Abstract
Background
Subcutaneous allergen-specific immunotherapy is a standard route for the immunotherapy of allergic diseases. It modulates the course of allergy and can generate long-term remission. However, subcutaneous allergen-specific immunotherapy can also induce anaphylaxis in some patients, and therefore additional routes of administration should be investigated to improve the safety and tolerability of immunotherapy.
Objective
We sought to determine whether epicutaneous treatment with antigen in the presence of a Toll-like receptor 9 agonist can suppress TH2-mediated responses in an antigen-specific manner.
Methods
Epicutaneous immunization was performed by applying a skin patch soaked with ovalbumin (OVA) plus CpG, and its suppressor activity was determined by using the mouse model of atopic dermatitis. Finally, adoptive cell transfers were implemented to characterize the regulatory cells that are induced by epicutaneous immunization.
Results
Epicutaneous immunization with OVA and CpG reduces the production of OVA-specific IgE and increases the synthesis of OVA-specific IgG2a antibodies in an antigen-specific manner. Moreover, eosinophil peroxidase activity in the skin and production of IL-4, IL-5, IL-10, and IL-13 are suppressed. The observed reduction of IgE synthesis is transferable with T-cell receptor (TCR) αβ+CD4+CD25− cells, whereas IgG2a production is dependent on both TCRαβ+ and TCRγδ+ T cells. Further experiments show that the described phenomenon is myeloid differentiation primary response 88, IFN-γ, and IL-17A dependent. Finally, the results suggest that epicutaneous immunization with OVA and CpG decreases the synthesis of OVA-specific IgE and skin eosinophil peroxidase activity in mice with ongoing skin allergy.
Conclusion
Epicutaneous application of protein antigen in the presence of adjuvant could be an attractive needle-free and self-administered immunotherapy for allergic diseases.
Keywords: Epicutaneous immunization, atopic dermatitis, eosinophil peroxidase, IgE, IgG2a, Toll-like receptor 9, TH1, TH17
Allergic diseases develop as a consequence of immune responses to normally innocuous environmental antigens and now afflict approximately 25% of people in the developed world.1,2 It is believed that TH2 lymphocytes play a crucial role in these types of diseases. The phenotypes of TH2 cells are characterized by the coordinated production of IL-4, IL-5, and IL-13, which, in conjunction with other inflammatory mediators, direct the activation and tissue accumulation of eosinophils and basophils, as well as B-cell switching to pathogenic IgE.3
Allergy treatment includes allergen avoidance and pharmacotherapy. Commonly used symptomatic therapy of allergic diseases includes corticosteroids, antihistamines, and inhaled β2-adrenoreceptor agonists, which efficiently ameliorate IgE-mediated symptoms.2 However, this type of treatment does not treat disease per se because pharmacotherapy benefits are not sustained after treatment is discontinued. Thus numerous efforts have been made to develop a treatment able to control allergy-associated immune responses in a specific manner. Allergen immunotherapy induces symptomatic relief not only during treatment but also after its discontinuation.4
Allergen immunotherapy includes subcutaneous allergen-specific immunotherapy and sublingual immunotherapy. It is believed that subcutaneous or sublingual immunization with an antigen favors production of TH1 cytokines, such as IFN-γ, over TH2 cytokines and activates IL-10– and TGF-β–producing regulatory T (Treg) cells.5 Further efforts to find safer and more effective allergen immunotherapy have led scientists to study alternative routes of immunization. Both animal and clinical studies showed the effectiveness of intralymphatic administration of allergens (ie, intralymphatic immunotherapy [ILIT]) in allergic patients. It has been shown that ILIT strengthens the efficacy of immunization, causing production of high levels of allergen-specific IgG2a antibodies. Moreover, ILIT increased production of IL-2, IFN-γ, IL-4, and IL-10 when compared with subcutaneous allergen-specific immunotherapy, suggesting that ILIT does not polarize the response toward TH1, TH2, or Treg cells but rather generates an overall stronger response.6,7
More recently, epicutaneous allergen-specific immunotherapy (EPIT) has been used as a novel, needle-free, and self-administered treatment method.8 EPIT has shown encouraging results for inhaled and food allergies, but the mechanism of the observed symptom alleviation is currently unknown.6
At the end of the last millennium, Wang et al9 showed that epicutaneous application of the protein antigen ovalbumin (OVA) resulted in allergic dermatitis accompanied by the appearance of IL-4–secreting T lymphocytes.9 Additionally, Herrick et al10 showed that epicutaneous immunization with protein antigen induced a TH2-mediated model of asthma. These data suggested to us that, similar to mucosal immunization, deposition of protein antigens on the skin also might induce T cells producing anti-inflammatory cytokines that would suppress TH1-mediated responses.
Our work in TH1-mediated contact hypersensitivity showed that epicutaneous immunization of normal mice with different protein antigens applied on the skin in the form of a patch or cream induces a state of subsequent non–antigen-specific unresponsiveness caused by suppressor T cells that inhibit both sensitization and elicitation of effector T-cell responses.11 TGF-β plays a crucial role in the effector phase of skin-induced tolerance, whereas IL-4, IL-10, and TGF-β play an important role in the induction of epicutaneously induced suppression.12
Our further work then showed that epicutaneous exposure to protein antigen in the presence of Toll-like receptor (TLR) ligands reversed skin-induced suppression of contact hypersensitivity.13 The observed reversal of skin-induced suppression was found to be dependent on myeloid differentiation primary response gene 88 (MyD88), IL-12, and IFN-γ.14 These findings prompted us to investigate whether epicutaneous immunization with OVA in the presence of TLR ligands could inhibit TH2-dependent allergic responses.
In the current study we have used a mouse model of atopic dermatitis (AD) to demonstrate that epicutaneous immunization with OVA and TLR9 ligand CpG reduces the production of OVA-specific IgE and increases the synthesis of OVA-specific IgG2a antibodies.
METHODS
A description of the materials and methods used in this study can be found in the Methods section in this article’s Online Repository at www.jacionline.org.
RESULTS
Epicutaneous immunization with OVA and TLR9 ligand CpG modulates production of OVA-specific antibodies and reduces eosinophil peroxidase activity in the skin
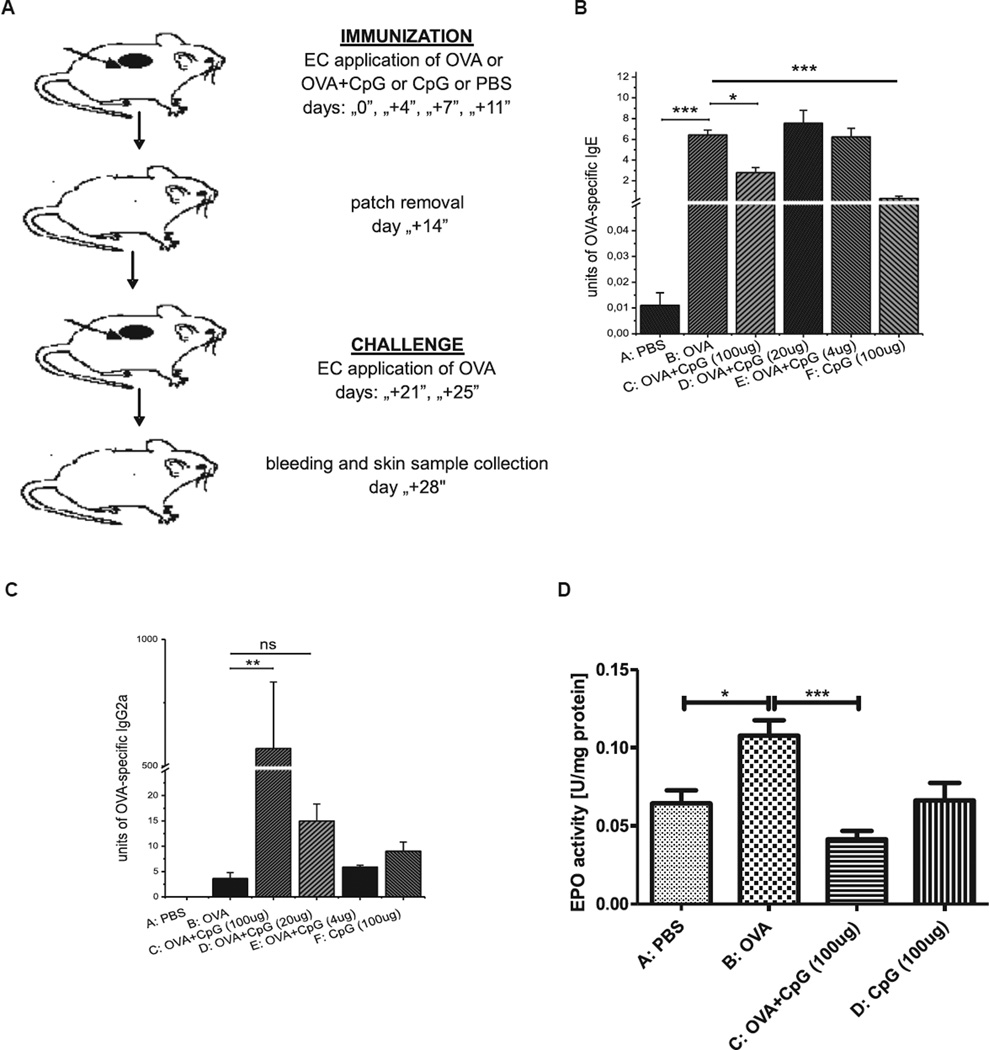
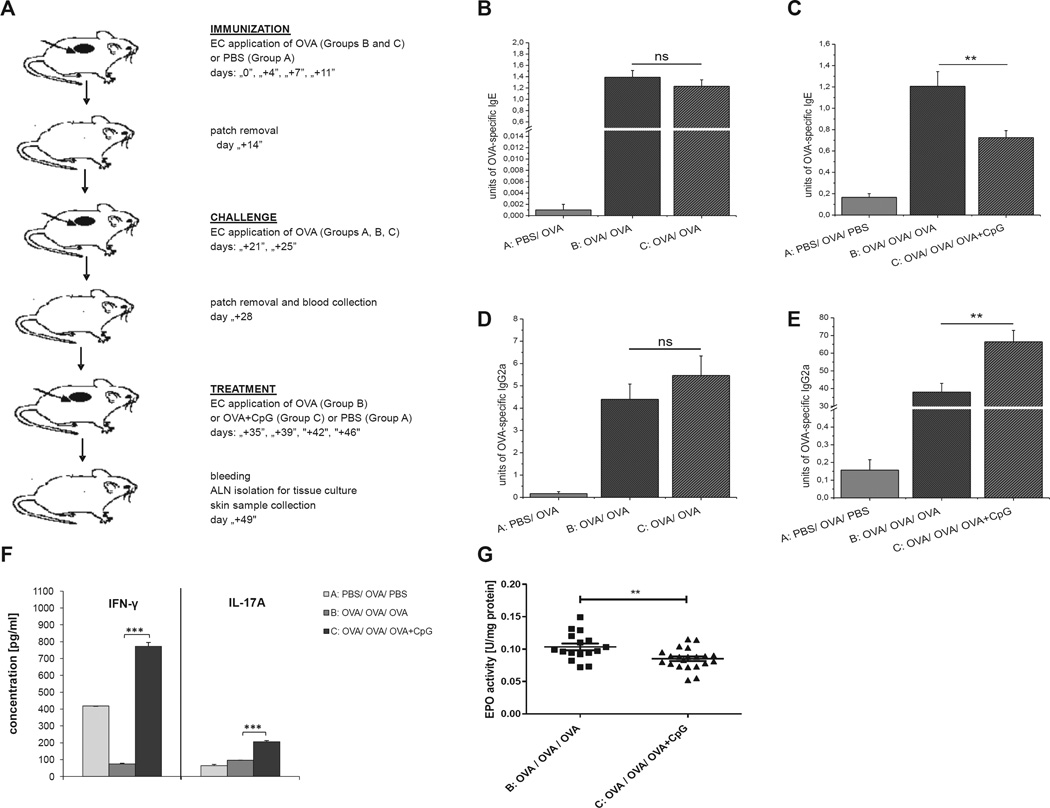
BALB/c mice were epicutaneously immunized and challenged, as described in the Methods section in this article’s Online Repository and Fig 1, A, to test the influence of epicutaneous immunization with OVA plus CpG on the production of OVA-specific IgE and IgG2a antibodies, as well as on eosinophil peroxidase (EPO) activity in the skin.
FIG 1.
Study design. BALB/c mice were epicutaneously (EC) immunized for 2 weeks with OVA alone or OVA plus CpG or CpG alone or PBS. A, After a 1-week interval, all animals were challenged with OVA for 1 week before bleeding and skin sample collection. B, Reduction of OVA-specific IgE production in mice EC immunized with OVA and CpG. C, Increase of OVA-specific IgG2a secretion in mice EC immunized with OVA plus CpG. D, Inhibition of EPO activity in skin homogenates from mice EC immunized with OVA and CpG. Fig 1, B and C, n = 3–5; Fig 1, D, n = 8–20. ns, Not significant. *P < .05, **P < .01, and ***P < .001.
The data presented in Fig 1, B and C, show that epicutaneous immunization with OVA plus CpG (group C) and subsequent challenge with OVA significantly decreased production of OVA-specific IgE antibodies and increased production of OVA-specific IgG2a antibodies when compared with that in mice in the positive control group (group B). We also observed that regulation of antibody production by epicutaneous immunization depends on the CpG dose. The significant suppression of OVA-specific IgE (Fig 1, B, group C vs B) and upregulation of OVA-specific IgG2a antibody production was observed only when 100 µg of CpG per animal was used. Additionally, Fig 1, D, shows that epicutaneous treatment with OVA and CpG before OVA challenge significantly reduced EPO activity in the skin when compared with that in mice treated with OVA alone before challenge (group C vs B). Data presented in Fig E1 in this article’s Online Repository at www.jacionline.org show CpG concentration in mouse serum after OVA plus CpG immunization measured at 3 different time points. We found no significant difference in CpG concentrations between mice treated with OVA plus CpG (group C) and animals patched with OVA alone (group B), suggesting minimal systemic absorption of CpG during patching.
Epicutaneous immunization with OVA and CpG induces antigen-specific cells that reduce IgE production
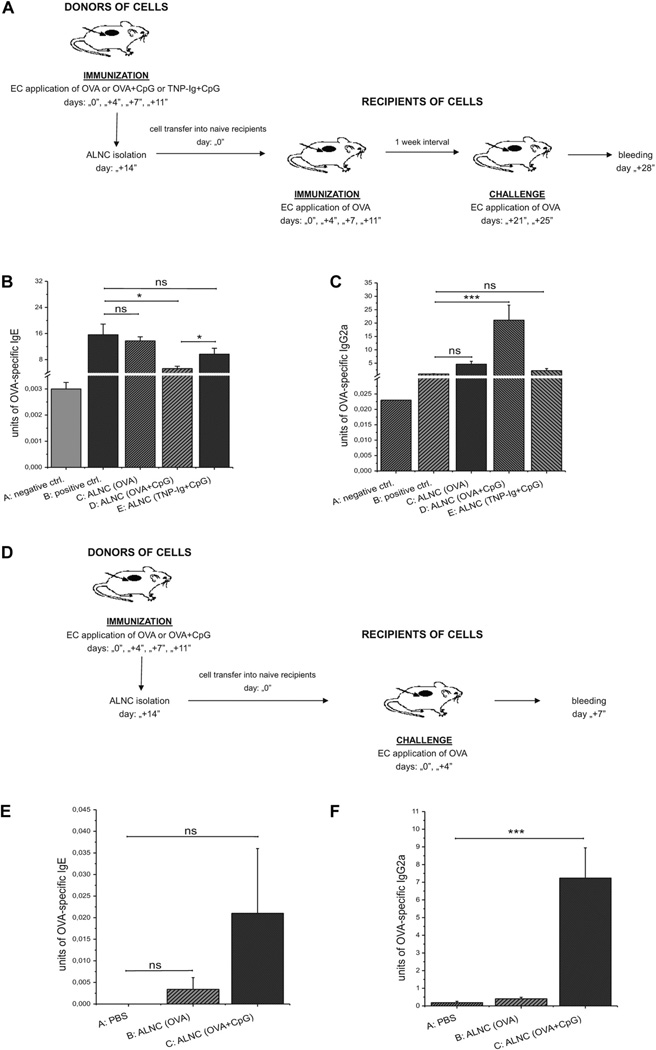
To determine whether the observed regulation of antibody production can be transferred with lymphoid cells, we used a “transfer-in” protocol whereby axillary and inguinal lymph node cells (ALNCs) isolated from donors patched with OVA plus CpG (group D), 2,4,6-trinitrophenyl–conjugated mouse immunoglobulin (TNP-Ig) plus CpG (group E), or OVA alone (Group C) were injected into naive recipients that then were epicutaneously immunized and challenged with OVA (Fig 2, A). In these experiments the positive control group consisted of mice that were immunized and challenged with OVA but did not receive any cells (group B).
FIG 2.
A–C, Transfer of ALNCs from donors immunized with OVA alone, OVA plus CpG, or TNP-Ig plus CpG into recipients subsequently immunized and challenge with OVA (Fig 2, A) and their effects on production of OVA-specific IgE (Fig 2, B) and IgG2a (Fig 2, C). D–F, Transfer of ALNCs from donors immunized with OVA alone or OVA and CpG into recipients subsequently challenged with OVA (Fig 2, D) and secretion of OVA-specific IgE (Fig 2, E) and IgG2a (Fig 2, F). Fig 2, B and C, n = 4–5; Fig 2, D and E, n = 6–8. ns, Not significant. *P < .05 and ***P < .001. EC, Epicutaneous.
We found that transfer of ALNCs derived from mice immunized with OVA and CpG reduces the production of OVA-specific IgE antibodies (Fig 2, B, group D vs B) and increases the synthesis of OVA-specific IgG2a antibodies (Fig 2, C, group D vs B) in recipients that then were immunized and challenged with OVA. This effect was not observed when mice received transfer of ALNCs from donors patched with either OVA alone or TNP-Ig plus CpG (Fig 2, B and C, groups C and E, respectively).
In addition, we also observed that ALNCs isolated from mice immunized either with OVA or OVA plus CpG (Fig 2, D) did not cause a significant production of OVA-specific IgE antibodies when transferred to naive recipients that were only challenged with OVA (Fig 2, E, groups B and C vs group A). However, injection of ALNCs isolated from donors immunized with OVA plus CpG caused a significant increase of OVA-specific IgG2a production in OVA-challenged recipients (Fig 2, F, group C vs A). These data strongly suggest that epicutaneous treatment with OVA plus CpG induces antigen-specific cells that reduce IgE production and promote IgG2a synthesis. Additionally, data presented in Fig E2 in this article’s Online Repository at www.jacionline.org show that only ALNCs from donors patched with OVA and CpG could regulate production of OVA-specific IgE (see Fig E2, B, group C vs B) and IgG2a (see Fig E2, C, group C vs B), whereas ALNCs from donors treated with CpG alone had no effect on antibody production (see Fig E2, B and C, group D vs B).
Epicutaneous immunization with OVA plus CpG induces T-cell receptor αβ+CD4+CD25− lymphocytes that reduce IgE production
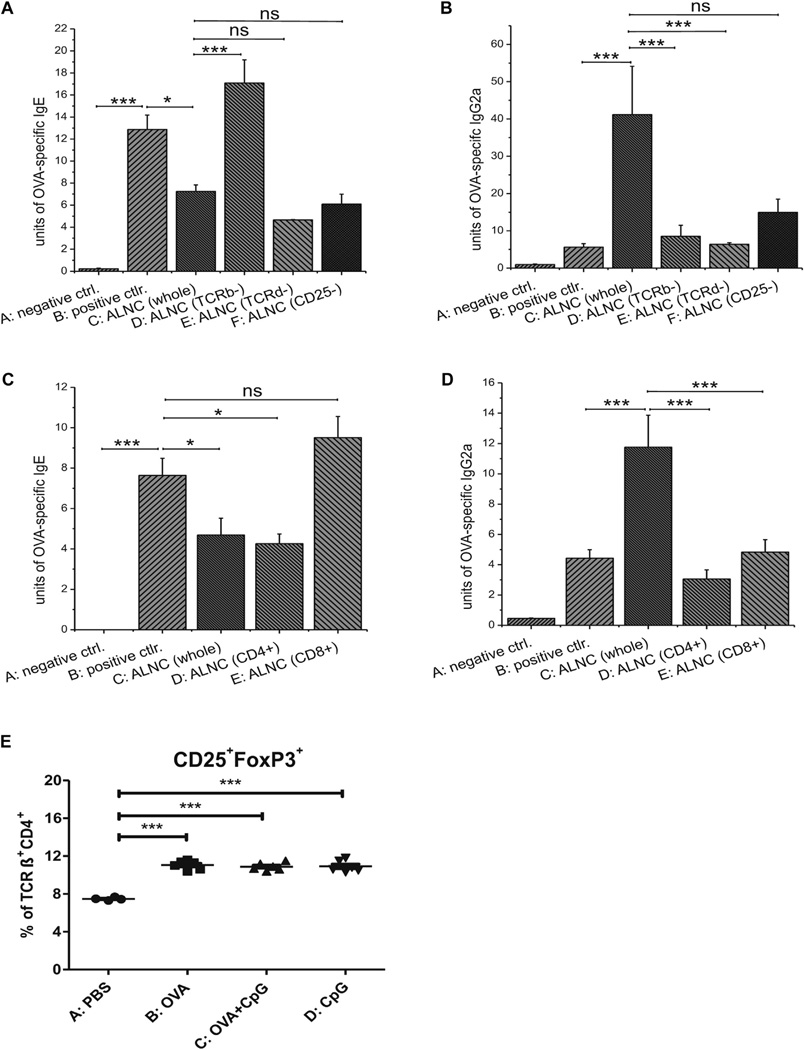
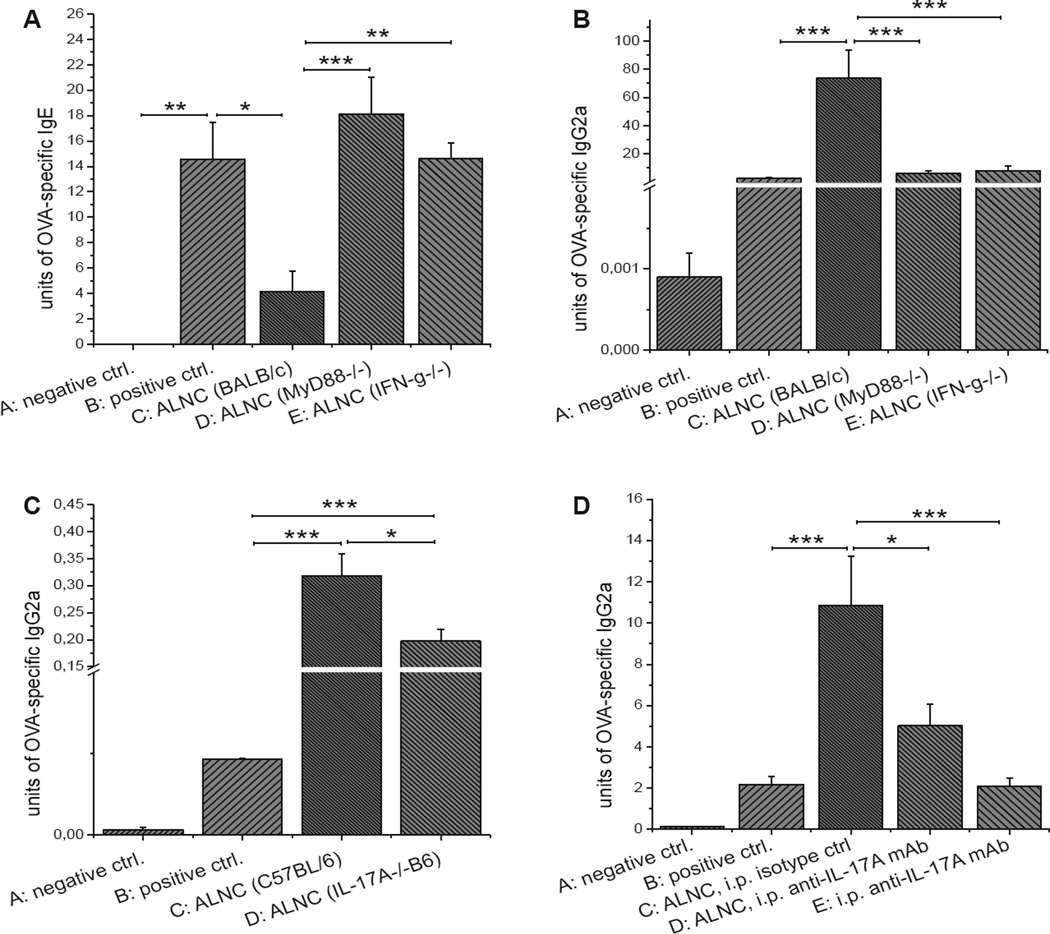
We found that ALNCs depleted of T-cell receptor (TCR) αβ+ lymphocytes lost their ability to suppress production of OVA-specific IgE antibodies (Fig 3, A, group D vs C). This was not observed when either TCRγδ+ or CD25+ cells were depleted from ALNCs (groups E and F, respectively). When analyzing for IgG2a production, we found that ALNCs depleted of either TCRαβ+ or TCRγδ+ T cells could no longer support production of OVA-specific IgG2a antibodies (Fig 3, B, groups D and E vs C). In contrast, removal of CD25+ cells reduced IgG2a production, but this was not significant when compared with whole ALNCs (Fig 3, B, group F vs C).
FIG 3.
A–D, Transfer of unseparated ALNCs or depleted or sorted cell populations isolated from donors epicutaneously (EC) immunized with OVA and CpG and their effects on production of OVA-specific IgE (Fig 3, A and C) and IgG2a (Fig 3, B and D) in recipients subsequently immunized and challenged with OVA. E, Percentage of Treg cells in mice EC treated with PBS, OVA, OVA and CpG, or CpG alone for 2 weeks before axillary and inguinal lymph node (ALN) isolation. Fig 3, A and B, n = 4–5; Fig 3, C and D, n = 7–10; Fig 3, E, n = 4–6. ns, Not significant. *P < .05 and ***P < .001.
CD4+ and CD8+ T cells were purified by means of positive selection from ALNCs isolated from animals epicutaneously immunized with OVA and CpG to determine the role of CD4+ and CD8+ T cells in the epicutaneously induced regulation of antibody response. We found that CD4+ T cells reduce production of OVA-specific IgE antibodies (Fig 3, C, group D vs B), whereas the CD8+ T-cell population did not have this effect (Fig 3, C, group E vs B). Moreover, the adoptive transfer of CD4+ and CD8+ cells separately could no longer support the production of OVA-specific IgG2a antibodies (Fig 3, D, groups D and E vs C), suggesting that both populations are involved in the synthesis of OVA-specific IgG2a antibodies in this model. Finally, fluorescence-activated cell sorting (FACS) analysis of ALNCs showed no difference in the proportion of TCRαβ+CD4+CD25+ forkhead box P3 (FoxP3)+ Treg cells between mice epicutaneously immunized with OVA alone or OVA plus CpG (Fig 3, E, and see Fig E3 in this article’s Online Repository at www.jacionline.org).
Epicutaneous immunization with OVA and CpG reduces production of TH2 cytokines and increases in vitro production of TH1/TH17 cytokines
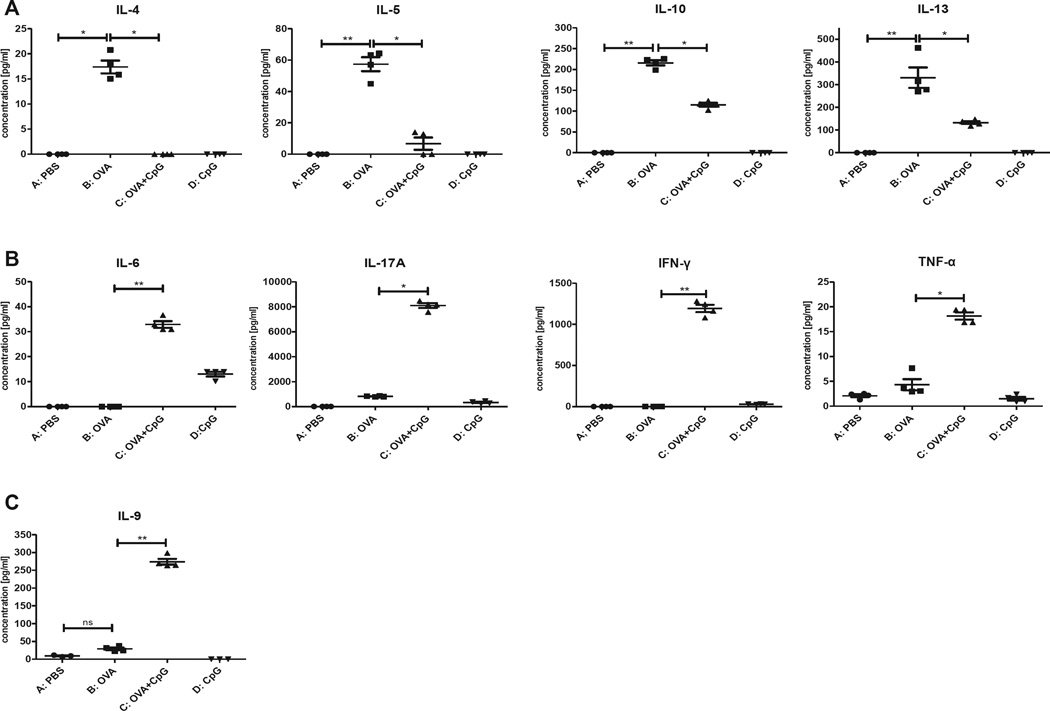
To investigate whether cytokines are involved in the regulation of IgE and IgG2a during epicutaneous immunization with OVA and CpG, we harvested ALNCs from immunized and control mice and cultured them in the presence of OVA for 48 hours. We found that mice epicutaneously immunized with OVA and CpG for 2 weeks showed suppressed IL-4, IL-5, IL-10, and IL-13 production (Fig 4, A, group C vs B) but with no difference in TGF-β production between the groups (data not shown). In addition, the data presented in Fig 4, B, show that epicutaneous immunization with OVA and CpG for 2 weeks resulted in increased production of IL-6, IL-17A, IFN-γ, and TNF-α (group C vs B). Moreover, we also found that IL-9 levels were increased after epicutaneous immunization with OVA and CpG relative to the control group (Fig 4, C, group C vs B). We found no significant differences in IL-12p70 production between mice epicutaneously immunized with OVA and OVA plus CpG tested either 4 or 7 days after immunization (see Fig E4 in this article’s Online Repository at www.jacionline.org).
FIG 4.
Cytokine production by ALNCs isolated from mice epicutaneously (EC) immunized with PBS, OVA, OVA and CpG, or CpG alone for 2 weeks before culture in the presence of OVA. IL-4, IL-5, IL-10, IL-13 (A), IL-6, IL-17A, IFN-γ, TNF-α (B), IL-9 (C). n = 4. *P < .05 and **P < .01.
Suppression of IgE production after epicutaneous immunization with OVA and CpG is MyD88 and IFN-γ dependent
The data presented in Fig 4 suggest that TH1/TH17-type cytokines might be involved in the regulation of antibody production in our epicutaneous immunization model. To further elucidate this pathway, we purified ALNCs from OVA and CpG epicutaneously immunized mice deficient in MyD88 or IFN-γ, respectively, and transferred them into recipients before immunization and challenge with OVA. We found that OVA-specific IgE was not suppressed in mice transferred with ALNCs deficient in MyD88 and IFN-γ (Fig 5, A, groups D and E vs B), suggesting that the reduction of IgE production in OVA and CpG-immunized mice is dependent on both IFN-γ and MyD88. In contrast, there was reduced synthesis of OVA-specific IgG2a in mice transferred with MyD88- and IFN-γ–deficient ALNCs in comparison with mice treated with wild-type ALNCs (Fig 5, B, groups D and E vs C). Therefore our results suggest that the increased synthesis of anti-OVA IgG2a in our model is also dependent on MyD88 and IFN-γ.
FIG 5.
A and B, Transfer of ALNCs from BALB/c, MyD88−/−, or IFN-γ−/− mice epicutaneously (EC) treated with OVA and CpG and its effect on production of OVA-specific IgE (Fig 5, A) and IgG2a (Fig 5, B) in recipients that underwent subsequent EC immunization and challenge with OVA. C and D, Role of IL-17A in OVA-specific IgG2a production: transfer of ALNCs from C57BL/6 or IL-17A−/− B6 donors EC immunized with OVA and CpG (Fig 5, C) and neutralization of IL-17A in recipients receiving ALNCs from BALB/c mice EC treated with OVA and CpG (Fig 5, D). Fig 5, A and B, n = 4–5; Fig 5, C, n = 7; Fig 5, D, n = 6–11. i.p ., Intraperitoneal. *P < .05, **P < .01, and ***P < .001.
We also assessed the role of IL-17A in the production of OVA-specific IgG2a. The transfer of ALNCs from IL-17A−/−B6 donors epicutaneously immunized with OVA and CpG only partially supported IgG2a production in OVA-immunized and challenged recipients (Fig 5, C, group D vs C). Moreover, a similar observation was made when recipients of ALNCs isolated from BALB/c mice epicutaneously treated with OVA and CpG were treated with anti–IL-17A neutralizing antibody (Fig 5, D, group D vs C). Therefore our data suggest that IL-17Ais a key cytokine involved in switching to IgG2a in our model.
Immunization with OVA plus CpG increases the number of IFN-γ– and IL-17A–producing cells in ALNCs
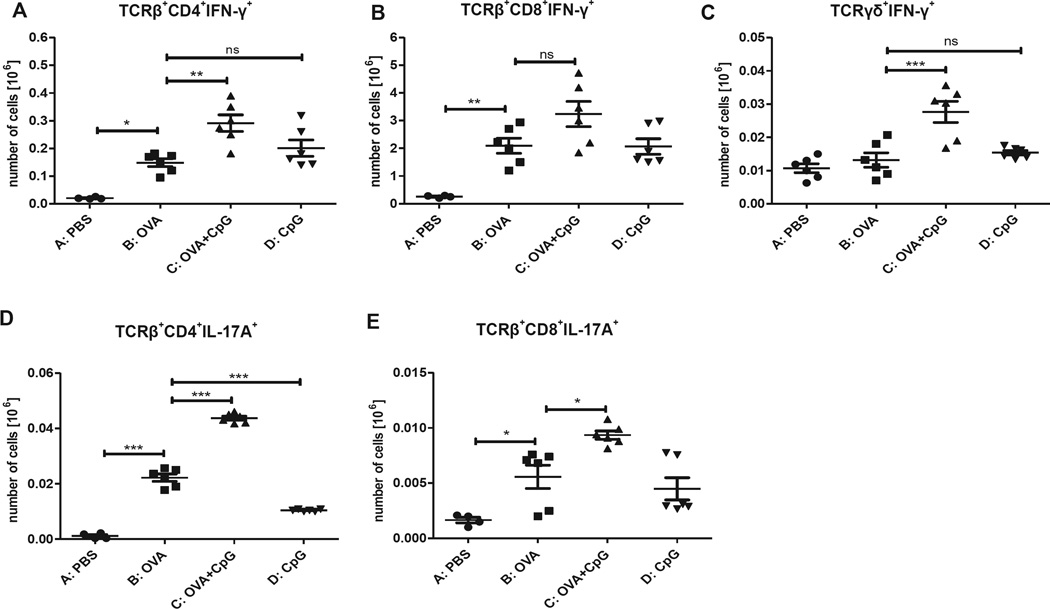
The data presented in Fig 3 show that TCRαβ+CD4+CD25− lymphocytes are involved in negative regulation of OVA-specific IgE antibodies, whereas upregulated production of OVA-specific IgG2a antibodies was mediated by TCRαβ+CD4+, TCRαβ+CD8+, and TCRγδ+ cells. Additionally, analysis of culture supernatants for cytokine production suggests that IFN-γ and IL-17A might be potential regulators of antibody production in this model. Different T-cell populations were stained for the presence of these cytokines and analyzed by means of flow cytometry to determine which cell population is responsible for IFN-γ and IL-17A production. Data presented in Fig 6 show that epicutaneous immunization with OVA plus CpG results in significantly increased absolute numbers of TCRαβ+CD4+IFN-γ+ (Fig 6, A) and TCRγδ+IFN-γ+ (Fig 6, C) lymphocytes. The increase in numbers of TCRαβ+CD8+IFN-γ+ (Fig 6, B) cells was not significant. Additionally, patching with OVA and CpG produces higher numbers of TCRαβ+CD4+IL-17A+ (Fig 6, D) and TCRαβ+CD8+IL-17A+ (Fig 6, E) cells.
FIG 6.
Intracellular staining of ALNCs isolated from mice epicutaneously (EC) immunized with PBS, OVA, OVA and CpG, or CpG alone for 2 weeks before FACS analysis. Absolute numbers of TCRβ+CD4+IFN-γ+ (A), TCRβ+CD8+IFN-γ+ (B), TCRγδ+IFN-γ+ (C), TCRβ+CD4+IL-17A+ (D), TCRβ+CD8+IL-17A+ (E) cells are shown. Results are expressed as means ± SEs (n = 4–6). ns, Not significant. *P < .05, **P < .01, and ***P < .001.
Transfer of cells induced by means of epicutaneous immunization with OVA plus CpG before either immunization or challenge reduces IgE production
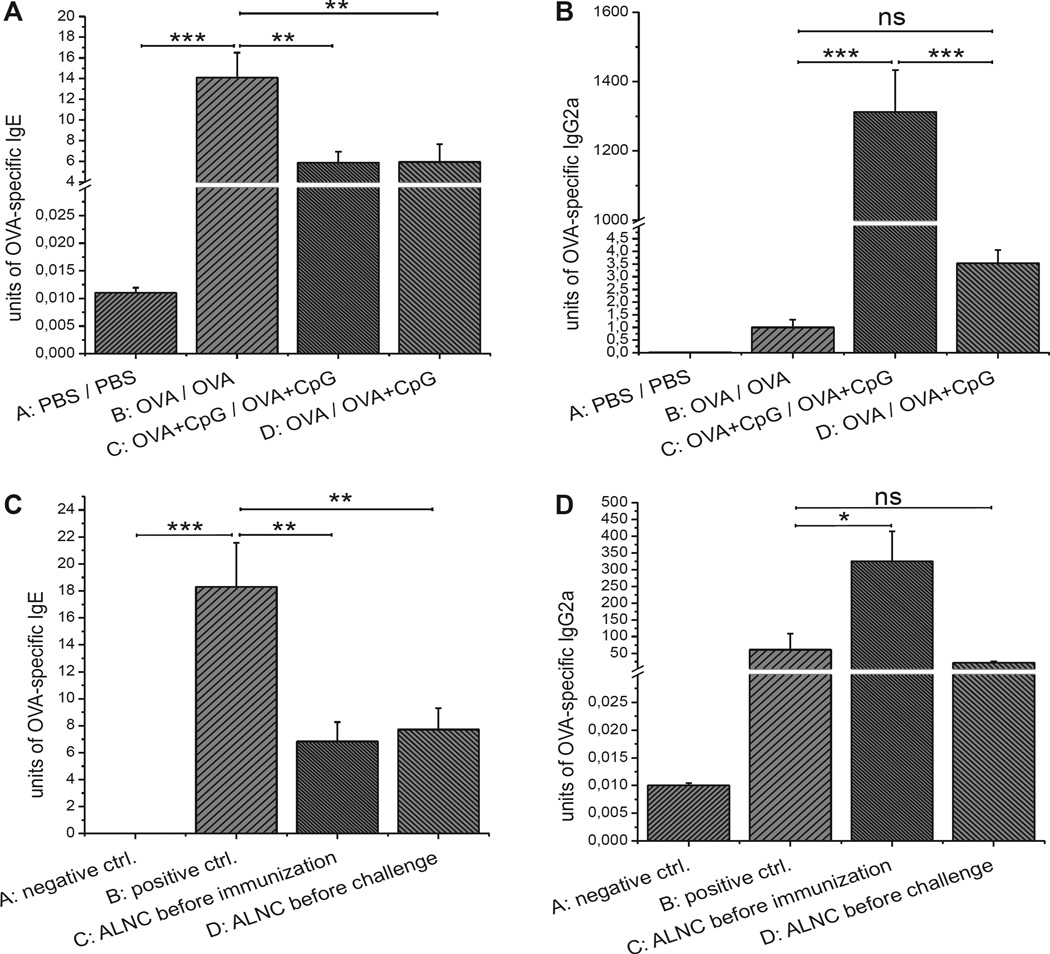
The aim of the current experiment was to investigate whether application of OVA in the presence of CpG can modify an ongoing OVA-specific TH2 response in vivo. Mice were epicutaneously immunized with OVA alone (group B) or OVA plus CpG (group C) for 2 weeks or with OVA alone for 1 week and then with OVA plus CpG for the following week (group D). Data presented in Fig 7, A, show that epicutaneous immunization with OVA plus CpG strongly reduces production of OVA-specific IgE antibodies when compared with the positive control (group C vs B). Moreover, mice epicutaneously immunized with OVA and then epicutaneously treated with OVA plus CpG produce less OVA-specific IgE antibodies when compared with the positive control (group D vs B). Furthermore, Fig 7, B, shows that epicutaneous treatment with OVA plus CpG after previous skin application of OVA alone still enhances production of OVA-specific IgG2a, but the difference is not significant when compared with the positive control (groups D vs B).
FIG 7.
A and B, OVA-specific IgE (Fig 7, A) and IgG2a (Fig 7, B) production in mice epicutaneously (EC) treated with OVA and CpG after EC immunization with OVA alone. C and D, OVA-specific IgE (Fig 7, C) and IgG2a (Fig 7, D) secretion in recipients injected with regulatory ALNCs before immunization or before challenge with OVA. Fig 7, A and B, n = 6–8; Fig 7, C and D, n = 4–5. ns, Not significant. *P < .05, **P < .01, and ***P < .001.
ALNCs isolated from mice epicutaneously immunized with OVA plus CpG were transferred into recipients on the day of epicutaneous immunization with OVA (day 0) or just before epicutaneous challenge (day +21) to determine at what stage of the TH2 response regulation occurs (Fig 7, C and Fig D, groups C and D, respectively). Data presented in Fig 7, C, show that cells transferred either on the day of immunization or on the day of challenge could significantly reduce production of OVA-specific IgE antibodies when compared with the positive control (groups C and D vs B). Additionally, Fig 7, D, shows that transfer of ALNCs on the day of epicutaneous immunization with OVA significantly increased production of OVA-specific IgG2a antibodies (group C vs B), whereas transfer of ALNCs on the day of challenge did not have such an effect (group D vs B).
Epicutaneous treatment with OVA plus CpG can modulate production of OVA-specific antibodies and reduce skin EPO activity in mice with an ongoing TH2-mediated reaction in the skin
BALB/c mice were epicutaneously immunized and challenged according to the protocol described in the Methods section in this article’s Online Repository and Fig 8, A, to test whether the switch from OVA to OVA plus CpG can suppress IgE production and reduce skin EPO activity in mice with ongoing AD. Data presented in Fig 8, B, show that epicutaneous immunization and challenge with OVA results in production of OVA-specific IgE antibodies (groups B and C vs A). Subsequent patching with OVA plus CpG caused significant reduction of OVA-specific IgE antibodies compared with subsequent patching with OVA alone (Fig 8, C, group C vs B). Additionally, we found that the switch from OVA to OVA plus CpG in previously OVA-immunized and challenged mice significantly increased production of OVA-specific IgG2a antibodies (Fig 8, E, group C vs B) and increased production of IFN-γ and IL-17A by ALNCs (Fig 8, F, group C vs B). EPO activity was measured in skin homogenates to test whether a later switch from OVA to OVA plus CpG alleviates skin pathology. Data presented in Fig 8, G, show that the switch from OVA to OVA plus CpG results in decreased EPO activity in skin homogenates, which is consistent with reduced eosinophil skin infiltration (group C vs B).
FIG 8.
Study design. A, Epicutaneous (EC) treatment with OVA and CpG after EC immunization and challenge with OVA alone. B and C, OVA-specific IgE production before (Fig 8, B) and after (Fig 8, C) EC treatment with OVA plus CpG. D and E, OVA-specific IgG2a secretion before (Fig 8, D) and after (Fig 8, E) EC treatment with OVA plus CpG. F and G, Increase of IFN-γ and IL-17A production by ALNCs (Fig 8, F) and decrease of EPO activity in the skin (Fig 8, G) isolated from mice EC treated with OVA and CpG after immunization and challenge with OVA. n = 11–21. ns, Not significant. **P < .01 and ***P < .001.
DISCUSSION
It has been shown previously that the skin is an attractive immunization route to both induce9,10,15 and manipulate16 the immune response. Moreover, it has been demonstrated that epicutaneous immunization with OVA can be used as a model to investigate allergic dermatitis and TH2-mediated asthma in mice.17 Our earlier studies have demonstrated that epicutaneous immunization with TNP-Ig in the presence of TLR ligands shifts the immune response from TH2 to TH1 in a mouse model of contact hypersensitivity.13,14 Therefore we theorize that epicutaneous immunization with OVA and a TLR agonist might reduce TH2-mediated immune responses in an animal model of AD. In this study we have confirmed our hypothesis and demonstrated that epicutaneous immunization with OVA and the TLR9 agonist CpG before challenge with OVA reduces the production of OVA-specific IgE antibodies and strongly upregulates the synthesis of OVA-specific IgG2a. Moreover, epicutaneous application of OVA and CpG decreases the EPO activity in skin homogenates, which is consistent with reduced eosinophil skin infiltration.18 Our results are in line with the work of others showing that parenteral or mucosal exposure to antigen and CpG inhibits TH2-mediated allergy in mice.19–21 A more recent double-blind, placebo-controlled, phase II clinical trial demonstrates that subcutaneous injection of ragweed pollen antigen conjugated to a phosphorothioate oligodeoxyribodinucleotide immunostimulatory sequence of DNA containing a CpG motif suppresses the seasonal increase in antigen-specific IgE antibody.22
Although these studies are promising, it should be noted that injections containing CpG can cause several adverse reactions. The primary adverse symptoms are dose-dependent local injection reactions (eg, erythema, pain, swelling, induration, and pruritus) or systemic flu-like reactions (eg, headache, rigors, myalgia, pyrexia, nausea, and vomiting).23 Thus the approach of epicutaneous immunizations might be a safer and alternative mode of treatment because it is likely to avoid the adverse effects of other forms of administration, especially systemic reactions observed after CpG injections. In our study we find that there is no significant difference in CpG concentrations in serum between mice epicutaneously treated with OVA plus CpG compared with those treated with OVA alone, suggesting minimal systemic absorption of CpG and a likely lower risk of undesirable side effects.
Our adoptive transfer experiments address the question of whether epicutaneous treatment with OVA and CpG induces cells that shift the immune response from TH2 to TH1 or induces regulatory cells that modulate the skin immune response. The data presented in Fig 2 show that transfer of ALNCs from donors epicutaneously treated with OVA and CpG to naive recipients before OVA patch immunization and challenge reduces production of OVA-specific IgE antibodies and increases synthesis of OVA-specific IgG2a antibodies. Transfer of ALNCs from donors immunized with OVA and CpG to naive recipients who were challenged but not immunized does not affect IgE production but does increase IgG2a synthesis. Therefore we believe that epicutaneous immunization with OVA and CpG shifts the response from TH2 to TH1 rather than induces regulatory cells. Using 2 non–cross-reacting antigens, OVA and TNP-Ig, we show that the observed regulation of humoral response is antigen specific. Furthermore, ALNC transfer experiments with depleted cell populations show that TCRαβ+CD4+CD25− lymphocytes induced through epicutaneous immunization with OVA and CpG are involved in the suppression of OVA-specific IgE production and might belong to the population of TH1 lymphocytes that reduce TH2-mediated immunity and support IgG2a antibody production. This observation is supported by our data showing that epicutaneous immunization with OVA and CpG results in increased production of IFN-γ. We also find that epicutaneous exposure to CpG results in IL-12 production, which is consistent with data published by Hemmi et al.24 Production of IL-12 after epicutaneous immunization with OVA and CpG is higher than that observed after treatment with OVA alone, but the difference between groups was not significant. In addition, we find that treatment with OVA and CpG increases the absolute number of TCRαβ+CD4+IFN-γ+ cells within axillary and inguinal lymph nodes.
Our findings are in line with those of previous reports showing that CpG promotes the development of a strong TH1-mediated adaptive immune response.25 The observed suppression of IgE production in mice patched with OVA and CpG correlates with reduced production of the TH2 cytokines IL-4, IL-5, and IL-13, which supports our data suggesting a shift from a TH2 to TH1 response.
It has been shown previously that CpG stimulation, apart from inducing TH1 immune responses, could also be involved in Treg cell induction.26 However, our experiments did not show significant differences in the percentages of TCRαβ+ CD4+CD25+FoxP3+ Treg cells between animals treated with OVA, OVA plus CpG, or CpG alone. In addition, depletion of CD25+ T cells did not affect the suppression of IgE production by transferred ALNCs isolated from donors patched with OVA and CpG. Thus these 2 experiments suggest that TCRαβ+ CD4+CD25+FoxP3+ Treg cells are not involved in negative regulation of OVA-specific IgE production.
On the other hand, the mechanism of increased production of OVA-specific IgG2a antibodies is more complex because isotype switching toward IgG2a is supported by both TCRαβ+ and TCRγδ+ cells. Our findings that TCRγδ+ cells are involved in the production of OVA-specific IgG2a is supported by our FACS data showing that patching with OVA and CpG increases the number of IFN-γ–producing TCRγδ lymphocytes in axillary and inguinal lymph nodes, which is consistent with previous studies showing that IFN-γ+TCRγδ cells support antibody responses.27 Furthermore, our adoptive transfer experiments showed that both CD4+ and CD8+ cell populations are required to upregulate production of OVA-specific IgG2a antibodies. Our results are in line with previous reports showing that CpG, apart from induction of CD4+ T cells, can induce CD8+ T cells.25 Our results also show that epicutaneous immunization with OVA and CpG increases the number of TCRαβ+CD4+IFN-γ+ but not TCRαβ+CD8+IFN-γ+ lymphocytes, suggesting that IFN-γ–producing TCRαβ+CD4+ cells are involved in the suppression of IgE production and support of IgG2a synthesis. Finally, the transfer experiment using IFN-γ−/− and MyD88−/− donor mice in our system confirms the involvement of IFN-γ and MyD88 in IgE/IgG2a regulation.
Our further experiments show that epicutaneous immunization with OVA and CpG increases TNF-α, IL-6, and IL-17A production by ALNCs. These data suggest that increased production of IL-6 and TNF-α together with TGF-β might promote TH17 cell development.28 Indeed, we find that patching with OVA and CpG increases the numbers of both TCRαβ+CD4+IL-17A+ and TCRαβ+CD8+IL-17A+ lymphocytes, suggesting that both populations might be involved in regulation of IgG2a production. This is supported by experiments with IL-17A−/− mice and IL-17A neutralizing antibodies. Our results showing the involvement of IL-17A in IgG2a production are in agreement with those of previous reports, which found that this cytokine drives isotype class-switching to IgG2a and IgG3 subtypes.29 In addition, it is also possible that IL-17A, apart from promoting IgG2a production, might also be involved in suppression of IgE production. Our findings are supported by observations in patients with AD, in whom there is a significant negative correlation between serum IgE levels and the percentage of IFN-γ−IL-17+ lymphocytes.30
It has been proposed that the ideal adjuvant for EPIT should shift the immune response toward TH1-type immunity, as well as induce IL-10–producing Treg cells and allergen-specific IgG2a antibodies.31 Our work shows that epicutaneous immunization with OVA and CpG shifts the immune response to a TH1/TH17 response, suppresses TH2-type cytokine release, reduces OVA-specific IgE production and EPO activity in the skin, and increases the synthesis of OVA-specific IgG2a antibodies. However, this approach does not increase the percentage of Treg cells or IL-10 levels.
A plethora of publications over the past 2 decades have demonstrated that IL-9–producing TH9 lymphocytes promote the development of allergic and autoimmune diseases.32 Our results show that epicutaneous immunization with OVA and CpG results in increased IL-9 production. Our data are in line with previous findings showing that patching with OVA and various pathogen-associated molecular patterns induces varying amounts of IL-9. However, there is no observed correlation between TH2 cytokine levels and IL-9 concentration.33
From a clinical point of view, the treatment of an ongoing inflammatory response is more important and realistic than prevention. Our approaches using an active immunization model and an adoptive transfer model test whether it is possible to control ongoing TH2-medated immune responses. The active immunization model proves that epicutaneous immunization with OVA plus CpG after 1 week’s exposure to OVA alone could reduce the production of OVA-specific IgE and upregulate the synthesis of OVA-specific IgG2a antibodies. Finally, transfer of ALNCs isolated from donors epicutaneously treated with OVA and CpG either before OVA immunization or challenge significantly suppresses IgE production. However, increased production of IgG2a is only observed when ALNCs are transferred before OVA immunization but do not affect IgG2a synthesis when transferred just before challenge. These data suggest that cells induced through epicutaneous immunization with OVA and CpG can immediately reduce TH2-mediated immune responses, whereas induction of TH1/TH17-dependent IgG2a production requires more time. Finally, we show the effectiveness of epicutaneous immunization with OVA and CpG in the treatment of ongoing AD. We find that epicutaneous treatment with OVA and CpG decreases the synthesis of OVA-specific IgE and increases the production of OVA-specific IgG2a in mice with ongoing skin allergy. The observed changes in antibody production correlate with lower EPO activity in the skin of animals treated with OVA plus CpG after AD development.
In summary, epicutaneous immunization with OVA and CpG shifts the immune response toward aTH1/TH17 phenotype, reduces production of TH2-type cytokines, suppresses secretion of antigen-specific IgE, and increases synthesis of antigen-specific IgG2a antibodies. Noninvasive immunization through epicutaneous application of protein antigen in the presence of adjuvant could be an attractive therapeutic method to control unwanted TH2-mediated immune responses in patients with allergic disease.
METHODS
Mice
Female 6- to 8-week-old BALB/c mice were either purchased from the Jackson Laboratory (Bar Harbor, Me) or obtained from the breeding unit of the Department of Medical Biology, Jagiellonian University, College of Medicine. MyD88−/− mice on a BALB/c (H2d) background were a kind gift of Dr D. R. Goldstein from the Section of Cardiovascular Medicine, Yale University School of Medicine (New Haven, Conn). IFN-γ−/− mice on a BALB/c (H2d) background were from Taconic (New York, NY) or the Jackson Laboratory. IL-17A−/− mice on a C57BL/6 (H2b) background were kindly provided by Dr R. A. Flavell, whereas control animals were purchased from the Jackson Laboratory. Mice were fed autoclaved food and water. All experiments were conducted according to guidelines of both the Jagiellonian University College of Medicine and Yale University School of Medicine. All experiments were carried out 2 to 4 times.
Reagents
OVA (grade V) was purchased from Sigma (St Louis, Mo). Low-tox rabbit complement (Pel-Freeze Biologicals, Brown Deer, Wis) was obtained from the manufacturer. The CpG oligodeoxynucleotide TCCATGACGTTCCT GACGTT was from the Keck Oligonucleotide Synthesis Facility at Yale University. Mouse immunoglobulins were prepared from CBA/J mouse sera and conjugated with 2,4,6-trinitrophenyl (TNP) hapten.E1,E2 A single preparation of TNP-Ig conjugate with approximately 40 TNP molecules per immunoglobulin (TNP40-Ig) was used throughout the study.
mAbs and hybridomas
The following hybridomas were cultured: anti-TCRβ (clone H57-597) from Dr R. Kubo (Cytel, La Jolla, Calif) and anti-TCRδ (clone UC7-13D5) from Dr J. Bluestone, University of California, San Francisco. The culture supernatants were purified on Protein A, as described previously.E3 In addition, purified rat anti-mouse CD25 mAb (clone 3C7), anti-mouse IL-17A mAb (clone TC11-18H10), and rat IgG1 isotype control were purchased from BD PharMingen (San Diego, Calif).
Epicutaneous immunization with OVA and TLR ligands
Epicutaneous immunization of mice was performed according to the method described by Spergel et alE4 and Wang et alE5 with our modifications. Briefly, immunization was performed by applying a 1-cm2 gauze patch soaked with a solution containing 100 µg of OVA and 100 µg of CpG in a volume of 100 µL of PBS to the shaved skin of the mouse dorsum on day 0. Mice in control groups were patched with OVA alone or CpG without antigen or PBS alone. The patches were secured by adhesive tape wrapped around the midsection. The patches were left in place from day 0 until day+4, when fresh patches were replaced and kept to day +7. The refreshed patches were then replaced on day+7 and kept until day+11 and replaced once again with fresh patches that were kept until day+14. On day+14, the patches were removed, and the mice were rested for 1 week. The animals were then epicutaneously challenged by patching with 100 µg of OVA in sterile PBS on day +21. Four days later (day+25), patches were replaced with fresh ones and left until day +28. After that, patches were removed, mice were bled, and sera were used to measure OVA-specific IgE and IgG2a levels. In addition, skin punches were collected and homogenized, and EPO activity was measured in tissue homogenates. The scheme of immunization and challenge is described in Fig 1, A.
Adoptive cell transfer of regulatory cells (transfer in protocol)
The scheme of the cell transfer is presented in Fig 2, A. Briefly, donors of regulatory cells were epicutaneously immunized with OVA plus CpG for 2 weeks, as previously described. Axillary and inguinal lymph nodes were harvested on day +14, and a single-cell suspension was prepared (ALNC). The cells were washed and counted, and 5 × 107 ALNCs isolated from donors epicutaneously immunized with OVA plus CpG or OVA alone were injected intravenously into naive syngeneic recipients. The recipient mice were epicutaneously immunized with 100 µg of OVA for 2 weeks, rested for a week, and then challenged with 100 µg of OVA according to the scheme in Fig 2, A. Positive control mice did not receive any cell transfer, but they were epicutaneously immunized and then challenged with OVA. Before immunization and challenge with OVA, recipient mice were transferred with 5 × 107 ALNCs isolated from donors patched with OVA plus CpG or TNP-Ig plus CpG to determine the antigen specificity of regulatory cells induced through epicutaneous immunization. At the end of the experiment, mice were bled, and levels of OVA-specific IgE and IgG2a antibodies were measured in sera.
Phenotype of cells involved in regulation of humoral response
ALNCs (5 × 107) isolated from mice patched with 100 µg of OVA plus 100 µg of CpG were incubated in PBS on ice with purified anti-TCRβ, anti-TCRδ, or anti-CD25 mAbs (1 µg of antibody/106 cells) or PBS alone for 45 minutes to determine the phenotype of epicutaneously induced regulatory cells. Cells were then washed and incubated with a predetermined dilution of low-tox rabbit complement for 60 minutes at 37°C and then washed and resuspended in PBS. Afterward, aliquots of treated cells were transferred intravenously into naive recipients that were immunized and challenged with OVA, as described in Fig 2, A.
CD4+ and CD8+ T cells were isolated with mouse CD4+ or CD8+ cell isolation kits and separated on an LS column (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of the isolated cells was tested by using FACS and reached 98%. Then 3.3 × 106 CD4+ or 1.3 × 106 CD8+ lymphocytes or 5 × 107 unsorted T cells (whole ALNCs) were transferred intravenously into naive recipients that underwent epicutaneous immunization, followed by challenge with OVA to induce a TH2-mediated immune response (Fig 2, A).
IL-17A neutralization in vivo
ALNCs (5 × 107) isolated from BALB/c mice epicutaneously immunized with OVA plus CpG were transferred into naive BALB/c recipients that were treated intraperitoneally with isotype control or purified anti-mouse IL-17A mAb 1 day before and again 1 day after epicutaneous immunization with OVA, followed by challenge.
Epicutaneous treatment with OVA and CpG after epicutaneous immunization with OVA
To test whether an already induced TH2 response with OVA could be inhibited by immunization with OVA plus CpG, mice were patched with OVA alone for 1 week and, on day +7, were subsequently epicutaneously treated with OVA plus CpG for 7 days. After a 1-week break from treatment, animals were then challenged with OVA and tested for IgE and IgG2a production.
Epicutaneous treatment of mice with ongoing AD
To test whether the maneuver of epicutaneous immunization with OVA and CpG could treat ongoing disease in our model, we performed an experiment in which mice were patched with PBS alone or 100 µg of OVA for 2 weeks. On day +14, the patches were removed, and the mice were rested for 1 week. Then the animals were epicutaneously challenged by patching with 100 µg of OVA in sterile PBS for 1 week. Once the patches were removed, on day +28, blood samples were collected from all the mice, and sera were used for measurement of OVA-specific IgE and IgG2a antibodies. After that, the mice were rested for 1 week. On day +35, half of the OVA-immunized and challenged mice were treated with OVA alone, and the other half were epicutaneously treated with OVA plus CpG for 2 weeks. Mice in the control group that were epicutaneously treated with PBS and then challenged with OVA were patched with PBS alone for 2 weeks. On day +49, animals were bled, and axillary and inguinal lymph nodes were isolated to evaluate cytokine production by ALNCs. Skin samples were taken on day +49, homogenized, and analyzed as described in Fig 8, A, to test whether a later switch from OVA to OVA plus CpG alleviates skin pathology.
Measurement of OVA-specific IgE and IgG2a antibodies
OVA-specific antibodies were measured according to the method described by Spergel et al,E4 with our modifications. Briefly, 96-well microtiter plates were coated with 50 µg/mL OVA in 0.1 mol/L NaHCO3 at 4°C overnight. The plates were washed with PBS–Tween 20 (0.05%) and then blocked with 10% PBS-FCS for 1 hour at room temperature. Serial dilutions of sera in 10% PBS-FCS were then added to the wells and incubated for 2 hours at room temperature. After washing with PBS–Tween 20, a mixture of biotinylated anti-mouse isotype–specific antibody and streptavidinhorseradish peroxidase (BD PharMingen) was added, and plates were incubated for 1 hour at room temperature. The plates were then washed with PBS-Tween and developed with 3,3′,5,5′ tetramethylbenzidine substrate (BD PharMingen) for 30 minutes. The reaction was then stopped, and absorption was measured at 450 nm with a wavelength correction at 570 nm subtracted from these values. Results were expressed as IgE-20 and IgG2a-40 arbitrary units.
Cytokine immunoassay
ALNCs (3 × 106) from mice epicutaneously treated with PBS (negative control), OVA, OVA plus CpG, or CpG alone were cultured in 1 mL of RPMI 1640 medium supplemented with 10% FCS in the presence of 100 µg/mL OVA in flat 24-well Falcon plates. After 48 hours, culture supernatants were collected, and samples were tested for cytokine concentrations. IL-4, IL-5, IL-6, IL-10, IL-12p70, TNF-α, and IFN-γ concentrations in culture supernatants were measured with mouse ELISA sets purchased from BD PharMingen. Additionally, IL-17A and IL-9 concentrations were evaluated with a mouse ELISA Ready-SET-Go kit (eBioscience, San Diego, Calif). The 3,3′,5,5′ tetramethylbenzidine reagent set was used as the substrate in our ELISAs (BD PharMingen). IL-13 levels were measured with a mouse IL-13 immunoassay (R&D Systems, Minneapolis, Minn).
CpG immunoassay
BALB/c mice were patched with PBS alone or 100 µg of OVA or 100 µg of OVA and 100 µg of CpG in a volume of 100 µL of PBS for 2 weeks starting on day 0 to measure the amount of CpG absorbed through the skin. On day +14, the patches were removed, and the mice were rested for 1 week. The animals were then epicutaneously challenged by patching with 100 µg of OVA in sterile PBS for 1 week starting on day +21. Blood samples were collected at the following time points: days +7, +14, and +28. Sera were tested for CpG concentration by using an ELISA kit (MyBioSource, San Diego, Calif).
EPO activity assay
Eosinophil infiltration in inflamed skin was indirectly quantitated with an EPO assay, as described previously.E6 Skin was removed, and a 6-mmdiameter skin punch was made. Biopsy specimens were homogenized in 0.5% hexadecyltrimethylammonium bromide (pH 6.0). The homogenates were freeze-thawed 3 times and then centrifuged at 40,000g. After that, the supernatants were collected. Aliquots (50 µL) of the supernatants suspended in 100 µL of 50 mmol/L TRIS-HCl buffer (pH 8.0) containing 0.1% Triton X-100 (Sigma), 1 mmol/L o-phenylenediamine (Sigma), and 500 mmol/L hydrogen peroxide (Sigma) were incubated for 30 minutes at room temperature. The reaction was stopped by adding 2 mol/L sulfuric acid (50 µL), and absorbance at 490 nm was measured with a microplate reader. Serial dilutions of EPO protein (MyBioSource) were performed to create a standard curve. The protein concentration in supernatants was determined by using the bicinchoninic acid method. EPO activity was expressed in units per protein concentration (units per milligram of protein).
Treg cell identification by means of flow cytometry
ALNCs from mice epicutaneously treated with PBS, OVA, OVA plus CpG, or CpG alone were stained for surface markers with anti-CD4– peridinin-chlorophyll proteins conjugated with cyanine 5.5 (PerCP-Cy5.5), anti-TCRβ– fluorescein isothiocyanate, and anti-CD25–allophycocyanin (APC) mAbs (BD Bioscience, San Jose, Calif). Samples were then intracellulary stained with anti-FoxP3–R-phycoerythrin mAb (BioLegend, San Diego, Calif) by using a mouse Treg staining kit (eBioscience). Stained samples were then assessed by using flow cytometry with a FACSCalibur (BD Biosciences), and data were analyzed with BD FACSDiva Software 6.1.
Staining for intracellular cytokines
For intracellular cytokine staining, ALNCs were cultured for 5 hours with GolgiPlug (eBioscience), phorbol 12-myristate 13-acetate, and ionomycin (both from Sigma). Then cells were stained for surface markers with anti-TCRβ–fluorescein isothiocyanate, anti-CD4–PerCP-Cy5.5, and anti-TCRγδ–APC mAbs (eBioscience) and further processed by using a fixation buffer and permeabilization buffer (both from eBioscience). Cells were then stained with anti-IL-17A–R-phycoerythrin and anti-IFN-γ–APC or anti-IFN-γ–PerCP-Cy5.5 mAbs. Samples were then assessed by using flow cytometry with a FACSCalibur (BD Biosciences), and data were analyzed with BD FACSDiva Software 6.1.
Statistics
Data in graphs are shown as means ± SEs. Based on our previous observations in a mouse model of AD, the results in measurement of OVA-specific antibodies in serum after OVA or OVA plus CpG treatment satisfy a normality test (data not shown). Results were compared by using ANOVA, followed by Tukey multiple comparison, and the Kruskal-Wallis test, followed by Dunn multiple comparison, for parametric and nonparametric data, respectively. Statistical significance was set at a P value of less than .05.
Supplementary Material
Key messages.
Epicutaneous immunization with OVA and CpG shifts the immune response toward a TH1/TH17 phenotype in mice.
Epicutaneous immunization with OVA and CpG reduces TH2-type cytokine levels, suppresses secretion of antigen-specific IgE, and increases synthesis of antigen-specific IgG2a antibodies in mouse serum.
Our findings suggest that epicutaneous immunization with OVA and CpG is effective in the treatment of established AD in mice.
Epicutaneous application of protein antigen in the presence of adjuvant could be an attractive therapeutic method to control unwanted TH2-mediated immune responses in patients with allergic disease.
Acknowledgments
Supported by research grants from the Ministry of Science and Higher Education (N N401 3553 33 to M.S.) and the National Science Center (2011/03/B/NZ6/00821 to M.M.-S. and UMO-2012/05/B/NZ6/00997 to M.S.).
M. Szczepanik receives research funding from MNISW and NCN. M. Majewska-Szczepanik receives research funding from NCN.
We thank Dr Andrew Hutchinson for helpful comments and rereading of the manuscript.
Abbreviations used
- AD
Atopic dermatitis
- ALNC
Axillary and inguinal lymph node cell
- APC
Allophycocyanin
- EPIT
Epicutaneous allergen-specific immunotherapy
- EPO
Eosinophil peroxidase
- FACS
Fluorescence-activated cell sorting
- FoxP3
Forkhead box P3
- ILIT
Intralymphatic immunotherapy
- MyD88
Myeloid differentiation primary response gene 88
- OVA
Ovalbumin
- PerCP-Cy5.5
Peridinin-chlorophyll proteins conjugated with cyanine 5.5
- TCR
T-cell receptor
- TLR
Toll-like receptor
- TNP-Ig
2,4,6-Trinitrophenyl–conjugated mouse immunoglobulin
- Treg
Regulatory T
Footnotes
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 3.van Panhuys N, Le Gros G, McConnell MJ. Epigenetic regulation of Th2 cytokine expression in atopic diseases. Tissue Antigens. 2008;72:91–97. doi: 10.1111/j.1399-0039.2008.01068.x. [DOI] [PubMed] [Google Scholar]
- 4.Marogna M, Spadolini I, Massolo A, Canonica GW, Passalacqua G. Long-lasting effects of sublingual immunotherapy according to its duration: a 15-year prospective study. J Allergy Clin Immunol. 2010;126:969–975. doi: 10.1016/j.jaci.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 5.Broide DH. Immunomodulation of allergic disease. Annu Rev Med. 2009;60:279–291. doi: 10.1146/annurev.med.60.041807.123524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox L, Compalati E, Kundig T, Larche M. New directions in immunotherapy. Curr Allergy Asthma Rep. 2013;13:178–195. doi: 10.1007/s11882-012-0335-7. [DOI] [PubMed] [Google Scholar]
- 7.Johansen P, von Moos S, Mohanan D, Kündig TM, Senti G. New routes for allergen immunotherapy. Hum Vaccin Immunother. 2012;8:1525–1533. doi: 10.4161/hv.21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senti G, von Moos S, Tay F, Graf N, Sonderegger T, Johansen P, et al. Epicutaneous allergen-specific immunotherapy ameliorates grass pollen-induced rhinoconjunctivitis: a double-blind, placebo-controlled dose escalation study. J Allergy Clin Immunol. 2012;129:128–135. doi: 10.1016/j.jaci.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 9.Wang LF, Lin JY, Hsieh KH, Lin RH. Epicutaneous exposure of protein antigen induces a predominant Th2-like response with high IgE production in mice. J Immunol. 1996;156:4077–4082. [PubMed] [Google Scholar]
- 10.Herrick CA, MacLeod H, Glusac E, Tigelaar RE, Bottomly K. Th2 responses induced by epicutaneous or inhalational protein exposure are differentially dependent on IL-4. J Clin Invest. 2000;105:765–775. doi: 10.1172/JCI8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ptak W, Szczepanik M, Bryniarski K, Tutaj M, Ptak M. Epicutaneous application of protein antigens incorporated into cosmetic cream induces antigen-nonspecific unresponsiveness in mice and affects the cell-mediated immune response. Int Arch Allergy Immunol. 2002;128:8–14. doi: 10.1159/000057998. [DOI] [PubMed] [Google Scholar]
- 12.Szczepanik M, Bryniarski K, Tutaj M, Ptak M, Skrzeczynska J, Askenase PW, et al. Epicutaneous immunization induces αβ T-cell receptor CD4 CD8 double-positive non-specific suppressor T cells that inhibit contact sensitivity via transforming growth factor-β. Immunology. 2005;115:42–54. doi: 10.1111/j.1365-2567.2005.02127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ptak W, Bryniarski K, Ptak M, Majewska M, Gamian A, Lobo FM, et al. Toll-like receptor ligands reverse suppression of contact hypersensitivity reactions induced by epicutaneous (EC) immunization with protein antigen. Int Arch Allergy Immunol. 2006;139:188–200. doi: 10.1159/000091164. [DOI] [PubMed] [Google Scholar]
- 14.Ptak W, Majewska M, Bryniarski K, Ptak M, Lobo FM, Zajac K, et al. Epicutaneous immunization with protein antigen in the presence of TLR4 ligand induces TCR alpha beta+CD4+ T contrasuppressor cells that reverse skin-induced suppression of Th1-mediated contact sensitivity. J Immunol. 2009;182:837–850. doi: 10.4049/jimmunol.182.2.837. [DOI] [PubMed] [Google Scholar]
- 15.Askenase PW, Szczepanik M, Itakura A, Kiener C, Campos RA. Extravascular T-cell recruitment requires initiation begun by Vα14+ NKT cells and B-1 B cells. Trends Immunol. 2004;25:441–449. doi: 10.1016/j.it.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Zhang Z, Tian R, Zhang K. Skin as a novel route for allergen-specific immunotherapy. Curr Pharm Des. 2014;20:886–891. doi: 10.2174/13816128113199990051. [DOI] [PubMed] [Google Scholar]
- 17.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Ghea RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakata A, Kaminuma O, Ogawa K, Fujimura H, Fushimi K, Kikkawa H, et al. Correlation between eosinophilia induced by CD4(+) T cells and bronchial hyper-responsiveness. Int Immunol. 2001;13:329–339. doi: 10.1093/intimm/13.3.329. [DOI] [PubMed] [Google Scholar]
- 19.Santeliz JV, Van Nest G, Traquina P, Larsen E, Wills-Karp M. Amb a 1-linked CpG oligodeoxynucleotides reverses established airway hyperresponsiveness in a murine model of asthma. J Allergy Clin Immunol. 2002;109:455–462. doi: 10.1067/mai.2002.122156. [DOI] [PubMed] [Google Scholar]
- 20.Kootiratrakarn T, Fujimura T, Sano K, Okuyama R, Aiba S, Tagami H, et al. Development of a novel Ag-specific immunotherapy using CpG oligonucleotides in a new, unique mouse cutaneous eosinophilic inflammation model. Eur J Immunol. 2005;35:3277–3286. doi: 10.1002/eji.200526274. [DOI] [PubMed] [Google Scholar]
- 21.Kitagaki K, Businga TR, Kline JN. Oral administration of CpG-ODNs suppresses antigen-induced asthma in mice. Clin Exp Immunol. 2006;143:249–259. doi: 10.1111/j.1365-2249.2005.03003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, et al. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–1455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 23.Krieg AM. CpG still rocks! Update on an accidental drug. Nucleic Acid Ther. 2012;22:77–89. doi: 10.1089/nat.2012.0340. [DOI] [PubMed] [Google Scholar]
- 24.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;7:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 25.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 26.Gupta GK, Agrawal DK. CpG oligodeoxynucleotides as TLR9 agonists: therapeutic application in allergy and asthma. Bio Drugs. 2010;24:225–235. doi: 10.2165/11536140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Maloy KJ, Odermatt B, Hengartner H, Zinkernagel RM. Interferon γ-producing γδT cell-dependent antibody isotype switching in the absence of germinal center formation during virus infection. Proc Natl Acad Sci U S A. 1998;95:1160–1165. doi: 10.1073/pnas.95.3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamane H, Paul WE. Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunol Rev. 2013;252:12–23. doi: 10.1111/imr.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitsdoerffer M, Lee Y, Jäger A, Kim HJ, Korn T, Kolls JK, et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashida S, Uchi H, Moroi Y, Furue M. Decrease in circulating Th17 cells correlates with increased levels of CCL17, IgE and eosinophils in atopic dermatitis. J Dermatol Sci. 2011;61:180–186. doi: 10.1016/j.jdermsci.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 32.Schmitt E, Klein M, Bopp T. Th9 cells, new players in adaptive immunity. Trends Immunol. 2014;35:61–68. doi: 10.1016/j.it.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Lin JY, Chen JS, Hsu CJ, Miaw SC, Liu CY, Lee SJ, et al. Epicutaneous sensitization with protein antigen induces Th9 cells. J Invest Dermatol. 2012;132:739–741. doi: 10.1038/jid.2011.382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.