Abstract
Leptin is a peptide hormone secreted by adipocytes. It has been shown to modulate production and clearance of amyloid beta (Aβ) in rodent models. We sought to determine if serum leptin was associated with cognitive decline in the elderly.
We studied 2871 well-functioning elders, aged 70–79, who were enrolled in a prospective study. Serum leptin concentrations were measured at baseline and analyzed by mean ± 1 SD. Clinically significantly cognitive decline over 4 years was defined as ≥ 5 point drop on the Modified Mini Mental State Exam (3MS).
Compared to those in the lower leptin groups, elders in the high leptin group had less cognitive decline, 20.5% vs. 24.7% (OR=0.79 95% CI 0.61–1.02, p=0.07). After adjustment for demographic and clinical variables, including body mass index and total percent body fat, those in the high leptin group had significantly less likelihood of cognitive decline, OR=0.66 (95% CI 0.48–0.91).
We conclude that in elderly individuals, higher serum leptin appears to protect against cognitive decline, independent of comorbidites and body fat.
Keywords: Leptin, cognition, learning, memory, elderly, aging, dementia, Alzheimer’s disease, obesity, hyperleptinemia, leptin resistance, metabolic syndrome
1. Introduction
Leptin is a protein, secreted predominantly by adipocytes, that regulates appetite, energy balance, and neuroendocrine function. It has also been implicated in bone and brain development (Harvey, 2003). A growing body of research suggests that leptin may play a role in learning and cognition.
Leptin receptors and mRNA are widely expressed in the human brain, including the hippocampus and neocortex (Funahashi et al., 2003). In animal models, leptin facilitates learning, spatial memory and long term potentiation (Li et al., 2002), and has been shown to enhance NMDA receptor function and modulate synaptic plasticity in the hippocampus (Shanley et al., 2001). Recent work by Fewlass et al. (2004) reveals that leptin may be linked to Alzheimer’s disease (AD) through modulation of Aβ production and clearance. Leptin was found to reduce production of Aβ, apparently through a reduction in β-secretase activity, as well as to increase apolipoprotein E (ApoE)-mediated clearance of Aβ fibrils. Most interestingly, leptin administered to AD transgenic mice led to a significant reduction in total brain Aβ load.
Aging is associated with declining serum leptin levels, independent of body mass index (BMI), as well as with the development of leptin resistance (Isidori et al., 2000; Ma et al., 2002; Nogalska et al., 2003; Scarpace et al., 2001; Wang et al., 2001). In case-controlled studies, patients with AD have lower leptin levels than controls, also independent of body mass index (Power et al., 2001). Weight loss is a common feature of AD, and is found to occur before the onset of dementia (Barrett-Connor et al., 1998), suggesting an underlying metabolic abnormality in the pathogenesis of AD.
The present study sought to determine the relationship between serum leptin level and cognition in humans, as there have been no studies to date addressing this directly. Given the compelling findings potentially linking leptin to AD pathogenesis, our hypothesis was that higher leptin would be associated with less cognitive decline.
2. Methods
2.1 Study population
Participants were part of the Health, Aging and Body Composition (Health ABC) Study, a prospective cohort study, beginning in 1997, of 3075 community-dwelling elders then aged 70–79 years old and living in Memphis, TN or Pittsburgh, PA. To identify potential participants, a random sample of white and all the black Medicare-eligible elders within designated zip code areas were contacted. To be eligible for the study, participants had to report no difficulties with walking a quarter of a mile or climbing 10 steps without resting, and no difficulty with mobility-associated activities of daily living. They also had to be free of life-threatening cancers and could not be intending to move out of the study area for at least 3 years. All elders participating in the study signed an informed written consent, approved by the institutional review boards at the clinical sites. This study was approved by the University of California San Francisco Committee of Human Research.
Of the 3075 Health ABC participants, 204 were missing serum leptin concentration data at baseline, leaving 2871 participants in our analytic cohort. Those without leptin data tended to be older, of Black race, and have higher BMI at baseline.
2.2 Measurements
The Modified Mini-Mental State Examination (3MS) was administered to all participants during the baseline visit and repeated at the Year 3 and 5 follow-up visits. It is a brief, general cognitive battery with components for orientation, concentration, language, praxis, and immediate and delayed memory (Teng and Chui, 1987). The maximum (best) score is 100. Cognitive decline was defined as a decline of 5 or more points at either follow-up visit as has been previously recommended (Yaffe et al., 2003).
Venous blood samples were collected in the morning from fasting subjects at baseline, and leptin concentrations were measured in duplicate using the Sensitive Human Leptin RIA Kit (product number SHL-81K) from Linco Research, Inc. (St. Charles, MO). The assay is a competitive radioimmunoassy in which the concentration of leptin is determined by competition with 125I-Human Leptin, with a maximum detectable leptin level of 50ng/ml. The intra-assay CV is 3.7 – 7.5 % and the inter-assay CV is 3.2 – 8.9%.
Potential covariates included variables previously shown in the literature to be associated with cognitive function or leptin levels. Participant age, race, gender, and whether or not they achieved a high-school level of education were self-reported at baseline. BMI was calculated by dividing a direct weight measurement (in kilograms) by the squared average of at least two height measurements (in millimeters converted to meters). Total body mass (total weight) and body composition were measured at baseline by using fan-beam DXA (QDR4500A) with DXA software (version 8.21) (both: Hologic, Bedford, MA), allowing for determination of percent body fat. The presence of diabetes mellitus and hypertension, and history of myocardial infarction (MI), stroke or transient ischemic attack (TIA) were determined using a combination of self-reported physician diagnoses, clinic data and medication use. The number of days that people spent in the hospital in the five-year period prior to baseline was included as an indication of health status, and determined from Medicare records. Depressive symptoms were assessed with the Center for Epidemiologic Studies-Depression Scale (CES-D), with higher scores indicating greater number of symptoms and a score ≥ 16 consistent with possible depression (Orme et al., 1986). Apolipoprotein-E (APOE) genotype was analyzed using standard techniques and coded as APOE ε4 or no ε4 (Hixson and Vernier, 1990).
2.3 Statistical analyses
We coded participants with less than one standard deviation (SD) below the mean level as having low leptin level, those within one SD of the mean as having medium leptin level, and those with more than one SD above the mean as having high leptin level. We tested for trend associations between leptin group and baseline characteristics using linear regression for those that were normally distributed, and an extension of the Wilcoxon rank sum test (Cuzick, 1985) for all other characteristics.
To test for an association between leptin group and development of cognitive decline, we used logistic regression models. Leptin values were divided into three categories, low, medium, and high, based on the mean ± 1 SD of the log transformed leptin values. Multivariate logistic regression models were used to adjust for characteristics shown to be associated with leptin and cognitive decline in bivariate analyses (p<0.05). These were age, race, gender, education, hypertension, diabetes and MI history. In addition, we adjusted for baseline cognitive score. Because we were interested in examining how BMI and percent body fat may mediate the relationship with leptin and cognitive decline, we added these two variables to the multivariate logistic analyses in the fully adjusted model.
3. Results
The mean age of the study population was 73.7 years (SD = 2.9 years); 40.6% were black and 51% were women. The mean serum leptin level for the study population was 13.0 ng/ml (SD= 10.6 ng/ml). The lowest leptin group was composed of 479 individuals with a mean leptin level of 2.3 ng/ml (SD = 1.0 ng/ml) and range of 0–3.7 ng/ml. The middle group was composed of 1915 individuals with a mean leptin level of 10.9 ng/ml (SD = 5.2 ng/ml) and range of 3.7–22.8 ng/ml. The highest group was composed of 476 individuals, and had a mean leptin level of 32.3 ng/ml (SD = 8.0 ng/ml) and range of 22.8–54.7 ng/ml.
Bivariate analysis revealed that several demographic and clinical factors varied significantly among the leptin groups (Table 1). In general, higher leptin was associated with younger age (p<0.01), black race (p<0.01), female gender (p<0.01), less education (p<0.01), greater BMI (p<0.01) and total percent body fat (p<0–.01), and the presence of hypertension (p<0.01), diabetes (p=0.02), and a prior MI (p<0.01). ApoE ε4 carrier status, depression, a history of stroke or TIA, the number of days spent in the hospital during the 5 years prior to enrollment, and baseline 3MS score were not significantly associated with leptin group. At five years, 14% of those in the low leptin group, 8% of those in the middle leptin group, and 6% of those in the high leptin group had died.
Table 1.
Baseline characteristics of the Health ABC participants by leptin group
| Leptin Group | ||||
|---|---|---|---|---|
|
| ||||
| Characteristic | Low (n=479) | Middle (n=1915) | High (n=476) | P-Value* |
|
| ||||
| Age (mean, SD) | 73.8 (2.9) | 73.7 (2.9) | 73.2 (2.8) | < 0.01 |
| Black (%) | 36.7 | 37.7 | 56.1 | < 0.01 |
| Female (%) | 16.1 | 47.3 | 89.5 | < 0.01 |
| Education ≤ high school (%) | 54.4 | 56.6 | 64.1 | < 0.01 |
| Depression score ≥ 16 (%) | 3.8 | 6.1 | 5.3 | 0.31 |
| Body mass index, kg/m2 (mean, SD) | 23.3 (3.1) | 27.0 (4.0) | 31.1 (4.8) | < 0.01 |
| Total % body fat (mean, SD) | 25.3 (4.6) | 34.6 (5.8) | 43.0 (4.9) | <0.01 |
| Hypertension (%) | 38.7 | 50.8 | 60.9 | < 0.01 |
| Diabetes (%) | 10.5 | 15.4 | 18.2 | 0.02 |
| Myocardial infarction history (%) | 15.0 | 12.1 | 7.6 | <0.01 |
| Stroke/TIA history (%) | 7.0 | 7.9 | 5.1 | 0.25 |
| Number of days in hospital (%) | ||||
| 0–7 | 84.3 | 82.8 | 84.2 | 0.59 |
| >7 | 15.7 | 17.2 | 15.8 | |
| ApoE ε4 Carrier (%) | 28.5 | 28.7 | 26.5 | 0.50 |
| Baseline 3MS score (mean, sd) | 92.2 (7.4) | 93.0 (6.3) | 92.7 (6.3) | 0.31 |
P-value for trend.
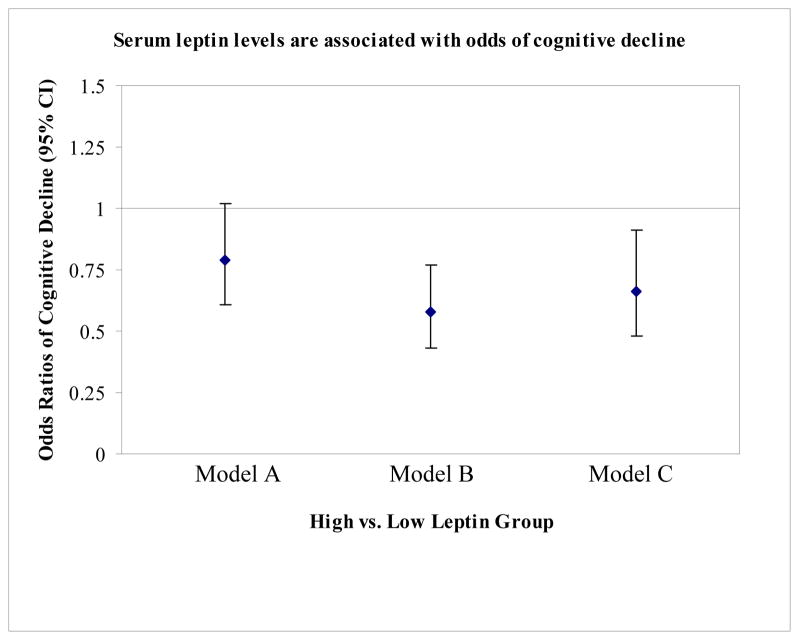
There was no difference in cognitive decline between the low and medium leptin groups, so these groups were combined to form a single low leptin group. Compared to those in the low leptin group, people in the high leptin group had less cognitive decline (20.5% vs. 24.7%, unadjusted OR=0.79 95% CI 0.61–1.02, p=0.07). After adjustment for age, race, gender, education, baseline cognitive score, hypertension, prior MI, diabetes and number of days spent in the hospital in the prior 5 years, those in the high leptin group had almost half the likelihood of developing cognitive decline than those in the lower group (OR=0.58; 95% CI 0.43–0.77) (Figure 1). When BMI and total percent body fat were added to the multivariate logistic regression, the association between leptin group and cognitive decline was attenuated slightly but remained statistically significant (OR=0.66 95% CI 0.48–0.91) (Figure 1). We tested for interactions between gender and leptin and between race and leptin on risk of cognitive decline but did not find statistically significant interactions.
Figure 1.
Serum leptin levels are associated with odds of cognitive decline: Model A is unadjusted; Model B is adjusted for race, gender, education, baseline cognitive score, hypertension, prior MI, diabetes, and number of days spent in the hospital in the prior 5 years; and Model C is adjusted for BMI and percent body fat in addition to all of the factors in Model B.
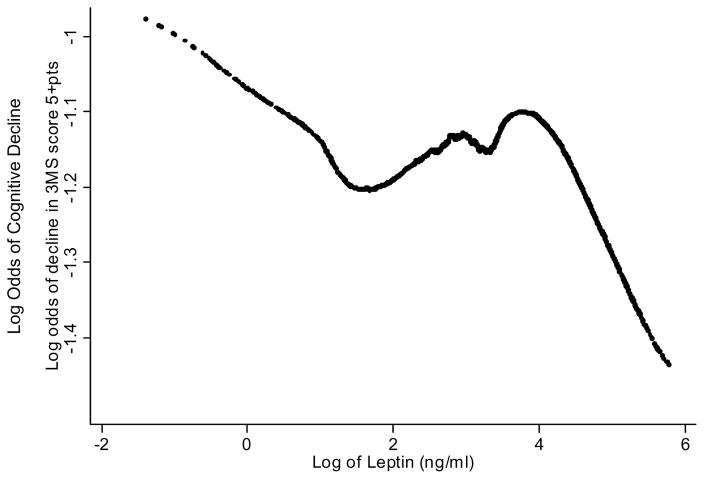
A lowess plot of leptin level versus log odds of cognitive decline confirmed that it was at the extremes of leptin distribution that the relationship between leptin and cognition took on distinct patterns, and that there was no clear threshold for the association between leptin and cognition (Figure 2).
Figure 2.
A lowess plot of leptin level versus log odds of cognitive decline
4. Discussion
We examined the relationship between serum leptin level and cognitive decline in a large population of well-functioning, community-dwelling, elderly individuals. At five years, we observed less cognitive decline in individuals with high leptin versus low leptin levels. After adjustment for potential confounding factors, this association remained and was statistically significant, with the high leptin group nearly 50% less likely to develop cognitive decline compared to the low leptin group.
To our knowledge, there have been no studies to date examining leptin and cognition in humans. However, our findings are in line with the basic research literature, which generally supports a positive role for leptin in memory and learning processes in rodents. Molecular mechanisms implicated in this process include actions that modulate synaptic plasticity. Genetically obese rodents with dysfunctional leptin receptors have impaired hippocampal long-term potentiation (LTP) and long-term depression (LTD), and perform poorly on spatial memory tasks (Gerges et al., 2003; Li et al., 2002). Moreover, direct administration of leptin into the dentate gyrus enhances LTP in rats (Wayner et al., 2004), while direct leptin administration into the CA1 region of the hippocampus improves spatial memory and learning in mice (Farr et al., 2006). At the cellular level, leptin has been shown to enhance NMDA receptor function (Shanley et al., 2001), possibly through rapid trafficking of NMDA receptors to the plasma membrane (Harvey et al., 2006), in a manner analogous to insulin (Skeberdis et al., 2001). Thus leptin may enhance memory and learning through mechanisms that modulate synaptic plasticity in the hippocampus.
In addition to leptin’s role in modulating synaptic plasticity, recent work by Fewlass et al. (2004) demonstrates that leptin alters brain Aβ levels through modulation of ApoE-mediated uptake of Aβ fibrils. Moreover, leptin decreased the total brain Aβ load in rodents by up to 50%. These findings suggest that leptin levels may play a role in the pathogenesis of AD. As noted previously, patients with AD have been found to have lower serum leptin levels compared to controls, independent of BMI (Power et al., 2001). Interestingly, in a 20-year prospective study of 299 community-dwelling older adults, Barrett-Connor et al. (1998) found that weight loss precedes mild and moderate dementia, suggesting that early weight loss is unlikely to be a consequence of AD itself, and that AD may be linked to underlying metabolic abnormalities.
The potential link between leptin, nutrition and cognition was examined in a recent study by Dagon and others (Dagon et al., 2005), in which it was observed that leptin reverses the deleterious effects of severe diet restriction, modulating cognitive ability through AMPK signaling pathways. Indeed, leptin has been found to act as an anti-apoptotic hormone under stress conditions (Russo et al., 2004). A role for leptin in neurogenesis and neurodevelopment is strengthened by the observations of Udagaw and others (Udagawa et al., 2006) that leptin stimulates the proliferation of precursor cells committed to neuronal differentiation, and promotes neuronal terminal differentiation. Recent work by Guo et al (2007) suggests that leptin serves a neurotrophic function in the developing and adult hippocampus by enhancing mitochondrial resistance to apoptosis and excitoxicity. Further supporting leptin’s antiapoptic function, leptin has been shown to be neuroprotective against ischemic cell injury as well as dopaminergic cell death (Weng et al., 2007; Zhang et al., 2007). Leptin’s anorexic action in the hypothalamus has been shown to be enhanced by high systemic estrogen levels in rats (Clegg et al., 2006). The possibility that estrogen may potentiate leptin signaling in cognitive processes is particularly intriguing.
In our study, leptin levels were positively correlated with BMI and percent body fat, an unsurprising result consistent with previous work (Sinha and Caro, 1998). This raises the question that low leptin might simply reflect a state of undernourishment or comorbid medical illness, and that it is these latter conditions that are impacting cognitive performance. However, when BMI and percent body fat were included in our multivariate analysis, the association of cognitive decline with leptin levels remained statistically significant, suggesting that additional factors may play a role in the association of leptin and cognition. Similarly, our results remained significant after adjusting for medical comorbidities.
In this population, both diabetes and hypertension were more prevalent in individuals with high serum leptin levels. In prior studies, insulin resistance, diabetes, and the metabolic syndrome have been associated with elevated leptin levels, independent of fat indices (Armellini et al., 2000; Li et al., 2004; Wauters et al., 2003; Zamboni et al., 2004; Zoico et al., 2004). The direct effects of leptin on glucose metabolism and insulin signaling have not yet been fully elucidated. However, in vivo studies have shown that leptin improves insulin sensitivity (Ebihara et al., 2001; Lin et al., 2002; Ogawa et al., 1999; Yamauchi et al., 2001) and normalize glucose metabolism in rodents (Chinookoswong et al., 1999; Masuzaki et al., 1999). Transgenic expression of neuron-specific leptin receptor in receptor-deficient mice leads to amelioration of diabetes (de Luca et al., 2005). Additionally, leptin has been shown to have an antidiabetic function through control of intracellular fatty acid metabolism, maintenance of glucose sensitivity, and prevention of islet lipotoxicity (Shimabukuro et al., 1997; Shimokawa and Higami, 2001; Unger, 2005). Peripheral insulin resistance and hyperleptinemia are associated with leptin resistance and reduced expression of leptin receptor mRNA in aging rats (Fernandez-Galaz et al., 2001). Obesity and aging are also associated with hyperleptinemia and leptin resistance (Chu et al., 2001; Ma et al., 2002). Considering this constellation of findings, it would appear that the association of diabetes and hyperleptinemia is likely a function of some degree of leptin resistance, but we have no ability to confirm this with our data. It is interesting to note that recent work has linked diabetes to mild cognitive impairment (Arvanitakis et al., 2006; Bent et al., 2000; Gregg et al., 2000; Nguyen et al., 2002; Yaffe et al., 2004; Yaffe et al., 2006). Indeed, after adjustment for the presence of diabetes, the association between high serum leptin level and less cognitive decline becomes more robust, arguing that diabetes functions as a negative confounder in this study. This also suggests that leptin resistance may play a role in the cognitive impairment noted in diabetes.
Our study has several limitations, including an inability to measure possible leptin resistance in our study subjects. Recent work has linked leptin resistance to both reduced leptin transport across the blood brain barrier as well as reduced leptin signaling (Arch, 2005; Banks et al., 2004; Banks and Farrell, 2003; Feng, 2006; Steinberg et al., 2006). However, the presence of leptin resistance in our study population would most likely serve to negatively confound our analysis, masking an even more robust association between higher leptin levels and reduced likelihood of cognitive decline. Another limitation of this study is the lack of clinical evaluation of the type of cognitive decline measured by the 3MS, which does not provide a complete assessment of all cognitive domains. Also, although we adjusted for age, race, gender, education, several medical comorbidities, and both percent body fat and BMI, we cannot rule out the presence of residual confounding.
5. Conclusion
This study is the first population-based study examining the association between serum leptin level and cognitive decline in older persons. Our main finding, that individuals with high serum leptin level have reduced odds of cognitive decline, is in line with rodent models and cellular studies. Further work is needed to corroborate a definitive role for leptin in human cognition.
Acknowledgments
Dr. Yaffe is supported by the Paul Beeson Faculty Scholar in Aging Program and NIH grant R01 AG021918-01. This research was supported in part by the Intramural Research program of the NIA, National Institute of Aging, as well as NIA contracts N01-AG-6-2101, N01-AG-6-2103 and N01-AG-6-21.
Footnotes
Disclosure statement
The authors have no actual or potential conflicts of interest to disclose.
References
- Arch JR. Central regulation of energy balance: inputs, outputs and leptin resistance. Proc Nutr Soc. 2005;64:39–46. doi: 10.1079/pns2004407. [DOI] [PubMed] [Google Scholar]
- Armellini F, Zamboni M, Bosello O. Hormones and body composition in humans: clinical studies. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S18–21. doi: 10.1038/sj.ijo.0801270. [DOI] [PubMed] [Google Scholar]
- Arvanitakis Z, Wilson RS, Li Y, Aggarwal NT, Bennett DA. Diabetes and function in different cognitive systems in older individuals without dementia. Diabetes Care. 2006;29:560–565. doi: 10.2337/diacare.29.03.06.dc05-1901. [DOI] [PubMed] [Google Scholar]
- Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- Banks WA, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. Am J Physiol Endocrinol Metab. 2003;285:E10–15. doi: 10.1152/ajpendo.00468.2002. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Edelstein S, Corey-Bloom J, Wiederholt W. Weight loss precedes dementia in community-dwelling older adults. J Nutr Health Aging. 1998;2:113–114. [PubMed] [Google Scholar]
- Bent N, Rabbitt P, Metcalfe D. Diabetes mellitus and the rate of cognitive ageing. Br J Clin Psychol. 2000;39( Pt 4):349–362. doi: 10.1348/014466500163356. [DOI] [PubMed] [Google Scholar]
- Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes. 1999;48:1487–1492. doi: 10.2337/diabetes.48.7.1487. [DOI] [PubMed] [Google Scholar]
- Chu NF, Stampfer MJ, Spiegelman D, Rifai N, Hotamisligil GS, Rimm EB. Dietary and lifestyle factors in relation to plasma leptin concentrations among normal weight and overweight men. Int J Obes Relat Metab Disord. 2001;25:106–114. doi: 10.1038/sj.ijo.0801468. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- Dagon Y, Avraham Y, Magen I, Gertler A, Ben-Hur T, Berry EM. Nutritional status, cognition, and survival: a new role for leptin and AMP kinase. J Biol Chem. 2005;280:42142–42148. doi: 10.1074/jbc.M507607200. [DOI] [PubMed] [Google Scholar]
- de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara K, Ogawa Y, Masuzaki H, Shintani M, Miyanaga F, Aizawa-Abe M, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Gavrilova O, Reitman ML, Nakao K. Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes. 2001;50:1440–1448. doi: 10.2337/diabetes.50.6.1440. [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420–1425. doi: 10.1016/j.peptides.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Feng GS. Shp2 as a therapeutic target for leptin resistance and obesity. Expert Opin Ther Targets. 2006;10:135–142. doi: 10.1517/14728222.10.1.135. [DOI] [PubMed] [Google Scholar]
- Fernandez-Galaz C, Fernandez-Agullo T, Campoy F, Arribas C, Gallardo N, Andres A, Ros M, Carrascosa JM. Decreased leptin uptake in hypothalamic nuclei with ageing in Wistar rats. J Endocrinol. 2001;171:23–32. doi: 10.1677/joe.0.1710023. [DOI] [PubMed] [Google Scholar]
- Funahashi H, Yada T, Suzuki R, Shioda S. Distribution, function, and properties of leptin receptors in the brain. Int Rev Cytol. 2003;224:1–27. doi: 10.1016/s0074-7696(05)24001-9. [DOI] [PubMed] [Google Scholar]
- Gerges NZ, Aleisa AM, Alkadhi KA. Impaired long-term potentiation in obese zucker rats: possible involvement of presynaptic mechanism. Neuroscience. 2003;120:535–539. doi: 10.1016/s0306-4522(03)00297-5. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Yaffe K, Cauley JA, Rolka DB, Blackwell TL, Narayan KM, Cummings SR. Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of Osteoporotic Fractures Research Group. Arch Intern Med. 2000;160:174–180. doi: 10.1001/archinte.160.2.174. [DOI] [PubMed] [Google Scholar]
- Harvey J. Leptin: a multifaceted hormone in the central nervous system. Mol Neurobiol. 2003;28:245–258. doi: 10.1385/MN:28:3:245. [DOI] [PubMed] [Google Scholar]
- Harvey J, Solovyova N, Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res. 2006;45:369–378. doi: 10.1016/j.plipres.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- Isidori AM, Strollo F, More M, Caprio M, Aversa A, Moretti C, Frajese G, Riondino G, Fabbri A. Leptin and aging: correlation with endocrine changes in male and female healthy adult populations of different body weights. J Clin Endocrinol Metab. 2000;85:1954–1962. doi: 10.1210/jcem.85.5.6572. [DOI] [PubMed] [Google Scholar]
- Li M, Wu CY, Zhan ZW, Li XG, Zhang K, Xiang HD. Leptin and clustering of the components of risk factors for metabolic syndrome. Zhonghua Yu Fang Yi Xue Za Zhi. 2004;38:226–230. [PubMed] [Google Scholar]
- Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–615. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Lin CY, Higginbotham DA, Judd RL, White BD. Central leptin increases insulin sensitivity in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2002;282:E1084–1091. doi: 10.1152/ajpendo.00489.2001. [DOI] [PubMed] [Google Scholar]
- Ma XH, Muzumdar R, Yang XM, Gabriely I, Berger R, Barzilai N. Aging is associated with resistance to effects of leptin on fat distribution and insulin action. J Gerontol A Biol Sci Med Sci. 2002;57:B225–231. doi: 10.1093/gerona/57.6.b225. [DOI] [PubMed] [Google Scholar]
- Masuzaki H, Ogawa Y, Aizawa-Abe M, Hosoda K, Suga J, Ebihara K, Satoh N, Iwai H, Inoue G, Nishimura H, Yoshimasa Y, Nakao K. Glucose metabolism and insulin sensitivity in transgenic mice overexpressing leptin with lethal yellow agouti mutation: usefulness of leptin for the treatment of obesity-associated diabetes. Diabetes. 1999;48:1615–1622. doi: 10.2337/diabetes.48.8.1615. [DOI] [PubMed] [Google Scholar]
- Nguyen HT, Black SA, Ray LA, Espino DV, Markides KS. Predictors of decline in MMSE scores among older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2002;57:M181–185. doi: 10.1093/gerona/57.3.m181. [DOI] [PubMed] [Google Scholar]
- Nogalska A, Pankiewicz A, Goyke E, Swierczynski J. The age-related inverse relationship between ob and lipogenic enzymes genes expression in rat white adipose tissue. Exp Gerontol. 2003;38:415–422. doi: 10.1016/s0531-5565(02)00210-3. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Masuzaki H, Hosoda K, Aizawa-Abe M, Suga J, Suda M, Ebihara K, Iwai H, Matsuoka N, Satoh N, Odaka H, Kasuga H, Fujisawa Y, Inoue G, Nishimura H, Yoshimasa Y, Nakao K. Increased glucose metabolism and insulin sensitivity in transgenic skinny mice overexpressing leptin. Diabetes. 1999;48:1822–1829. doi: 10.2337/diabetes.48.9.1822. [DOI] [PubMed] [Google Scholar]
- Orme JG, Reis J, Herz EJ. Factorial and discriminant validity of the Center for Epidemiological Studies Depression (CES-D) scale. J Clin Psychol. 1986;42:28–33. doi: 10.1002/1097-4679(198601)42:1<28::aid-jclp2270420104>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Power DA, Noel J, Collins R, O’Neill D. Circulating leptin levels and weight loss in Alzheimer’s disease patients. Dement Geriatr Cogn Disord. 2001;12:167–170. doi: 10.1159/000051252. [DOI] [PubMed] [Google Scholar]
- Russo VC, Metaxas S, Kobayashi K, Harris M, Werther GA. Antiapoptotic effects of leptin in human neuroblastoma cells. Endocrinology. 2004;145:4103–4112. doi: 10.1210/en.2003-1767. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Tumer N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience. 2001;104:1111–1117. doi: 10.1016/s0306-4522(01)00142-7. [DOI] [PubMed] [Google Scholar]
- Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21:RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro M, Koyama K, Chen G, Wang MY, Trieu F, Lee Y, Newgard CB, Unger RH. Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc Natl Acad Sci U S A. 1997;94:4637–4641. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa I, Higami Y. Leptin signaling and aging: insight from caloric restriction. Mech Ageing Dev. 2001;122:1511–1519. doi: 10.1016/s0047-6374(01)00284-6. [DOI] [PubMed] [Google Scholar]
- Sinha MK, Caro JF. Clinical aspects of leptin. Vitam Horm. 1998;54:1–30. doi: 10.1016/s0083-6729(08)60919-x. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV. Insulin promotes rapid delivery of N-methyl-D-aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci U S A. 2001;98:3561–3566. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg GR, McAinch AJ, Chen MB, O’Brien PE, Dixon JB, Cameron-Smith D, Kemp BE. The suppressor of cytokine signalling 3 (SOCS3) inhibits leptin activation of AMP-kinase in cultured skeletal muscle of obese humans. J Clin Endocrinol Metab. 2006 doi: 10.1210/jc.2006-0638. [DOI] [PubMed] [Google Scholar]
- Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- Udagawa J, Hashimoto R, Suzuki H, Hatta T, Sotomaru Y, Hioki K, Kagohashi Y, Nomura T, Minami Y, Otani H. The role of leptin in the development of the cerebral cortex in mouse embryos. Endocrinology. 2006;147:647–658. doi: 10.1210/en.2005-0791. [DOI] [PubMed] [Google Scholar]
- Unger RH. Longevity, lipotoxicity and leptin: the adipocyte defense against feasting and famine. Biochimie. 2005;87:57–64. doi: 10.1016/j.biochi.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Wang ZW, Pan WT, Lee Y, Kakuma T, Zhou YT, Unger RH. The role of leptin resistance in the lipid abnormalities of aging. Faseb J. 2001;15:108–114. doi: 10.1096/fj.00-0310com. [DOI] [PubMed] [Google Scholar]
- Wauters M, Considine RV, Yudkin JS, Peiffer F, De Leeuw I, Van Gaal LF. Leptin levels in type 2 diabetes: associations with measures of insulin resistance and insulin secretion. Horm Metab Res. 2003;35:92–96. doi: 10.1055/s-2003-39054. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Armstrong DL, Phelix CF, Oomura Y. Orexin-A (Hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo. Peptides. 2004;25:991–996. doi: 10.1016/j.peptides.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Weng Z, Signore AP, Gao Y, Wang S, Zhang F, Hastings T, Yin XM, Chen J. Leptin protects against 6-hydroxydopamine-induced dopaminergic cell death via mitogen-activated protein kinase signaling. J Biol Chem. 2007 doi: 10.1074/jbc.M705426200. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Blackwell T, Whitmer RA, Krueger K, Barrett Connor E. Glycosylated Hemoglobin Level and Development of Mild Cognitive Impairment or Dementia in OlderWomen. J Nutr Health Aging. 2006;10:293–295. [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Zamboni M, Zoico E, Fantin F, Panourgia MP, Di Francesco V, Tosoni P, Solerte B, Vettor R, Bosello O. Relation between leptin and the metabolic syndrome in elderly women. J Gerontol A Biol Sci Med Sci. 2004;59:396–400. doi: 10.1093/gerona/59.4.m396. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang S, Signore AP, Chen J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke; a journal of cerebral circulation. 2007;38:2329–2336. doi: 10.1161/STROKEAHA.107.482786. [DOI] [PubMed] [Google Scholar]
- Zoico E, Di Francesco V, Mazzali G, Vettor R, Fantin F, Bissoli L, Guariento S, Bosello O, Zamboni M. Adipocytokines, fat distribution, and insulin resistance in elderly men and women. J Gerontol A Biol Sci Med Sci. 2004;59:M935–939. doi: 10.1093/gerona/59.9.m935. [DOI] [PubMed] [Google Scholar]