Abstract
Mural cells (vascular smooth muscle cells and pericytes) play an essential role in the development of the vasculature, promoting vascular quiescence and long-term vessel stabilization through their interactions with endothelial cells. However, the mechanistic details of how mural cells stabilize vessels are not fully understood. We have examined the emergence and functional role of mural cells investing the dorsal aorta during early development using the zebrafish. Consistent with previous literature, our data suggest that cells ensheathing the dorsal aorta emerge from a sub-population of cells in the adjacent sclerotome. Inhibition of mural cell recruitment to the dorsal aorta through disruption of pdgfr signaling leads to a reduced vascular basement membrane, which in turn results in enhanced dorsal aorta vessel elasticity and failure to restrict aortic diameter. Our results provide direct in vivo evidence for a functional role for mural cells in patterning and stabilization of the early vasculature through production and maintenance of the vascular basement membrane to prevent abnormal aortic expansion and elasticity.
KEY WORDS: Vascular smooth muscle, Pericyte, Zebrafish, PDGFR signaling, Vascular basement membrane
Summary: Vascular smooth muscle cells are required to help maintain the vascular basement membrane and promote vessel stabilization during development.
INTRODUCTION
Mural cells – vascular smooth muscle cells (vSMCs) and pericytes – play crucial roles in establishment and long-term stabilization of blood vessels (Bergers and Song, 2005; Abraham et al., 2008; Owens, 1995; Armulik et al., 2011a; Jain, 2003). vSMCs are recruited to large-caliber vessels, namely arteries, arterioles and veins, where they concentrically wrap the endothelium, fully encasing the vessels in multi-layered smooth muscle sheaths. Interactions between endothelial cells (ECs) and vSMCs are thought to strengthen the maturing vascular wall to increases in flow and shear stress while helping to regulate vessel contractility and tone. Pericytes are associated with smaller-caliber vessels, in particular capillary beds and post-capillary venules. Pericytes associate with these smaller-caliber vessels in lower numbers, with pericyte/EC ratios between 1:1 and 1:10, localizing primarily along endothelial cell-cell junctions, branch points, and in areas that need to maintain high levels of barrier function [such as the blood-brain barrier (BBB)] (Ando et al., 2016; Owens, 1995; Daneman et al., 2010; Armulik et al., 2010, 2011a,b). PDGF-BB/PDGFRβ signaling is required for recruitment of vSMCs and pericytes to vessels (Hellstrom et al., 1999; Fortuna et al., 2015; Lindblom et al., 2003; Lindahl et al., 1997; Hirschi et al., 1999; Lehti et al., 2005; Benjamin et al., 1998), although a variety of other signaling pathways have been shown to contribute to mural cell differentiation, recruitment and stabilization, in particular TGFβ, Notch and EGF signaling among others (Lee et al., 2007; Higashiyama et al., 1993; Iivanainen et al., 2003; Krymskaya et al., 1997; Wang et al., 2014, 2012; Kofler et al., 2015; Fortuna et al., 2015; Kennard et al., 2008; Hirschi et al., 2003; Aplin et al., 2010; Stratman et al., 2010; Zaucker et al., 2013).
Mouse lineage tracing and chick/quail chimera studies have led to the adoption of a multi-site hypothesis for the ontogeny of mural cells (reviewed in Majesky, 2007; Pouget et al., 2008; Wiegreffe et al., 2007; Wasteson et al., 2008; Armulik et al., 2011a). In summary, vSMCs in the head and central nervous system appear to be derived from the neural crest, while smooth muscle cells in the gut, liver and lung seem to be of mesothelial origin. The developmental origins of mural cells investing the dorsal aorta are less well described, with at least two main sites proposed based on chick, mouse and zebrafish data as possible contributors of aortic vSMCs: the neural crest and the sclerotome/somite. In general, all studied models of vSMC ontogeny suggest an epithelial to mesenchymal transition (EMT) for mesothelial cells as they delaminate and then migrate to various anatomical locations.
EC-mural cell interactions have been implicated in production and maintenance of the vascular basement membrane to help promote blood vessel stabilization and quiescence (Jain, 2003; Davis and Senger, 2005; Hynes, 2007). EC-pericyte co-culture assays in vitro and quail chick chorioallantoic membrane (CAM) assays have provided evidence that pericytes help to maintain extracellularly deposited basement membrane proteins (Stratman and Davis, 2012; Stratman et al., 2009, 2010; Zhao et al., 2015). Although ECs appear to have the capacity to synthesize basement membrane proteins on their own, they do not appear to be properly deposited to form an EC-associated basement membrane in the absence of mural cells, or mural cells/astrocytes in the case of the BBB (Abraham et al., 2008; Armulik et al., 2010, 2011b; Yao et al., 2014; Chen et al., 2013). Although these assays suggest that EC-mural cell interactions are important for building and maintaining the vascular basement membrane, a full analysis of the requirement for both cell types in formation and stabilization of the basement membrane in the developing vasculature of an intact organism, in particular around larger-caliber vessels such as the dorsal aorta, has not been carried out.
Here, we show data suggesting that vSMCs associated with the dorsal aorta are derived from a sub-population of the sclerotome, and further demonstrate that recruitment of vSMCs to the dorsal aorta in the fish is dependent on PDGFRβ signaling. Reduced vSMC recruitment to the dorsal aorta following disrupted PDGFRβ signaling leads to decreased aortic accumulation of basement membrane proteins, along with increased aortic diameter and increased aortic wall elasticity. These data show that mural cell recruitment to the developing aorta is essential for proper assembly and maintenance of the developing vascular wall in vivo.
RESULTS
Mural cells invest the dorsal aorta in the developing zebrafish
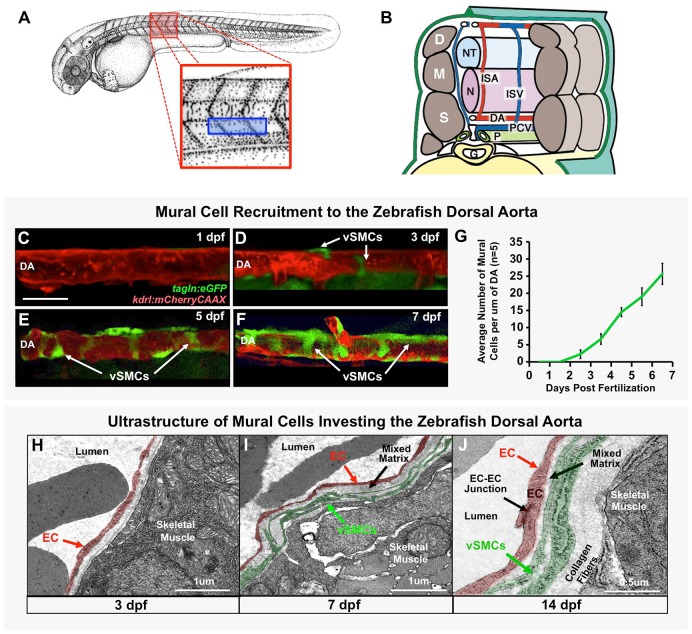
The dorsal aorta is the major trunk axial artery, and is one of the first vessels to assemble during early development in all vertebrates. The dorsal aorta forms immediately below the notochord and immediately above the posterior cardinal vein, the major trunk axial vein (Fig. 1A,B). In mammals, the dorsal aorta becomes heavily invested with supporting vSMCs beginning early in embryogenesis (Owens, 1995; Armulik et al., 2011a).
Fig. 1.
Vascular smooth muscle cells associate with the zebrafish dorsal aorta. (A) Camera lucida drawing of a 2 dpf zebrafish embryo (Kimmel et al., 1995), with the magnified red boxed region shown in B and blue boxed region imaged in C-F. (B) Schematic (‘cut-away’) diagram showing the anatomy of the zebrafish trunk and its blood vessels at approximately 2 days post-fertilization. Trunk circulation flows through the dorsal aorta (DA), posterior cardinal vein (PCV) and intersegmental arteries (ISA) and intersegmental veins (ISV). The vessels are shown relative to adjacent tissues and structures in the mid-trunk including the gut (G), notochord (N), neural tube (NT), left pronephric duct (P), dermamyotomes (D), myotomes (M) and sclerotomes (S). (C-F) Representative confocal images of the dorsal aorta in Tg(tagln:egfp), Tg(kdrl:mCherryCAAX) double transgenic zebrafish at 1 dpf (C), 3 dpf (D), 5 dpf (E), or 7 dpf (F), with red fluorescent vascular endothelium and green fluorescent vSMCs, showing accumulation of vSMCs on the dorsal aorta. (G) Quantification of vSMC accumulation on the first 6-somite segments of the dorsal aorta at 1-7 dpf. Values are mean±s.e.m.; n=5 fish. (H-J) Vascular smooth muscle cell investment of the DA was confirmed by electron microscopy. Representative images of the dorsal aorta wall at 3 dpf (H), 7 dpf (I) and 14 dpf (J) are shown. ECs and vSMCs are pseudo-colored red and green, respectively. Panel B is adapted from Isogai et al. (2003). Scale bar: 50 µm (C-F).
We used the experimentally accessible, optically clear zebrafish embryo to study the ontogeny of smooth muscle (mural) cells contributing to the wall of the dorsal aorta. A number of previous studies have provided solid documentation that the zebrafish dorsal aorta acquires a vSMC-containing vascular wall similar to that found in other vertebrates, and have suggested a somitic origin for these cells (Santoro et al., 2009; Whitesell et al., 2014; Fortuna et al., 2015; Wang et al., 2014; Ando et al., 2016; Zaucker et al., 2013). We crossed a previously reported zebrafish Tg(tagln:egfp) smooth muscle cell transgenic reporter line (Seiler et al., 2010; Yang et al., 2003) to a Tg(kdrl:mCherryCAAX) red fluorescent vascular endothelial-specific transgenic reporter line (Fujita et al., 2011) and employed confocal microscopy to examine the time course of mural/vSMC recruitment to the dorsal aorta during early development (Fig. 1C-G, Fig. S1). At 1 day post-fertilization (dpf) no vSMCs are observed along the mid-trunk dorsal aorta (Fig. 1C), but by 3 dpf, a small number of GFP-positive vSMCs are clearly associated with this vessel (Fig. 1D). Dorsal aorta-associated vSMCs continue to increase in number and begin to wrap around the vessel at the 5 dpf and 7 dpf time points (Fig. 1E-G). Rostral portions of the dorsal aorta become invested with vSMCs earlier than more caudal portions of the dorsal aorta (Fig. S2A-E). vSMC investment of the trunk intersegmental vessels lags behind that of the dorsal aorta, while the posterior cardinal vein lags even further behind (Fig. S2F-I).
We used electron microscopy (EM) to show that dorsal aorta-associated GFP-positive cells represent bona fide perivascular mural cells. Tg(tagln:egfp) transgenic animals and Tg(kdrl:egfp) controls were collected separately at 3, 7 and 14 dpf and processed for conventional transmission EM and immuno-EM. Representative TEM images at 3, 7 and 14 dpf are shown with the endothelium pseudocolored red and vSMCs pseudocolored green (Fig. 1H-J). The identity of these cells was evident from their anatomical location and morphological features, but more directly confirmed by immuno-EM. Anti-GFP immuno-EM showed that the presumptive endothelium of the dorsal aorta was labeled with nano-gold particles in samples from Tg(kdrl:egfp) fish, while presumptive vSMCs were labeled in samples from Tg(tagln:egfp) animals (Fig. S3). Our EM results also confirmed that there are few vSMCs associated with the dorsal aorta at 3 dpf (Fig. 1H), but that these cells accumulate in number by 7 dpf (Fig. 1I), and even more so by 14 dpf (Fig. 1J), at which time a few tightly associated vSMC layers are apparent along the length of the dorsal aorta that resemble those found on developing arteries of other vertebrates (Whitesell et al., 2014; Hellstrom et al., 1999; Santoro et al., 2009).
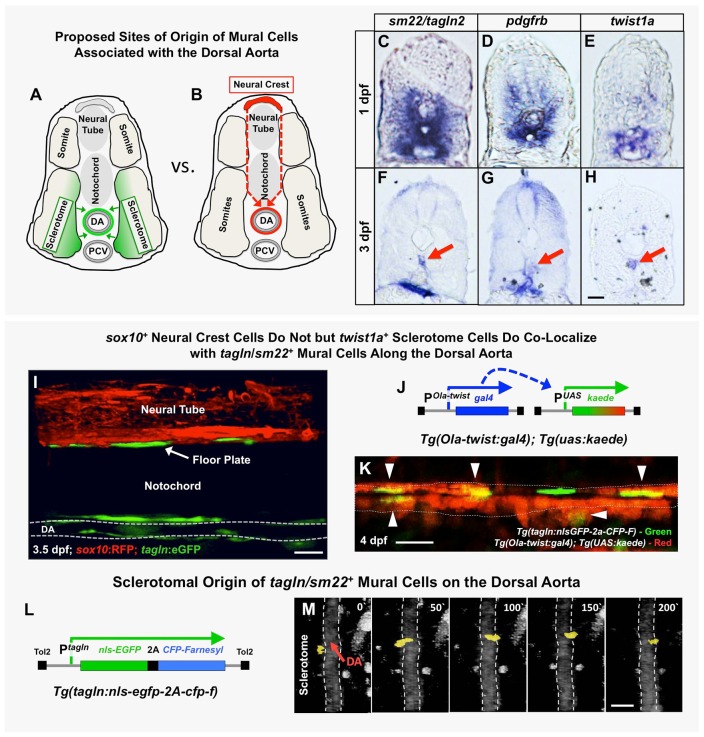
Various sites of origin have been proposed for the progenitors of vSMCs in the trunk, including the somites and the neural crest (Fig. 2A,B), with recent work in the zebrafish and other species suggesting that the majority of trunk vSMCs are mesothelial derived (Ando et al., 2016; Majesky, 2007; Pouget et al., 2008; Wiegreffe et al., 2007; Wasteson et al., 2008; Armulik et al., 2011a). Prior to the onset of vSMC recruitment to the dorsal aorta, vSMC markers tagln (sm22) and pdgfrb are expressed fairly broadly in the ventral-medial quadrant of the somites (Fig. 2C,D), with the highest expression concentrated in twist1a-positive sclerotome adjacent to the dorsal aorta (Fig. 2E). By 3 dpf, trunk expression of tagln and pdgfrb is restricted mainly to presumptive SMCs localized on or near the dorsal aorta and the developing gut (Fig. 2F,G), with twist1a expression also largely restricted to the vicinity of the dorsal aorta (Fig. 2H), consistent with a possible sclerotomal origin for dorsal aorta vSMCs.
Fig. 2.
The origins of trunk vSMCs. (A,B) Schematic diagrams illustrating two alternative models for the origin and migration of vSMCs recruited to the dorsal aorta, either from the sclerotome (A) or from the trunk neural crest (B). (C-H) Transverse sections of the trunk from 1 dpf (C-E) or 3 dpf (F-H) animals subjected to whole-mount in situ hybridization expression analysis, probed with tagln2 (C,F), pdgfrb (D,G) or twist1a (E,H). All three markers are expressed more broadly in the sclerotome at 1 dpf (C-E), but by 3 dpf their expression becomes restricted to SMCs of the DA (red arrows) and gut (F,G) or DA alone (H). (I) Confocal lateral view image of the mid-trunk of a 3.5 dpf Tg(tagln:gfp), Tg(sox10:rfp) double transgenic animal. The lack of RFP expression along the dorsal aorta suggests tagln+ vSMCs are not of neural crest origin. DA, dorsal aorta. (J) Schematic diagram of the Tg(Ola-twist:gal4) and Tg(uas:kaede) transgenes. In Tg(Ola-twist:gal4), Tg(uas:kaede) double transgenic fish, the medaka (Oryzias latipes) twist promoter (Ola-twist) is used to drive expression of Gal4 from the Ola-twist:gal4 transgene, which then activates expression of green to red photoconvertible kaede from the uas:kaede transgene. (K) Confocal lateral view image of the dorsal aorta in a 4 dpf Tg(Ola-twist:gal4), Tg(uas:kaede), Tg(tagln:nlsGFP-2a-CFP-F) triple-transgenic animal in which sclerotome-expressed kaede was photoconverted to red fluorescence at 4 dpf to mark twist1-positive cells. Numerous double-positive tagln+ (green nucleus), twist+ (red cytoplasm) cells are clearly evident on the dorsal aorta (arrowheads). A total of five fish were imaged in two rounds of photoconversion experiments with equivalent results. (L) Schematic diagram of the Tg(tagln:nlsgfp-2a-cfp-f) transgene, with the tagln promoter driving expression of nuclear-localized GFP and membrane-localized farnesyl CFP, joined by a 2A peptide linker. (M) Selected frames from a confocal time-lapse image series collected from a Tg(tagln:nlsgfp-2a-cfp-f), Tg(kdrl:mCherryCAAX) double-transgenic fish shows the movement of a tagln+ nucleus (yellow) from the adjacent sclerotome to the dorsal aorta (demarcated by dashed lines). The image reconstructions shown are ventral views, with rostral to the top. Confocal images were acquired every 10 min and the displayed stills are shown at approximately 50 min intervals. In total, seven tagln+ nuclei investing the DA were traced to the sclerotome in five separate time-lapse experiments. Scale bars: 50 µm.
To rule out neural crest as a source of mural cell progenitors (Fig. 2B), we examined the offspring of Tg(sox10:rfp);Tg(tagln:egfp) double-transgenic animals (Fig. 2I). Previous reports have documented Tg(sox10:rfp) transgene expression in both pre-migratory neural crest and neural crest-derived cells (Kucenas et al., 2008), and examination of double transgenic animals showed that, as previously described, the Tg(sox10:RFP) transgene was strongly expressed in the neural tube and neural crest (Fig. 2I). Although we could also easily detect green fluorescent Tg(tagln:egfp) transgene-positive cells along the dorsal aorta at 3.5 dpf, none of these cells were red fluorescent (Fig. 2I), confirming that this population of vSMCs was not of neural crest origin.
To examine whether the sclerotome serves as a source of aortic vSMCs, we utilized a previously reported zebrafish Tg(ola-twist1:gal4) transgenic line with a medaka Twist1 promoter (ola-twist1) expressing Gal4 in the sclerotome (Lee et al., 2013; Yasutake et al., 2004; Yeo et al., 2009; Germanguz et al., 2007) to drive expression of a UAS:kaede transgene (Fig. 2J). Examination of the progeny of double transgenic Tg(ola-twist1:gal4); Tg(UAS:kaede) animals or triple transgenic Tg(ola-twist1:gal4); Tg(UAS:kaede); Tg(kdrl:mCherryCAAX) animals showed that the sclerotome was indeed expressing kaede at early time points (Fig. S4A) and at later time points twist1-positive cells were observed along the dorsal aorta (Fig. S4B,C). We crossed Tg(ola-twist1:gal4);Tg(UAS:kaede) double transgenics to the Tg(tagln:nls-egfp-2a-cfp-f)y450 line, photoconverted the sclerotomally expressed kaede to red fluorescence at 4 dpf, and then examined the mural cell coverage of the dorsal aorta of photoconverted animals. We found that most GFP-positive vSMC nuclei on the dorsal aorta overlapped with cells containing photoconverted twist1-driven red kaede (Fig. 2K, arrowheads), consistent with a sclerotomal origin for these cells.
To examine more directly whether sclerotome-derived cells contribute to aortic vSMCs (Fig. 2A), we used two-photon time-lapse imaging of Tg(tagln:nls-egfp-2a-cfp-f)y450 transgenic animals in which the tagln promoter drives expression of both nuclear-targeted EGFP and membrane-targeted farnesylated CFP (Fig. 2L) in order to track the nuclei of mural cells contributing to the dorsal aorta. We collected 1- to 2-day-long time-lapse sequences beginning at approximately 2.5 dpf. Although most dorsal aorta-juxtaposed EGFP-positive nuclei appeared to move little if at all during time lapse imaging, in a few particularly striking examples the cells could be observed migrating from the sclerotome on one side of the aorta to take up position on the other side of the aorta (Fig. 2M, Movie 1). Other aorta-associated EGFP-positive cells were lost to analysis for technical reasons (i.e. difficulty in following the nuclei because of low fluorescence, too many adjacent cells to distinguish nuclei, lack of movement, etc.). In no case however were any cells traced to tissues or sites of origin other than adjacent to the aorta in and/or near the sclerotome. Interestingly, despite lack of substantial movement of most aorta-associated nuclei in Tg(tagln:nls-egfp-2a-cfp-f)y450 transgenic animals, it was relatively easy to observe bulk movement of EGFP-positive cell membranes from the sclerotome to become more closely associated with the adjacent dorsal aorta in Tg(tagln:egfp) transgenic animals (Fig. S4D,E, Movie 2). Taken together, these data suggest that vSMC precursors are moving relatively small distances from the adjacent sclerotome to become associated with the dorsal aorta, with nuclei that may even remain relatively stationary. Once the initial population of vSMCs become associated with the dorsal aorta and intersegmental vessels, further increases in mural cell coverage of the trunk vasculature appear to occur, at least in part, via proliferation of the initial cell population (Fig. S5). The findings we describe above are entirely consistent with a study recently published by Ando et al. (2016).
Disrupting PDGF signaling interferes with mural cell recruitment
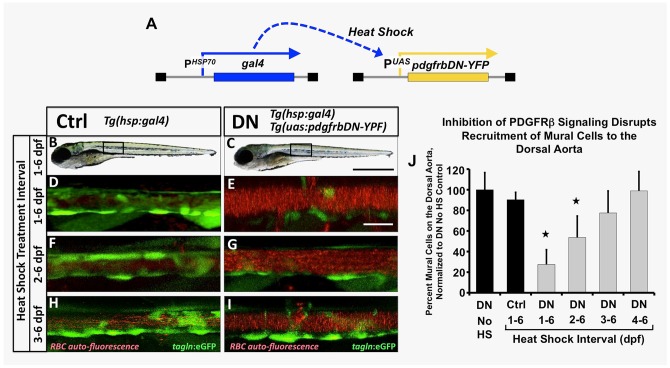
In order to examine the functional role of dorsal aorta-associated mural cells in the early embryo and larva, we inhibited their recruitment to the developing dorsal aorta by disrupting PDGF signaling (Lindblom et al., 2003; Lindahl et al., 1997; Hellstrom et al., 1999; Armulik et al., 2011a). We utilized a Tg(UAS:pdgfrbDN-YFP) transgenic zebrafish to inducibly express a PDGFRβ construct containing intact N-terminal PDGF ligand binding and transmembrane domains but with the intracellular signaling domain replaced by YFP. This C-terminally truncated PDGFRβ has previously been shown to act in a dominant-negative fashion and to have effects on vSMC recruitment to anterior regions of the dorsal aorta when injected as a mosaic construct (Wiens et al., 2010; Fortuna et al., 2015).
We began by driving heat shock-inducible global expression of the dominant-negative PDGFRβ by crossing the Tg(UAS:pdgfrbDN-YFP) transgenic line to the Tg(hsp70:gal4) driver line (Scheer and Campos-Ortega, 1999) (Fig. 3A). By also including the Tg(tagln:egfp) transgenic background and using auto-fluorescence of circulating red blood cells to mark the vascular compartment we could easily assess mural cell recruitment to the dorsal aorta in these animals. Heat shock induction of Tg(hsp70:gal4)/+, Tg(UAS:pdgfrbDN-YFP)/+ double transgenic embryos or control Tg(hsp70:gal4)/+ siblings was initiated beginning at 1, 2, 3 or 4 dpf, with daily heat shock cycles continuing until vSMC coverage was assessed at 6 dpf by confocal imaging (Fig. 3B-J). Early heat shock-inducible global expression of the pdgfrbDN led to strongly reduced vSMC recruitment to the zebrafish dorsal aorta, with fewer defects noted if heat shock was initiated later, after vSMCs have started to migrate towards the dorsal aorta at 2 to 3 dpf (Fig. 3E,G,I,J). No gross morphological defects (Fig. 3B,C) or vascular patterning defects (Fig. S6) were noted in heat-shocked double-transgenic animals. Non-heat shocked Tg(hsp70:gal4)/+, Tg(UAS:pdgfrbDN-YFP)/+ double transgenic animals (Fig. 3J, first column) or heat-shocked control Tg(hsp70:gal4)/+ transgenic siblings (Fig. 3B,D,F,H, and J, second column) showed no defects in vSMC recruitment.
Fig. 3.
Inhibition of pdgfr signaling leads to loss of mural cell coverage on the dorsal aorta. (A) Schematic diagram showing the Tg(HSP70:gal4 and UAS:pdgfrbDN-YFP) double transgenes used for heat shock-inducible expression of pdgfrbDN. These fish were outcrossed to Tg(tagln:egfp) transgenic fish to generate triple heterozygotes, heat shocked daily for the indicated time frame and imaged for analysis of mural cell coverage at 6 dpf. (B-I) Representative confocal images of 6 dpf heat shocked Tg(tagln:egfp), Tg(HSP70:gal4) control (Ctrl, B,D,F,H) or Tg(tagln:egfp), Tg(HSP70:gal4), Tg(UAS:pdgfrbDN-YFP) dominant negative-expressing (DN, C,E,G,I) animals are shown. Fish were heat shocked daily from 1-6 dpf (B-E), 2-6 dpf (F-G) or 3-6 dpf (H-I) and then imaged at 6 dpf. The tagln/sm22+ vSMCs associated with the dorsal aorta are shown in green; red blood cell autofluorescence showing the vascular compartment is in red. (J) Quantification of the number of vSMCs covering the dorsal aorta following the indicated heat shock interval. Tg(tagln:egfp), Tg(HSP70:gal4), Tg(UAS:pdgfrbDN-YFP) heat shock-inducible dominant negative PDGFRB (DN) or Tg(tagln:egfp), Tg(HSP70:gal4) control (Ctrl) fish were heat shocked as indicated, imaged at 6 dpf and the number of tagln/sm22+ cells per 3-somite segment length of dorsal aorta counted. Values were normalized to the tagln/sm22+ cell counts from non-heat shocked DN fish. Values are mean±s.e.m.; *P≤0.05 significance compared with non-heat shocked DN fish. Data are presented from a single experiment of n=5 fish; two experimental replicates for 2, 3, 4 day HS experiments and four experimental replicates from 1-6 day HS were carried out as validation showing consistent results. In more absolute values, we typically see approximately 20 vSMCs recruit to a 3-somite segment of dorsal aorta under control conditions, and ∼7-8 vSMCs recruit to a 3-somite length of dorsal aorta under DN activation conditions. Scale bars:1 mm (B,C) and 50 µm (D-I).
To further examine the requirement for PDGF signaling more specifically in vSMCs and minimize potential off-target or pleiotropic effects, we generated a new Tg(tagln:EcRgal4)y449 transgenic line in order to drive tebufenozide-inducible expression of pdgfrbDN in vSMCs when crossed to the Tg(UAS:pdgfrbDN-YFP) line (Fig. S7A). Tebufenozide was added to Tg(tagln:EcRgal4)/+, Tg(UAS:pdgfrbDN-YFP)/+ double-transgenic animals in the Tg(tagln:egfp) background beginning at 0.5 dpf with daily replacement of the drug. vSMC association with the dorsal aorta was analyzed at 6 dpf, utilizing red blood cell auto-fluorescence to visualize the vascular compartment. Animals to which tebufenozide was added to induce expression of pdgfrbDN showed strongly reduced numbers of tagln:egfp-positive vSMCs associated with the dorsal aorta (Fig. S7C,E,G, and H, light gray column) compared with their non-tebufenozide treated sibling controls (Fig. S7B,D,F, and H, black column). Tebufenozide treatment of Tg(tagln:EcRgal4)/+ single-transgenic siblings also has no effect on vSMC coverage (Fig. S7H, dark gray column). These data support a role for mural cell-autonomous PDGF signaling in the development and stabilization of the vascular wall.
Mural cell recruitment restricts aortic diameter and promotes assembly of vascular basement membranes
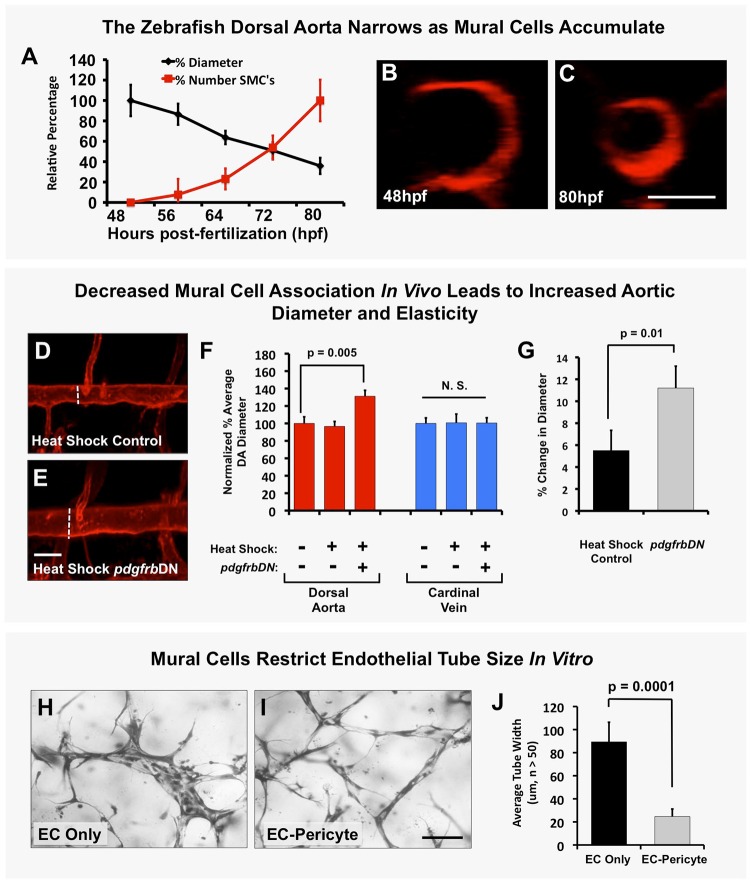
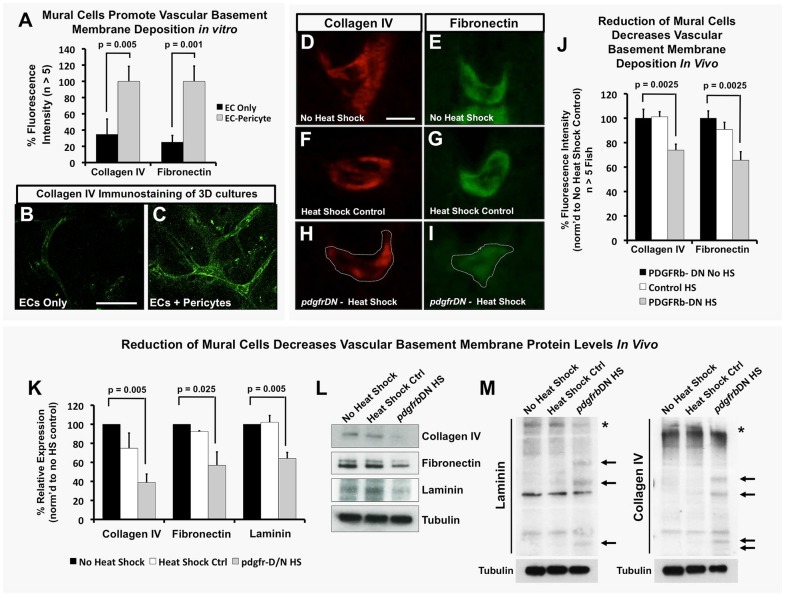
Previous studies have shown that the diameter of the dorsal aorta (DA) and posterior cardinal vein (PCV) decreases as development proceeds in the zebrafish (Bagatto and Burggren, 2006). During normal development, aortic diameter is inversely correlated with accumulation of mural cells over time (Fig. 4A-C), suggesting a possible relationship between the two phenotypes. To examine whether mural cell coverage plays a role in restricting aortic diameter, we examined aortas in heat shock control versus Tg(hsp70:gal4)/+, Tg(UAS:pdgfrbDN-YFP)/+, Tg(kdrl:mCherry) triple transgenic animals (Fig. 4D-F) and in tebufenozide-treated versus untreated Tg(tagln:EcRgal4)/+, Tg(UAS:pdgfrbDN-YFP)/+ double-transgenic animals (Fig. S7I). The diameter of the dorsal aorta was significantly larger in animals in which pdgfrbDN was induced by heat shock (Fig. 4D-F) or tebufenozide treatment (Fig. S7I), while the diameter of the posterior cardinal vein (which becomes invested by mural cells mostly after 6 dpf – see Fig. S1) was not significantly increased (Fig. 4F, Fig. S7I). This phenomenon was also readily modeled in vitro, where ECs seeded alone in collagen type I gels formed tubes that continued to increase in diameter over time, while ECs seeded together with pericytes formed tubes that recruited pericytes and maintained a restricted diameter (Fig. 4H-J).
Fig. 4.
Mural cells regulate aortic diameter and elasticity. (A) Accumulation of vSMCs on the dorsal aorta inversely correlates with reduced dorsal aorta diameter over time during zebrafish vascular development. Dorsal aorta diameter is normalized to starting 48 hpf value and vSMC number is normalized to final 80 hpf value. n=3 fish. (B,C) Representative 2 dpf (B) and 3.25 dpf (C) transverse reconstructions of confocal images of the dorsal aorta in Tg(kdrl:mCherryCAAX) transgenic zebrafish, shown at the same magnification. (D,E) Loss of mural cell coverage leads to expansion of the zebrafish dorsal aorta. Representative 5 dpf confocal images of the mid-trunk dorsal aorta in Tg(kdrl:mCherryCAAX), Tg(tagln:egfp), Tg(HSP70:gal4) control (D) or Tg(kdrl:mCherryCAAX), Tg(tagln:egfp), Tg(HSP70:gal4), Tg(UAS:pdgfrbDN-YFP) dominant negative pdgfrbDN transgene-expressing (E) embryos that were heat shocked for a full 1-5 day time course. (F) Quantification of dorsal aorta diameter, demonstrating marked expansion of the dorsal aorta in heat shock pdgfrbDN fish compared with non-heat shocked or heat shocked control siblings. No effects were noted on the diameter of the cardinal vein. A minimum of three fish were measured per sample, with ten separate dorsal aorta measurements per fish. Experiments were repeated three times showing consistent results; N.S., not significant. (G) Loss of vSMC coverage leads to increased elasticity of the dorsal aorta in the developing zebrafish embryo. Heat shock control (maintains vSMC coverage) versus heat shock pdgfrbDN (demonstrates marked loss of vSMC coverage) fish were imaged using SPIM. Images were acquired at 22 frames per second to capture the movement of the wall of the dorsal aorta (‘elasticity’) as it expands and retracts in accordance with the animal's heartbeat. Data are reported as a percentage diameter change [(maximal width of the aorta-minimal width of the aorta)/minimal width of the aorta×100]. Five fish were measured per sample, with three separate dorsal aorta measurements taken per fish. Mean±s.e.m. (H-J) Regulation of EC tube diameter by mural cells can be modeled in 3D collagen matrix assays in vitro. Representative images of EC-only culture (H) and EC-pericyte co-cultures (I) are shown. (J) Quantification of endothelial tube diameter in EC-only cultures and EC-pericyte co-cultures established in 3D collagen matrices, showing a marked reduction in EC tube diameter in the presence of mural cells (pericytes). n=5 images from separate collagen plugs, ten measurements per image. Scale bars: 50 µm. Values are mean±s.d.
Heat shocked Tg(hsp70:gal4)/+, Tg(UAS:pdgfrbDN-YFP)/+, Tg(fli:egfp) animals also showed significantly greater vessel wall elasticity compared with their heat shocked control siblings (Fig. 4G; Movie 3). Using single plane illumination microscopy (SPIM) to measure the magnitude of the rapid changes in vessel diameter occurring in conjunction with the beating of the heart, we showed that heat shocked Tg(hsp70:gal4)/+, Tg(UAS:pdgfrbDN-YFP)/+, Tg(fli:egfp) had a nearly 12% change in dorsal aorta diameter with each heartbeat compared with less than 6% in heat shocked Tg(hsp70:gal4)/+, Tg(fli:egfp) sibling control animals (Fig. 4G; Movie 3).
Since previous evidence suggests that basement membrane formation is required to help restrict vessel expansion (Davis and Senger, 2005; Hynes, 2007; Jain, 2003; Stratman et al., 2009, 2010; Stratman and Davis, 2012) and that production/stabilization of basement membrane proteins is in part mediated by EC-mural cell interactions, we examined whether EC-mural cell interaction could be modulating the assembly of the vascular basement membrane of the developing zebrafish dorsal aorta. In vitro cultures of ECs alone show a lack of extracellular deposition of basement membrane matrix structural components, such as collagen IV and fibronectin, compared with cultures seeded with both ECs and pericytes (Fig. 5A-C), suggesting that the presence of both cell types is required to maintain a vascular basement membrane. We used our vSMC-deficient zebrafish models to examine whether this is similarly the case in vivo. In the 5 dpf zebrafish trunk, deposition of structural components of the vascular basement membrane, including laminin, fibronectin and collagen IV, is concentrated around the developing dorsal aorta, as assessed by immunofluorescence staining of transverse trunk tissue sections with specific antibodies (Fig. S8A and Fig. S9). Heat shock treatment of Tg(hsp70:gal4)/+, Tg(UAS:pdgfrbDN-YFP)/+ double-transgenic animals significantly reduced the amount of antibody-positive immunostaining for collagen IV and fibronectin present around the 5 dpf dorsal aorta (Fig. 5D-J). Protein lysates from these same animals showed significant decreases in the full-length forms of collagen IV, laminin and fibronectin (Fig. 5K,L). By collecting protein lysates under non-reducing conditions, we could also show an increase in fragmented forms of collagen IV and laminin proteins in heat-shocked Tg(hsp70:gal4)/+, Tg(UAS:pdgfrbDN-YFP)/+ double-transgenic animals compared with heat shocked control or no heat shock double transgenic animals (Fig. 5M).
Fig. 5.
EC-mural cell interactions promote deposition and maintenance of the vascular basement membrane. (A-C) Mural cells promote vascular basement membrane deposition in vitro. (A) Quantification of basement membrane components collagen IV and fibronectin in 3D collagen matrix assays with ECs alone (black) or EC-pericyte co-cultures (gray) using detergent-free immunostaining protocols to assess only the extracellular deposition of these proteins. Representative images of collagen IV staining in EC only (B) and EC-pericyte co-cultures (C); n>5 individual immunostained collagen plugs. (D-J) vSMCs promote vascular basement membrane deposition and stability in vivo. (D-I) Representative images of collagen IV (D,F,H) or fibronectin (E,G,I) immunostaining of the dorsal aorta in transverse paraffin sections of 5 dpf no heat shock double transgenic Tg(HSP70:gal4; UAS:pdgfrbDN-YFP) animals (D,E), 1-5 dpf heat-shocked control Tg(HSP70:gal4) animals (F,G) or 1-5 dpf heat-shocked pdgfrbDN-expressing double transgenic Tg(HSP70:gal4; UAS:pdgfrbDN-YFP) animals (H,I). (J) Quantification of 5 dpf dorsal aorta collagen IV or fibronectin immunostaining intensity using the relative basement membrane staining intensity of sections such as those shown in D-I. All data are shown as a percentage of the no heat shock control condition. n=10 fish total, combined data from three individual experiments. (K) Quantification of basement membrane protein levels by western blot analysis (n=3 blots, from three individual experiments) of each of the conditions shown in D-J. Data are represented as a percentage of the no heat shock control condition. (L) Representative western blot images are shown versus a tubulin loading control. (M) The ability of mural cells to regulate maintenance of full-length basement membrane components was analyzed by collecting protein lysates under non-reducing conditions. No heat shock control, heat shock control (not carrying the pdgfrbDN cassette) and pdgfrbDN, heat shock treatment lysates were collected for analysis of protein fragmentation levels of collagen IV and laminin proteins. Representative western blots are shown, with increased fragmentation (highlighted by arrows) of the proteins noted only in the pdgfrbDN heat shock condition, where mural cell coverage has been disrupted. Location of the full-length proteins is marked with an asterisk. Representative western blots from lysates of ten pooled zebrafish embryos and two individual experiments. Scale bars: 50 µm. Values are mean±s.e.m.
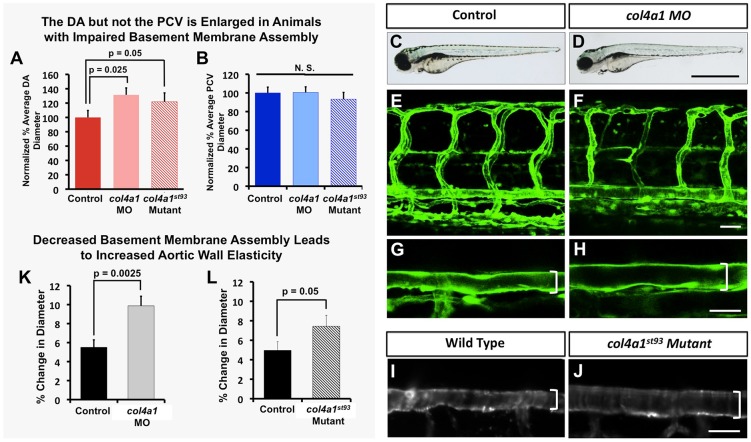
To further examine whether the basement membrane restricts aortic diameter and elasticity, we disrupted a key structural component of the basement membrane, collagen IV (col4a1 in zebrafish). Animals that were either injected with col4a1 translation-blocking morpholino or homozygous for the col4a1st93/st93 mutation displayed increased aortic diameter (Fig. 6A,C-J) similar to that seen in vSMC-deficient animals (Fig. 4D-F, Fig. S7I). Overall vascular patterning (Fig. 6E,F) and blood flow (Movie 4) were not significantly altered in any of the animals analyzed for these studies. Using SPIM to measure changes in vessel wall elasticity, we showed that there was a ∼5-6% change in dorsal aorta diameter with each beat of the heart in control animals, whereas in col4a1 morpholino-treated animals or col4a1st93/st93 mutants there was an approximate 10% and 7-8% change in diameter with each heartbeat, respectively (Fig. 6K,L Movie 4).
Fig. 6.
The vascular basement membrane functionally regulates aortic diameter and stability. Impaired basement membrane assembly leads to increased aortic diameter and elasticity. (A,B) Quantification of dorsal aorta (A) and posterior cardinal vein (B) diameter in 4 dpf col4a1 morpholino-injected or col4a1st93/st93 mutant animals compared with control wild-type animals. A minimum of four fish were measured per sample, with ten separate dorsal aorta measurements per fish. N.S., not significant. (C-J) Representative transmitted light (C,D) and confocal (E-J) images of 4 dpf control (C,E,G,I), col4a1 morpholino-injected (D,F,H), or col4a1st93 mutant (J) animals, showing lateral views of entire animal (C,D) lateral views of the trunk vasculature (E,F) and higher-magnification lateral images of the dorsal aorta (G-J). Brackets indicate diameter of dorsal aorta. (K,L) Quantification of dorsal aorta elasticity in 4 dpf col4a1 morpholino-injected (K) or col4a1st93 mutant (L) animals compared with control wild-type animals, using SPIM. Data are reported as percentage diameter change. A minimum of four fish were measured per sample, with three separate dorsal aorta measurements made per fish. Scale bars: 50 µm. Values are mean±s.e.m.
DISCUSSION
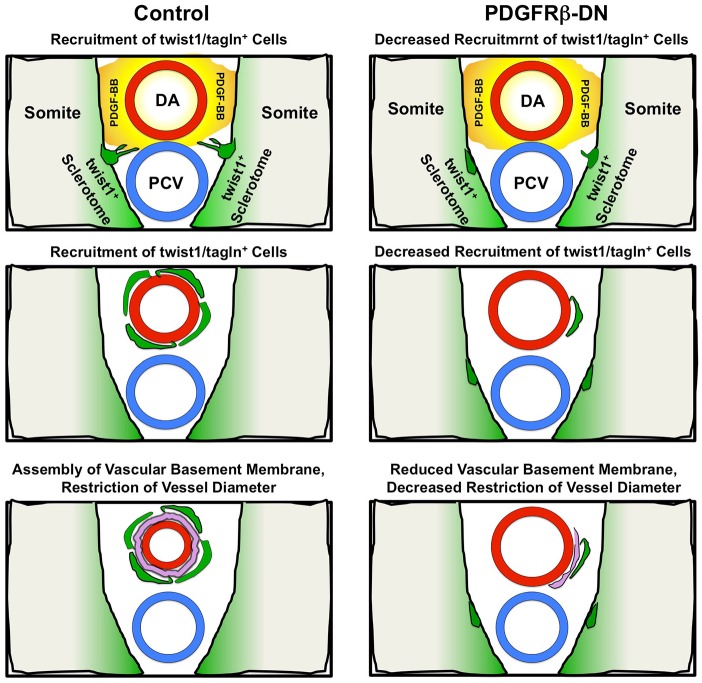
We have examined the ontogeny and functional role of vSMCs surrounding the dorsal aorta during early stages of zebrafish development. Using a combination of molecular markers, transgenic lines and live imaging, we show that a subpopulation of cells from the sclerotome appears to delaminate and differentiate into vSMCs recruited to the dorsal aorta. These cells serve an important functional purpose: to restrict aortic diameter by facilitating assembly and maintenance of the vascular basement membrane. Reducing vSMC recruitment by disrupting PDGF signaling results in defects in basement membrane formation around the dorsal aorta, failure to restrict dorsal aorta diameter and increased dorsal aorta elasticity, as measured using SPIM. Dorsal aorta diameter and elasticity are also increased by directly disrupting the vascular basement membrane component col4a1, supporting a crucial role for vSMC-promoted basement membrane assembly in regulating the growth and size of vessels during development (Fig. 7).
Fig. 7.
Mural cells regulate vascular outgrowth and stability through deposition of the basement membrane. Schematic representation of the role mural cells play in stabilization of blood vessels. Under control conditions, mural cells (green) delaminate from the sclerotome to associate with the dorsal aorta (red). These cells interact with the endothelium to promote basement membrane protein deposition and maintenance (pink). Under conditions in which mural cell recruitment is inhibited (i.e. pdgfrbDN) vascular stabilization is markedly disrupted and reduced basement membrane deposition is noted.
Ontogeny of vSMCs associated with the zebrafish dorsal aorta
Previous reports from the zebrafish and studies from chick/quail and murine models have supported a model whereby mesodermal-derived cells contribute to the host population of cells that become vSMCs along the dorsal aorta (Bergers and Song, 2005; Majesky, 2007; Pouget et al., 2008; Wiegreffe et al., 2007; Wasteson et al., 2008; Armulik et al., 2011a). Through use of transgenic reporters and nuclear tracking studies, we demonstrate that the majority of vSMCs that end up resident on the dorsal aorta in the zebrafish trunk are twist1+ cells derived from the sclerotome, not sox10+ neural crest-derived cells. Our findings are entirely consistent with previous mammalian reports and with a well-documented recent report in the zebrafish from Ando et al. (2016) that identified the paraxial and lateral plate mesoderm as the source of mural cells in the trunk. Together with those of Ando et al. (2016), our data suggest that most early dorsal aorta vSMC progenitors emerge from the immediately adjacent twist1+ sclerotomal compartment of the somites, and in most cases appear to move little distance before investing the dorsal aorta. As with any experiments designed to mark and track vSMCs to determine their origins, it is difficult to completely exclude any contribution from other anatomical sites of origin, particularly as many of the transgenic markers used to mark vSMCs tend to start being expressed just as these cells are undergoing differentiation, complicating execution and interpretation of these experiments. Although our data and those of Ando et al. (2016) strongly implicate sclerotomal progenitors as the major source of vSMCs investing the dorsal aorta, and demonstrate clearly that neural crest-derived cells do not contribute to aortic vSMCs, additional rigorous lineage-tracing experiments would be needed to fully rule out alternative sources for vSMCs in the trunk. While not the main focus of the present study, further more comprehensive examination of the ontogeny of vSMCs and migratory patterns and pathways used by early trunk vSMC progenitors would be useful.
A large body of work has exhaustively documented the crucial role that PDGF-BB/PDGFRβ signaling plays in mural cell recruitment to blood vessels in a variety of models. Consistent with these studies (Hellstrom et al., 1999; Lindahl et al., 1997; Lindblom et al., 2003; Bergers and Song, 2005; Stratman et al., 2010; Hirschi et al., 1999; Lehti et al., 2005; Fortuna et al., 2015; Armulik et al., 2011a), our work also suggests that PDGF signaling is required for proper vSMC recruitment to the dorsal aorta. Global (Fig. 3) or vSMC-autonomous (Fig. S7) inducible expression of a dominant-negative pdgfrbDN transgene results in defects in vSMC recruitment to the dorsal aorta without evidence of any significant effects on gross anatomy or vessel growth. The inhibition of vSMC recruitment is not complete in our pdgrfrbDN experiments. This raises the possibility that other mechanisms besides PDGF signaling may also be used to help vSMC ‘home’ to vessels (Lee et al., 2007; Higashiyama et al., 1993; Iivanainen et al., 2003; Krymskaya et al., 1997; Wang et al., 2014, 2012; Kofler et al., 2015; Fortuna et al., 2015; Kennard et al., 2008; Hirschi et al., 2003; Aplin et al., 2010; Stratman et al., 2010; Zaucker et al., 2013). However, it is also possible that we are simply not able to sufficiently eliminate vSMC PDGF signaling using this method and that residual vSMC recruitment reflects this ‘bleed through’. Further studies will be needed to determine which of these possibilities is true. Although previous reports utilizing this pdgfrbDN line suggest alterations in ISV outgrowth (Wiens et al., 2010), we did not note these phenotypes in our heat shock experiments (we initiated heat shock no earlier than 24 hpf, Fig. S6). Additionally, we took the extra step of generating a new Tg(tagln:EcRgal4)y449 tebufenozide-inducible vSMC driver line to provide stronger evidence that the results we obtained reflected vSMC-autonomous effects. Previous reports have described pdgfrb zebrafish mutants lacking marked effects on vSMC recruitment (Kok et al., 2015; Ando et al., 2016). However, other studies utilizing mosaic injection of the pdgfrbDN construct and the PDGFRβ inhibitor, inhibitor V, in zebrafish (in addition to the data shown in our study) have documented significant effects of activation of this construct on vSMC recruitment to the dorsal aorta (Fortuna et al., 2015; Fig. 3, Fig. S7). The discrepancy between vSMC phenotypes in zebrafish pdgfrbDN expression studies and mouse PDGF-BB and PDGFRβ knockout/knock-in studies as compared with the lack of phenotype in zebrafish pdgfrb mutants might reflect genetic compensation in zebrafish pdgfrb mutants (Rossi et al., 2015). The pdgfrb and pdgfra receptors are known to dimerize, and it is likely that the pdgfrbDN dominant-negative construct would prevent compensation of one receptor by the other, something that is much less likely to occur in the pdgfrb zebrafish mutants. Although further work will be needed to fully unravel the roles of specific PDGF receptors and ligands in vSMC development in the fish, it is clear from our data and other previous reports that the pdgfrbDN construct provides a useful tool to disrupt vSMC recruitment to the dorsal aorta as a way to functionally study the consequences of loss of this cell population on vascular stabilization.
The crucial function of the vascular basement membrane during development
Assembly of the vascular basement membrane is contingent on two fundamental processes: (1) synthesis and deposition of all of the individual components, and (2) maintenance of these components (Yurchenco, 2011; Francis et al., 2002; George et al., 1993; Jain, 2003; Davis and Senger, 2005; Hynes, 2007). A variety of reports have suggested that cooperation between ECs and vSMCs/pericytes is needed to properly synthesize and maintain all components of the vascular basement membrane niche (Stratman et al., 2009, 2010; Abraham et al., 2008; Smola et al., 1998; Stratman and Davis, 2012). A number of in vitro studies have shown that while ECs can synthesize the majority of basement membrane proteins in an autonomous fashion, vSMCs and pericytes provide a number of proteolytic inhibitors, such as TIMPs, that can prevent the basement membrane from undergoing degradation by EC-generated MMPs (Saunders et al., 2006; Stratman et al., 2009; Mascall et al., 2012; Castoldi et al., 2003; Stratman and Davis, 2012). Pericytes have also been reported to generate a high abundance of proteins, such as nidogen-1, and proteoglycans that serve to crosslink and stabilize many of the main structural components of basement membranes, such as laminin, collagen IV and fibronectin (Yurchenco, 2011; Perkins et al., 1979; Jain, 2003; Breitkreutz et al., 2004; Hynes, 2007; Stratman et al., 2009). Although additional studies are needed to further understand the molecular mechanisms involved, including the use of cell type-specific gene suppression strategies in vivo, the data suggest strongly that ECs and mural cells are required in tandem to build and maintain the vascular basement membrane.
Our findings using experimental manipulation of intact animals and live imaging in the zebrafish now provides evidence that EC-mural cell interactions are indeed essential for maintenance of the vascular basement membrane. We show that loss of vSMC coverage leads to marked decreases in stabilization of full-length collagen IV and fibronectin and decreased accumulation of these proteins around the dorsal aorta (Fig. 5). Loss of proper basement membrane formation translates to changes in blood vessel morphology and dynamics, including increased diameter of the dorsal aorta and increased elasticity of the vascular wall (Figs 4 and 6, Fig. S7). These changes might also be expected to lead to alterations in blood pressure, shear stress and even flow directionality in vivo, analogous to phenotypes that present in many disease states such as stroke and atherosclerosis, suggesting that disruption of these fundamental cell-cell interactions could be a factor in the vascular dysfunction in these pathologies.
Concluding remarks
Our study and other recent work (Ando et al., 2016; Fortuna et al., 2015; Santoro et al., 2009; Wang et al., 2014; Whitesell et al., 2014; Seiler et al., 2010; Miano et al., 2006; Vanhollebeke et al., 2015; Zaucker et al., 2013) demonstrate the utility of the zebrafish model for studying the recruitment and function of mural cells during vascular development. In addition to the transgenic lines and constructs described and validated in this report, a number of other recently generated tools have made it relatively easy to visualize and study this crucial cell population within an intact organism (Seiler et al., 2010; Whitesell et al., 2014; Santoro et al., 2009; Miano et al., 2006; Vanhollebeke et al., 2015). Furthermore, a number of functional studies have begun to assess the vital signaling pathways and functionality of mural cells during vascular development in the fish model. Ando et al. (2016) have generated a number of highly useful reporter constructs in addition to their work on studying mural cell ontogeny in the fish. Wang et al. (2014) carried out innovative work studying the combined roles of Notch signaling, mural cells and vascular integrity in the brain. The new tools developed for all of these studies will prove highly useful for future functional work focused on understanding vSMC development, recruitment and contribution to vascular stabilization.
MATERIALS AND METHODS
Zebrafish
Zebrafish (Danio rerio) embryos were raised and maintained as described (Westerfield, 1995; Kimmel et al., 1995). New transgenic lines generated include: Tg(tagln:nls-egfp-2a-CFP-F)y450 and Tg(tagln:EcRgal4)y449. For details of established transgenic lines, see supplementary Materials and methods. Zebrafish husbandry and research protocols were reviewed and approved by the NICHD Animal Care and Use Committee at the National Institutes of Health. All animal studies were carried out according to NIH-approved protocols, in compliance with the Guide for the Care and use of Laboratory Animals.
Reagents
Antibodies for immunostaining and western blot analysis include: collagen IV (AbCAM, ab6586); fibronectin (AbCAM, ab6328); laminin (AbCAM, 11575); tubulin (Sigma, T6199); GFP (AbCAM, ab6556). Morpholino antisense oligonucleotides (Genetools) utilized include: 4 ng/μl col4a1 translation blocking MO: ACACATGGAAGCCGCATC TTCACAC.
Generation of col4a1st93 allele
Transcription activator-like effector nucleases (TALENs; Sanjana et al., 2012) were employed to generate the st93 allele. Sequencing identified st93 as a 2 bp deletion in the ORF of col4a1, resulting in a frameshift from the wild-type sequence of 5′-CCT GGT CTA GAA GGA-3′ to 5′-CCT GGT CT--A AGG AGC-3′. This frameshift is predicted to change the protein sequence after the leucine residue at position 66 from PGLEGA to PGLRST and introduce a premature STOP codon at position 86 in the mutant allele. Details on generation of the allele can be found in supplementary Materials and methods.
TEM and immuno-EM
Zebrafish embryos (heads and tails removed) were fixed with 4% paraformaldehyde (PFA) with 0.5% glutaraldehyde in PBS. After fixation, the embryos were rinsed with PBS, dehydrated in ethanol and infiltrated in LR White resin, medium grade (Electron Microscopy Sciences, Hatfield, PA) and finally embedded in 100% LR White resin. Polymerization of the resin was achieved using a Pelco UVC2 Cryo Chamber overnight at 4°C (Ted Pella, Redding, CA) as described in supplementary Materials and methods.
Time-lapse imaging and microscopy
Time-lapse images were collected utilizing either a Leica SP5 II or Olympus FV1000 confocal microscope starting at 56 hpf for tagln lines and 30 hpf for Ola-twist lines. Embryos were immobilized in buffered MS-222 and embedded in 0.8% low melting point agarose, with their heads and tail freed to facilitate continued growth. Images were acquired every 10 min for the desired time course. General microscopy for signal time points utilized the same confocal microscopes.
Heat shock and EcRgal4-driven pdgfrbDN activation
A daily 12 h heat shock program was developed to elicit maximal activation of the pdgfrbDN construct with minimal side effects on the growth and development of the zebrafish embryos. Further information on heat shock treatments can be found in supplementary Materials and methods. For EcRgal4-driven pdgfrbDN activation, tebufenozide resuspended in ethanol was added to the fish water at 100 μM. Water and drug were changed daily. Quantification of vSMC cell number was done by acquiring images of the zebrafish trunk starting at the 3-4 ISV and quantifying a 3-somite segment of dorsal aorta. Dorsal aorta width measurements were made using ImageJ by taking 10 individual measurements per image of the distance between the two walls of the aorta over a three somite segment length. Data are reported as a percentage of the control±s.e.m.
Endothelial cell culture and assays
HUVECs (Lonza) were cultured in bovine hypothalamus extract, 0.01% heparin and 20% FBS in M199 base medium (Gibco) on 1 mg/ml gelatin-coated tissue culture flasks. HUVECs were used from passages 3-6. Human pericytes (ScienCell) were cultured in 10% FBS in Advanced DMEM base medium (Gibco) on 1 mg/ml gelatin-coated tissue culture flasks. Pericytes were used from passages 3-10. Additional cell culture information can be found in the supplementary Materials and methods.
Immunostaining and western blot analysis
Zebrafish and 3D collagen assays utilized for immunostaining analysis were fixed in 4% or 2% PFA respectively at 4°C overnight. Zebrafish embryos were dehydrated in ethanol and embedded in paraffin for sectioning. Zebrafish samples for western blot analysis were de-yolked and directly lysed in 2× Laemmli sample buffer containing 5% b-ME and a PhosSTOP tablet (Roche), 10 μl per embryo unless otherwise indicated. Samples collected under native, non-reducing conditions did not contain β-ME. For further details, see supplementary Materials and methods.
qPCR and RNA extraction
Zebrafish embryos were collected at the indicated time points in Trizol and RNA purified using a double chloroform extraction protocol. cDNA was generated using a Bio-Rad I-Script cDNA synthesis kit from 500 ng RNA. SyberGreen qPCR protocols were utilized to generate relative expression data. Primer sets are available in the in the supplementary Materials and methods.
Dorsal aorta elasticity measurement methods
High-speed imaging of dorsal aorta movements were obtained using a lab-built light sheet microscope constructed using the Open-SPIM (Pitrone et al., 2013) wiki (www.openspim.org) with a VersaLase laser (Vortran Laser Technology) (488 nm/150 mW, 20% power), a Hamamatsu Orca R2 digital CCD camera, and a Picard Industries USB 4D Stage controlled by Fiji Micro-Manager open source software (Edelstein et al., 2010). Full details are available in in the supplementary Materials and methods.
Statistics
Statistical analysis of data was done using Microsoft Excel. Statistical significance was set at a minimum of P≤0.05 and is indicated in individual figures. Student's t-tests were used when analyzing two groups within a single experiment.
Acknowledgements
The authors would like to thank members of the Weinstein laboratory for their critical comments on this manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
A.N.S., S.A.P., O.M.F., D.C., M.G.B., H.S. and L.E.D. performed experiments; A.N.S., S.A.P., O.M.F., D.C., L.E.D., W.S.T. and B.M.W. analyzed results and made the figures; A.N.S., S.A.P., O.M.F., D.C. and B.M.W. designed the research and wrote the paper.
Funding
This work was supported by the intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (ZIA-HD001011 and ZIA-HD008915 to B.M.W.), the National Institutes of Health (R01NS050223 to W.S.T.), an Agency for Science, Technology and Research (A*STAR) fellowship (to H.S.) and a National Heart, Lung, and Blood Institute/NIH K99 Pathway to Independence Award (to A.N.S.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.143131.supplemental
References
- Abraham S., Kogata N., Fassler R. and Adams R. H. (2008). Integrin beta1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ. Res. 102, 562-570. 10.1161/CIRCRESAHA.107.167908 [DOI] [PubMed] [Google Scholar]
- Ando K., Fukuhara S., Izumi N., Nakajima H., Fukui H., Kelsh R. N. and Mochizuki N. (2016). Clarification of mural cell coverage of vascular endothelial cells by live imaging of zebrafish. Development 143, 1328-1339. 10.1242/dev.132654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin A. C., Fogel E. and Nicosia R. F. (2010). MCP-1 promotes mural cell recruitment during angiogenesis in the aortic ring model. Angiogenesis 13, 219-226. 10.1007/s10456-010-9179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A., Genové G., Mäe M., Nisancioglu M. H., Wallgard E., Niaudet C., He L., Norlin J., Lindblom P., Strittmatter K. et al. (2010). Pericytes regulate the blood-brain barrier. Nature 468, 557-561. 10.1038/nature09522 [DOI] [PubMed] [Google Scholar]
- Armulik A., Genove G. and Betsholtz C. (2011a). Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193-215. 10.1016/j.devcel.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Armulik A., Mäe M. and Betsholtz C. (2011b). Pericytes and the blood-brain barrier: recent advances and implications for the delivery of CNS therapy. Ther. Deliv. 2, 419-422. 10.4155/tde.11.23 [DOI] [PubMed] [Google Scholar]
- Bagatto B. and Burggren W. (2006). A three-dimensional functional assessment of heart and vessel development in the larva of the zebrafish (Danio rerio). Physiol. Biochem. Zool. 79, 194-201. 10.1086/498185 [DOI] [PubMed] [Google Scholar]
- Benjamin L. E., Hemo I. and Keshet E. (1998). A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 125, 1591-1598. [DOI] [PubMed] [Google Scholar]
- Bergers G. and Song S. (2005). The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 7, 452-464. 10.1215/S1152851705000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitkreutz D., Mirancea N., Schmidt C., Beck R., Werner U., Stark H.-J., Gerl M. and Fusenig N. E. (2004). Inhibition of basement membrane formation by a nidogen-binding laminin gamma1-chain fragment in human skin-organotypic cocultures. J. Cell Sci. 117, 2611-2622. 10.1242/jcs.01127 [DOI] [PubMed] [Google Scholar]
- Castoldi G., di Gioia C. R. T., Pieruzzi F., D'Orlando C., Van, De Greef W. M. M., Busca G., Sperti G. and Stella A. (2003). ANG II increases TIMP-1 expression in rat aortic smooth muscle cells in vivo. Am. J. Physiol. Heart Circ. Physiol. 284, H635-H643. 10.1152/ajpheart.00986.2001 [DOI] [PubMed] [Google Scholar]
- Chen Z.-L., Yao Y., Norris E. H., Kruyer A., Jno-Charles O., Akhmerov A. and Strickland S. (2013). Ablation of astrocytic laminin impairs vascular smooth muscle cell function and leads to hemorrhagic stroke. J. Cell Biol. 202, 381-395. 10.1083/jcb.201212032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R., Zhou L., Kebede A. A. and Barres B. A. (2010). Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562-566. 10.1038/nature09513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G. E. and Senger D. R. (2005). Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 97, 1093-1107. 10.1161/01.RES.0000191547.64391.e3 [DOI] [PubMed] [Google Scholar]
- Edelstein A., Amodaj N., Hoover K., Vale R. and Stuurman N. (2010). Computer control of microscopes using microManager. Curr. Protoc. Mol. Biol. 92, 14.20.1-14.20.17 10.1002/0471142727.mb1420s92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna V., Pardanaud L., Brunet I., Ola R., Ristori E., Santoro M. M., Nicoli S. and Eichmann A. (2015). Vascular mural cells promote noradrenergic differentiation of embryonic sympathetic neurons. Cell Rep. 11, 1786-1796. 10.1016/j.celrep.2015.05.028 [DOI] [PubMed] [Google Scholar]
- Francis S. E., Goh K. L., Hodivala-Dilke K., Bader B. L., Stark M., Davidson D. and Hynes R. O. (2002). Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler. Thromb. Vasc. Biol. 22, 927-933. 10.1161/01.ATV.0000016045.93313.F2 [DOI] [PubMed] [Google Scholar]
- Fujita M., Cha Y. R., Pham V. N., Sakurai A., Roman B. L., Gutkind J. S. and Weinstein B. M. (2011). Assembly and patterning of the vascular network of the vertebrate hindbrain. Development 138, 1705-1715. 10.1242/dev.058776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George E. L., Georges-Labouesse E. N., Patel-King R. S., Rayburn H. and Hynes R. O. (1993). Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 119, 1079-1091. [DOI] [PubMed] [Google Scholar]
- Germanguz I., Lev D., Waisman T., Kim C.-H. and Gitelman I. (2007). Four twist genes in zebrafish, four expression patterns. Dev. Dyn. 236, 2615-2626. 10.1002/dvdy.21267 [DOI] [PubMed] [Google Scholar]
- Hellstrom M., Kalen M., Lindahl P., Abramsson A. and Betsholtz C. (1999). Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047-3055. [DOI] [PubMed] [Google Scholar]
- Higashiyama S., Abraham J. A. and Klagsbrun M. (1993). Heparin-binding EGF-like growth factor stimulation of smooth muscle cell migration: dependence on interactions with cell surface heparan sulfate. J. Cell Biol. 122, 933-940. 10.1083/jcb.122.4.933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi K. K., Rohovsky S. A., Beck L. H., Smith S. R. and D'Amore P. A. (1999). Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ. Res. 84, 298-305. 10.1161/01.RES.84.3.298 [DOI] [PubMed] [Google Scholar]
- Hirschi K. K., Burt J. M., Hirschi K. D. and Dai C. (2003). Gap junction communication mediates transforming growth factor-beta activation and endothelial-induced mural cell differentiation. Circ. Res. 93, 429-437. 10.1161/01.RES.0000091259.84556.D5 [DOI] [PubMed] [Google Scholar]
- Hynes R. O. (2007). Cell-matrix adhesion in vascular development. J. Thromb. Haemost. 5 Suppl. 1, 32-40. 10.1111/j.1538-7836.2007.02569.x [DOI] [PubMed] [Google Scholar]
- Iivanainen E., Nelimarkka L., Elenius V., Heikkinen S.-M., Junttila T. T., Sihombing L., Sundvall M., Määttä J. A., Laine V. J., Ylä-Herttuala S. et al. (2003). Angiopoietin-regulated recruitment of vascular smooth muscle cells by endothelial-derived heparin binding EGF-like growth factor. FASEB J. 17, 1609-1621. 10.1096/fj.02-0939com [DOI] [PubMed] [Google Scholar]
- Isogai S., Lawson N. D., Torrealday S., Horiguchi M. and Weinstein B. M. (2003). Angiogenic network formation in the developing vertebrate trunk. Development 130, 5281-5290. 10.1242/dev.00733 [DOI] [PubMed] [Google Scholar]
- Jain R. K. (2003). Molecular regulation of vessel maturation. Nat. Med. 9, 685-693. 10.1038/nm0603-685 [DOI] [PubMed] [Google Scholar]
- Kennard S., Liu H. and Lilly B. (2008). Transforming growth factor-beta (TGF- 1) down-regulates Notch3 in fibroblasts to promote smooth muscle gene expression. J. Biol. Chem. 283, 1324-1333. 10.1074/jbc.M706651200 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B. and Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310. 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- Kofler N. M., Cuervo H., Uh M. K., Murtomaki A. and Kitajewski J. (2015). Combined deficiency of Notch1 and Notch3 causes pericyte dysfunction, models CADASIL, and results in arteriovenous malformations. Sci. Rep. 5, 16449 10.1038/srep16449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok F. O., Shin M., Ni C.-W., Gupta A., Grosse A. S., van Impel A., Kirchmaier B. C., Peterson-Maduro J., Kourkoulis G., Male I. et al. (2015). Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev. Cell 32, 97-108. 10.1016/j.devcel.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krymskaya V. P., Hoffman R., Eszterhas A., Ciocca V. and Panettieri R. A. Jr (1997). TGF-beta 1 modulates EGF-stimulated phosphatidylinositol 3-kinase activity in human airway smooth muscle cells. Am. J. Physiol. 273, L1220-L1227. [DOI] [PubMed] [Google Scholar]
- Kucenas S., Takada N., Park H.-C., Woodruff E., Broadie K. and Appel B. (2008). CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat. Neurosci. 11, 143-151. 10.1038/nn2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-W., Lin C.-C., Lin W.-N., Liang K.-C., Luo S.-F., Wu C.-B., Wang S.-W. and Yang C.-M. (2007). TNF-alpha induces MMP-9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF-kappaB/p300 binding in human tracheal smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L799-L812. 10.1152/ajplung.00311.2006 [DOI] [PubMed] [Google Scholar]
- Lee R. T. H., Knapik E. W., Thiery J. P. and Carney T. J. (2013). An exclusively mesodermal origin of fin mesenchyme demonstrates that zebrafish trunk neural crest does not generate ectomesenchyme. Development 140, 2923-2932. 10.1242/dev.093534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehti K., Allen E., Birkedal-Hansen H., Holmbeck K., Miyake Y., Chun T.-H. and Weiss S. J. (2005). An MT1-MMP-PDGF receptor-beta axis regulates mural cell investment of the microvasculature. Genes Dev. 19, 979-991. 10.1101/gad.1294605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P., Johansson B. R., Leveen P. and Betsholtz C. (1997). Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277, 242-245. 10.1126/science.277.5323.242 [DOI] [PubMed] [Google Scholar]
- Lindblom P., Gerhardt H., Liebner S., Abramsson A., Enge M., Hellstrom M., Backstrom G., Fredriksson S., Landegren U., Nyström H. C. et al. (2003). Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 17, 1835-1840. 10.1101/gad.266803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W. (2007). Developmental basis of vascular smooth muscle diversity. Arterioscler. Thromb. Vasc. Biol. 27, 1248-1258. 10.1161/ATVBAHA.107.141069 [DOI] [PubMed] [Google Scholar]
- Mascall K. S., Small G. R., Gibson G. and Nixon G. F. (2012). Sphingosine-1-phosphate-induced release of TIMP-2 from vascular smooth muscle cells inhibits angiogenesis. J. Cell Sci. 125, 2267-2275. 10.1242/jcs.099044 [DOI] [PubMed] [Google Scholar]
- Miano J. M., Georger M. A., Rich A. and De Mesy Bentley K. L. (2006). Ultrastructure of zebrafish dorsal aortic cells. Zebrafish 3, 455-463. 10.1089/zeb.2006.3.455 [DOI] [PubMed] [Google Scholar]
- Owens G. K. (1995). Regulation of differentiation of vascular smooth muscle cells. Physiol. Rev. 75, 487-517. [DOI] [PubMed] [Google Scholar]
- Perkins M. E., Ji T. H. and Hynes R. O. (1979). Cross-linking of fibronectin to sulfated proteoglycans at the cell surface. Cell 16, 941-952. 10.1016/0092-8674(79)90109-0 [DOI] [PubMed] [Google Scholar]
- Pitrone P. G., Schindelin J., Stuyvenberg L., Preibisch S., Weber M., Eliceiri K. W., Huisken J. and Tomancak P. (2013). OpenSPIM: an open-access light-sheet microscopy platform. Nat. Methods 10, 598-599. 10.1038/nmeth.2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget C., Pottin K. and Jaffredo T. (2008). Sclerotomal origin of vascular smooth muscle cells and pericytes in the embryo. Dev. Biol. 315, 437-447. 10.1016/j.ydbio.2007.12.045 [DOI] [PubMed] [Google Scholar]
- Rossi A., Kontarakis Z., Gerri C., Nolte H., Hölper S., Krüger M. and Stainier D. Y. R. (2015). Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 524, 230-233. 10.1038/nature14580 [DOI] [PubMed] [Google Scholar]
- Sanjana N. E., Cong L., Zhou Y., Cunniff M. M., Feng G. and Zhang F. (2012). A transcription activator-like effector toolbox for genome engineering. Nat. Protoc. 7, 171-192. 10.1038/nprot.2011.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M. M., Pesce G. and Stainier D. Y. (2009). Characterization of vascular mural cells during zebrafish development. Mech. Dev. 126, 638-649. 10.1016/j.mod.2009.06.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders W. B., Bohnsack B. L., Faske J. B., Anthis N. J., Bayless K. J., Hirschi K. K. and Davis G. E. (2006). Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J. Cell Biol. 175, 179-191. 10.1083/jcb.200603176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer N. and Campos-Ortega J. A. (1999). Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech. Dev. 80, 153-158. 10.1016/S0925-4773(98)00209-3 [DOI] [PubMed] [Google Scholar]
- Seiler C., Abrams J. and Pack M. (2010). Characterization of zebrafish intestinal smooth muscle development using a novel sm22alpha-b promoter. Dev. Dyn. 239, 2806-2812. 10.1002/dvdy.22420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smola H., Stark H.-J., Thiekötter G., Mirancea N., Krieg T. and Fusenig N. E. (1998). Dynamics of basement membrane formation by keratinocyte-fibroblast interactions in organotypic skin culture. Exp. Cell Res. 239, 399-410. 10.1006/excr.1997.3910 [DOI] [PubMed] [Google Scholar]
- Stratman A. N. and Davis G. E. (2012). Endothelial cell-pericyte interactions stimulate basement membrane matrix assembly: influence on vascular tube remodeling, maturation, and stabilization. Microsc. Microanal. 18, 68-80. 10.1017/S1431927611012402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratman A. N., Malotte K. M., Mahan R. D., Davis M. J. and Davis G. E. (2009). Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 114, 5091-5101. 10.1182/blood-2009-05-222364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratman A. N., Schwindt A. E., Malotte K. M. and Davis G. E. (2010). Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood 116, 4720-4730. 10.1182/blood-2010-05-286872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhollebeke B., Stone O. A., Bostaille N., Cho C., Zhou Y., Maquet E., Gauquier A., Cabochette P., Fukuhara S., Mochizuki N. et al. (2015). Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/beta-catenin pathway during brain angiogenesis. Elife 4, 1-25. 10.7554/eLife.06489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhao N., Kennard S. and Lilly B. (2012). Notch2 and Notch3 function together to regulate vascular smooth muscle development. PLoS ONE 7, e37365 10.1371/journal.pone.0037365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Pan L., Moens C. B. and Appel B. (2014). Notch3 establishes brain vascular integrity by regulating pericyte number. Development 141, 307-317. 10.1242/dev.096107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson P., Johansson B. R., Jukkola T., Breuer S., Akyurek L. M., Partanen J. and Lindahl P. (2008). Developmental origin of smooth muscle cells in the descending aorta in mice. Development 135, 1823-1832. 10.1242/dev.020958 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (1995). The Zebrafish Book. Eugene, OR: University of Oregon Press. [Google Scholar]
- Whitesell T. R., Kennedy R. M., Carter A. D., Rollins E.-L., Georgijevic S., Santoro M. M. and Childs S. J. (2014). An alpha-smooth muscle actin (acta2/alphasma) zebrafish transgenic line marking vascular mural cells and visceral smooth muscle cells. PLoS ONE 9, e90590 10.1371/journal.pone.0090590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegreffe C., Christ B., Huang R. and Scaal M. (2007). Sclerotomal origin of smooth muscle cells in the wall of the avian dorsal aorta. Dev. Dyn. 236, 2578-2585. 10.1002/dvdy.21279 [DOI] [PubMed] [Google Scholar]
- Wiens K. M., Lee H. L., Shimada H., Metcalf A. E., Chao M. Y. and Lien C.-L. (2010). Platelet-derived growth factor receptor beta is critical for zebrafish intersegmental vessel formation. PLoS ONE 5, e11324 10.1371/journal.pone.0011324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.-Y., Yao J.-H., Cheng L., Wei D.-W., Xue J.-L. and Lu D.-R. (2003). Molecular cloning and expression of a smooth muscle-specific gene SM22alpha in zebrafish. Biochem. Biophys. Res. Commun. 312, 741-746. 10.1016/j.bbrc.2003.10.185 [DOI] [PubMed] [Google Scholar]
- Yao Y., Chen Z. L., Norris E. H. and Strickland S. (2014). Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat. Commun. 5, 3413 10.1038/ncomms4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasutake J., Inohaya K. and Kudo A. (2004). Twist functions in vertebral column formation in medaka, Oryzias latipes. Mech. Dev. 121, 883-894. 10.1016/j.mod.2004.03.008 [DOI] [PubMed] [Google Scholar]
- Yeo G. H., Cheah F. S. H., Winkler C., Jabs E. W., Venkatesh B. and Chong S. S. (2009). Phylogenetic and evolutionary relationships and developmental expression patterns of the zebrafish twist gene family. Dev. Genes Evol. 219, 289-300. 10.1007/s00427-009-0290-z [DOI] [PubMed] [Google Scholar]
- Yurchenco P. D. (2011). Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect. Biol. 3, a004911 10.1101/cshperspect.a004911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaucker A., Mercurio S., Sternheim N., Talbot W. S. and Marlow F. L. (2013). notch3 is essential for oligodendrocyte development and vascular integrity in zebrafish. Dis. Model. Mech. 6, 1246-1259. 10.1242/dmm.012005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N., Koenig S. N., Trask A. J., Lin C.-H., Hans C. P., Garg V. and Lilly B. (2015). MicroRNA miR145 regulates TGFBR2 expression and matrix synthesis in vascular smooth muscle cells. Circ. Res. 116, 23-34. 10.1161/CIRCRESAHA.115.303970 [DOI] [PMC free article] [PubMed] [Google Scholar]