Abstract
P-glycoprotein (P-gp), Breast cancer resistance protein (BCRP) and Multidrug resistance-associated protein 2 (MRP2) residing at the blood-brain barrier (BBB) and the blood-spinal cord barrier (BSCB) are major obstacles for drug delivery to the Central Nervous System (CNS). Disease-induced changes of these xenobiotic transporters at the CNS barriers have been previously documented. Changes in the functional expression of these transporters at the CNS barriers would limit the clinical efficacy of therapeutic agents targeting the CNS. In this study, we characterized the changes in expression and efflux activity of P-gp, BCRP and MRP2 at the BBB and BSCB of an amyotrophic lateral sclerosis (ALS) SOD1-G93A transgenic rat model across the three stages of disease progression: pre-onset, onset and symptomatic. Up-regulation of P-gp and BCRP at the BBB and BSCB during disease progression of ALS would reduce drug entry to the CNS, while any decreases in transport activity would increase drug entry. In SOD rats at the ALS symptomatic stage, we observed increases in both P-gp transport activity and expression compared to age-matched wildtypes. BCRP and MRP2 levels were unchanged in these animals. Immunohistochemical analysis in brain and spinal cord capillaries of SOD rats from all three ALS stages and age-matched wildtypes showed no differences in nuclear localization of a known P-gp regulator, nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB). It suggests that NFκB may have a limited role during P-gp induction observed in our study and additional signaling pathways could be responsible for this response. Our observations imply that novel pharmacological approaches for treating ALS require selecting drugs that are not P-gp substrates in order to improve therapeutic efficacy in the CNS during ALS progression.
Keywords: P-glycoprotein, Breast cancer resistance protein, Multidrug resistance-associated protein 2, Blood-brain barrier, Blood-spinal cord barrier, Amyotrophic lateral sclerosis
Introduction
The blood-brain barrier (BBB) and the blood-spinal cord barrier (BSCB) are capillary-based barriers that separate brain and spinal cord tissue, respectively, from peripheral blood circulation [1, 2]. The two barriers are morphologically similar in that they both reside within the non-fenestrated capillary endothelium, which is sealed together by tight junctions and adhesion molecules. Although BSCB is believed to have a higher junctional permeability than BBB, these two barriers tightly regulate the paracellular and transcellular exchange of nutrients, endogenous chemicals, metabolites and xenobiotics into and out of the Central Nervous System (CNS) [1, 2]. Hence, they play an important role in maintaining homeostasis of the CNS microenvironment, which is essential for proper neuronal function. In addition, both barriers highly express a wide array of xenobiotic efflux transport pumps that are members of the ATP-Binding Cassette (ABC) transporter superfamily [8, 20]. The luminal capillary expression of several xenobiotic transporters, such as P-glycoprotein (P-gp), Breast cancer resistance protein (BCRP) and Multidrug resistance-associated protein 2 (MRP2), is a major obstacle for drug delivery to the brain and spinal cord due to their concentrative efflux activity that pumps drugs from the barrier endothelial plasma membrane or cytosol compartment back into the blood for subsequent clearance [20]. Changes to the expression levels of these transporters at both barriers can alter drug concentrations in brain and spinal cord tissues. Therefore, an understanding of transporter activities at the BBB and BSCB is essential to predict drug pharmacokinetics and pharmacodynamics in the CNS more accurately. Furthermore, this knowledge will allow new drug designs that avoid or exploit drug interactions with these transporters to enhance CNS drug delivery or neuroprotection.
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease caused by progressive damage of cortical, brainstem and spinal motor neurons. Patients living with the disease face long-term paralysis and disability. Riluzole is the only FDA-approved ALS drug in the clinic, yet it only modestly slows ALS progression during early disease stages in some patients and does not halt or reverse ALS [6]. Since riluzole is a substrate for two ABC xenobiotic efflux transporters, P-gp and BCRP, the full therapeutic efficacy of riluzole in the CNS could be limited by these efflux transporters at the BBB and BSCB [16, 18, 19]. Recent in vivo studies using mouse models of ALS showed that P-gp and BCRP transport activity and expression were induced in CNS barriers during the late stage of disease progression [15, 17]. These inductions could potentially further limit the therapeutic efficacy of riluzole in the CNS [14]. Therefore, an understanding of how and when transporter activity changes at the BBB and BSCB in animal models presenting clinical ALS symptoms could provide insights on drug development and therapeutic window necessary to improve CNS delivery of riluzole. In our study, we characterized for the first time an expression profile for three major xenobiotic efflux transporters (i.e., P-gp, BCRP and MRP2) at the BBB and BSCB of a copper zinc superoxide dismutase 1 (SOD1) transgenic rat model (available from Taconic Model 2148) across three stages of ALS progression: pre-onset, onset and symptomatic. The use of this novel SOD rat model offers advantages for future mechanistic studies. Firstly, rats provide a more robust yield of brain and spinal cord capillaries than other models such as mice. Secondly, several regulatory pathways that drive transporter activity at the BBB and BSCB have been characterized in rats [20]. Our results show that P-gp transport activity and protein expression were unchanged in SOD rats at ALS pre-onset and onset stages compared to age-matched wildtypes, however they were significantly increased (1.6 to 2-fold) in SOD rats at ALS symptomatic stage compared to age-matched wildtypes. P-gp induction was not associated with an increase of NFκB nuclear localization in capillaries that showed an induction. Conversely, BCRP and MRP2 transport activity and protein expression remained unchanged at the BBB and BSCB of SOD rats across all stages of ALS progression.
Materials and methods
Chemicals
NBD-CSA was custom synthesized [3, 8, 12]. Bodipy-Prazosin was purchased from Life Technologies (Grand Island, NY). Sulforhodamine 101 free acid (Texas Red), 4 % paraformaldehyde, 0.2% glutaraldehyde, Triton X-100 and D-glucose were purchased from Sigma-Aldrich (St. Louis, MO). Valspodar (PSC-833), KO143 and MK571 were purchased from Tocris Bioscience (Ellisville, MO). All the reagents used in this study were analytical or best available pharmaceutical grade.
Animals
The ALS rat model expressing a copper zinc superoxide dismutase 1 (SOD1) G93A mutation (SOD rat model, #2148) was purchased from Taconic and housed under a 12 h light and dark cycle in temperature controlled facility with access to ad libitum food and water. Animals were purchased at 56–70 days of age, weighted every 2–3 days, and allowed to mature and develop clinical ALS-related symptoms, such as muscle weight loss and clear-cut motor deficits. Motor deficits were defined by any observable muscle weakness (i.e., kicking of the hind limbs), paralysis (i.e., inability to move a limb), dragging hind limbs and inability to stand up or right itself when it is laid to the side. In our hands, this model developed initial observable weight loss at 147.8 ± 7.9 of age, and progressed to have a clear motor dysfunction, such as muscle weakness, paralyzed or dragging limbs at approximately 156.6 ± 7.3 days of age. All studies were conducted in accordance with the Animal Care and Use Committee at National Institute of Environmental Health Sciences.
Capillary Isolation
Brain capillary isolation protocol was adopted previously with the following minor modification [12]. Cerebral cortex grey matter and spinal cord tissues were homogenized 40 up-and-down strokes using a tissue grinder (clearance: 150–230 µm) rotating at 50 rpm in ice-cold incubation buffer [2.7 mM KCl, 1.46 mM KH2PO4, 136.9 mM NaCl, 8.1 mM Na2HPO4, 0.9 mM CaCl2, 0.5 mM MgCl2, 5 mM D-Glucose and 1 mM Na+-pyruvate at pH 7.4].
Transport Assays
Confocal microscopy-based transport assay was performed on isolated rat brain capillaries as described previously [3, 8, 12]. Briefly, freshly isolated capillaries were exposed to incubation buffer containing 2 µM NBD-CSA, Bodipy-Prazosin, or Texas Red for 45 minutes. A Zeiss LSM 510 inverted confocal laser scanning microscope with a 40X 1.2 numeric aperture water-immersion objective and the ZEN microscope software (Carl Zeiss, Thornwood, NY) was used to capture fluorescence images. Luminal fluorescence of each capillary luminal area was quantified using ImageJ (Version 1.49i, NIH, Bethesda, MD). Image acquisition parameters for capillary luminal fluorescence were identical between SOD rats at each ALS stage and its respective age-matched group of wildtype rats. The initial acquisition parameters were validated by a range indicator function to ensure images were within detectable range and not under- or over-saturated. Specific transport activity was calculated by taking the difference between total luminal fluorescence [measured in the absence of the corresponding transporter inhibitor (10 µM PSC-833, 10 µM KO143 or 15 µM MK571)] and the background luminal fluorescence (measured in the presence of the inhibitor). Percent change (normalized to age-matched wildtype) of specific transport activity are presented with S.E.M..
Immunohistochemistry
Immunohistochemistry on capillaries were performed as described previously [3, 8, 12]. The fixed capillaries were incubated with 0.7 µg/mL anti-P-glycoprotein antibody (C219, ThermoFisher Scientific, Rochester, NY) or 1.2 µg/mL anti-BCRP antibody (BXP53, Enzo Life Sciences, Farmingdale, NY) or 1.0 µg/mL anti-MRP2 antibody (M2III-6, Santa Cruz Biotechnology, Dallas, TX) or 10 µg/mL anti-NFkB antibody (ab16502, Abcam, Cambridge, MA) in PBS overnight at 4°C. After rinsing with PBS, capillaries were incubated with Alexa Fluor®568 goat anti-mouse IgG secondary antibody for P-glycoprotein and MRP2 or Alexa Fluor®568 goat anti-rat for BCRP or Alexa Fluor®488 goat anti-rabbit for NFkB (Life Technologies, Grand Island, NY) diluted 1:1000 in PBS, for 90 min at 37°C. Nuclear staining was performed in some of the samples using DRAQ5 (Cell Signaling Technology, Beverly, MA). Immunostaining images of transporters and light contrast images of capillary morphology were captured using Zeiss LSM 510 confocal microscopy.
Western Blotting
Plasma membrane protein preparation and western blotting protocol were performed as previously described [3, 8]. 10 µg of plasma membrane lysate was loaded for each sample. After transfer, PVDF membrane was immunoblotted overnight at 4°C in PBS containing primary antibodies against P-gp (C219, 0.7 µg/mL), BCRP (BXP53, 2.5 µg/mL), MRP2 (M2 III-6, 2 µg/mL) and Actin (Anti-β-Actin Clone AC-15, 1:10000, Sigma) followed by incubation for an hour at room temperature with the corresponding infrared dye-conjugated anti-mouse or anti-rat secondary antibody (0.1 µg/mL, Li-Cor Biosciences). The membrane was imaged and protein band intensity was measured using a Li-Cor Biosciences Odyssey Infrared Imaging System.
Statistical Analysis
Data are expressed as mean ± standard error of mean (S.E.M.). Comparisons between groups were performed using paired, two-tailed Student’s t-test, One-Way Analyses of Variance (ANOVA) with Dunnett’s multiple comparisons post-hoc test. Data were analyzed by GraphPad Prism 6 (La Jolla, CA) and post-hoc test with a p value < 0.05 was considered to be statistically significant.
Results
SOD and age-matched wildtype rats were purchased at 56–70 days of age and allowed to mature. Peak bodyweight was identified to best indicate onset of ALS progression before the first sign of motor deficits in rats [22]. SOD rats at ALS pre-onset stage exhibited consistent weight gain and no motor deficits. SOD rats at ALS onset stage exhibited a noticeable decrease in body weight (>5% of total body weight), but no sign of motor deficits (i.e., muscle weakness, paralysis, dragging limbs and/or inability to stand up or right itself). SOD rats in the ALS symptomatic stage exhibited both symptoms: weight loss of more than 15% total body weight and motor deficits
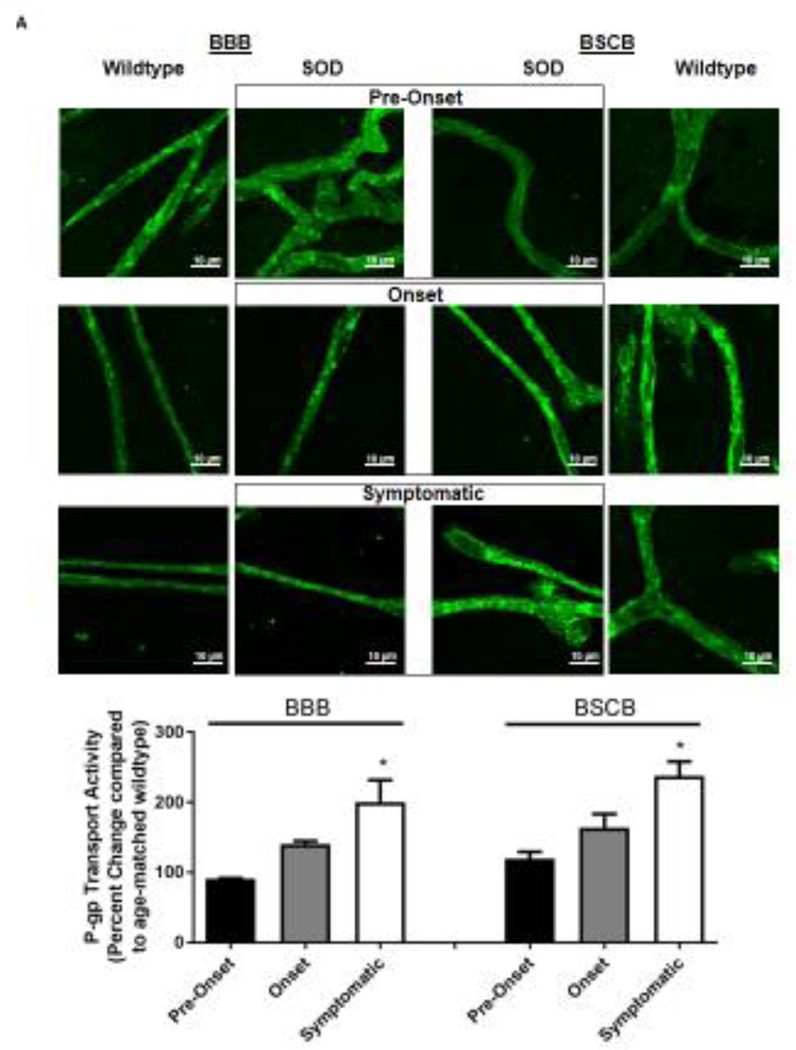
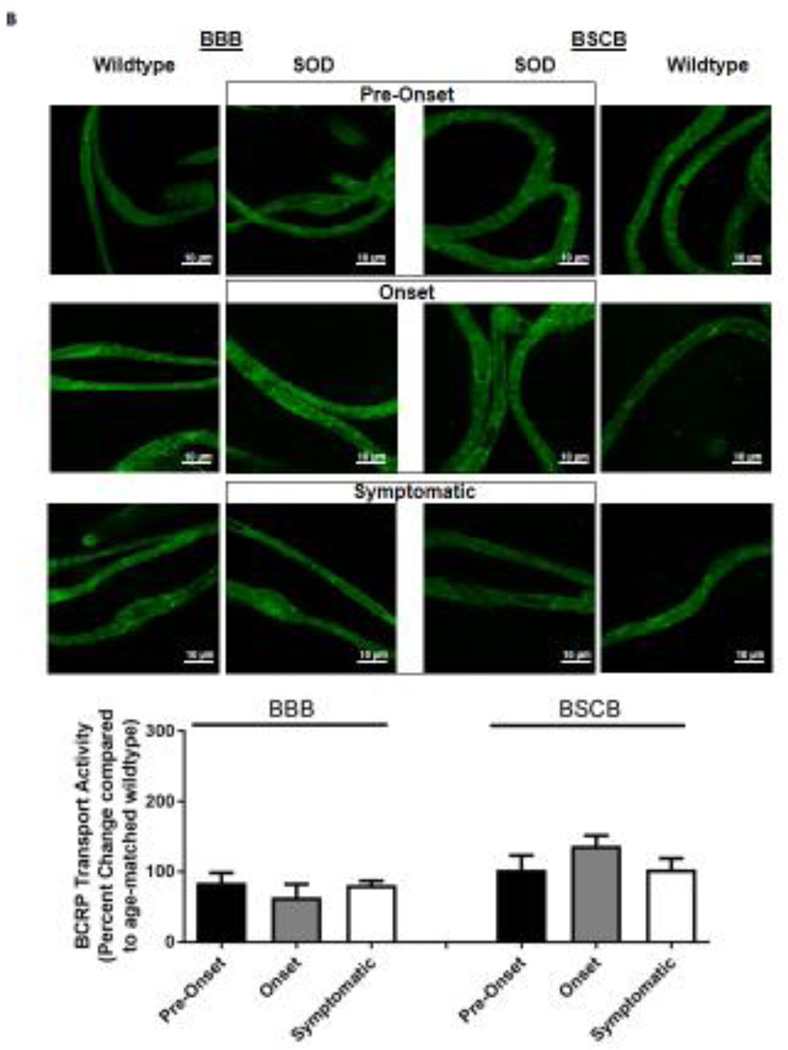
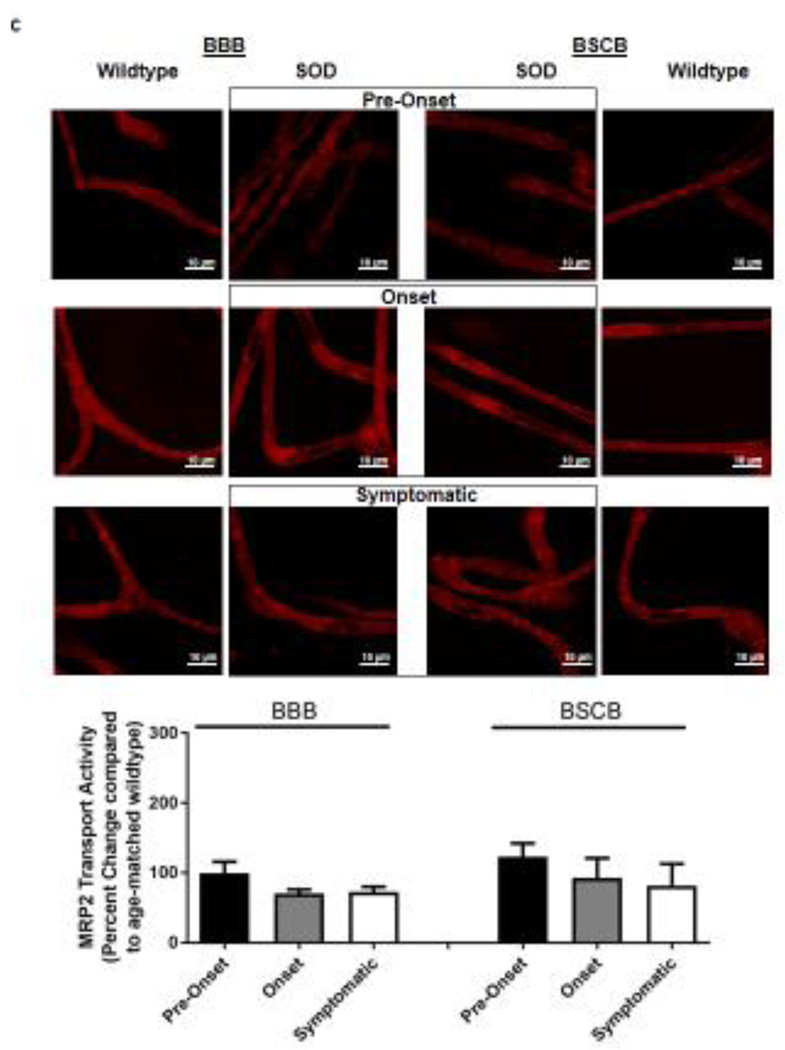
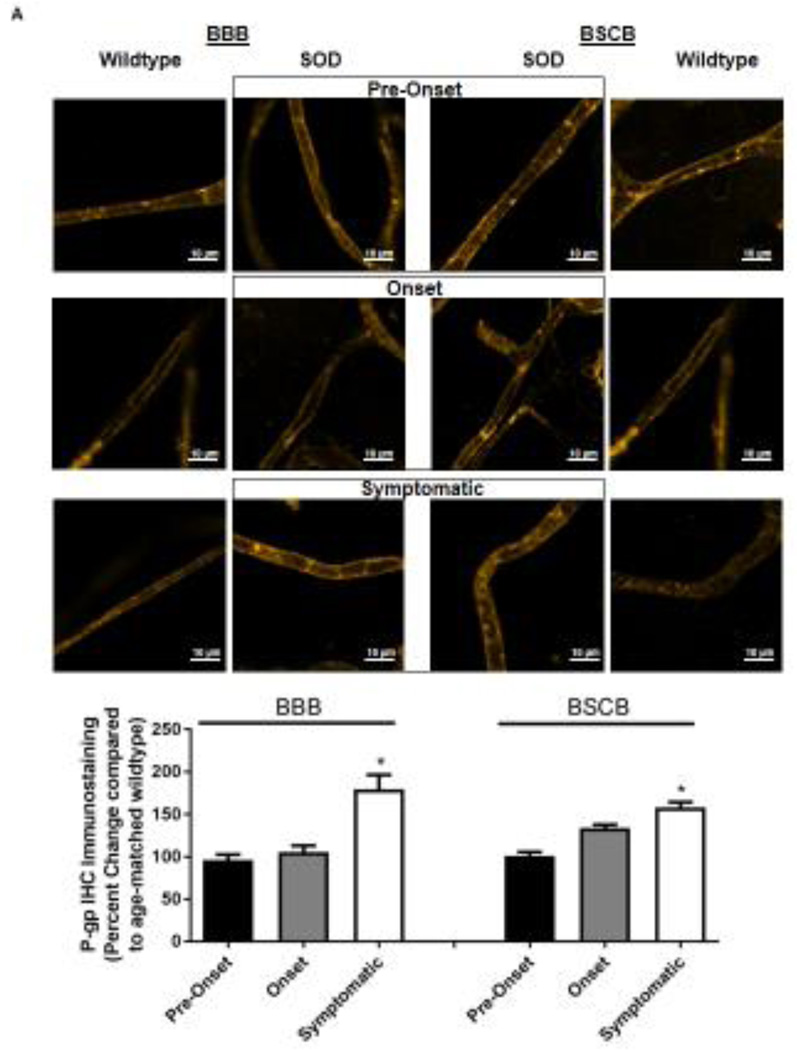
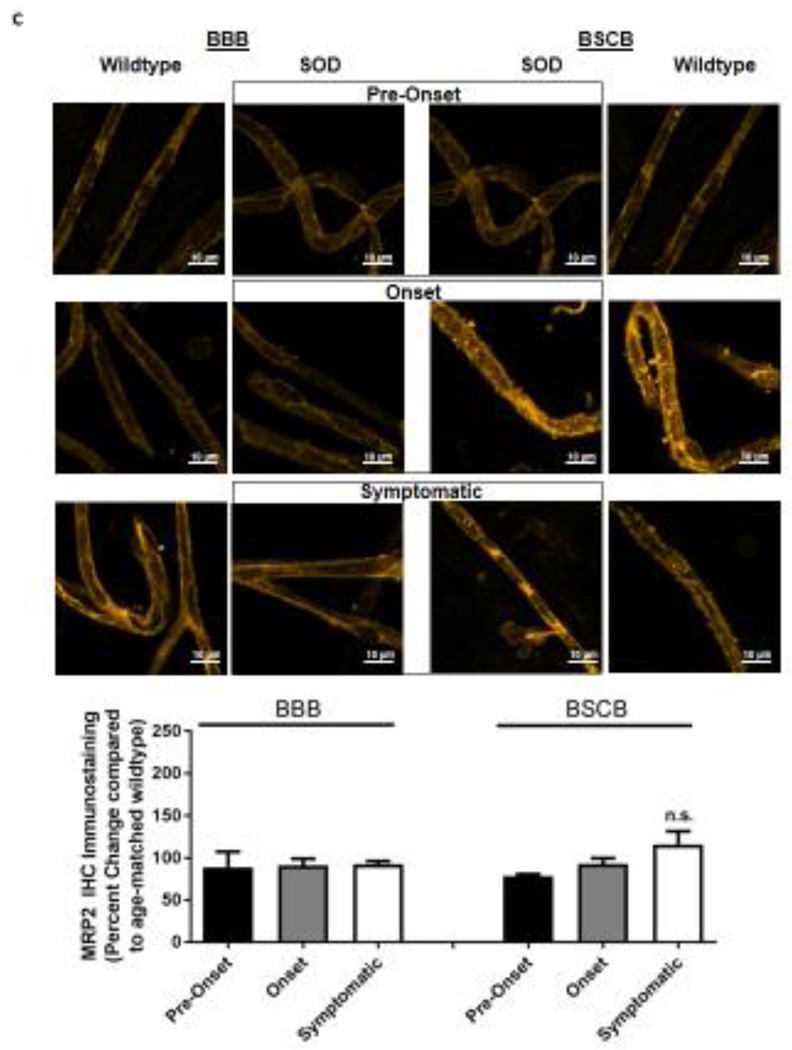
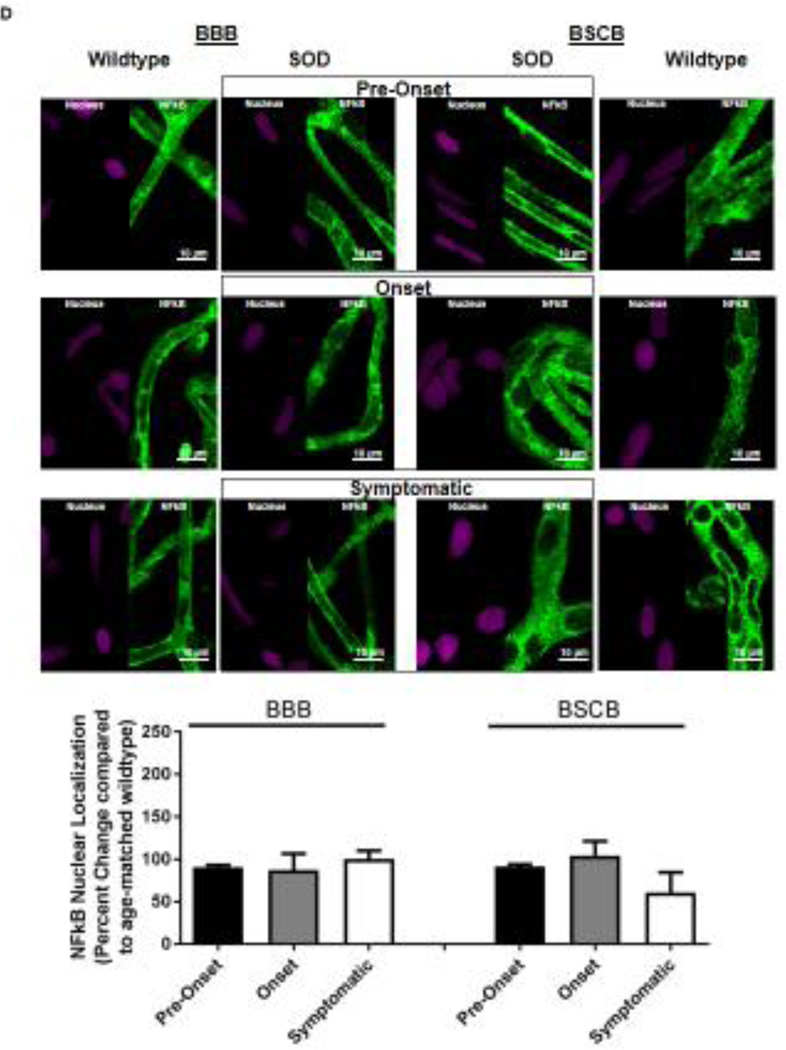
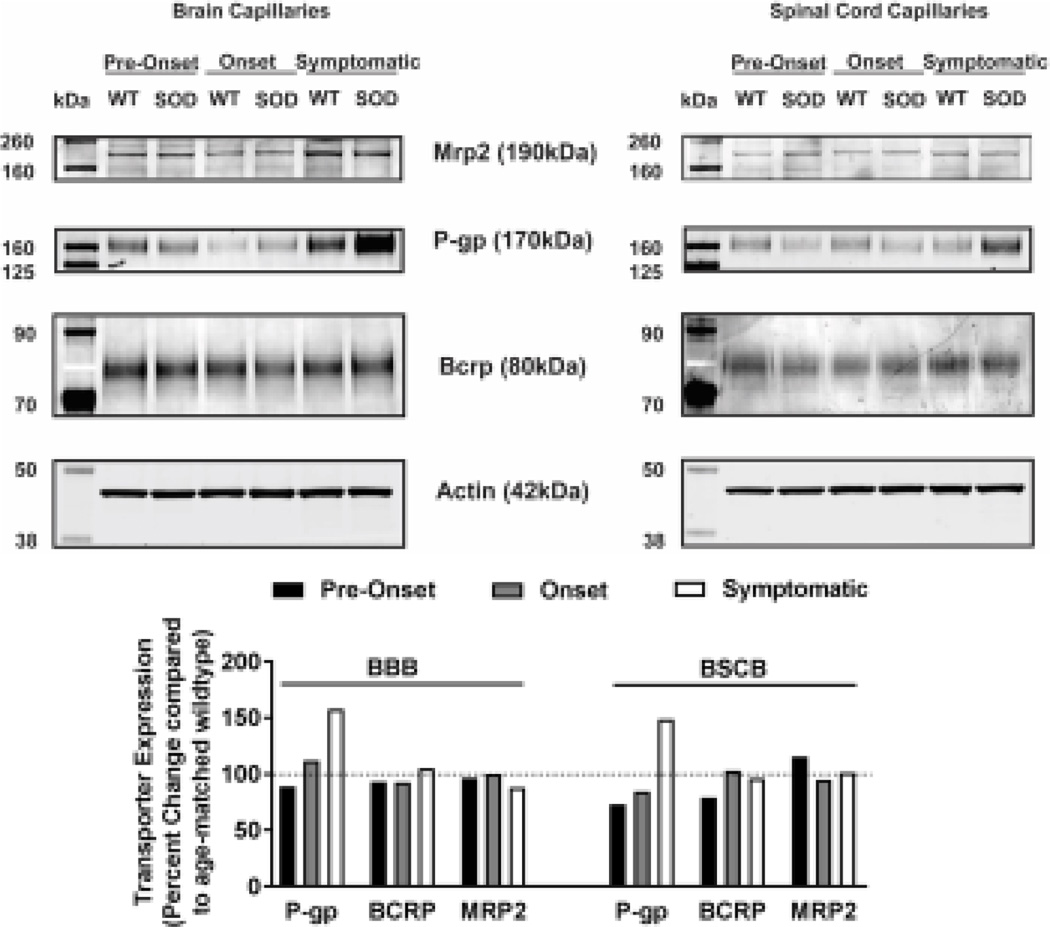
Knowing that ABC xenobiotic efflux transporters are highly regulated by post-transcriptional and post-translational processes, we investigated two important biological endpoints: the steady state protein levels and transport activity. We performed ex vivo transport assay to investigate transport activity of P-gp, Bcrp and Mrp2 in isolated rat brain and spinal cord capillaries obtained from SOD rats across all three stages and wildtype rats. To compare the age-independent changes of P-gp, BCRP and MRP2 transport activity in SOD rats across different ALS stages, data from each ALS stage were normalized to those measured from age-matched wildtype rats to generate a percent change. Across the three ALS stages, SOD rats at the symptomatic stage had a significant 2-fold higher percent change of P-gp transport activity in brain capillaries (Mean=200, S.E.M.±31.5) compared to SOD rats at ALS pre-onset stages (Mean=91.1, S.E.M.±0.70) (Figure 1A). In addition, SOD rats at the symptomatic stage also had a significant 2-fold higher percent change of P-gp transport activity in spinal cord capillaries (Mean=238, S.E.M.±20.8) compared to SOD rats at ALS pre-onset stages (Mean=120, S.E.M.± 9.85) (Figure 1A). Conversely, percent change of BCRP and MRP2 transport activities in brain and spinal cord capillaries was not altered compared among all three ALS stages (Figures 1B&C). Having established that P-gp transport activity increases in SOD rats at ALS symptomatic stage, we next measured transporter expression in the luminal membranes of brain and spinal cord capillaries from these rats using immunohistochemistry. In agreement with the transport activity results, SOD rats at ALS symptomatic stage had approximately 1.6-fold higher P-gp expression in brain (Mean=180, S.E.M.±17.0) and spinal cord capillaries (Mean=148, S.E.M.±16.1) compared to those measured in brain (Mean=96.0, S.E.M.±6.60) and spinal cord capillaries (Mean=101, S.E.M.±4.62) of SOD rats at pre-onset stage (Figure 2A). In contrast, no significant changes in the levels of BCRP and MRP2 expression was detected in SOD rats across all three ALS stages. (Figures 2B&C). As shown in our previous publications, NFkB is a nuclear transcription factor known to induce P-gp expression in rat brain capillaries [23]. Using immunohistochemistry, we observed no significant difference of NFkB nuclear localization in brain and spinal cord capillaries from SOD rats compared among all three ALS stages (Figure 2D). Next, we used Western blot analysis to quantify P-gp protein induction at the BBB and BSCB of SOD rats across all three ALS stages and wildtype rats (Figure 3). We found that P-gp protein expression in brain and spinal cord capillaries of SOD rats at ALS symptomatic stages was approximately 50% higher compared to age-matched wildtype rats. This induction was not observed in pre-onset and onset SOD rats. In contrast, BCRP and MRP2 protein expression levels in brain and spinal cord capillaries of SOD rats from all three ALS stages were no different than their respective age-matched wildtype rats.
Figure 1. Transport activities of P-gp, BCRP and MRP2 in brain and spinal cord capillaries of SOD rats at ALS pre-onset, symptomatic onset or symptomatic stage and age-matched wildtype rats.
Representative confocal images showing luminal accumulation of (A) NBD-CSA, (B) Bodipy-prazosin and (C) Texas Red in brain and spinal cord capillaries. At least 10 capillaries from at least two capillary preparations (pooled tissue from at least four rats) were analyzed from each ALS stage of SOD and age-matched wildtype rats. Percent change (normalized to agematched wildtypes) of specific transport activity are presented with S.E.M.. Scale bars in confocal images indicates 10 µm. One-way ANOVA with Dunnett’s multiple comparisons post-hoc test was performed. Post-hoc test with p <0.05 was considered a significant difference compared to capillaries of SOD rats at pre-onset stage.
Figure 2. Immunohistochemical staining of P-gp, BCRP, Mrp 2 and NFkB in brain and spinal cord capillaries of SOD rats at ALS pre-onset, symptomatic onset and symptomatic stage and age-matched wildtype rats.
Representative confocal images and percent expression change of (A) P-gp, (B) BCRP, (C) MRP2, and D) NFkB in brain and spinal cord capillaries. Nuclear staining was achieved using DRAQ5 (left side of each representative image). For each protein, at least 10 capillaries per at least two capillary preparations (pooled tissue from at least four rats) were analyzed from each ALS stage of SOD rats and age-matched wildtype rats. Percent change (normalized to age-matched wildtypes) of protein immunohistochemical staining are presented with S.E.M.. Scale bars in confocal images indicates 10 µm. One-way ANOVA with Dunnett’s multiple comparisons post-hoc test was used. *Post-hoc test with p <0.05 was considered a significant difference compared to capillaries of SOD rats at pre-onset stage. n.s. = nonsignificant compared to capillaries exposed to pre-onset rats.
Figure 3. P-gp, BCRP and Mrp 2 protein expression in brain and spinal cord capillaries of SOD rats at ALS pre-onset, onset and symptomatic stage and age-matched wildtype rats.
Representative westernblots (top) and densitometric analysis (bottom) of transporter expression in 10 µg of plasma membrane lysates of brain and spinal cord capillaries. Four rat brains and spinal cords (pooled samples from two to three capillary preparations) were utilized in each SOD group and their respective wildtype (WT) groups. Data represent the percent change in transporter expression first normalized to actin expression and then normalized to age-matched wildtypes.
Discussion
The current study investigates changes of major xenobiotic efflux transporters, i.e., P-gp, BCRP and MRP2, at the BBB and BSCB of an ALS (SOD1G93A) transgenic rat model across three ALS stages: Pre-onset, onset and symptomatic. To our knowledge, no previous report has characterized these transporters at the BBB and BSCB in the current model. Our results show that P-gp transport activity and protein expression were increased at the BBB and BSCB of SOD rats at ALS symptomatic stage. Conversely, both BCRP and MRP2 transport activity and protein expression were unchanged at the BBB and BSCB of SOD rats among all ALS stages. Our work implies that P-gp induction at the CNS barrier should be considered during development of effective ALS pharmacotherapies.
To date, the exact cellular mechanisms of ALS progression are unknown and treatment options are limited to riluzole, the single FDA-approved agent shown to slow disease progression in some patients. The lack of effective ALS pharmacotherapy can be contributed by the limited drug entry to the brain, which can lead to disease-driven pharmacoresistance. This study suggests that ALS-driven pharmacoresistance may result in part from the up-regulation of a major ABC xenobiotic efflux transporter, P-gp, at the luminal membranes of capillary endothelial cells at the BBB and BSCB. Increased P-gp transport activity contributes to riluzole and other potential ALS drugs reduced entry, and increased removal from the CNS compartments. Therefore, investigating ALS-mediated changes of xenobiotic transporters at the BBB and BSCB is of primary importance to the development of effective pharmacotherapy for ALS and other CNS disorders. Recently, two in vivo studies using transgenic mice with a SOD1 mutation revealed ALS-mediated changes in xenobiotic transporters at the BBB and BSCB [15, 17]. The first study showed that transgenic male mice with the SOD1-G86R mutation at ALS pre-symptomatic stage had increased P-gp transport activity and protein levels in capillaries isolated from whole brain compared to wildtype controls. However, BCRP levels remained unchanged [17]. This P-gp induction was associated with a decrease in brain/plasma ratios of riluzole and a selective P-gp substrate, digoxin [17]. In the second study, two transgenic male mouse models, the SOD1-G93A and SOD1-A315T mutants, at ALS symptomatic stage showed mRNA and protein induction of P-gp and BCRP in brain cortex and spinal cord tissue lysates compared to wildtypes. The transport activities of both P-gp and BCRP, measured ex vivo, were also increased in capillaries isolated from brain cortex and spinal cord tissues of SOD1-G93A transgenic mice compared to wildtype controls [15]. In our current study, we measured P-gp transport activity and protein expression at the BBB and BSCB of SOD rats across three ALS stages and found that they were increased in SOD rats at the ALS symptomatic stage, which is consistent with previous in vivo studies utilizing SOD mouse models. Conversely, BCRP transport activity and protein expression were unchanged at these sites. Moreover, our data are consistent with P-gp induction observed in whole tissue homogenates isolated from the lumbar spinal cords of human ALS specimens [15]. BCRP expression profile we provided and those reported previously in vivo are different, possibly because of the use of different transgenic models, i.e., SOD1-G86R versus SOD1-G93A, and species differences between mouse and rat. Therefore, future in vivo ALS-driven pharmacoresistance studies should consider carefully the potential differences in transporter expression profiles among these rodent models. Nonetheless, the usefulness of our rat model and other mouse models for ALS drugs pre-clinical testing remains to be evaluated as more data on P-gp and BCRP expression profile at the human BBB and BSCB becomes available from clinical studies. In addition, we did not observe changes of MRP2 transport activity and protein expression at the BBB and BSCB of SOD rats compared among all three ALS stages. Transport activity of MRP2 was measured by a small molecular weight fluorescence substrate, Texas Red. Unchanged Mrp2 transport activity also indicates an intact non-leaky barrier; a leaky barrier would not permit fluorescent substrate accumulation in the capillaries.
Previous studies at the rat BBB showed that P-gp, BCRP and MRP2 are regulated by several signaling pathways. For example, peroxisome proliferator activated receptor alpha (PPARα) regulates all three transporters [13, 21], while pregnane X receptor (PXR) primarily regulates P-gp and MRP2 [5, 9], and estrogen receptors (ERs)α and β primarily regulate BCRP [11]. Similarly, inflammation-related signaling pathways involving long-term exposure of tumor necrosis factor α (TNFα) increase P-gp transport activity and expression; however, this pathway decreases MRP2 expression and has no effect on BCRP [4]. In our current study, induction at CNS barriers was selective for P-gp and no changes were observed for the other two major xenobiotic transporters, BCRP and MRP2. Thus, we speculate that a unique or several new additional regulatory pathways may be involved during ALS-driven induction of P-gp at the CNS barriers.
Next, we examined the role of NFκB for P-gp induction in SOD rats, because this transcription factor was shown to increase P-gp at the rat BBB [23] and was recently shown to play a role in microglia-induced motor neuron death in an ALS mouse model with SOD1-G93A mutation [10]. We observed no major changes of NFκB nuclear localization in capillaries of the brain and spinal cords from SOD rats at all ALS stages compared to wildtype. Our data suggests several possible explanations: First, NFκB already residing within the nucleus could be sufficient to induce P-gp in SOD rats, or second, small changes of NFκB nuclear localization are difficult to be detected in our study, or thirdly, NFκB may not play a role in P-gp induction at the BBB and BSCB during disease progression in this SOD rat model. Additional studies are required to fully elucidate the role of NFκB for P-gp induction observed in the current ALS SOD rat model. If NFκB is shown to induce P-gp expression at the BBB and BSCB during ALS progression, the use of NFκB inhibitors could be beneficial to prevent P-gp induction and increase CNS effectiveness of riluzole and other future ALS drugs that are P-gp substrates. One under consideration is activated protein C, a NFκB inhibitor with neurological protective properties at the BBB, BSCB, and motor neurons. [24–26]. On the other hand, it is equally important to study other signaling pathways that can play a role in P-gp induction during ALS progression. In particular, signaling pathway involving TNFα is of great interest because this proinflammatory cytokine was shown to induce P-gp expression at the BBB [4] and was recently associated with inflammatory processes in human ALS spinal cords [7].
In conclusion, we reported here that P-gp transport activity and protein expression were increased in SOD rats at ALS symptomatic stage compared to pre-onset and onset stages. Conversely, BCRP and MRP2 levels remained unchanged in these animals. In brain and spinal cord capillaries of SOD rats at ALS symptomatic stage, there was no increase in the nuclear localization of NFκB corresponding with P-gp induction, possibly indicating that additional signaling pathways are involved. Future studies using the current SOD rat model will expand our knowledge on cellular mechanisms that regulate xenobiotic efflux transporters at the CNS barriers during ALS progression.
Given the findings of this study and others, P-gp induction at CNS barriers in some patients with ALS is possible and might explain the difficulty in identifying effective ALS pharmacotherapy. Additionally, the only current FDA-approved drug for ALS management, riluzole, is suggested to be a P-gp and BCRP substrate [16, 18, 19]. ALS-induced P-gp upregulation could further restrict riluzole permeability across CNS barriers and reduce its concentration at neuronal target sites, thereby lowering its therapeutic efficacy. In this case, proper adjustments on dosage or therapeutic window of CNS pharmacotherapeutics that are substrates for P-gp should be studied throughout ALS progression in patients who are expected to have P-gp induction at the CNS barriers. Taken together, pharmacological interventions to prevent induction or substrate interactions of P-gp could be useful for improving therapeutic efficacy in CNS disorders showing P-gp induction at the CNS barriers, such as ALS.
Highlights.
Expression and activity profiles of drug efflux xenobiotic transporters at the blood-brain and blood-spinal cord barriers was characterized in an ALS SOD1 rat model across three disease stages: ALS Pre-onset, onset and symptomatic.
P-glycoprotein transport activity and expression levels were increased at the CNS barriers in rats at the ALS symptomatic stage.
P-glycoprotein induction can restrict CNS delivery of current and new ALS pharmacotherapies that are substrates for this transporter.
Acknowledgments
The authors thank Joyce Blaisdell and staff at the NIEHS animal facility for their assistance in providing excellent care to all rats used in this study. This research was supported (in part) by Target ALS and the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Abbreviations
- ABC
ATP-Binding Cassette
- ALS
Amyotrophic lateral sclerosis
- BBB
Blood-brain barrier
- BCRP
Breast cancer resistance protein
- BSCB
Blood-spinal cord barrier
- CNS
Central Nervous System
- MRP2
Multidrug resistance-associated protein 2
- P-gp
P-glycoprotein
- SOD1
Superoxide dismutase 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. Journal of Inherited Metabolic Disease. 2013;36:437–449. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 2.Bartanusz V, Jezova D, Alajajian B, Digicaylioglu M. The blood-spinal cord barrier: morphology and clinical implications. Ann Neurol. 2011;70:194–206. doi: 10.1002/ana.22421. [DOI] [PubMed] [Google Scholar]
- 3.Bauer B, Hartz AMS, Lucking JR, Yang X, Pollack GM, Miller DS. Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTπ, at the blood-brain barrier. Journal of Cerebral Blood Flow and Metabolism. 2008;28:1222–1234. doi: 10.1038/jcbfm.2008.16. [DOI] [PubMed] [Google Scholar]
- 4.Bauer B, Hartz AMS, Miller DS. Tumor necrosis factor α and endothelin-1 increase p-glycoprotein expression and transport activity at the blood-brain barrier. Molecular Pharmacology. 2007;71:667–675. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- 5.Bauer B, Yang X, Hartz AMS, Olson ER, Zhao R, Kalvass JC, Pollack GM, Miller DS. In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through p-glycoprotein up-regulation. Molecular Pharmacology. 2006;70:1212–1219. doi: 10.1124/mol.106.023796. [DOI] [PubMed] [Google Scholar]
- 6.Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 7.Brohawn DG, O'Brien LC, Bennett JP., Jr RNAseq Analyses Identify Tumor Necrosis Factor-Mediated Inflammation as a Major Abnormality in ALS Spinal Cord. PLoS One. 2016;11:e0160520. doi: 10.1371/journal.pone.0160520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campos CR, Schröter C, Wang X, Miller DS. ABC transporter function and regulation at the blood-spinal cord barrier. Journal of Cerebral Blood Flow and Metabolism. 2012;32:1559–1566. doi: 10.1038/jcbfm.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan GNY, Hoque MT, Cummins CL, Bendayan R. Regulation of P-glycoprotein by orphan nuclear receptors in human brain microvessel endothelial cells. Journal of Neurochemistry. 2011;118:163–175. doi: 10.1111/j.1471-4159.2011.07288.x. [DOI] [PubMed] [Google Scholar]
- 10.Frakes AE, Ferraiuolo L, Haidet-Phillips AM, Schmelzer L, Braun L, Miranda CJ, Ladner KJ, Bevan AK, Foust KD, Godbout JP, Popovich PG, Guttridge DC, Kaspar BK. Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron. 2014;81:1009–1023. doi: 10.1016/j.neuron.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartz AMS, Madole EK, Miller DS, Bauer B. Estrogen receptor β signaling through phosphatase and tensin homolog/phosphoinositide 3-kinase/Akt/glycogen synthase kinase 3 down-regulates blood-brain barrier breast cancer resistance protein. Journal of Pharmacology and Experimental Therapeutics. 2010;334:467–476. doi: 10.1124/jpet.110.168930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartz AMS, Zhong Y, Wolf A, LeVine H, III, Miller D, Bauer B. Aβ40 reduces p-glycoprotein at the blood–brain barrier through the ubiquitin–proteasome pathway. Journal of Neuroscience. 2016;36:1930–1941. doi: 10.1523/JNEUROSCI.0350-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoque MT, Shah A, More V, Miller DS, Bendayan R. In vivo and ex vivo regulation of breast cancer resistant protein (Bcrp) by peroxisome proliferator-activated receptor alpha (PPARα) at the blood-brain barrier. Journal of Neurochemistry. 2015;135:1113–1122. doi: 10.1111/jnc.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jablonski M, Miller DS, Pasinelli P, Trotti D. ABC transporter-driven pharmacoresistance in Amyotrophic Lateral Sclerosis. Brain Res. 2015;1607:1–14. doi: 10.1016/j.brainres.2014.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jablonski MR, Jacob DA, Campos C, Miller DS, Maragakis NJ, Pasinelli P, Trotti D. Selective increase of two ABC drug efflux transporters at the blood-spinal cord barrier suggests induced pharmacoresistance in ALS. Neurobiol Dis. 2012;47:194–200. doi: 10.1016/j.nbd.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jablonski MR, Markandaiah SS, Jacob D, Meng NJ, Li K, Gennaro V, Lepore AC, Trotti D, Pasinelli P. Inhibiting drug efflux transporters improves efficacy of ALS therapeutics. Ann Clin Transl Neurol. 2014;1:996–1005. doi: 10.1002/acn3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milane A, Fernandez C, Dupuis L, Buyse M, Loeffler JP, Farinotti R, Meininger V, Bensimon G. P-glycoprotein expression and function are increased in an animal model of amyotrophic lateral sclerosis. Neurosci Lett. 2010;472:166–170. doi: 10.1016/j.neulet.2010.01.078. [DOI] [PubMed] [Google Scholar]
- 18.Milane A, Fernandez C, Vautier S, Bensimon G, Meininger V, Farinotti R. Minocycline and riluzole brain disposition: interactions with p-glycoprotein at the blood-brain barrier. J Neurochem. 2007;103:164–173. doi: 10.1111/j.1471-4159.2007.04772.x. [DOI] [PubMed] [Google Scholar]
- 19.Milane A, Vautier S, Chacun H, Meininger V, Bensimon G, Farinotti R, Fernandez C. Interactions between riluzole and ABCG2/BCRP transporter. Neuroscience Letters. 2009;452:12–16. doi: 10.1016/j.neulet.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 20.Miller DS. Regulation of ABC Transporters Blood-Brain Barrier. The Good, the Bad, and the Ugly. Advances in Cancer Research. 2015;125:43–70. doi: 10.1016/bs.acr.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 21.More VR, Campos CR, Evans RA, Oliver KD, Chan GN, Miller DS, Cannon RE. PPAR-alpha, a lipid-sensing transcription factor, regulates blood-brain barrier efflux transporter expression. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016 doi: 10.1177/0271678X16650216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thonhoff JR, Jordan PM, Karam JR, Bassett BL, Wu P. Identification of early disease progression in an ALS rat model. Neurosci Lett. 2007;415:264–268. doi: 10.1016/j.neulet.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Campos CR, Peart JC, Smith LK, Boni JL, Cannon RE, Miller DS. Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood-brain and blood-spinal cord barriers. Journal of Neuroscience. 2014;34:8585–8593. doi: 10.1523/JNEUROSCI.2935-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winkler EA, Sengillo JD, Sagare AP, Zhao Z, Ma Q, Zuniga E, Wang Y, Zhong Z, Sullivan JS, Griffin JH, Cleveland DW, Zlokovic BV. Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc Natl Acad Sci U S A. 2014;111:E1035–E1042. doi: 10.1073/pnas.1401595111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong Z, Ilieva H, Hallagan L, Bell R, Singh I, Paquette N, Thiyagarajan M, Deane R, Fernandez JA, Lane S, Zlokovic AB, Liu T, Griffin JH, Chow N, Castellino FJ, Stojanovic K, Cleveland DW, Zlokovic BV. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J Clin Invest. 2009;119:3437–3449. doi: 10.1172/JCI38476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zlokovic BV, Griffin JH. Cytoprotective protein C pathways and implications for stroke and neurological disorders. Trends Neurosci. 2011;34:198–209. doi: 10.1016/j.tins.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]