Abstract
The evolutionarily conserved enzyme encoded by the leucine-rich repeat kinase 2 gene, LRRK2, harbors both a Rab-like GTPase domain and a serine/threonine protein kinase domain. Pathogenic mutations in either the GTPase or kinase domain can cause neurodegeneration and Parkinson disease. No high-resolution structure of the human LRRK2 kinase domain is available but the most common mutation, G2019S in the kinase domain, is predicted to alter the ATP-binding pocket structure and interaction with divalent cations. Here we find that the manganese-bound kinase domain acquires a robust ability to utilize both GTP as well as ATP in autophosphorylation of the GTPase domain and phosphorylation of peptide substrates in vitro. The G2019S LRRK2 mutation increases the efficiency of GTP-mediated kinase activity ten-fold compared to WT LRRK2 activity. Moreover, GTP-dependent phosphorylation alters autophosphorylation-site preference in vitro. While additional studies are required to determine the physiological relevance of these observations, LRRK2 is one of the only known kinases to be able to utilize GTP as a phospho-donor at physiological levels in vitro, and thus one of the only known proteins to be able to hydrolyze GTP in two distinct domains within the same protein.
Keywords: PARK8, ROCO4, dardarin, enzyme kinetics, manganese, nucleotide-binding pocket
Introduction
The multi-domain leucine-rich repeat kinase 2 (LRRK2) protein is one of the few enzymes discovered with both GTPase and kinase domains encoded in the same protein [1]. The complex juxtaposition of the two domains together with linker domains and other protein-interaction domains is incredibly conserved through evolution as LRRK2 homologues can be found in some single celled organisms, most notably ROCO4 in slime-mold [2]. ROCO4 bears striking sequence homology but also conserved functional and enzymatic action in both autophosphorylation and intra-molecular interactions [3, 4]. Pathogenic missense mutations in either the GTPase or kinase domain in LRRK2 leads to neurodegeneration and late-onset Parkinson disease, and are one of the most common known causes of neurodegeneration [5]. Of the known pathogenic mutations in LRRK2, the most prevalent, G2019S, resides in the activation loop of the kinase domain and increases the rate of hydrolysis of ATP and transfer of phosphate to many different protein substrates in vitro [6–8]. In contrast, pathogenic LRRK2 mutations localized to the GTPase domain, for example R1441C, decrease the hydrolysis rate of GTP in vitro [9–11]. The intramolecular interaction between the ATP-binding pocket of the kinase domain and the GTP-binding pocket of the GTPase domain can lead to phosphorylation of nearly every threonine residue in the GTP-binding pocket through the process of LRRK2 autophosphorylation [12].
A high-resolution structure of the human LRRK2 kinase domain is currently not available, but alignments to other kinases and in silico models clearly show that the G2019S mutation alters the canonical DFG metal binding motif found in most protein kinases (is DYG in human LRRK2) to DYS, adjacent to the residues interacting with nucleotides [8]. Some evidence suggests the pathogenic DYS variant stabilizes the metal binding pocket into a “DYG-in” conformation, based largely on the crystal structure of a G1179S mutant ROCO4, where the human LRRK2 S2019 equivalent, S1179 on ROCO4, forms a hydrogen bond with the R1077 αC helix and therefore stabilizes and primes the DFG loop for an interaction with Mg2+ [13, 14]. Other kinases like MST3 show some ATP-binding pocket similarities to LRRK2 [15] and can bind Zn2+ as a cofactor [16, 17]. LRRK2 can use both Mn2+ and Mg2+ as co-factors and the kcat of G2019S LRRK2 is 7-fold higher than WT LRRK2 in the presence of Mn2+, compared to just 2-fold higher in the presence of Mg2+ [18]. However, whether the G2019S mutation could affect the nucleotide binding pocket apart from metal interactions remains unknown. Our previous study showed that ATP competitive inhibitors can be designed to target specifically G2019S or WT LRRK2, suggesting that the G2019S mutation also causes a broader conformational change in the pocket [4].
In the purification and analysis of LRRK2 it is common to include GTP or GDP to help maintain stability in biochemical and structural studies [9, 19, 20]. We noted a few past reports that demonstrated protein kinases PKC and CK2 can utilize GTP as a phospho-donor through minor conformational changes in their respective nucleotide binding pockets [21]. We wondered whether a fluctuated nucleotide binding pocket associated with the LRRK2 kinase domain, further altered by the pathogenic G2019S mutation, might show similar behavior. Indeed, we could measure and study the kinetics of GTP-dependent kinase activity of LRRK2. The G2019S LRRK2 mutation greatly enhanced the efficiency of GTP-mediated phosphorylation with respect to both cis and trans phosphorylation in vitro. Kinetic measurements (e.g., the km of GTP) suggest this phenomenon is well within the physiological range of GTP concentration if LRRK2 is in a Mn2+-bound state. Moreover, we found that GTP and divalent metals could alter substrate preference for LRRK2 autophosphorylation. LRRK2 is one of the few known kinases to be able to utilize GTP as a substrate and the only kinase to be able to hydrolyze GTP at two distinct domains in complex, the GTPase domain and the kinase domain.
Methods and Materials
Kinase assays for autophosphorylation
Recombinant LRRK2 proteins were purchased from Life technologies. 1 μM GST-[Δ970]LRRK2WT, GST-[Δ970] LRRK2G2019S or GST-[Δ970] LRRK2D1994A, or full-length FLAG-LRRK2 proteins were mixed with 2 μCi of [γ-32P] GTP or [γ-32P] ATP (PerkinElmer) in the presence of cold nucleotides as indicated (purchased from Sigma), along with Mg2+ or Mn2+ in reaction buffers at the indicated concentrations. Reaction buffer included 150 mM NaCl, 50 mM Tris pH 7.5, and 1 mM dithiothreitol. For kinetic studies, 100 μCi of [γ-32P]GTP was mixed with cold GTP for a 10 mM GTP solution. 10 μCi of [γ-32P] ATP was mixed with cold ATP for a 1 mM solution. 1 μM GST-[Δ970]LRRK2WT, GST-[970] LRRK2G2019S or GST-[970] LRRK2D1994A protein was mixed with 0–4 mM (as indicated) of labeled nucleotide solutions in the presence of MgCl or MnCl. Kinase reactions were incubated at 30 °C and heat inactivated at 90 °C for 2 minutes. Some kinase reactions were loaded into slot-blot apparatus (Bio-Rad) fitted with nitrocellulose membranes, or processed on TGX polyacrylamide gels (BioRad) and transferred to membranes. Membranes were washed using buffer consisting of 20 mM Tris pH 7.5 and 150 mM NaCl, and bands analyzed by liquid scintillation counting. Counts were compared to standard curves always run in parallel to correct for counter efficiencies and calculate Pi incorporation.
Antibodies to total LRRK2, FLAG-tag, GST-tag, pSer1292, pThr1410, pThr1491, pThr1503, pThr2483-LRRK2 were purchased from Abcam. Signal intensities produced by immunoblot with these antibodies were quantified using ImageLab software (Bio-Rad).
Peptide phosphorylation
10 μM in-house synthesized peptide was included in some kinase reactions which were applied to Whatman P81 phosphocellulose membranes using a slot blot apparatus. Membranes were washed using 5% phosphoric acid. Phosphate incorporation was determined by liquid scintillation as above.
In silico analysis of ATP binding pockets
3D models of WT and G2019S human-LRRK2 binding pocket were extrapolated using SWISS-MODEL homology modeling server based on the crystal structure of WT and G1179S ROCO4(PDB ID 4F0F, 4F1M) and MLK3(PDB ID 3DTC) as indicated [14]. The GTP-bound kinase model was generated by substitution of the bound ATP analog AppCp in ROCO4 crystal structure with GTP using Pymol.
Statistics
Each data point represents the mean value of at least three independent experiments. Kinetics, statistical analyses, and graphs were generated using GraphPad Prism 5.0. Figures were arranged in Adobe Illustrator 9.0.
Results
Mn2+-bound LRRK2 can use GTP as a phospho-donor for kinase activity
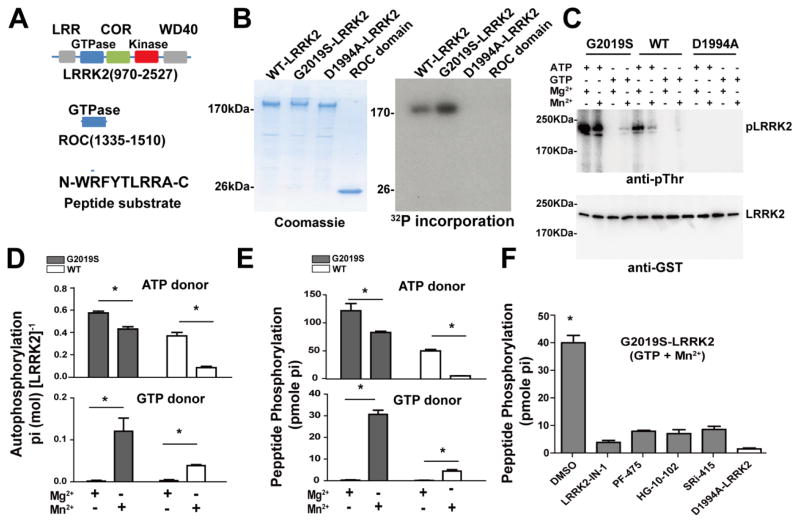
LRRK2 is a ~280 kDa protein in humans and truncation of the N-terminus permits expression and purification of a highly active ~200 kDa fragment from insect cells (Figure 1A). The kinase domain of LRRK2 phosphorylates the nucleotide-binding pocket of its own GTPase domain, termed ROC [12, 22]. It was previously suggested that some E. coli GTPase proteins, as well as a Ras GTPase, might show physiologically-relevant intrinsic autophosphorylation activity [23–25]. Given the extent of phosphorylated residues detected in ROC, we wondered whether the LRRK2 GTPase domain may also demonstrate this rare property. In kinase assays using radionucleotides, we tested autophosphorylation activity of the 200 kDa LRRK2 fragment as well as the isolated LRRK2 ROC GTPase domain, but no autophosphorylation could be detected with the isolated ROC domain (Figure 1B). In contrast, both the isolated ROC domain and the truncated protein are well-known to bind and hydrolyze GTP via ROC GTPase activity [3].
Figure 1. GTP is a phospho-donor for LRRK2 kinase activity.
A) Schematic of the human GST-[Δ970]LRRK2 and isolated ROC domain with annotation relative to the full length LRRK2 protein (NCBI entry NP_940980.3), and a LRRK2 small-peptide kinase substrate. B) WT, G2019S (kinase over-active) and D1994A (kinase-dead) LRRK2 incubated with 1 mM GTP and 1.7 nM [γ-32P]-GTP in the presence of 10 mM MnCl2. Coomassie staining showed the purity of the recombinant proteins. 32P incorporation is demonstrated with autoradiography. C) WT, G2019S and D1994A recombinant LRRK2 proteins were incubated in kinase assays with 1 mM (cold) ATP or GTP in the presence of 10 mM Mg2+ or Mn2+, as indicated. LRRK2 autophosphorylation was measured by a phospho-threonine specific antibody from 30 min kinase reactions. A representative immunoblot is shown. Total recombinant protein in the reaction was measured using a GST antibody. D) The absolute amount of Pi incorporated during LRRK2-autophosphorylation was calculated by measuring by [γ-32P] ATP (top panels) or GTP (bottom panels, as indicated) incorporation into LRRK2 protein (G2019S, gray bars, WT, white bars) in the presence of 1 mM ATP or GTP (cold) with 1.7 nM [γ-32P] ATP or [γ-32P] GTP and 10 mM MnCl2 or MgCl2 (as indicated), followed by liquid scintillation and normalization to standard curves of Pi that correct for efficiency in detection. Of note, all autophosphorylation signal in reactions with GTP and Mg2+ were not detectable above baseline (background). E) LRRK2 trans phosphorylation of a small peptide substrate (1 nmole per reaction) was measured in reactions supplemented with 1.7 nM [γ-32P] ATP or GTP with 1 mM (cold) ATP or GTP. F) G2019S-LRRK2 incubated with peptide substrate and 1 mM GTP supplemented with 1.7nM [γ-32P] GTP in the presence of 1 μM of the indicated ATP-competitive LRRK2 kinase inhibitor, or DMSO control (0.1% of reaction volume), with kinase dead (D1994A) LRRK2 that fails to produce significant peptide phosphorylation. All graphs depict the mean value from three independent experiments with S.E.M error bars. * p<0.05, two-way ANOVA with Tukey’s post-hoc test.
LRRK2 autophoshorylation can also be measured using phospho-threonine antibodies since both fragments (Figure 1A) do not show appreciable phospho-threonine pre-kinase reaction, or with phospho-specific antibodies to the ROC autophosphorylation sites [12]. We incubated purified WT, G2019S, and D1994A (kinase dead) LRRK2 fragments with 2 mM ATP or GTP in the presence of 5 mM MgCl2 or MnCl2 and measured autophosphorylation using a phospho-threonine antibody (Figure 1C). We found that G2019S LRRK2 can use GTP as a substrate for autophosphorylation, with notable increases in the presence of MnCl2 compared to MgCl2, although the overall autophosphorylation level that could be achieved with GTP was always lower than reactions with ATP included. The D1994A-kinase dead LRRK2 showed no GTP or ATP-mediated autophosphorylation.
LRRK2 kinase activity can also be measured directly using radionucleotides to estimate overall phosphate incorporation in cis (autophosphorylation) as well as to small peptides in trans [26]. LRRK2 autophosphorylation was not efficient with GTP in the Mg2+ bound state, but was robust with GTP and Mn2+ (Figure 1D). G2019S LRRK2 showed an increase in GTP-dependent activity compared to WT LRRK2 (Figure 1D). Similar to GTP-mediated autophosphorylation, phosphorylation of a small peptide substrate occurred with both WT and G2019S LRRK2 with GTP as a phospho-donor (Figure 1E). In these experiments, the G2019S mutation caused a five-fold increase in GTP-dependent kinase activity compared to WT LRRK2 activity (Figure 1E).
To localize GTP-dependent kinase activity to the ATP-binding pocket of the LRRK2 kinase domain, we performed kinase assays with numerous potent and highly specific ATP-competitive LRRK2 kinase inhibitors in the presence of 1,000-fold excess GTP [4, 27–29]. Under these conditions, all of the inhibitors blocked GTP-dependent kinase activity (Figure 1F). Since these structurally distinct molecules would not all bind to the GTPase domain in such a potent way, we conclude the novel LRRK2 GTP-dependent kinase activity is derived from the ATP-binding pocket of the LRRK2 kinase domain.
The G2019S LRRK2 mutation increases the efficiency of GTP-mediated kinase activity
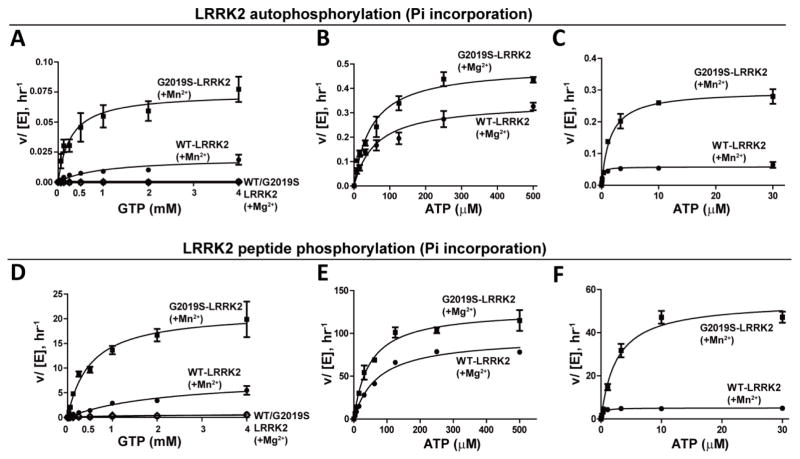
To assess kinetic values for GTP-dependent peptide phosphorylation, we titrated GTP in autophosphorylation assays with WT or G2019S LRRK2 in the presence of 5 mM Mg2+ or Mn2+ (Figure 2A). The phosphate incorporated in LRRK2 was measured after a one-hour kinase reaction. GTP-mediated kinase activity for both WT or G2019S LRRK2 was low in the presence of 5 mM Mg2+, but both WT and G2019S LRRK2 showed robust autophosphorylation activity with 5 mM Mn2+. The specific constant (kcat · km−1) of a kinase may relate to the efficiency of transfer from the donor to the substrate. G2019S LRRK2 demonstrated a ~13-fold higher efficiency than WT LRRK2 in GTP-mediated autophosphorylation activity (Table 1), highlighting the large difference of the G2019S mutation in adopting a conformation amenable to GTP docking. Changes in kinase efficiency for ATP-dependent autophosphorylation between WT and G2019S LRRK2 were only nominal, with G2019S LRRK2 showing a 1.8-fold increased efficiency for the Mg2+ bound state associated with the highest levels of ATP-dependent kinase activity (Table 1, Figure 2B, C).
Figure 2. Kinetics of LRRK2 activity with GTP compared to ATP as a phospho-donor.
LRRK2 protein (WT or kinase-overactive G2019S) was incubated in a kinase assay with the indicated concentration of ATP or GTP in the presence of Mg2+ or Mn2+. A) GTP-dependent autophosphorylation with Mg2+ or Mn2+. With Mn2+, kcat= 0.020hr−1 and 0.074 hr−1, and km= 985.5 and 280.1 μM were recorded for WT and G2019S-LRRK2, respectively. Negligible activities were recorded for GTP-mediated autophosphorylation with Mg2+. B) Mg2+-mediated ATP-dependent autophosphorylation, kcat= 0.343 hr−1 and 0.492 hr−1, km= 60.9 and 49.6 μM were recorded for WT and G2019S-LRRK2, respectively. C) Mn2+ mediated ATP-dependent autophosphorylation, kcat= 0.058 hr−1 and 0.298 hr−1, km= 0.1931 and 1.544 μM were recorded for WT and G2019S-LRRK2, respectively. D) GTP-dependent peptide phosphorylation activity with Mg2+ or Mn2+. With Mn2+, kcat= 8.148 hr−1 and 21.42 hr−1, and km= 2166 and 498 μM, for WT and G2019S-LRRK2, respectively. Negligible activities were recorded for GTP-mediated peptide phosphorylation in the presence of Mg2+. E) Mg2+ mediated ATP-dependent peptide phosphorylation, kcat= 95.4 hr−1 and 127.5 hr−1, and km= 71.46 and 47.22 μM were recorded for WT and G2019S-LRRK2, respectively. F) Mn2+ mediated ATP dependent peptide phosphorylation, kcat= 5.1 hr−1 and 54.5 hr−1, and km= 0.2802 and 2.575 μM were recorded for WT and G2019S-LRRK2, respectively. Data points depict the mean value from three independent experiments with error bars depicting S.E.M.
Table 1.
Kinetic values related to LRRK2 kinase activity.
| WT ATP-Mg2+ |
G2019S ATP-Mg2+ |
WT ATP-Mn2+ |
G2019S ATP-Mn2+ |
WT GTP-Mg2+ |
WT GTP-Mn2+ |
G2019S GTP-Mg2+ |
G2019S GTP-Mn2+ |
||
|---|---|---|---|---|---|---|---|---|---|
| Auto phosphorylation | kcat (hr−1) | 0.34±0.02 | 0.49±0.02 | 0.058±0.003 | 0.30±0.011 | - | 0.020±0.003 | - | 0.074±0.007 |
| Km (μM) | 60.9±12.8 | 49.6±7.6 | 0.19±0.05 | 1.5±0.22 | - | 985.5±310 | - | 280.1±93 | |
| kcat · Km−1 | 0.0056 | 0.0099 | 0.30 | 0.19 | - | 2.03×10−5 | - | 2.64×10−4 | |
| Peptide phosphorylation | kcat (hr−1) | 95.4±2.9 | 128±5.4 | 5.10±0.1 | 54.5±2.3 | - | 8.20±1.1 | 0.056±0.01 | 21.4±1.5 |
| Km (μM) | 71.5±6.5 | 47.2±6.6 | 0.280±0.024 | 2.60±0.38 | - | 2166±410 | 838.2±89.8 | 497.9±105 | |
| kcat · Km−1 | 1.34 | 2.70 | 18.20 | 21.16 | - | 0.0038 | 6.7×10−5 | 0.043 |
±S.E.M. are indicated
To measure trans-peptide kinase efficiency for GTP-dependent kinase activity, we titrated GTP in peptide phosphorylation assays in the presence of 5 mM Mg2+ or Mn2+ (Figure 2D–F). Little peptide phosphorylation could be detected at any concentration of GTP in the Mg2+ bound state. In the presence of 5 mM Mn2+, G2019S LRRK2 demonstrated an ~11-fold increase in the efficiency of GTP-mediated peptide phosphorylation versus WT LRRK2 (Table 1). The efficiency of ATP-mediated kinase activity for WT and G2019S LRRK2 both increased by ~14 and ~8 fold, respectively, compared to Mg2+ reactions (Figure 2E, F). These results are consistent with previous observations for the effects of Mn2+ on LRRK2 kinase activity [18, 30]. Therefore, Mn2+-bound G2019S LRRK2 showed a large increase (>10-fold) in kinase activity for both autophosphorylation and peptide phosphorylation when either GTP or ATP are used as phospho-donors.
GTP utilization alters substrate preference
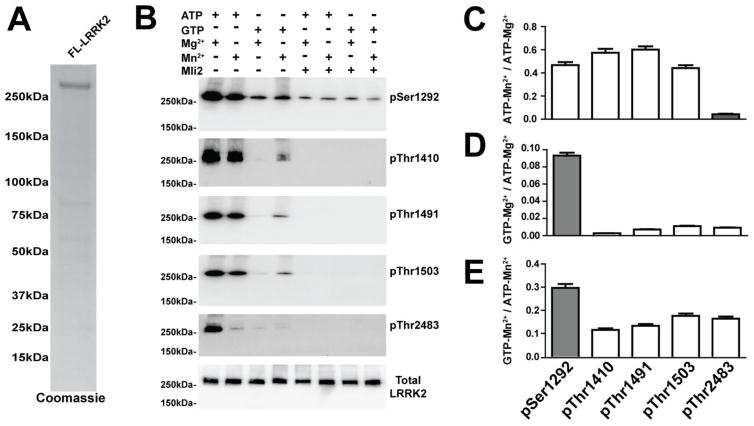
It was previously suggested that the few kinases known to utilize GTP as a phospho-donor may show different substrate specificity with GTP versus ATP [31, 32]. To study whether LRRK2 substrate specificity is altered in a GTP and Mn2+ bound state, we analyzed the incorporation of phosphates into specific cis autophosphorylation sites in full-length LRRK2 protein using phospho-specific antibodies pThr1410, pThr1491, pThr1503 and pThr2483 antibodies. All antibodies detected robust GTP-dependent autophosphorylation (Figure 3A, B). Kinase reactions with the potent ATP competitive inhibitor LRRK2 inhibitor Mli2 efficiently blocked any additional phosphorylation above what could be detected de novo from the full length recombinant LRRK2.
Figure 3. Nucleotide and divalent metal utilization by LRRK2 can alter substrate preference.
A) Purity of full-length human LRRK2 protein demonstrated by Coomassie stain. B) A representative (medium-intensity exposure) immunoblot of LRRK2 proteins 30 minutes post-kinase reaction with 2 mM ATP, 2 mM GTP, 10 mM MgCl2, 10 mM MnCl2, or 1 μM Mli2 LRRK2 inhibitor included, as indicated. In vitro LRRK2 autophosphorylation was measured using antibodies generated towards pSer1292, pThr1410, pThr1491, pThr1503, pThr2483. Total LRRK2 was measured using anti-Flag antibody. C–E) Comparative analysis of autophosphorylation under different phospho-donor and metal co-factor conditions with all results presented as ratiometric values (see ordinate labels in panels C–E). In all cases, background signal derived from conditions that included the kinase inhibitor Mli2 were subtracted. Results as displayed in column graphs that depict the mean value from three independent experiments with S.E.M error bars. Gray bars represent a condition in each graph that are significantly different from all other conditions (white bars), p<0.05, one-way ANOVA with Tukey’s post-hoc test.
To directly compare the effects of GTP versus ATP-mediate autophosphorylation, the intensities of each autophosphorylation site were calculated as a ratio of autophosphorylation produced in either a Mg2+ or Mn2+ bound state, with either ATP or GTP as a phospho-donor (Figure 3B). Mn2+ bound LRRK2 could not efficiently phosphorylate the pThr2483 residue with GTP as a donor, as compared to the other phosphorylation sites (Figure 3B, C). In the presence of the potent LRRK2 kinase inhibitor MLi2, phospho-S1292 signal was distinctly detected on full length LRRK2 protein and the increments in phospho-S1292 levels in the presence of GTP were nominal (Figure 3D). Phospho-T1410, phospho-T1491 and phospho-T1503 levels were undetectable in the presence of MLi2, and therefore the increase in these phosphorylation sites in the presence of GTP was much higher, suggesting these sites as preferential (e.g., compared to phospho-T2483) for GTP-mediated phosphorylation (Figure 3E). These results suggest that nucleotide and divalent metals can induce limited conformational changes that may affect substrate preference and recognition.
Discussion
Our results center on three novel observations. First, both WT and G2019S LRRK2 can utilize GTP as a phospho-donor for both peptide phosphorylation and autophosphorylation in vitro. This property is extremely rare in protein kinases, having only been described a few times in other kinases in vitro. Future studies are required to determine whether this can occur in cells and tissues that express LRRK2. Second, pathogenic G2019S LRRK2 shows a much higher kinase enhancement relative to WT LRRK2 when GTP is the phospho-donor versus ATP, although the overall kinase activity is lower (but higher efficiency) than ATP-mediated kinase activity. Third, nucleotide and divalent metals can change substrate preference in vitro. The added complexity of GTP-binding in two distinct domains in LRRK2 adds another layer of complexity in enzymatic function, at least in test-tubes, and opens the possibility of GTP-dependent kinase activity in cells.
Kinases are known to be differentially affected by Mn2+ and Mg2+ binding, for example CK2 which has been reported to utilize GTP as a phospho-donor when bound to Mn2+ [21]. LRRK2 kinase activity has previously been reported with both Mn2+ and Mg2+, where Mn2+ significantly increases the enzyme efficiency of ATP-dependent LRRK2 kinase activity compared to Mg2+ [18]. We therefore substituted Mn2+ for Mg2+ and with this increased efficiency we could develop robust GTP-mediated kinase activity. Further, in our experiments using a protein fragment of LRRK2 that lacks the N-terminal domain, we were able to replicate a previously reported finding in full-length LRRK2 protein that G2019S LRRK2 in the Mn2+ bound state shows a much larger increase in kinase activity relative to WT LRRK2 than Mg2+ bound LRRK2 [30].
Michaelis-Menten kinetic values of Mg2+ and Mn2+ mediated ATP and GTP-dependent peptide phosphorylation and autophosphorylation revealed Mn2+ significantly increases the enzyme efficiency for both ATP and GTP-dependent kinase activity, although the mechanisms are different. For ATP-dependent kinase activity, Mn2+ decreases both the kcat and km for WT and G2019S LRRK2, suggesting Mn2+ increases the ATP-binding affinity of LRRK2 while interrupting the conformation and electron distribution around the catalytic center. For GTP-dependent kinase activity, Mn2+ significantly increases the kcat while decreasing the km, suggesting Mn2+ increases the GTP-binding affinity and improves the conformation and electron distribution around the catalytic center. The kcat values for GTP-dependent kinase activity are significantly lower than with ATP while the km values are significantly higher than the corresponding values for ATP-dependent kinase activity. These results show that GTP is a weaker binding ligand compared to ATP. However, the km-GTP for Mn2+-mediated G2019S-LRRK2 peptide phosphorylation and autophosphorylation is 497.9μM and 280.1 μM respectively, comparable to the GTP concentration in cells (~500 μM), suggesting G2019S-LRRK2, but perhaps not WT-LRRK2, can utilize GTP for autophosphorylation and substrate peptide phosphorylation in cells. Kinase-domain GTP-affinity may be further influenced by donor activity of the adjacent ROC GTPase domain that also binds GTP with weak affinity and has weak GTPase activity. Further studies are required to parse out this potential relationship.
Previously molecular dynamics studies showed LRRK2 kinase domain might undergo a conformational change in different stages of kinase activation, suggesting a fluctuated structure [13]. This was further supported by the finding that small molecule inhibitors can be designed to target specifically cis autophosphorylation or trans peptide phosphorylation [4]. Moreover, different nucleotides are known to cause conformational changes of dual-substrate kinases such as CK2 and PKC [21, 32], resulting in conformational changes hypothesized to direct substrate specificity. For LRRK2 we could not find evidence that GTP and Mn2+ overtly change substrate specificity, but there are clear shifts in preference for the autophosphorylation site pSer1292. In contrast, the phosphorylation of Thr2483 is significantly inhibited. These results may imply that LRRK2 undergoes complicated conformational changes when interacting with different ligands as well as substrates.
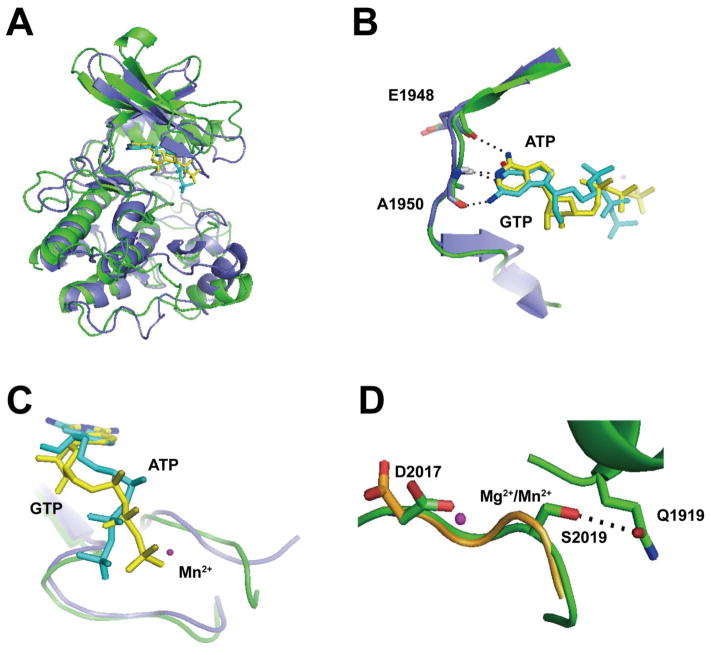
Without a high-resolution structure of the LRRK2 kinase domain, it is difficult to understand the unusual property of GTP-binding to an ATP pocket associated with a kinase domain. Homology modeling may provide some insights in this regard. Superimposition of LRRK2 sequence in the ROCO4 and MLK3 ATP-binding pockets suggests that ATP may form a critical hydrogen bond with the main chain oxygen on the E1948 LRRK2 residue (Figure 4A), but with GTP the carbonyl oxygen on C6 must shift away from the main chain oxygen on E1948. Instead, the amino group on C2 might form a hydrogen bond with the main chain oxygen with the A1950 residue. As a result of the purine ring shift, the ribose group must also shift, allowed by the lack of a ribose anchoring point in the LRRK2 pocket. Thus, the apparent decreased activity in GTP versus ATP-mediated kinase reactions may be due to a necessary shift in the phosphate groups away from the catalytic metal ion.
Figure 4. In silico prediction of a novel GTP-bound LRRK2 isoform.
A) Hypothetical model of ATP (yellow) and GTP (cyan)-bound LRRK2 kinase domain based on human MLK3 crystal structure (PDBID: 3DTC, slate) overlaid with the LRRK2 kinase domain constructed from a model based on amoeba ROCO4 crystal structure (PDBID, 4F0F, green). LRRK2 overlaid on either the MLK3 or ROCO4 nucleotide pockets overall resembles each other with an overall root-mean-square deviation of 1.5 Å, and B) in this hypothetical model, the amino group on C6 of ATP could form a hydrogen bond with the main chain oxygen on E1948 (hLRRK2 sequence). The N1 atom could form a hydrogen bond with the main chain amide group of A1950. The N1 atom of GTP could form a hydrogen bond with the main chain amide group on A1950. The amino group on C2 of GTP might form a hydrogen bond with the main chain oxygen of A1950. C) Overlay of bound ATP (yellow) and GTP (cyan) showed the triphosphate tail of GTP must shift away from the bound Mn2+. D) The overlay model of WT (orange) and G2019S (green) LRRK2 kinase domain bound with Mg2+ or Mn2+ showed that in WT LRRK2, D2017 is directed away from the Mn2+. However, in G2019S LRRK2 the Ser2019 residue might form a hydrogen bond with Q1919, necessarily dragging D2017 closer to the Mn2+ atom. Important residues are shown as sticks. Metals are shown as spheres. Atoms involved in key interactions are color coded (blue: nitrogen, red: oxygen). Black dotted lines denote the atoms involved in potential hydrogen bonding.
Mg2+ and Mn2+ are similar to each other in term of charge and ion radius and our in silico modeling cannot predict a conformational difference between Mg2+ and Mn2+ bound protein (Figure 4B), despite the clear differences we observed here. However, Mn2+ might increase the pKa of the γ-phosphate [33]. The imperfect conformation of the GTP γ-phosphate would be compensated by the increased pKa induced by Mn2+, causing increased GTP-dependent phospho transfer activity compared to Mg2+.
Finally, modeling may provide some insights to explain the effects of the G2019S LRRK2 mutation in our experiments. The DYG motif in WT LRRK2 may be altered to a more flexible DYS structure in G2019S LRRK2 [13]. An in silico model based on MLK3 and ROCO4 both show the WT LRRK2 D2017 residue as pointed away from the Mg2+ ion (Figure 4C). In G2019S LRRK2, the S2019 residue might form a novel hydrogen bond with Q1919, securing the DYG motif in a conformation where the D2017 residue now points toward the divalent metal binding site (Figure 4C). This effect may remediate the Mn2+ -mediated GTP-dependent γ-phosphate transfer that is otherwise in a non-optimal conformation. Potentially supporting this model, mutation of Q1919 to alanine abolished G2019S dependent increase of LRRK2 kinase activity [14].
Metal exposures, particularly manganese, are identified as important risk factors for susceptibility to PD [34–36]. Mn2+ has a host of deleterious effects on cells including damaging mitochondria, disrupting vesicle transport, altering proteasomal pathways, and increasing α-synuclein aggregation [37]. The enzymes that underlie all of these changes have not been identified. In vitro, we showed that in the presence of Mn2+, LRRK2 kinase becomes more active and this includes possible utilization of GTP, particularly when LRRK2 is mutated with G2019S. We believe our results warrant in vivo studies to determine how LRRK2 kinase activity may change in cells after Mn2+ exposures.
Highlights.
The LRRK2 kinase domain, bound to Mn2+, can use GTP as phospho-donor for both autophosphorylation and trans peptide phosphorylation activity.
The pathological mutation G2019S increased GTP-dependent kinase activity.
LRRK2 utilization of GTP as a phospho-donor changes the preferences of LRRK2 autophosphorylation.
Acknowledgments
This work was supported by NIH/NINDS grant R01 NS064934.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bosgraaf L, Van Haastert PJ. Roc, a Ras/GTPase domain in complex proteins. Biochim Biophys Acta. 2003;1643:5–10. doi: 10.1016/j.bbamcr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 2.van Egmond WN, van Haastert PJ. Characterization of the Roco protein family in Dictyostelium discoideum. Eukaryot Cell. 2010;9:751–761. doi: 10.1128/EC.00366-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, Mobley JA, DeLucas LJ, Kahn RA, West AB. LRRK2 autophosphorylation enhances its GTPase activity. FASEB J. 2015 doi: 10.1096/fj.15-277095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z, Galemmo RA, Jr, Fraser KB, Moehle MS, Sen S, Volpicelli-Daley LA, DeLucas LJ, Ross LJ, Valiyaveettil J, Moukha-Chafiq O, Pathak AK, Ananthan S, Kezar H, White EL, Gupta V, Maddry JA, Suto MJ, West AB. Unique functional and structural properties of the LRRK2 protein ATP-binding pocket. J Biol Chem. 2014;289:32937–32951. doi: 10.1074/jbc.M114.602318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 6.West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anand VS, Braithwaite SP. LRRK2 in Parkinson’s disease: biochemical functions. FEBS J. 2009;276:6428–6435. doi: 10.1111/j.1742-4658.2009.07341.x. [DOI] [PubMed] [Google Scholar]
- 8.West AB, Moore DJ, Choi C, Andrabi SA, Li X, Dikeman D, Biskup S, Zhang Z, Lim KL, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet. 2007;16:223–232. doi: 10.1093/hmg/ddl471. [DOI] [PubMed] [Google Scholar]
- 9.Deng J, Lewis PA, Greggio E, Sluch E, Beilina A, Cookson MR. Structure of the ROC domain from the Parkinson’s disease-associated leucine-rich repeat kinase 2 reveals a dimeric GTPase. Proc Natl Acad Sci U S A. 2008;105:1499–1504. doi: 10.1073/pnas.0709098105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Tan YC, Poulose S, Olanow CW, Huang XY, Yue Z. Leucine-rich repeat kinase 2 (LRRK2)/PARK8 possesses GTPase activity that is altered in familial Parkinson’s disease R1441C/G mutants. J Neurochem. 2007;103:238–247. doi: 10.1111/j.1471-4159.2007.04743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis PA, Greggio E, Beilina A, Jain S, Baker A, Cookson MR. The R1441C mutation of LRRK2 disrupts GTP hydrolysis. Biochem Biophys Res Commun. 2007;357:668–671. doi: 10.1016/j.bbrc.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webber PJ, Smith AD, Sen S, Renfrow MB, Mobley JA, West AB. Autophosphorylation in the leucine-rich repeat kinase 2 (LRRK2) GTPase domain modifies kinase and GTP-binding activities. J Mol Biol. 2011;412:94–110. doi: 10.1016/j.jmb.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu M, Bender SA, Cuny GD, Sherman W, Glicksman M, Ray SS. Type II kinase inhibitors show an unexpected inhibition mode against Parkinson’s disease-linked LRRK2 mutant G2019S. Biochemistry. 2013;52:1725–1736. doi: 10.1021/bi3012077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilsbach BK, Ho FY, Vetter IR, van Haastert PJ, Wittinghofer A, Kortholt A. Roco kinase structures give insights into the mechanism of Parkinson disease-related leucine-rich-repeat kinase 2 mutations. Proc Natl Acad Sci U S A. 2012;109:10322–10327. doi: 10.1073/pnas.1203223109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson JL, Kormos BL, Hayward MM, Coffman KJ, Jasti J, Kurumbail RG, Wager TT, Verhoest PR, Noell GS, Chen Y, Needle E, Berger Z, Steyn SJ, Houle C, Hirst WD, Galatsis P. Discovery and preclinical profiling of 3-[4-(morpholin-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-5-yl]benzonitrile (PF-06447475), a highly potent, selective, brain penetrant, and in vivo active LRRK2 kinase inhibitor. J Med Chem. 2015;58:419–432. doi: 10.1021/jm5014055. [DOI] [PubMed] [Google Scholar]
- 16.Lu TJ, Huang CY, Yuan CJ, Lee YC, Leu TH, Chang WC, Lu TL, Jeng WY, Lai MD. Zinc ion acts as a cofactor for serine/threonine kinase MST3 and has a distinct role in autophosphorylation of MST3. J Inorg Biochem. 2005;99:1306–1313. doi: 10.1016/j.jinorgbio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Turski ML, Brady DC, Kim HJ, Kim BE, Nose Y, Counter CM, Winge DR, Thiele DJ. A novel role for copper in Ras/mitogen-activated protein kinase signaling. Mol Cell Biol. 2012;32:1284–1295. doi: 10.1128/MCB.05722-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovitt B, Vanderporten EC, Sheng Z, Zhu H, Drummond J, Liu Y. Differential effects of divalent manganese and magnesium on the kinase activity of leucine-rich repeat kinase 2 (LRRK2) Biochemistry. 2010;49:3092–3100. doi: 10.1021/bi901726c. [DOI] [PubMed] [Google Scholar]
- 19.Guaitoli G, Raimondi F, Gilsbach BK, Gomez-Llorente Y, Deyaert E, Renzi F, Li X, Schaffner A, Jagtap PK, Boldt K, von Zweydorf F, Gotthardt K, Lorimer DD, Yue Z, Burgin A, Janjic N, Sattler M, Versees W, Ueffing M, Ubarretxena-Belandia I, Kortholt A, Gloeckner CJ. Structural model of the dimeric Parkinson’s protein LRRK2 reveals a compact architecture involving distant interdomain contacts. Proc Natl Acad Sci U S A. 2016;113:E4357–4366. doi: 10.1073/pnas.1523708113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu M, Dobson B, Glicksman MA, Yue Z, Stein RL. Kinetic mechanistic studies of wild-type leucine-rich repeat kinase 2: characterization of the kinase and GTPase activities. Biochemistry. 2010;49:2008–2017. doi: 10.1021/bi901851y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niefind K, Putter M, Guerra B, Issinger OG, Schomburg D. GTP plus water mimic ATP in the active site of protein kinase CK2. Nat Struct Biol. 1999;6:1100–1103. doi: 10.1038/70033. [DOI] [PubMed] [Google Scholar]
- 22.Greggio E, Taymans JM, Zhen EY, Ryder J, Vancraenenbroeck R, Beilina A, Sun P, Deng J, Jaffe H, Baekelandt V, Merchant K, Cookson MR. The Parkinson’s disease kinase LRRK2 autophosphorylates its GTPase domain at multiple sites. Biochem Biophys Res Commun. 2009;389:449–454. doi: 10.1016/j.bbrc.2009.08.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibbs JB, Ellis RW, Scolnick EM. Autophosphorylation of v-Ha-ras p21 is modulated by amino acid residue 12. Proc Natl Acad Sci U S A. 1984;81:2674–2678. doi: 10.1073/pnas.81.9.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sood P, Lerner CG, Shimamoto T, Lu Q, Inouye M. Characterization of the autophosphorylation of Era, an essential Escherichia coli GTPase. Mol Microbiol. 1994;12:201–208. doi: 10.1111/j.1365-2958.1994.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh A, Dutta D, Bandyopadhyay K, Parrack P. Characterization of the autophosphorylation property of HflX, a ribosome-binding GTPase from Escherichia coli. FEBS open bio. 2016;6:651–659. doi: 10.1002/2211-5463.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sen S, Webber PJ, West AB. Dependence of leucine-rich repeat kinase 2 (LRRK2) kinase activity on dimerization. J Biol Chem. 2009;284:36346–36356. doi: 10.1074/jbc.M109.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng X, Dzamko N, Prescott A, Davies P, Liu Q, Yang Q, Lee JD, Patricelli MP, Nomanbhoy TK, Alessi DR, Gray NS. Characterization of a selective inhibitor of the Parkinson’s disease kinase LRRK2. Nat Chem Biol. 2011;7:203–205. doi: 10.1038/nchembio.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estrada AA, Liu X, Baker-Glenn C, Beresford A, Burdick DJ, Chambers M, Chan BK, Chen H, Ding X, DiPasquale AG, Dominguez SL, Dotson J, Drummond J, Flagella M, Flynn S, Fuji R, Gill A, Gunzner-Toste J, Harris SF, Heffron TP, Kleinheinz T, Lee DW, Le Pichon CE, Lyssikatos JP, Medhurst AD, Moffat JG, Mukund S, Nash K, Scearce-Levie K, Sheng Z, Shore DG, Tran T, Trivedi N, Wang S, Zhang S, Zhang X, Zhao G, Zhu H, Sweeney ZK. Discovery of highly potent, selective, and brain-penetrable leucine-rich repeat kinase 2 (LRRK2) small molecule inhibitors. J Med Chem. 2012;55:9416–9433. doi: 10.1021/jm301020q. [DOI] [PubMed] [Google Scholar]
- 29.Volpicelli-Daley LA, Abdelmotilib H, Liu Z, Stoyka L, Daher JP, Milnerwood AJ, Unni VK, Hirst WD, Yue Z, Zhao HT, Fraser K, Kennedy RE, West AB. G2019S-LRRK2 Expression Augments alpha-Synuclein Sequestration into Inclusions in Neurons. J Neurosci. 2016;36:7415–7427. doi: 10.1523/JNEUROSCI.3642-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Covy JP, Giasson BI. The G2019S pathogenic mutation disrupts sensitivity of leucine-rich repeat kinase 2 to manganese kinase inhibition. J Neurochem. 2010;115:36–46. doi: 10.1111/j.1471-4159.2010.06894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan CJ, Huang CY, Graves DJ. Phosphorylase kinase, a metal ion-dependent dual specificity kinase. J Biol Chem. 1993;268:17683–17686. [PubMed] [Google Scholar]
- 32.Gschwendt M, Kittstein W, Kielbassa K, Marks F. Protein kinase C delta accepts GTP for autophosphorylation. Biochem Biophys Res Commun. 1995;206:614–620. doi: 10.1006/bbrc.1995.1087. [DOI] [PubMed] [Google Scholar]
- 33.Schweins T, Scheffzek K, Assheuer R, Wittinghofer A. The role of the metal ion in the p21ras catalysed GTP-hydrolysis: Mn2+ versus Mg2+ J Mol Biol. 1997;266:847–856. doi: 10.1006/jmbi.1996.0814. [DOI] [PubMed] [Google Scholar]
- 34.Chen P, Chakraborty S, Mukhopadhyay S, Lee E, Paoliello MM, Bowman AB, Aschner M. Manganese homeostasis in the nervous system. J Neurochem. 2015;134:601–610. doi: 10.1111/jnc.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montes S, Rivera-Mancia S, Diaz-Ruiz A, Tristan-Lopez L, Rios C. Copper and copper proteins in Parkinson’s disease. Oxid Med Cell Longev. 2014;2014:147251. doi: 10.1155/2014/147251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuschl K, Mills PB, Clayton PT. Manganese and the brain. Int Rev Neurobiol. 2013;110:277–312. doi: 10.1016/B978-0-12-410502-7.00013-2. [DOI] [PubMed] [Google Scholar]
- 37.Roth JA. Correlation between the biochemical pathways altered by mutated parkinson-related genes and chronic exposure to manganese. Neurotoxicology. 2014;44:314–325. doi: 10.1016/j.neuro.2014.08.006. [DOI] [PubMed] [Google Scholar]