at least some common pathways for the inheritance of social behavior, mediated in both cases by the parental care experienced by the young. Whereas in rats this care is the sole purview of the mother, in prairie voles and other socially monogamous species (and including humans, regardless of whether you consider them to be monogamous), fathers are also often active participants in infant care. Current evidence suggests that maternal care in prairie vole and rat mothers appears to be trait-like, but responsive to early experience. It is possible that prairie vole fathers may be able to adjust their investment to either match or compensate for the mother, and therefore give additional flexibility to the expression of social behavior in offspring (Perkeybile et al., 2013). In captive groups of cooperatively breeding marmoset and tamarins, males are more frequently noted to change the level of their investment in offspring than females (Bales et al., 2000), although wild female golden lion tamarins (Leontopithecus rosalia) significantly altered investment based on number of helpers and on their own body condition (Bales et al., 2002). Independent experimental manipulations of maternal and paternal care would be valuable in distinguishing these contingencies.
Why might a system for maintenance of individual variation in social behavior exist in prairie voles? For this, it is necessary to return to the basic ecological and population dynamics of their natural habitat. As far back as 1978, Lowell Getz hypothesized that social structure of microtine rodents varied according to habitat, and suggested that these social structures could then be used to explain the observed cyclic changes in population density (Getz, 1978). Essentially, he argued that prairie voles lived in large, contiguous, relatively stable habitats. In low density years (due to high juvenile mortality), monogamy is more common; reproductive suppression and alloparenting would serve to reduce the possibility of inbreeding (important with a home range size of only 10 m) or overuse of the resources. However, with lower juvenile mortality, there might be sufficient encounters between strangers to override the suppression, initiate breeding and cause rapid population growth and the characteristic cycles. In this context, the maintenance of both high contact and low contact pairs in the population makes a lot of sense. Presumably, high contact offspring would be more successful under stable conditions with higher juvenile mortality, while low contact offspring would be more successful under ‘unstable’ conditions of lower juvenile mortality. In the wild, offspring from large communal groups tend to disperse less than offspring from monogamous pairs or single female breeding groups, and the females that did leave from these communal groups dispersed at older ages (McGuire et al., 1993). This is consistent with our observations that high contact offspring were more reluctant to explore away from their natal group, at least at an earlier age.
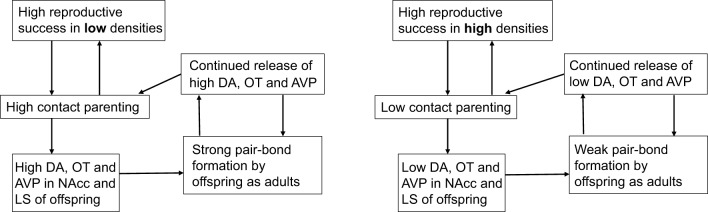
Current research has focused primarily on how parenting behavior can predispose offspring to display different strength or types of social behavior. Within the context of social monogamy, however, we do not yet know what consequences varying early care has on development of a pair bond and displays of biparental care of offspring. We have previously detailed the roles DA and OT have in maternal behavior in rats, where dams that engage in higher amounts of licking/grooming care show increases in DA activity in the NAcc and increases in OT-ir and OTR binding in maternal behavior circuitry (Champagne et al., 2001; Shahrokh et al., 2010). These same patterns of hormone activity are found in offspring reared by high licking/grooming dams, suggesting a point of influence of early experience on an animal's later behavior and physiology. These hormones have also been clearly linked to the formation and, in the case of DA D1 receptors, maintenance of pair bonds in both male and female prairie voles (DA: Aragona et al., 2003, 2006; Gingrich et al., 2000; Wang et al., 1999; OT: Cho et al., 1999; Liu and Wang, 2003), even acting in similar regions, such as the NAcc and LS, to regulate both maternal behavior and pair bonding. Based on findings in rats as well as our own data in voles, it is clear these systems are sensitive to early experiences. In Fig. 1, we hypothesize that increased parental care in voles is driven by a similar increase in DA and OT as is seen in regards to maternal behavior circuitry in rats, and that this increase in early care acts to increase DA and OT activity, as well as the closely related neuropeptide AVP, in offspring, resulting in the formation of a stronger pair bond in high contact offspring. This would mean that adults with higher levels of DA, OT and AVP would display increases in parental care of offspring as well as stronger pair bonds with their partner. Their offspring would then receive greater amounts of early care themselves, which would upregulate their own DA, OT and AVP, leading to a greater propensity to form a strong social bond as an adult and to engage in greater amounts of parental care of offspring. Preliminary evidence (see ‘Selective social bonds’, above) would suggest that at least the aspect of pair-bond strength is true, where decreased early care leads to decreased partner preference formation. An initial step in the prairie vole system might be to characterize pair bonding in established high contact versus low contact breeders; while experimental manipulations of pair bonding, and their effects on offspring, could be follow-ups.
Fig. 1.
Potential pathways for intergenerational transmission of social bonds. AVP, arginine vasopressin; DA, dopamine; LS, lateral septum; NAcc, nucleus accumbens; OT, oxytocin.
Working to understand the neural mechanisms of this difference in behavior may give insight into the sustained variation we see in pair bonding in this species. For instance, are high contact offspring more likely to choose other high contact offspring as partners? If they instead mate with a low contact offspring, does one animal tend to dictate parenting style or do they instead tend to average each other out and become medium contact parents with a moderately strong pair bond? Addressing these questions will help us understand how variation in social behavior in general, and social bonds in particular, are maintained in this species and what role early experience plays in shaping social monogamy behavior.
Acknowledgements
K.L.B. would like to thank Joel Levine and Daniel Kronauer for the invitation to participate in the Evolution of Social Behavior meeting in March 2016, and to Michaela Handel for her excellent organization.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
K.L.B. was supported during the writing of this paper by the National Institutes of Health (NIH) [HD071998 and MH110014 to K.L.B.; MH108319 to Nirao Shah and K.L.B.; OD011107 to the California National Primate Research Center] and the Good Nature Institute. A.M.P. is supported by NIH grant HD075750 to C. Sue Carter. Deposited in PMC for release after 12 months.
References
- Ahern T. H. and Young L. J. (2009). The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster). Front. Behav. Neurosci. 3, 17 10.3389/neuro.08.017.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth M. D. S., Blehar M. C., Waters E. and Wall S. (1978). Patterns of Attachment. Hillsdale, NJ: Erlebaum. [Google Scholar]
- Aragona B. J., Liu Y., Curtis T., Stephan F. K. and Wang Z. X. (2003). A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J. Neurosci. 23, 3483-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona B. J., Liu Y., Yu Y. J., Curtis J. T., Detwiler J. M., Insel T. R. and Wang Z. X. (2006). Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat. Neurosci. 9, 133-139. 10.1038/nn1613 [DOI] [PubMed] [Google Scholar]
- Arias del Razo R. and Bales K. L. (2016). Exploration in a dispersal task: effects of early experience and correlation with other behaviors in prairie voles (Microtus ochrogaster). Behav. Proc. 132, 66-75. 10.1016/j.beproc.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales K. L. and Carter C. S. (2003a). Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster). Behav. Neurosci. 117, 854-859. 10.1037/0735-7044.117.4.854 [DOI] [PubMed] [Google Scholar]
- Bales K. L. and Carter C. S. (2003b). Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster). Horm. Behav. 44, 178-184. 10.1016/S0018-506X(03)00154-5 [DOI] [PubMed] [Google Scholar]
- Bales K., Dietz J., Baker A., Miller K. and Tardif S. D. (2000). Effects of allocare-givers on fitness of infants and parents in callitrichid primates. Folia Primatol. 71, 27-38. 10.1159/000021728 [DOI] [PubMed] [Google Scholar]
- Bales K., French J. A. and Dietz J. M. (2002). Explaining variation in maternal care in a cooperatively breeding mammal. Anim. Behav. 63, 453-461. 10.1006/anbe.2001.1954 [DOI] [Google Scholar]
- Bales K. L., Kim A. J., Lewis-Reese A. D. and Carter C. S. (2004). Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm. Behav. 45, 354-361. 10.1016/j.yhbeh.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Bales K. L., Lewis-Reese A. D., Pfeifer L. A., Kramer K. M. and Carter C. S. (2007a). Early experience affects the traits of monogamy in a sexually dimorphic manner. Dev. Psychobiol 49, 335-342. 10.1002/dev.20216 [DOI] [PubMed] [Google Scholar]
- Bales K. L., Mason W. A., Catana C., Cherry S. R. and Mendoza S. P. (2007b). Neural correlates of pair-bonding in a monogamous primate. Brain Res. 1184, 245-253. 10.1016/j.brainres.2007.09.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales K. L., van Westerhuyzen J. A., Lewis-Reese A. D., Grotte N. D., Lanter J. A. and Carter C. S. (2007c). Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Horm. Behav. 52, 274-279. 10.1016/j.yhbeh.2007.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales K. L., Boone E., Epperson P., Hoffman G. and Carter C. S. (2011). Are behavioral effects of early experience mediated by oxytocin? Front. Psychiatry 2, 24 10.3389/fpsyt.2011.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. A., Moore B. M., Auger C. and Marler C. A. (2010). Paternal behavior increases testosterone levels in offspring of the California mouse. Horm. Behav. 58, 385-389. 10.1016/j.yhbeh.2010.03.019 [DOI] [PubMed] [Google Scholar]
- Bester-Meredith J. K. and Marler C. A. (2003a). The association between male offspring aggression and paternal and maternal behavior of Peromyscus mice. Ethology 109, 797-808. 10.1046/j.0179-1613.2003.00917.x [DOI] [Google Scholar]
- Bester-Meredith J. K. and Marler C. A. (2003b). Vasopressin and the transmission of paternal behavior across generations in mated, cross-fostered Peromyscus mice. Behav. Neurosci. 117, 455-463. 10.1037/0735-7044.117.3.455 [DOI] [PubMed] [Google Scholar]
- Bester-Meredith J. K. and Marler C. A. (2012). Naturally occurring variation in vasopressin immunoreactivity is associated with maternal behavior in female Peromyscus mice. Brain Behav. Evol. 80, 244-253. 10.1159/000341899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bester-Meredith J. K., Young L. J. and Marler C. A. (1999). Species differences in paternal behavior and aggression in Peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm. Behav. 36, 25-38. 10.1006/hbeh.1999.1522 [DOI] [PubMed] [Google Scholar]
- Boccia M. L. and Pedersen C. A. (2001). Brief vs. long maternal separations in infancy: contrasting relationships with adult maternal behavior and lactation levels of aggression and anxiety. Psychoneuroendocrinology 26, 657-672. 10.1016/S0306-4530(01)00019-1 [DOI] [PubMed] [Google Scholar]
- Bowlby J. (1969). Attachment and Loss. New York: Basic Books. [Google Scholar]
- Bowlby J. (1973). Attachment and Loss: Vol. 2. Separation: Anxiety and Anger. New York: Basic Books. [Google Scholar]
- Bowlby J. (1982). Attachment and Loss: Vol. 1. Attachment. New York: Basic Books. [Google Scholar]
- Branchi I., Santarelli S., D'Andrea I. and Alleva E. (2013). Not all stressors are equal: early social enrichment favors resilience to social but not physical stress in male mice. Horm. Behav. 63, 503-509. 10.1016/j.yhbeh.2013.01.003 [DOI] [PubMed] [Google Scholar]
- Bredy T. W., Lee A. W., Meaney M. J. and Brown R. E. (2004). Effect of neonatal handling and paternal care on offspring cognitive development in the monogamous California mouse (Peromyscus californicus). Horm. Behav. 46, 30-38. 10.1016/j.yhbeh.2003.09.017 [DOI] [PubMed] [Google Scholar]
- Burkett J. P., Andari E., Johnson Z. V., Curry D. C., de Waal F. B. and Young L. J. (2016). Oxytocin-dependent consolation behavior in rodents. Science 351, 375-378. 10.1126/science.aac4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C., Tannenbaum B., Sharma S., Francis D., Plotsky P. M. and Meaney M. J. (1998). Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl. Acad. Sci. USA 95, 5335-5340. 10.1073/pnas.95.9.5335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C., Francis D., Sharma S., Plotsky P. M. and Meaney M. J. (2000). The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology 22, 219-229. 10.1016/S0893-133X(99)00110-4 [DOI] [PubMed] [Google Scholar]
- Caldji C., Diorio J. and Meaney M. J. (2003). Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology 28, 1950-1959. 10.1038/sj.npp.1300237 [DOI] [PubMed] [Google Scholar]
- Caldji C., Diorio J., Anisman H. and Meaney M. J. (2004). Maternal behavior regulates benzodiazepine/GABA(A) receptor subunit expression in brain regions associated with fear in BALB/c and C57BL/6 mice. Neuropsychopharmacology 29, 1344-1352. 10.1038/sj.npp.1300436 [DOI] [PubMed] [Google Scholar]
- Cameron N., Del Corpo A., Diorio J., McAllister K., Sharma S. and Meaney M. J. (2008a). Maternal programming of sexual behavior and hypothalamic-pituitary-gonadal function in the female rat. PLoS ONE 3, e2210 10.1371/journal.pone.0002210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N. M., Fish E. W. and Meaney M. J. (2008b). Maternal influences on the sexual behavior and reproductive success of the female rat. Horm. Behav. 54, 178-184. 10.1016/j.yhbeh.2008.02.013 [DOI] [PubMed] [Google Scholar]
- Carp S. B., Rothwell E. S., Bourdon A., Freeman S. M., Ferrer E. and Bales K. L. (2015). Development of a partner preference test that differentiates between established pair bonds and other relationships in socially monogamous titi monkeys (Callicebus cupreus). Am. J. Primatol. 78, 326-339. 10.1002/ajp.22450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. S. and Getz L. L. (1993). Monogamy and the prairie vole. Sci. Am. 268, 100-106. 10.1038/scientificamerican0693-100 [DOI] [PubMed] [Google Scholar]
- Carter C. S., Boone E. M., Pournajafi-Nazarloo H. and Bales K. L. (2009). Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Dev. Neurosci. 31, 332-341. 10.1159/000216544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F. A. (2011). Maternal imprints and the origins of variation. Horm. Behav. 60, 4-11. 10.1016/j.yhbeh.2011.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F., Diorio J., Sharma S. and Meaney M. J. (2001). Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc. Natl. Acad. Sci. USA 98, 12736-12741. 10.1073/pnas.221224598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F. A., Francis D. D., Mar A. and Meaney M. J. (2003a). Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 79, 359-371. 10.1016/S0031-9384(03)00149-5 [DOI] [PubMed] [Google Scholar]
- Champagne F. A., Weaver I. C. G., Diorio J., Sharma S. and Meaney M. J. (2003b). Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology 144, 4720-4724. 10.1210/en.2003-0564 [DOI] [PubMed] [Google Scholar]
- Champagne F. A., Chretien P., Stevenson C. W., Zhang T. Y., Gratton A. and Meaney M. J. (2004). Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J. Neurosci. 24, 4113-4123. 10.1523/JNEUROSCI.5322-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F. A., Weaver I. C. G., Diorio J., Dymov S., Szyf M. and Meaney M. J. (2006). Maternal care associated with methylation of the estrogen receptor-alpha 1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology 147, 2909-2915. 10.1210/en.2005-1119 [DOI] [PubMed] [Google Scholar]
- Champagne F. A., Curley J. P., Keverne E. B. and Bateson P. P. G. (2007). Natural variations in postpartum maternal care in inbred and outbred mice. Physiol. Behav. 91, 325-334. 10.1016/j.physbeh.2007.03.014 [DOI] [PubMed] [Google Scholar]
- Cho M. M., DeVries A. C., Williams J. R. and Carter C. S. (1999). The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav. Neurosci. 113, 1071-1079. 10.1037/0735-7044.113.5.1071 [DOI] [PubMed] [Google Scholar]
- Curley J. P., Champagne F. A., Bateson P. and Keverne E. B. (2008). Transgenerational effects of impaired maternal care on behaviour of offspring and grandoffspring. Anim. Behav. 75, 1551-1561. 10.1016/j.anbehav.2007.10.008 [DOI] [Google Scholar]
- Curley J. P., Jensen C. L., Franks B. and Champagne F. A. (2012). Variation in maternal and anxiety-like behavior associated with discrete patterns of oxytocin and vasopressin 1a receptor density in the lateral septum. Horm. Behav. 61, 454-461. 10.1016/j.yhbeh.2012.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moura A. C., Lazzari V. M., Becker R. O., Gil M. S., Ruthschilling C. A., Agnes G., Almeida S., da Veiga A. B. G., Lucion A. B. and Giovenardi M. (2015). Gene expression in the CNS of lactating rats with different patterns of maternal behavior. Neurosci. Res. 99, 8-15. 10.1016/j.neures.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Denenberg V. H. (1964). Critical periods, stimulus input, and emotional reactivity: a theory of infantile stimulation. Psychol. Rev. 71, 335-351. 10.1037/h0042567 [DOI] [PubMed] [Google Scholar]
- DeVries A. C. and Carter C. S. (1999). Sex differences in temporal parameters of partner preference in prairie voles (Microtus ochrogaster). Can. J. Zool. 77, 885-889. 10.1139/z99-054 [DOI] [Google Scholar]
- Duvarci S., Bauer E. P. and Pare D. (2009). The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. J. Neurosci. 29, 10357-10361. 10.1523/JNEUROSCI.2119-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D., Diorio J., Liu D. and Meaney M. J. (1999). Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 286, 1155-1158. 10.1126/science.286.5442.1155 [DOI] [PubMed] [Google Scholar]
- Francis D. D., Champagne F. C. and Meaney M. J. (2000). Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J. Neuroendocrinol. 12, 1145-1148. 10.1046/j.1365-2826.2000.00599.x [DOI] [PubMed] [Google Scholar]
- Francis D. D., Young L. J., Meaney M. J. and Insel T. R. (2002). Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J. Neuroendocrinol. 14, 349-353. 10.1046/j.0007-1331.2002.00776.x [DOI] [PubMed] [Google Scholar]
- Francis D. D., Szegda K., Campbell G., Martin W. D. and Insel T. R. (2003). Epigenetic sources of behavioral differences in mice. Nat. Neurosci. 6, 445-446. 10.1038/nn1038 [DOI] [PubMed] [Google Scholar]
- Franks B., Champagne F. A. and Curley J. P. (2015). Postnatal maternal care predicts divergent weaning strategies and the development of social behavior. Dev. Psychobiol. 57, 809-817. 10.1002/dev.21326 [DOI] [PubMed] [Google Scholar]
- Frazier C. R. M., Trainor B. C., Cravens C. J., Whitney T. K. and Marler C. A. (2006). Paternal behavior influences development of aggression and vasopressin expression in male California mouse offspring. Horm. Behav. 50, 699-707. 10.1016/j.yhbeh.2006.06.035 [DOI] [PubMed] [Google Scholar]
- Fuentes A. (1999). Re-evaluating primate monogamy. Am. Anthropol. 100, 890-907. 10.1525/aa.1998.100.4.890 [DOI] [Google Scholar]
- Getz L. L. (1978). Speculation on social structure and population cycles of microtine rodents. Biologist 60, 134-147. [Google Scholar]
- Getz L. L. and Hofmann J. E. (1986). Social organization in free-living prairie voles, Microtus ochrogaster. Behav. Ecol. Sociobiol. 18, 275-282. 10.1007/BF00300004 [DOI] [Google Scholar]
- Getz L. L. and McGuire B. (1993). A comparison of living singly and in male-female pairs in the prairie vole, Microtus ochrogaster. Ethology 94, 265-278. 10.1111/j.1439-0310.1993.tb00444.x [DOI] [Google Scholar]
- Getz L. L. and McGuire B. (1997). Communal nesting in prairie voles (Microtus ochrogaster): formation, composition, and persistence of communal groups. Can. J. Zool. 75, 525-534. 10.1139/z97-065 [DOI] [Google Scholar]
- Getz L. L., Carter C. S. and Gavish L. (1981). The mating system of the prairie vole, Microtus ochrogaster: field and laboratory evidence for pair-bonding. Behav. Ecol. Sociobiol. 8, 189-194. 10.1007/BF00299829 [DOI] [Google Scholar]
- Getz L. L., Mcguire B., Pizzuto T., Hofmann J. E. and Frase B. (1993). Social organization of the prairie vole (Microtus ochrogaster). J. Mammal. 74, 44-58. 10.2307/1381904 [DOI] [Google Scholar]
- Gingrich B., Liu Y., Cascio C., Wang Z. X. and Insel T. R. (2000). Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav. Neurosci. 114, 173-183. 10.1037/0735-7044.114.1.173 [DOI] [PubMed] [Google Scholar]
- Gleason E. D. and Marler C. A. (2013). Non-genomic transmission of paternal behaviour between fathers and sons in the monogamous and biparental California mouse. Proc. R. Soc. B Biol. Sci. 280, 20130824 10.1098/rspb.2013.0824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobrogge K. and Wang Z. (2015). Neuropeptidergic regulation of pair-bonding and stress buffering: lessons from voles. Horm. Behav. 76, 91-105. 10.1016/j.yhbeh.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobrogge K. L., Liu Y., Jia S. and Wang Z. (2007). Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J. Comp. Neurol. 502, 1109-1122. 10.1002/cne.21364 [DOI] [PubMed] [Google Scholar]
- Goodson J. L. (2005). The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 48, 11-22. 10.1016/j.yhbeh.2005.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo A. J., Cushing B. S. and Carter C. S. (2007a). Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom. Med. 69, 149-157. 10.1097/PSY.0b013e31802f054b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo A. J., Gerena D., Huang J., Kumar N., Shah M., Ughreja R. and Carter C. S. (2007b). Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology 32, 966-980. 10.1016/j.psyneuen.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo A. J., Lamb D. G., Carter C. S. and Porges S. W. (2007c). Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol. Psychiatry 62, 1162-1170. 10.1016/j.biopsych.2007.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo A. J., Trahanas D. M., Zimmerman R. R., Porges S. W. and Carter C. S. (2009). Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology 34, 1542-1553. 10.1016/j.psyneuen.2009.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubernick D. J. and Alberts J. R. (1987). The biparental care system of the California mouse, Peromyscus californicus. J. Comp. Psychol. 101, 169-177. 10.1037/0735-7036.101.2.169 [DOI] [PubMed] [Google Scholar]
- Gubernick D. J. and Teferi T. (2000). Adaptive significance of male parental care in a monogamous mammal. Proc. R. Soc. B Biol. Sci. 267, 147-150. 10.1098/rspb.2000.0979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock S. D., Menard J. L. and Olmstead M. C. (2005). Variations in maternal care influence vulnerability to stress-induced binge eating in female rats. Physiol. Behav. 85, 430-439. 10.1016/j.physbeh.2005.05.007 [DOI] [PubMed] [Google Scholar]
- Hazan C. and Shaver P. (1987). Romantic love conceptualized as an attachment process. J. Pers. Soc. Psychol. 52, 511-524. 10.1037/0022-3514.52.3.511 [DOI] [PubMed] [Google Scholar]
- Hennessy M. B., Mendoza S. P., Mason W. A. and Moberg G. P. (1995). Endocrine sensitivity to novelty in squirrel monkeys and titi monkeys: species differences in characteristic modes of responding to the environment. Physiol. Behav. 57, 331-338. 10.1016/0031-9384(94)00250-9 [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. and LeVay S. (1977). Plasticity of ocular dominance columns in monkey striate cortex. Philos. Trans. R. Soc. B Biol. Sci. 278, 377 10.1098/rstb.1977.0050 [DOI] [PubMed] [Google Scholar]
- Insel T. R. and Hulihan T. J. (1995). A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav. Neurosci. 109, 782-789. 10.1037/0735-7044.109.4.782 [DOI] [PubMed] [Google Scholar]
- Jacobson L. and Sapolsky R. (1991). The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 12, 118-134. 10.1210/edrv-12-2-118 [DOI] [PubMed] [Google Scholar]
- Jarcho M. R., Mendoza S. P., Mason W. A., Yang X. and Bales K. L. (2011). Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes Brain Behav. 10, 375-383. 10.1111/j.1601-183X.2010.00677.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killackey H. P. and Dawson D. R. (1989). Expansion of the central hindpaw representation following fetal forelimb removal in the rat. Eur. J. Neurosci. 1, 210-221. 10.1111/j.1460-9568.1989.tb00790.x [DOI] [PubMed] [Google Scholar]
- Kleiman D. G. (1977). Monogamy in mammals. Q. Rev. Biol. 52, 39-69. 10.1086/409721 [DOI] [PubMed] [Google Scholar]
- Ladd C. O., Owens M. J. and Nemeroff C. B. (1996). Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology 137, 1212-1218. [DOI] [PubMed] [Google Scholar]
- Levine S. (1957). Infantile experience and resistance to physiological stress. Science 126, 405 10.1126/science.126.3270.405 [DOI] [PubMed] [Google Scholar]
- Levine S., Haltmeyer G. C., Kargs G. G. and Denenberg V. H. (1967). Physiological and behavioral effects of infantile stimulation. Physiol. Behav. 2, 55- 59 10.1016/0031-9384(67)90011-X [DOI] [Google Scholar]
- Lévy F. and Keller M. (2009). Olfactory mediation of maternal behavior in selected mammalian species. Behav. Brain Res. 200, 336-345. 10.1016/j.bbr.2008.12.017 [DOI] [PubMed] [Google Scholar]
- Lévy F., Keller M. and Poindron P. (2004). Olfactory regulation of maternal behavior in mammals. Horm. Behav. 46, 284-302. 10.1016/j.yhbeh.2004.02.005 [DOI] [PubMed] [Google Scholar]
- Lightman S. L. (2008). The neuroendocrinology of stress: a never ending story. J. Neuroendocrinol. 20, 880-884. 10.1111/j.1365-2826.2008.01711.x [DOI] [PubMed] [Google Scholar]
- Liu Y. and Wang Z. X. (2003). Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121, 537-544. 10.1016/S0306-4522(03)00555-4 [DOI] [PubMed] [Google Scholar]
- Liu D., Diorio J., Tannenbaum B., Caldji C., Francis D., Freedman A., Sharma S., Pearson D., Plotsky P. M. and Meaney M. J. (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277, 1659-1662. 10.1126/science.277.5332.1659 [DOI] [PubMed] [Google Scholar]
- Lonstein J. S. and De Vries G. J. (2000). Influence of gonadal hormones on the development of parental behavior in adult virgin prairie voles (Microtus ochrogaster). Behav. Brain Res. 114, 79-87. 10.1016/S0166-4328(00)00192-3 [DOI] [PubMed] [Google Scholar]
- Lonstein J. S. and De Vries G. J. (2001). Social influences on parental and nonparental responses toward pups in virgin female prairie voles (Microtus ochrogaster). J. Comp. Psychol. 115, 53-61. 10.1037/0735-7036.115.1.53 [DOI] [PubMed] [Google Scholar]
- Malkesman O., Maayan R., Weizman A. and Weller A. (2006). Aggressive behavior and HPA axis hormones after social isolation in adult rats of two different genetic animal models for depression. Behav. Brain Res. 175, 408-414. 10.1016/j.bbr.2006.09.017 [DOI] [PubMed] [Google Scholar]
- Mason W. A. and Mendoza S. P. (1998). Generic aspects of primate attachments: Parents, offspring and mates. Psychoneuroendocrinology 23, 765-778. 10.1016/S0306-4530(98)00054-7 [DOI] [PubMed] [Google Scholar]
- McGuire B. (1988). Effects of cross-fostering on parental behavior of meadow voles (Microtus pennsylvanicus). J. Mammal. 69, 332-341. 10.2307/1381383 [DOI] [PubMed] [Google Scholar]
- McGuire B., Getz L. L., Hofmann J. E., Pizzuto T. and Frase B. (1993). Natal dispersal and philopatry in prairie voles (Microtus ochrogaster) in relation to population density, season, and natal social environment. Behav. Ecol. Sociobiol. 32, 293-302. 10.1007/BF00183784 [DOI] [Google Scholar]
- Meaney M. J. and Aitken D. H. (1985). The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations: temporal parameters. Dev. Brain Res. 22, 301-304. 10.1016/0165-3806(85)90183-X [DOI] [PubMed] [Google Scholar]
- Meaney M. J., Aitken D. H., Viau V., Sharma S. and Sarrieau A. (1989). Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology 50, 597-604. 10.1159/000125287 [DOI] [PubMed] [Google Scholar]
- Meaney M. J., Diorio J., Francis D., Widdowson J., LaPlante P., Caldji C., Sharma S., Seckl J. R. and Plotsky P. M. (1996). Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Dev. Neurosci. 18, 49-60. 10.1159/000111395 [DOI] [PubMed] [Google Scholar]
- Menard J. L. and Hakvoort R. M. (2007). Variations of maternal care alter offspring levels of behavioural defensiveness in adulthood: evidence for a threshold model. Behav. Brain Res. 176, 302-313. 10.1016/j.bbr.2006.10.014 [DOI] [PubMed] [Google Scholar]
- Menard J. L., Champagne D. L. and Meaney M. J. P. (2004). Variations of maternal care differentially influence ‘fear’ reactivity and regional patterns of cFos immunoreactivity in response to the shock-probe burying test. Neuroscience 129, 297-308. 10.1016/j.neuroscience.2004.08.009 [DOI] [PubMed] [Google Scholar]
- Mendoza S. P. and Mason W. A. (1986). Contrasting responses to intruders and to involuntary separation by monogamous and polygynous New World monkeys. Physiol. Behav. 38, 795-801. 10.1016/0031-9384(86)90045-4 [DOI] [PubMed] [Google Scholar]
- Mendoza S. P., Capitanio J. P. and Mason W. A. (2000). Chronic social stress: studies in non-human primates. In Biology of Animal Stress: Basic Principles and Implications for Animal Welfare (ed. Moberg G. P. and Mench J. A.), pp. 227-247. New York: CABI Publishing. [Google Scholar]
- Ogawa T., Mikuni M., Kuroda Y., Muneoka K., Mori K. J. and Takahashi K. (1994). Periodic maternal deprivation alters stress response in adult offspring: potentiates the negative feedback regulation of restraint stress-induced adrenocortical response and reduces the frequencies of open field-induced behaviors. Pharmacol. Biochem. Behav. 49, 961-967. 10.1016/0091-3057(94)90250-X [DOI] [PubMed] [Google Scholar]
- Olazábal D. E. and Young L. J. (2006a). Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience 141, 559-568. 10.1016/j.neuroscience.2006.04.017 [DOI] [PubMed] [Google Scholar]
- Olazábal D. E. and Young L. J. (2006b). Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm. Behav. 49, 681-687. 10.1016/j.yhbeh.2005.12.010 [DOI] [PubMed] [Google Scholar]
- O'Leary D. D. M. and Sahara S. (2008). Genetic regulation of arealization of the neocortex. Curr. Opin. Neurobiol. 18, 90-100. 10.1016/j.conb.2008.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir A. G., Wolff J. O. and Phelps S. M. (2008). Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proc. Natl. Acad. Sci. USA 105, 1249-1254. 10.1073/pnas.0709116105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. L., Liu Y., Young K. A., Zhang Z. B. and Wang Z. X. (2009). Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci. Lett. 454, 67-71. 10.1016/j.neulet.2009.02.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent C. I. and Meaney M. J. (2008). The influence of natural variations in maternal care on play fighting in the rat. Dev. Psychobiol. 50, 767-776. 10.1002/dev.20342 [DOI] [PubMed] [Google Scholar]
- Parent C. I., Del Corpo A., Cameron N. M. and Meaney M. J. (2013). Maternal care associates with play dominance rank among adult female rats. Dev. Psychobiol. 55, 745-756. 10.1002/dev.21070 [DOI] [PubMed] [Google Scholar]
- Parker V. and Morinan A. (1986). The socially-isolated rat as a model for anxiety. Neuropharmacology 25, 663-664. 10.1016/0028-3908(86)90224-8 [DOI] [Google Scholar]
- Peña C. J., Neugut Y. D., Calarco C. A. and Champagne F. A. (2014). Effects of maternal care on the development of midbrain dopamine pathways and reward-directed behavior in female offspring. Eur. J. Neurosci. 39, 946-956. 10.1111/ejn.12479 [DOI] [PubMed] [Google Scholar]
- Perkeybile A. M. and Bales K. L. (2015a). Early rearing experience is associated with vasopressin immunoreactivity but not reactivity to an acute non-social stressor in the prairie vole. Physiol. Behav. 147, 149-156. 10.1016/j.physbeh.2015.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile A. M. and Bales K. L. (2015b). Early rearing experience is related to altered aggression and vasopressin production following chronic social isolation in the prairie vole. Behav. Brain Res. 283, 37-46. 10.1016/j.bbr.2015.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile A. M., Griffin L. L. and Bales K. L. (2013). Natural variation in early parental care correlates with social behaviors in adolescent prairie voles (Microtus ochrogaster). Front. Behav. Neurosci. 7, 21 10.3389/fnbeh.2013.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile A. M., Delaney-Busch N., Hartman S., Grimm K. J. and Bales K. L. (2015). Intergenerational transmission of alloparental behavior and oxytocin and vasopressin receptor distribution in the prairie vole. Front. Behav. Neurosci. 9, 191 10.3389/fnbeh.2015.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky P. M. and Meaney M. J. (1993). Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) messenger-RNA, median eminence CRF content and stress-induced release in adult rats. Mol. Brain Res. 18, 195-200. 10.1016/0169-328X(93)90189-V [DOI] [PubMed] [Google Scholar]
- Ragen B. J., Maninger N., Mendoza S. P., Jarcho M. R. and Bales K. L. (2013). Presence of a pair-mate regulates the behavioral and physiological effects of opioid manipulation in the monogamous titi monkey (Callicebus cupreus). Psychoneuroendocrinology 38, 2448-2461. 10.1016/j.psyneuen.2013.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragen B. J., Freeman S. M., Laredo S. A., Mendoza S. P. and Bales K. L. (2015a). µ and κ opioid receptor distribution in the monogamous titi monkey (Callicebus cupreus): implications for social behavior and endocrine functioning. Neuroscience 290, 421-434. 10.1016/j.neuroscience.2015.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragen B. J., Maninger N., Mendoza S. P. and Bales K. L. (2015b). The effects of morphine, naloxone, and κ opioid manipulation on endocrine functioning and social behavior in monogamous titi monkeys (Callicebus cupreus). Neuroscience 287, 32-42. 10.1016/j.neuroscience.2014.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez S. L. and Aragona B. J. (2013). Aversive motivation and the maintenance of monogamous pair bonding. Rev. Neurosci. 24, 51-60. 10.1515/revneuro-2012-0068 [DOI] [PubMed] [Google Scholar]
- Resendez S. L., Kuhnmuench M., Krzywosinski T. and Aragona B. J. (2012). K-opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. J. Neurosci. 32, 6771-6784. 10.1523/JNEUROSCI.5779-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez S. L., Dome M., Gormley G., Franco D., Nevarez N., Hamid A. A. and Aragona B. J. (2013). µ-Opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms. J. Neurosci. 33, 9140-9149. 10.1523/JNEUROSCI.4123-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribble D. O. (1991). The monogamous mating system of Peromyscus californicus as revealed by DNA fingerprinting. Behav. Ecol. Sociobiol. 29, 161-166. 10.1007/BF00166397 [DOI] [Google Scholar]
- Roberts R. L., Miller A. K., Taymans S. E. and Carter C. S. (1998). Role of social and endocrine factors in alloparental behavior of prairie voles (Microtus ochrogaster). Can. J. Zool. 76, 1862-1868. 10.1139/cjz-76-10-1862 [DOI] [Google Scholar]
- Ruscio M. G., Sweeny T., Hazelton J., Suppatkul P. and Carter C. S. (2007). Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Horm. Behav. 51, 54-61. 10.1016/j.yhbeh.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Sakhai S. A., Kriegsfeld L. J. and Francis D. D. (2011). Maternal programming of sexual attractivity in female Long Evans rats. Psychoneuroendocrinology 36, 1217-1225. 10.1016/j.psyneuen.2011.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R. M., Krey L. C. and McEwen B. S. (1984). Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc. Natl. Acad. Sci. USA 81, 6174-6177. 10.1073/pnas.81.19.6174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelke A. M., Perkeybile A. M., Grunewald R., Bales K. L. and Krubitzer L. A. (2016). Individual differences in cortical connections of somatosensory cortex are associated with parental rearing style in prairie voles (Microtus ochrogaster). J. Comp. Neurol. 524, 564-577. 10.1002/cne.23837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrokh D. K., Zhang T.-Y., Diorio J., Gratton A. and Meaney M. J. (2010). Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology 151, 2276-2286. 10.1210/en.2009-1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons D. J. and Land P. W. (1987). Early experience of tactile stimulation influences organization of somatic sensory cortex. Nature 326, 694-697. 10.1038/326694a0 [DOI] [PubMed] [Google Scholar]
- Smith A. S. and Wang Z. (2014). Hypothalamic oxytocin mediates social buffering of the stress response. Biol. Psychiatry 76, 281-288. 10.1016/j.biopsych.2013.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman W. P. and Bell R. W. (ed.) (1980). Maternal mediation of early experience. In Maternal Influence and Early Behavior. New York: Spectrum Publishing. [Google Scholar]
- Smotherman W. P., Brown C. P. and Levine S. (1977). Maternal responsiveness following differential pup treatment and mother–pup interactions. Horm. Behav. 8, 242-253. 10.1016/0018-506X(77)90041-1 [DOI] [PubMed] [Google Scholar]
- Starr-Phillips E. J. and Beery A. K. (2014). Natural variation in maternal care shapes adult social behavior in rats. Dev. Psychobiol. 56, 1017-1026. 10.1002/dev.21182 [DOI] [PubMed] [Google Scholar]
- Stearns S. C. (1976). Life-history tactics: a review of the ideas. Q. Rev. Biol. 51, 3-47. 10.1086/409052 [DOI] [PubMed] [Google Scholar]
- Sun P., Smith A. S., Lei K., Liu Y. and Wang Z. (2014). Breaking bonds in male prairie vole: long-term effects on emotional and social behavior, physiology, and neurochemistry. Behav. Brain Res. 265, 22-31. 10.1016/j.bbr.2014.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecot S. R., Singletary B. and Eadie E. (2016). Why “monogamy” isn't good enough. Am. J. Primatol. 78, 340-354. 10.1002/ajp.22412 [DOI] [PubMed] [Google Scholar]
- Thomas J. A. and Birney E. C. (1979). Parental care and mating system of the prairie vole, Microtus ochrogaster. Behav. Ecol. Sociobiol. 5, 171-186. 10.1007/BF00293304 [DOI] [Google Scholar]
- Uriarte N., Breigeiron M. K., Benetti F., Rosa X. F. and Lucion A. B. (2007). Effects of maternal care on the development, emotionality, and reproductive functions in male and female rats. Dev. Psychobiol 49, 451-462. 10.1002/dev.20241 [DOI] [PubMed] [Google Scholar]
- van Hasselt F. N., Tieskens J. M., Trezza V., Krugers H. J., Vanderschuren L. J. M. J. and Joëls M. (2012). Within-litter variation in maternal care received by individual pups correlates with adolescent social play behavior in male rats. Physiol. Behav. 106, 701-706. 10.1016/j.physbeh.2011.12.007 [DOI] [PubMed] [Google Scholar]
- Viau V., Sharma S., Plotsky P. M. and Meaney M. J. (1993). Increased plasma ACTH responses to stress in nonhandled compared with handled rats require basal levels of corticosterone and are associated with increased levels of ACTH secretagogues in the median eminence. J. Neurosci. 13, 1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. X. and Novak M. A. (1992). Influence of the social environment on parental behavior and pup development of meadow voles (Microtus pennsylvanicus) and prairie voles (Microtus ochrogaster). J. Comp. Psychol. 106, 163-171. 10.1037/0735-7036.106.2.163 [DOI] [Google Scholar]
- Wang Z. X., Yu G. Z., Cascio C., Liu Y., Gingrich B. and Insel T. R. (1999). Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): a mechanism for pair bonding? Behav. Neurosci. 113, 602-611. 10.1037/0735-7044.113.3.602 [DOI] [PubMed] [Google Scholar]
- Weaver I. C. G., Cervoni N., Champagne F. A., D'Alessio A. C., Sharma S., Seckl J. R., Dymov S., Szyf M. and Meaney M. J. (2004). Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847-854. 10.1038/nn1276 [DOI] [PubMed] [Google Scholar]
- Weiss I. C., Pryce C. R., Jongen-Rêlo A. L., Nanz-Bahr N. I. and Feldon J. (2004). Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav. Brain Res. 152, 279-295. 10.1016/j.bbr.2003.10.015 [DOI] [PubMed] [Google Scholar]
- Williams J. R., Catania K. C. and Carter C. S. (1992). Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm. Behav. 26, 339-349. 10.1016/0018-506X(92)90004-F [DOI] [PubMed] [Google Scholar]
- Winslow J. T., Hastings N., Carter C. S., Harbaugh C. R. and Insel T. R. (1993). A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365, 545-548. 10.1038/365545a0 [DOI] [PubMed] [Google Scholar]
- Xerri C., Stern J. M. and Merzenich M. M. (1994). Alterations of the cortical representation of the rat ventrum induced by nursing behavior. J. Neurosci. 14, 1710-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Cushing B. S., Kramer K. M., Epperson P. D., Hoffman G. E. and Carter C. S. (2004). Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience 125, 947-955. 10.1016/j.neuroscience.2004.02.028 [DOI] [PubMed] [Google Scholar]
- Yorgason J. T., España R. A., Konstantopoulos J. K., Weiner J. L. and Jones S. R. (2013). Enduring increases in anxiety-like behavior and rapid nucleus accumbens dopamine signaling in socially isolated rats. Eur. J. Neurosci. 37, 1022-1031. 10.1111/ejn.12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K. A., Gobrogge K. L., Liu Y. and Wang Z. X. (2011). The neurobiology of pair bonding: insights from a socially monogamous rodent. Front. Neuroendocrinol. 32, 53-69. 10.1016/j.yfrne.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]