ABSTRACT
Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacter cloacae has been recently recognized in the United States. Whole-genome sequencing (WGS) has become a useful tool for analysis of outbreaks and for determining transmission networks of multidrug-resistant organisms in health care settings, including carbapenem-resistant Enterobacteriaceae (CRE). We experienced a prolonged outbreak of CRE E. cloacae and K. pneumoniae over a 3-year period at a large academic burn center despite rigorous infection control measures. To understand the molecular mechanisms that sustained this outbreak, we investigated the CRE outbreak isolates by using WGS. Twenty-two clinical isolates of CRE, including E. cloacae (n = 15) and K. pneumoniae (n = 7), were sequenced and analyzed genetically. WGS revealed that this outbreak, which seemed epidemiologically unlinked, was in fact genetically linked over a prolonged period. Multiple mechanisms were found to account for the ongoing outbreak of KPC-3-producing E. cloacae and K. pneumoniae. This outbreak was primarily maintained by a clonal expansion of E. cloacae sequence type 114 (ST114) with distribution of multiple resistance determinants. Plasmid and transposon analyses suggested that the majority of blaKPC-3 was transmitted via an identical Tn4401b element on part of a common plasmid. WGS analysis demonstrated complex transmission dynamics within the burn center at levels of the strain and/or plasmid in association with a transposon, highlighting the versatility of KPC-producing Enterobacteriaceae in their ability to utilize multiple modes to resistance gene propagation.
KEYWORDS: carbapenem-resistant Enterobacteriaceae (CRE), Klebsiella pneumoniae carbapenemase (KPC), outbreak, health care-associated infection, whole-genome sequencing, burn patients
INTRODUCTION
Carbapenem-resistant Enterobacteriaceae (CRE) and more specifically carbapenemase-producing Enterobacteriaceae (CPE) are a major global health concern (1, 2). Infections with these pathogens have been associated with substantial mortality and are often difficult to treat (3, 4). CPE infections are frequently caused by Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae in the United States and are associated with the spread of K. pneumoniae sequence type 258 (ST258) (5, 6). Of particular concern, the blaKPC genes responsible for this resistance are transmissible to other Enterobacteriaceae via plasmids in association with the mobile transposon Tn4401 (6) and are now circulating in Enterobacter cloacae in the United States (e.g., Pennsylvania, North Dakota, and the upper midwestern United States) (7–9). Despite this, KPC-producing E. cloacae infections have been relatively infrequent (10). A 3-year outbreak of KPC-producing E. cloacae and K. pneumoniae occurred at a large academic burn center in North Carolina. Here, we report the results of a genomic investigation into this outbreak.
Whole-genome sequencing (WGS) has become a useful tool for analysis of outbreaks and transmission networks of multidrug-resistant organisms, including CRE, in health care settings, enabling us to more comprehensively describe resistance genes and more accurately determine genotypes among epidemiologically linked strains (11–13). In contrast to multidrug-resistant Acinetobacter baumannii or Pseudomonas aeruginosa outbreaks, reports of Enterobacter cloacae or CRE/CPE hospital outbreaks in this particularly vulnerable population are limited (11, 14–19). In addition, there are no reports of KPC-producing E. cloacae outbreaks among burn patients. In this study, we used WGS to explore details of this sequential CRE outbreak, including KPC-producing E. cloacae and K. pneumoniae. We assessed the genetic relatedness of CRE strains and uncovered complex and multimodal transmission dynamics of blaKPC in this prolonged hospital outbreak in the burn center. (This work was presented in part at the Society for Healthcare Epidemiology of America [SHEA] Spring 2016 Conference, Atlanta, GA [20].)
RESULTS
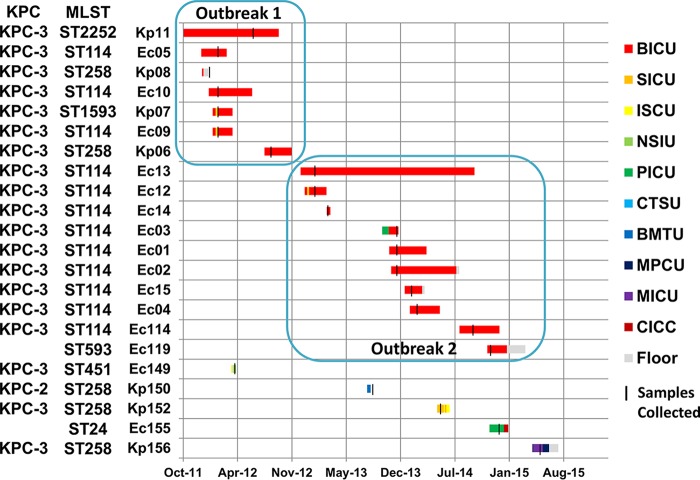
The timeline and location of this prolonged CRE outbreak are provided in Fig. 1. On the basis of epidemiological data, the first outbreak of CRE occurred in the burn center from January through November 2012. The index patient (defined as a patient at the earliest admission date in this CRE outbreak) had Kp11, a KPC-3-producing K. pneumoniae isolated from a urine culture in June 2012. After this index case, 5 additional patients with KPC-3-producing Enterobacteriaceae were identified: 2 patients with K. pneumoniae, 2 patients with E. cloacae, and a single patient with K. pneumoniae and E. cloacae (strain identification [ID] numbers Kp07 and Ec09). On the basis of hospital infection control surveillance, this outbreak seemed to end in November 2012, upon discharge of the last patient (Kp06). However, a second outbreak occurred in the burn center from January 2013 through March 2015. Ec13, a KPC-producing E. cloacae isolate, chronologically became the index case of the second outbreak. Nine patients with E. cloacae were subsequently identified, but there were no patients with K. pneumoniae. Our infection control plan included strict adherence to hospital policy, meticulous hand hygiene by health care personnel, contact precautions for patients infected or colonized with CRE, enhanced environmental cleaning, decontamination of equipment, and enhanced precautions in an outbreak setting (e.g., educating and cohorting staff and patients, implementing surveillance cultures, monitoring and reinforcing interventions frequently). In this outbreak, multiple K. pneumoniae and E. cloacae isolates were PCR positive for blaKPC. Three (Ec05, Ec09, and Ec10) and four (Ec1, Ec2, Ec3, and Ec13) E. cloacae isolates were identical based on pulsed-field gel electrophoresis.
FIG 1.
Timeline and location of prolonged transmission caused by carbapenem-resistant Enterobacteriaceae. Ec, E. cloacae; Kp, K. pneumoniae; BICU, burn intensive care unit; ISCU, intermediate surgical care unit; MICU, medicine intensive care unit; NSIU, neuroscience intensive care unit; SICU, surgery intensive care unit; PICU, pediatric intensive care unit; CTSU, cardiothoracic stepdown unit; MPCU, medicine progressive care unit; floor, general ward. Each strain ID corresponds to a case summarized in Table 1.
Clinical features of patients with CRE infection or colonization are summarized in Table 1. Briefly, 17 patients (81%) were diagnosed with a health care-associated infection (HAI), including respiratory tract infections (n = 12), urinary tract infections (n = 3), and bloodstream infections (n = 2). Underlying diseases of the CRE cases included burns (n = 12, 57%), Stevens-Johnson syndrome or toxic epidermal necrolysis (n = 2), and other diseases (n = 7). Six patients (29%), including 3 burn cases (25%), died during hospitalization despite appropriate treatment.
TABLE 1.
Clinical features of patients with carbapenem-resistant Enterobacteriaceae infection or colonization during a prolonged outbreak
| Strain ID | Organism | Specimen sourcea | Collection date | Accession no. | Age range (yrs) | Sexb | Underlying diseasec | HAId | Type of HAIe | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Ec01 | E. cloacae | BAL fluid | 2013 Nov | DRX055644 | 70–79 | M | SJS | Yes | VAP | Died |
| Ec02 | E. cloacae | Tracheal aspirate | 2013 Nov | DRX055645 | 30–39 | M | 55% TBSA burn | Yes | LRI | Survived |
| Ec03 | E. cloacae | Blood | 2013 Nov | DRX055646 | 20–29 | F | Desquamating rash complicated by multisystem organ failure | Yes | CLABSI | Survived |
| Ec04 | E. cloacae | BAL fluid | 2014 Feb | DRX055647 | 60–69 | F | 20–29% TBSA burn | Yes | LRI | Survived |
| Ec05 | E. cloacae | BAL fluid | 2012 Feb | DRX055648 | 70–79 | F | Inhalational injury and 20% TBSA burn | Yes | LRI | Died |
| Kp06 | K. pneumoniae | BAL fluid | 2012 Aug | DRX055649 | 40–49 | M | SJS/TENS | Yes | VAP | Survived |
| Kp07 | K. pneumoniae | BAL fluid | 2012 Feb | DRX055650 | 20–29 | M | Inhalational burn injury | Yes | VAP | Survived |
| Kp08 | K. pneumoniae | Urine | 2012 Jan | DRX055651 | 60–69 | M | Grease burn | No | Survived | |
| Ec09 | E. cloacae | Blood | 2012 Feb | DRX055652 | 20–29 | M | Inhalational burn injury | Yes | VAP | Survived |
| Ec10 | E. cloacae | Blood | 2012 Feb | DRX055653 | 30–39 | F | 30% flame burn | Yes | VAP | Survived |
| Kp11 | K. pneumoniae | Rectal | 2012 Jun | DRX055654 | 40–49 | F | 30% flame burn | No | Survived | |
| Ec12 | E. cloacae | Rectal | 2013 Jan | DRX055655 | 60–69 | M | >50% TBSA burn | No | Died | |
| Ec13 | E. cloacae | Wound surface | 2013 Jan | DRX055656 | 30–39 | M | Severe multitrauma | Yes | CAUTI | Survived |
| Ec14 | E. cloacae | Rectal | 2013 Mar | DRX055657 | 80–89 | F | 7% TBSA chemical burn | No | Survived | |
| Ec15 | E. cloacae | BAL fluid | 2014 Jan | DRX055658 | 40–49 | M | 9% TBSA flame burn | Yes | LRI | Survived |
| Ec114 | E. cloacae | Urine | 2014 Sept | DRX055660 | 20–29 | M | 64%TBSA flame burn | Yes | UTI | Died |
| Ec119 | E. cloacae | Lower respiratory tract | 2014 Nov | DRX055661 | 0–9 | M | 50% TBSA flame burn | No | Survived | |
| Ec149 | E. cloacae | Blood | 2012 Apr | DRX055666 | 50–59 | F | Metastatic colon cancer, septic shock | Yes | BSI | Died |
| Kp150 | K. pneumoniae | Tracheal aspirate | 2013 Sept | DRX055667 | 40–49 | F | Relapsed AML, septic shock | Yes | Pneumonia | Died |
| Kp152 | K. pneumoniae | Urine | 2014 May | DRX055669 | 60–69 | M | Multiple injury by motor vehicle accident | Yes | UTI | Survived |
| Ec155 | E. cloacae | Nasopharynx | 2014 Dec | DRX055672 | 0–9 | M | Congenital heart disease | Yes | LRI | Survived |
| Kp156 | K. pneumoniae | Tracheal aspirate | 2015 May | DRX055673 | 20–29 | F | MRSA bacteremia from i.v. drug use, endocarditis, necrotizing pneumonia | Yes | Pneumonia | Survived |
BAL, bronchoalveolar lavage.
M, male; F, female.
AML, acute myeloid leukemia; SJS, Stevens-Johnson syndrome; TBSA, total body surface area; TENS, toxic epidermal necrolysis; MRSA, methicillin-resistant Staphylococcus aureus infection; i.v., intravenous.
HAI, health care-associated infection.
BSI, bloodstream infection; CAUTI, catheter-associated urinary tract infection; CLABSI, central line-associated bloodstream infection; LRI, lower respiratory tract infection; SSI, surgical site infection; UTI, urinary tract infection; VAE, ventilator-associated event; VAP, ventilator-associated pneumonia.
WGS of 22 CRE isolates produced an average of 1,873,130 read pairs per isolate for K. pneumoniae and 2,073,037 read pairs per isolate for E. cloacae. Results of metagenomic sequence classification matched precisely with the results of conventional species identification techniques. After reference-guided mapping of K. pneumoniae isolates, all samples had at least 5× coverage at 90% of the genome or greater, with the majority of the genome covered to at least 25× coverage (see Fig. S1 in the supplemental material). For E. cloacae isolates, all samples had 5× coverage at nearly 80% of the genome, and most had 50× coverage at more than 50% of the genome (Fig. S1). In many genomic locations, and particularly in plasmid contig assemblies, we achieved coverage depths well over 200. Thus, the vast majority of both the K. pneumoniae and E. cloacae genome assemblies were suitable for variant calling.
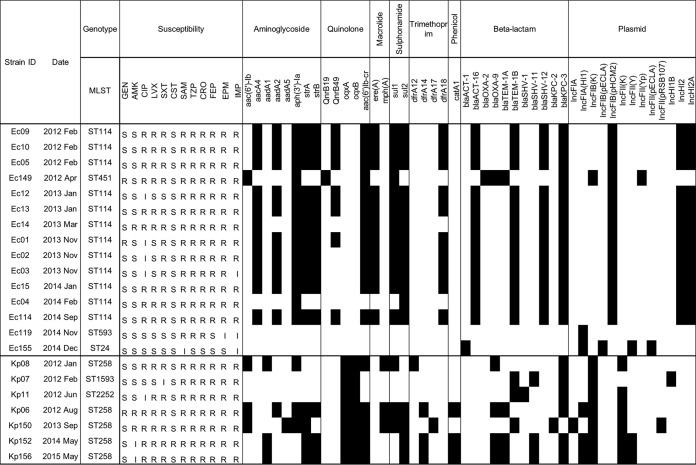
Antimicrobial susceptibility profiles and acquired resistance genes are shown in Fig. 2. All isolates were nonsusceptible to imipenem, but one was susceptible to ertapenem (Ec155, a non-KPC producer). Most were nonsusceptible to fluoroquinolones but were susceptible to aminoglycosides. All except one (Kp152) were susceptible to colistin, and none of them possessed mcr-1 and mcr-2. We detected the following carbapenemase genes in our CRE isolates: 13 (86.7%) E. cloacae strains were positive for blaKPC-3 and 6 (85.7%) K. pneumoniae strains were positive for blaKPC-3, as well as 1 (14.3%) K. pneumoniae strain positive for blaKPC-2. WGS analysis suggested distribution and intra- and interspecies dissemination of multiple resistance genes and plasmids in this prolonged outbreak, as well as potential association of blaKPC transmission with other β-lactamase genes and different classes of antimicrobial resistance (Fig. 2).
FIG 2.
Characterization of acquired resistance genes and genotypes among carbapenem-resistant Enterobacteriaceae strains. Antimicrobial susceptibility profiles: S, susceptible; I, intermediate; R, resistant. Presence (black) or absence (white) of resistance genes and plasmids for each isolate is also shown. SAM, ampicillin-sulbactam; TZP, piperacillin-tazobactam; CRO, ceftriaxone; FEP, cefepime; IMP, imipenem; EPM, ertapenem; GEN, gentamicin; AMK, amikacin; CIP, ciprofloxacin; LVX, levofloxacin; SXT, trimethoprim-sulfamethoxazole; CST, colistin.
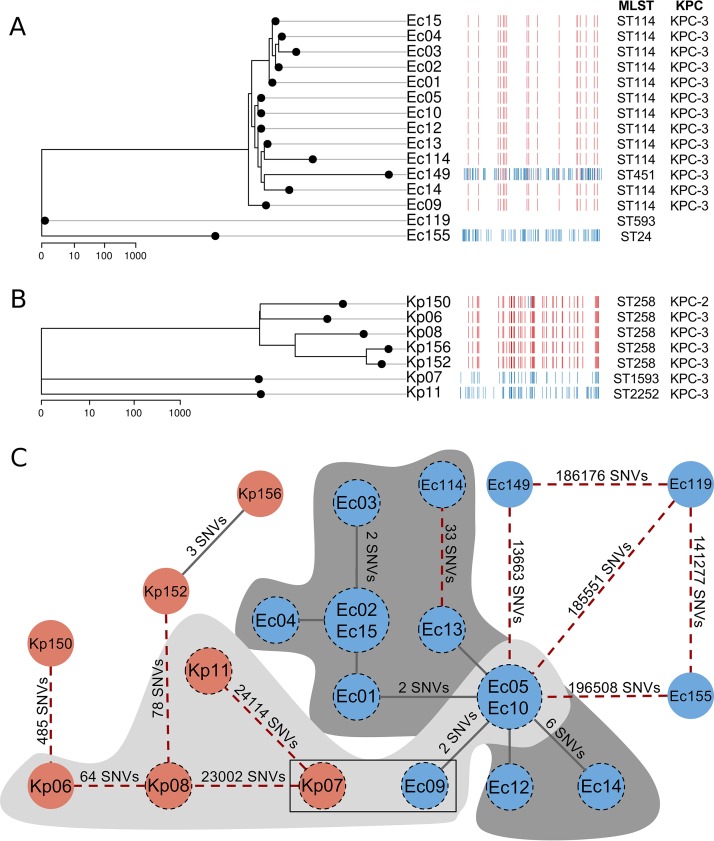
It is known that β-lactamase genes, including blaKPC, can spread through multiple mechanisms, including clonal strain expansion, plasmid transfer, and/or mobile genetic elements, such as transposons (6). First, we explored the strain-level similarity of isolates from this CRE outbreak. Overall, CRE isolates collected within the burn unit were genetically quite distinct from samples collected outside the burn unit on the basis of in silico multilocus sequence typing (MLST) and chromosomal single nucleotide variant (SNV) differences (Fig. 3). Ec119 and Kp11 represented strains novel to this study and were assigned the identifiers ST593 and ST2252, respectively. To resolve whether SNV differences within these outbreak strain types were due to recombination events or to point mutations, we constructed recombination-aware maximum-likelihood phylograms for E. cloacae and K. pneumoniae (Fig. 3A and B). Upon analysis of core genome variants, the 0-33 SNVs observed in pairwise comparisons among E. cloacae ST114 isolates from the burn unit were due to point mutations, while the 3-485 SNVs identified among K. pneumoniae ST258 isolates were due to both mutation and recombination events. SNV strain typing revealed that these outbreaks, which appeared distinct based on epidemiologic data, were in fact one prolonged outbreak. The first epidemiological CRE outbreak was caused by a clone (Ec_UNC) of E. cloacae (Ec05, Ec09, and Ec10, all within two SNV differences) and by four distinct strains of K. pneumoniae (Kp06, Kp07, Kp08, and Kp11). The second epidemiological CRE outbreak was driven by a clone (Ec_UNC, identical to the first outbreak clone) of E. cloacae (Ec01, Ec02, Ec03, Ec04, Ec12, Ec13, Ec14, and Ec15, all within 11 SNV differences) as well as by two distinct strains of E. cloacae (Ec114 and Ec119). Ec114 differed from Ec13 (Ec_UNC) by 33 SNVs only. All CRE isolates outside the burn center were genetically distinct from strains inside the burn center (Fig. 3C). Of 11 patients who had the Ec_UNC clone isolates, 9 (81.8%) had an HAI and 3 (27.3%) died. Two patients who had isolates of the clone Kp_UNC had an HAI and survived.
FIG 3.
(A and B) Maximum-likelihood (ML) phylogram and recombination map showing the relatedness between carbapenem-resistant Enterobacteriaceae isolates. The ML phylogeny was constructed based on genetic variation in nonrecombinant genomic regions. In the horizontal tracks, each column represents a single nucleotide in the reference genome and each row represents an individual clinical isolate. Red blocks mark recombination events occurring on an internal branch of the phylogeny, while blue blocks mark potential recombination events or extensive mutation occurring on a terminal node. The E. cloacae phylogram was rooted to the most genetically distinct isolate in our sample set (Ec155). The K. pneumoniae phylogram was similarly rooted (using Kp11). Phylogenies are drawn on a log scale, and the scale bar below each image represents the phylogenetic distance (in point mutations). (C) Variant difference network, demonstrating Tn4401b-blaKPC-3 transmission between species and between chromosomally divergent strains. Nodes represent CRE clinical isolates of E. cloacae (blue) and K. pneumoniae (red) from the burn unit outbreak. Small nodes represent single isolates, while large nodes represent two identical isolates. The two nodes within a rectangular outline represent two samples, Kp07 and Ec09, which were isolated from a single patient. All nodes with a dashed black perimeter represent samples bearing the common Tn4401b genetic element. Isolates without a dashed perimeter either had no Tn4401 element or had a non-b subtype (i.e., a or d). Lines (edges) indicate the extent of chromosomal difference between isolates. Solid lines bridging nodes indicate membership in a clonal group (defined as consecutive isolates with ≤12 variant differences; Ec_UNC for E. cloacae and Kp_UNC for K. pneumoniae), while red dashed lines connect less-related isolates. Lines are labeled with the number of SNVs that separate the clinical isolates if there are more than 2 SNVs. Line lengths are not proportional to genetic distance. Isolates depicted above a light gray matte and a dark gray matte were collected in outbreak 1 and outbreak 2 within the BICU, respectively, while isolates outside the mattes were collected outside the unit and are putatively unrelated to the outbreak.
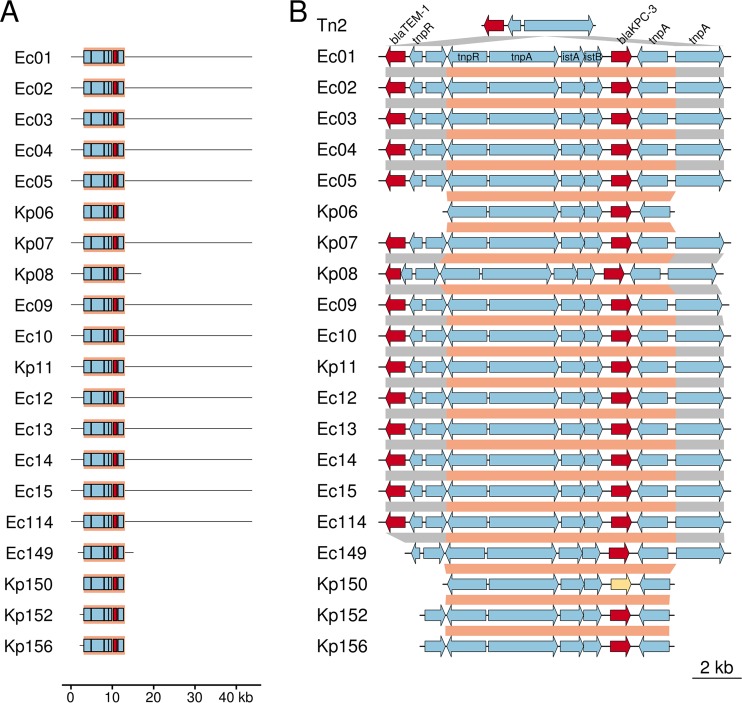
We next evaluated the genetic similarity of these isolates at the plasmid level. We observed high sequence homology between 3 of the 35 publicly available KPC-3-containing RefSeq plasmids (NZ_CP011575.1, NZ_CP010387.1, and NZ_CP008826.1) and the KPC-3-containing contigs from de novo assemblies of the 12 E. cloacae isolates from the burn unit as well as two K. pneumoniae strains (Kp07 and Kp11), suggesting that a highly similar blaKPC-3-containing entire or partial plasmid (pKPC-3_UNC, ∼45,000 bp) was shared (Fig. 4A; Fig. S2A). Both Kp07 and Ec09 from a single patient also shared this plasmid, which we termed pKPC-3_UNC, suggesting interspecies plasmid transmission. The quality of these de novo-assembled KPC-bearing plasmids was further validated by assembly against plasmid reference sequence NZ_CP008826 as well as reference-guided mapping of each isolate's raw reads to its own de novo-assembled KPC-containing contigs (Fig. S2B) (see Materials and Methods).
FIG 4.
Plasmid and transposon maps revealing significant gene-sharing among carbapenem-resistant Enterobacteriaceae outbreak isolates within the burn unit. (A) The blaKPC-3-containing contig from de novo read assemblies are depicted. In every case, blaKPC-3 was identified nested within a Tn4401 element, the boundaries of which are shown with a salmon background. Tn4401 accessory genes are shown in blue, and blaKPC-3 is highlighted in red. For each isolate, only the plasmid region homologous to the primary outbreak plasmid identified in most Ec and two Kp isolates are shown. (B) Structure of the Tn4401-blaKPC-3 composite genetic element as identified in each outbreak isolate. Tn4401 boundaries are marked in salmon, and Tn2-like element boundaries are marked in gray. Accessory genes are colored blue, while the β-lactamase genes blaTEM-1 and blaKPC-3 are highlighted in red. The single isolate bearing blaKPC-2 (Kp150) is marked in yellow. A generic Tn2-like element is shown on the top track, and isolate-specific tracks show insertion points of Tn4401 elements into the Tn2-like element.
Finally, we considered the immediate genetic context for blaKPC among KPC-producing E. cloacae and K. pneumoniae isolates during the prolonged outbreak. Findings are summarized in Table 2. On the basis of de novo sequence assembly, blaKPC-3 was, in all isolates, nested within a Tn4401 transposable element (Fig. 4B). All KPC-3-producing isolates within the burn unit except for one (Kp06, with Tn4401d), contained blaKPC-3 nested within the Tn4401b element, while isolates outside the burn unit contained a variety of Tn4401 isoforms (Table 2). All Tn4401b sequences harboring blaKPC-3 (882 bp) from pKPC-3_UNC were 100% concordant, except for Tn4401b within Kp08 (1 SNV) (Fig. S3). Thus, plasmid and Tn4401 transposon analyses suggested that the majority of blaKPC-3 genes were transmitted via an identical Tn4401b element on part of a common plasmid (Fig. 4; Fig. S2 and S3). On the other hand, Kp08, which was isolated within the burn unit, and Ec149, which was isolated outside the burn unit, both contained the Tn4401b-blaKPC-3 nested genetic element but did not share the pKPC-3_UNC plasmid. The KPC-3-bearing contigs from Kp06 and Kp08 likely represented different KPC-3-containing plasmids (IncFIA, IncI2) and different Tn4401 isoforms (d, b), in addition to being different strains of K. pneumoniae (64 SNVs). Two identical strains of K. pneumoniae (Kp152 and Kp156; 3 SNVs) outside the burn unit had the same Tn4401 isoform (d), which occurred on a plasmid other than pKPC-3_UNC. We found that the blaKPC-3-Tn4401b structure was nested within 5-bp flanking sequences on the Tn2-like element in every case where an isolate contained the structure (Fig. 4; Table 2).
TABLE 2.
Summary of the genetic context for blaKPC among KPC-producing Enterobacter cloacae and Klebsiella pneumoniae isolates during a prolonged outbreak
| Strain ID | Organism | KPC | MLST | Strain relatedness | Plasmid varianta | Tn4401 variantb | Flanking sequencesc | Composite Tn4401/Tn2-like structure |
|---|---|---|---|---|---|---|---|---|
| Ec01 | E. cloacae | blaKPC-3 | ST114 | Ec_UNC | pKPC-3_UNC | Tn4401b | GTTCT/GTTCT | Complete, identical |
| Ec02 | E. cloacae | blaKPC-3 | ST114 | Ec_UNC | pKPC-3_UNC | Tn4401b | GTTCT/GTTCT | Complete, identical |
| Ec03 | E. cloacae | blaKPC-3 | ST114 | Ec_UNC | pKPC-3_UNC | Tn4401b | GTTCT/GTTCT | Complete, identical |
| Ec04 | E. cloacae | blaKPC-3 | ST114 | Ec_UNC | pKPC-3_UNC | Tn4401b | GTTCT/GTTCT | Complete, identical |
| Ec05 | E. cloacae | blaKPC-3 | ST114 | Ec_UNC | pKPC-3_UNC | Tn4401b | GTTCT/GTTCT | Complete, identical |
| Kp06 | K. pneumoniae | blaKPC-3 | ST258 | Tn4401d | GTTCT/TCTCT | |||
| Kp07 | K. pneumoniae | blaKPC-3 | ST1593 | pKPC-3_UNC | Tn4401b | GTTCT/GTTCT | Complete, identical | |
| Kp08 | K. pneumoniae | blaKPC-3 | ST258 | Tn4401b** | GTTCT/GTTCT | Partial, identical | ||
| Ec09 | E. cloacae | blaKPC-3 | ST114 | Ec_UNC | pKPC-3_UNC | Tn4401b | GTTCT/GTTCT | Complete, identical |
| Ec10 | E. cloacae | blaKPC-3 | ST114 | Ec_UNC | pKPC-3_UNC | Tn4401b | GTTCT/GTTCT | Complete, identical |
| Kp11 | K. pneumoniae | blaKPC-3 | ST2252 | pKPC-3_UNC | Tn4401b | GTTCT/GTTCT | Complete, identical | |
| Ec12 | E. cloacae | blaKPC-3 | ST114 | Ec_UNC | pKPC-3_UNC | Tn4401b | GTTCT/GTTCT | Complete, identical |
| Ec13 | E. cloacae | blaKPC-3 | ST114 | Ec_UNC | pKPC-3_UNC | Tn4401b | GTTCT/GTTCT | Complete, identical |
| Ec14 | E. cloacae | blaKPC-3 | ST114 | Ec_UNC | pKPC-3_UNC | Tn4401b | GTTCT/GTTCT | Complete, identical |
| Ec15 | E. cloacae | blaKPC-3 | ST114 | Ec_UNC | pKPC-3_UNC | Tn4401b | GTTCT/GTTCT | Complete, identical |
| Ec114 | E. cloacae | blaKPC-3 | ST114 | pKPC-3_UNC* | Tn4401b | GTTCT/GTTCT | Complete, identical | |
| Ec149 | E. cloacae | blaKPC-3 | ST451 | Tn4401 novel*** | GTTCT/GTTCT | |||
| Kp150 | K. pneumoniae | blaKPC-2 | ST258 | Tn4401a | ATTGA/ATTGA | |||
| Kp152 | K. pneumoniae | blaKPC-3 | ST258 | Kp_UNC | Tn4401d | GTTCT/TCTCT | ||
| Kp156 | K. pneumoniae | blaKPC-3 | ST258 | Kp_UNC | Tn4401d | GTTCT/TCTCT |
Isolates with shared plasmid variants indicate possible plasmid-mediated blaKPC-3 transmission among isolates (pKPC-3_UNC). *, Ec114 differed from others by 1 SNV.
All isolates carried Tn4401b (except for one, marked with two asterisks, isolate Kp08 with 1 SNV) and are identical, and all isolates carrying Tn4401d are identical. “Tn4401 novel” (indicated with three asterisks, isolate Ec149) is an isoform of Tn4401 with a 91-bp deletion downstream of blaKPC.
Flanking sequences of Tn4401 are shown as 5-bp target site duplications in transposition. There was no evidence of target site duplication for Tn4401d.
DISCUSSION
In this study, we describe the genomic analysis of two sequential CRE outbreaks that occurred at a large academic burn center over a 3-year period. Although these outbreaks seemed epidemiologically unlinked, genome-wide sequence analysis provided strong evidence that they were in fact genetically linked. We found that both E. cloacae and K. pneumoniae strains causing this outbreak utilized multiple modes of blaKPC-3 gene transfer. A recent genetic surveillance study involving hundreds of KPC-producing Enterobacteriaceae isolates at a large academic medical center clearly demonstrated these mechanisms (21). In our study, we were surprised to find multiple transmission mechanisms for the blaKPC gene among strains and plasmids in association with transposons, even in the few CRE which comprised this outbreak (19 KPC-3-producing Enterobacteriaceae isolates), suggesting challenges for infection prevention and control measures against CRE in immunocompromised populations.
Although E. cloacae outbreaks were previously reported to be largely clonal (22, 23), recent genomic studies (21, 24) have identified a variety of molecular mechanisms of transmission in KPC- or New Delhi metallo-β-lactamase (NDM)-producing E. cloacae. We found that most cases in this outbreak were caused by a clonal strain of KPC-3-producing E. cloacae (Ec_UNC). Despite the importance of clonal strain expansion in the outbreak at our hospital, propagation of the outbreak was likely facilitated by plasmids in association with transposons. Specifically, we found that an E. cloacae strain circulating in the hospital likely acquired the blaKPC-3 gene via plasmid transfer from a K. pneumoniae isolate or vice versa. This potentially occurred among different species of isolates Kp07 and Ec09, which were isolated from a single patient.
Given the epidemiological timeline and location as well as the genetic relatedness, a majority of transmission routes in two outbreaks could represent direct transmissions. The index cases for each outbreak (Kp11 and Ec13) shared time and space with other in patients in the burn center during almost the whole outbreak period and could be the source of KPC-3. Remarkably, WGS analysis demonstrated possible transmission of KPC-3 producers in the burn center during ∼2-year periods at the strain (Ec_UNC) level (e.g., Ec05 isolated in February 2012 and Ec04 isolated in February 2014) and ∼2.5-year periods at the plasmid level (pKPC-3_UNC; Kp07 isolated in February 2012 and Ec114 isolated in September 2014). Although KPC-3-producing CRE isolates outside the burn center during this outbreak period initially seemed to be related to CRE isolates inside the burn center, WGS analysis clearly revealed differentiation of these strains; therefore, CRE isolates outside the burn center may have been sporadic cases in our hospital or independent introductions of blaKPC into our hospital.
There were no direct contacts between the first and second outbreaks (1 month between Kp06 from the last case of the first outbreak and Ec13 from the initial case of the second outbreak) in spite of persuasive genetic relatedness, likely due to transmission through health care personnel, colonization of another patient in the burn unit, or a contaminated environmental reservoir which was not identified in this investigation. Patient-to-patient transmission is an important mode for acquisition of carbapenem-resistant E. cloacae (23, 25), but transmission from the hospital environment may occur since room surfaces in surroundings of CRE carriers have been frequently identified as contaminated (26). On the other hand, our previous study showed that CRE (Enterobacter and Klebsiella species) survived poorly in a hospital environment for 24 h and was infrequently isolated from environmental surfaces in rooms housing CRE-colonized/infected patients (27); thus, the role of hospital environmental surfaces for CRE transmission is yet to be clarified. Some authors have described environmental reservoirs of CPE related to transmission in health care settings (28), including sinks (29, 30), soap dispensers (24), and hospital wastewater systems (31, 32).
The index patient with K. pneumoniae ST2252 producing KPC-3 (Kp11) was first admitted to the burn center in this outbreak and stayed there for ∼1 year, and then likely shared E. cloacae ST114 and other STs of K. pneumoniae, spreading KPC-3 among them. This transmission of KPC-3 producers was primarily maintained by E. cloacae ST114 with other β-lactamases, including ACT-16, TEM-1B, and SHV-12, as well as distribution of multiple classes of resistance determinants. Two molecular epidemiologic studies of E. cloacae isolates from Europe and other countries described that E. cloacae ST114 was one of the most common genotypes and was associated with producing CTX-M-15, although there were no strong correlations between a majority of E. cloacae STs and other β-lactamase profiles (33, 34). To our knowledge, this is the first outbreak due to KPC-3-producing E. cloacae ST114 among a burn patient population, and this strain was identified in all of three deceased burn patients.
WGS-based genotyping had greater sensitivity for distinguishing strains than did MLST. In particular, Ec114 belonged to ST114 but was different from Ec_UNC (33 SNVs). Of CRE isolates (Kp06, Kp08, Kp152, Kp156) belonging to ST258, Kp152 and Kp156 were a clone (Kp_UNC) based on SNV typing, but others were distinct (64 to 78 SNVs). When the relatedness in hospital outbreak strains of CRE has been investigated, WGS-based SNV typing has demonstrated differentiation of strains belonging to the same ST.
There are several limitations in this study. First, we were not able to identify specific reservoirs in this outbreak. Our transmission analysis also could be affected by isolates not detected nor analyzed during this outbreak period. Nucleic acid detection and WGS analysis through broad sampling of the hospital environment and items as well as multiple specimens from a single patient could be useful for obtaining a better understanding of transmission networks in health care outbreaks (24, 35). Second, using sequencing data, we found evidence of diverse plasmids and transposons. However, even with our high-coverage short reads, we are likely unable to describe the full extent of diversity among plasmids and mobile elements that harbor resistance genes. While our careful process of de novo assemblies and reference-guided mapping lent confidence to our current findings, the true picture of resistance gene propagation in this outbreak may be more complex than we found. Emerging long-read sequencing technologies will prove crucial in accurately describing the full extent of resistance gene transmission in health care settings (13, 21, 36).
In conclusion, our WGS analysis provided insight into complex genetic transmissions of KPC-3-producing E. cloacae and K. pneumoniae in this prolonged burn outbreak as well as the difficulty of infection prevention and control at the burn center. WGS analysis allowed us to uncover transmission dynamics regarding resistance genes, mobile elements, and strains in health care settings. Current phenotypical detection of CRE and implementation of strict standard and contact precaution for patients with CRE infection or colonization may help prevent transmission of CRE strains but would be difficult for gene-based transmission. Further outbreak investigations using WGS will be required to elucidate the full complexity of genetic mechanisms of resistance transmission and establish infection prevention and control strategies that are responsive to specific gene-based transmission.
MATERIALS AND METHODS
Bacterial strains and clinical data.
A total of 22 clinical isolates of CRE from 21 patients, including E. cloacae (n = 15) and K. pneumoniae (n = 7), between January 2012 and May 2015 at the University of North Carolina Hospitals (Chapel Hill, NC, USA), an 853-bed tertiary care academic facility, were analyzed (Table 1). A single isolate from the primary site of infection or colonization was used for each patient with CRE (K. pneumoniae and E. cloacae) at the North Carolina Jaycee Burn Center during this period. In addition, one patient was infected with both K. pneumoniae and E. cloacae, so an isolate from each species (Kp07 from bronchoalveolar lavage fluid [BAL] and Ec09 from blood) was sequenced. In addition to the isolates from the burn unit, 5 CRE isolates (two E. cloacae, Ec149 and Ec155, and three K. pneumoniae, Kp150, Kp152, and Kp156) from HAI collected during this period were included for comparison. All CRE strains were isolated from clinical specimens collected in UNC's McLendon Clinical Laboratories and were (i) nonsusceptible to one of the following carbapenems, doripenem, meropenem, or imipenem, and (ii) resistant to all of the following cephalosporins that were tested, ceftriaxone, cefotaxime, and ceftazidime, according to the 2012 Centers for Disease Control and Prevention (CDC) criteria (37).
Clinical and epidemiological data for CRE cases were obtained through chart review, an administrative database, and a comprehensive hospital-wide surveillance database, using a laboratory-based approach. HAI were prospectively ascertained using the CDC definitions (38). This study was approved by the Institutional Review Board of UNC at Chapel Hill (IRB number 06-0437).
Antimicrobial susceptibility testing.
Kirby-Bauer disk-diffusion susceptibility testing was performed, and the breakpoints for each drug were interpreted according to the 2015 Clinical and Laboratory Standards Institute guideline (colistin breakpoint for Pseudomonas aeruginosa and the others for Enterobacteriaceae) (39). Antibiotics tested included ampicillin-sulbactam, piperacillin-tazobactam, ceftriaxone, cefepime, imipenem, ertapenem, gentamicin, amikacin, ciprofloxacin, levofloxacin, trimethoprim-sulfamethoxazole, and colistin.
Whole-genome sequencing.
Bacterial strains were grown overnight in LB broth at 37°C. DNA from each isolate was extracted and purified using an UltraClean microbial DNA isolation kit (Mo Bio, Carlsbad, CA). Sequencing libraries were prepared using the NEBNext Ultra DNA library prep kit for Illumina (New England BioLabs, Ipswich, MA) per the manufacturer's instructions with NEBNext multiplex oligonucleotides (New England BioLabs). All libraries were pooled and sequenced on a single Illumina HiSeq2500 run using 125-base paired-end V4 chemistry at the UNC High-Throughput Sequencing Facility.
De novo sequence assembly.
Predicting accurate de novo haplotypes is crucial in an outbreak investigation where the genetic drivers are unknown. However, because predicting accurate de novo haplotypes from short read data can be a precarious endeavor (21), we adopted a very conservative approach to contig assembly and interpretation. We subjected our short-read WGS data to numerous de novo assemblers (those available at http://nucleotid.es/assemblers/), and we determined that the A5-miseq pipeline with default parameter settings produced the highest-quality assemblies, with the longest contig lengths and the highest N50 values (40). To further increase our certainty in the accuracy and completeness of our assembled blaKPC-3-containing contigs and to check for occult duplication events that can arise during de novo assembly (41), we constructed multistring Burrows-Wheeler transformations of raw sequencing data (https://github.com/andrewparkermorgan/snoop) and queried these structures for the presence/absence of relevant sequence strings (42, 43).
As a single contig bearing the blaKPC gene was identified in 20 of 22 UNC isolates, reads from these blaKPC-containing isolates (n = 20) were mapped pairwise against the blaKPC-containing contigs identified above (n = 20), for a total of n = 400 unique alignments. Salient comparisons and, in particular, self-alignments were examined closely for coverage gaps, misalignments, and evidence of plasmid closure in order to produce a hand-curated set of blaKPC-containing plasmids, one plasmid for each blaKPC-containing isolate (n = 20).
Reference-guided core genome mapping.
To better characterize the extent of genetic diversity within the bacterial core genomes, we mapped short reads to reference genomes: NCTC9394 (RefSeq accession number NC_021046.1) in the case of E. cloacae and MGH78578 (GenBank accession number CP000647.1) in the case of K. pneumoniae. Mapping was conducted using bwa mem, and coverage is reported in Fig. S1 in the supplemental material (44). Mapped reads were deduplicated and subjected to local realignment through high-entropic regions using Picard (http://broadinstitute.github.io/picard/) and the Genome Analysis toolkit (GATK v3.3) (45).
SNV discovery was performed by applying the GATK unified genotyper across all reference-aligned CRE isolates simultaneously. Variants were filtered stringently using cutoffs responsive to the underlying distribution of quality scores. The following parameters were used for filtering: quality-by-depth, ≥25; mapping quality, ≥59; Fisher score, ≤4; map quality rank sum, greater than or equal to −4.0, and read position rank sum, greater than or equal to −4.0. In addition, only biallelic variants with at least 5× coverage in 100% of isolates were used for further comparative analysis.
Genetic analysis.
First, species identification of individual isolates from short-read sequencing data was confirmed using Kraken (46). Acquired resistance genes were identified using ResFinder v2.1 (47) with a threshold of 98% identity and a minimum length of 60% on de novo assemblies. In silico MLST was performed and STs were determined from de novo assemblies using MLST v1.7 (48) and the PubMLST database (http://pubmlst.org/ecloacae/) for E. cloacae (49) and the Institut Pasteur MLST database (http://bigsdb.web.pasteur.fr/klebsiella/klebsiella.html) for K. pneumoniae (50). Plasmid types (e.g., incompatibility groups) were identified using PlasmidFinder 1.3 (51), with a threshold of 95% identity on de novo assemblies. We also compared blaKPC-containing contigs extracted from our de novo assemblies with all 35 publicly available RefSeq plasmids carrying blaKPC-3, which were searched on the NCBI GenBank nucleotide database using the keyword KPC-3 as well as the categories RefSeq and plasmid (http://www.ncbi.nlm.nih.gov/nuccore). The presence/absence of the RefSeq plasmids was determined with 95% identity at the nucleotide level and 90% coverage level by BLASTn (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The presence of Tn4401 was confirmed via BLASTn, and its isoform was determined as described previously (52). Tn4401b-containing KPC-3 isolates from the UNC outbreak were compared to publicly available Tn4401b reference sequences. These were identified within the NCBI GenBank nucleotide database using the search term Tn4401b. After nonaligning database sequences and duplicate entries were removed, a maximum-likelihood phylogeny was constructed using the remaining database-derived Tn4401b sequences and our de novo-assembled sequences.
An analysis of recombination was undertaken using the method of Gubbins (53). Potential blocks of horizontal gene transfer were identified by at least three base substitutions, and recombinogenic regions were masked during construction of a maximum-likelihood phylogenetic tree using RAxML (54). Pairwise SNV differences between isolates were calculated using bedtools (https://bedtools.readthedocs.io/en/latest/). We used criteria for relatedness based on SNVs among strains which were proposed by Salipante et al.: genomically indistinguishable, 3 or fewer SNVs; closely related (up to 12 SNVs); and unrelated (13 or more SNVs) (12). In this study, a clone (Ec_UNC or Kp_UNC) was defined as closely related strains with up to 12 SNVs.
Accession number(s).
All sequence data reported in this study have been deposited at the DDBJ/EMBL/GenBank Sequence Read Archive (SRA) under accession numbers DRX055644 to DRX055673 (see Table 1).
Supplementary Material
ACKNOWLEDGMENTS
We are very grateful to Rebecca Brooks, Lauren DiBiase, and Swathi Kodavanti for their assistance in data collection. We acknowledge Maria Gergen for her experimental support. We thank Tohru Miyoshi-Akiyama for curating Enterobacter cloacae MLST Databases and the team of curators of the Institut Pasteur MLST for curating the data and making them publicly available.
This study was supported by internal funding from UNC Health Care and UNC School of Medicine. H.K. received financial support from the Japan Society for the Promotion of Science (JSPS) Postdoctoral Fellowship for Research Abroad. C.M.P. was supported by F30 AI109979 and T32 GM008719.
We report no conflicts of interest relevant to this article.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01516-16.
REFERENCES
- 1.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. 2012. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doi Y, Paterson DL. 2015. Carbapenemase-producing Enterobacteriaceae. Semin Respir Crit Care Med 36:74–84. doi: 10.1055/s-0035-1544208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. 2014. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis 20:1170–1175. doi: 10.3201/eid2007.121004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauck C, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Scalera NM, Doi Y, Kaye KS, Evans S, Fowler VG Jr, Bonomo RA, van Duin D, Antibacterial Resistance Leadership Group. 2016. Spectrum of excess mortality due to carbapenem-resistant Klebsiella pneumoniae infections. Clin Microbiol Infect 22:513–519. doi: 10.1016/j.cmi.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guh AY, Limbago BM, Kallen AJ. 2014. Epidemiology and prevention of carbapenem-resistant Enterobacteriaceae in the United States. Expert Rev Anti Infect Ther 12:565–580. doi: 10.1586/14787210.2014.902306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathers AJ, Peirano G, Pitout JD. 2015. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin Microbiol Rev 28:565–591. doi: 10.1128/CMR.00116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahn C, Syed A, Hu F, O'Hara JA, Rivera JI, Doi Y. 2014. Microbiological features of KPC-producing Enterobacter isolates identified in a U.S. hospital system. Diagn Microbiol Infect Dis 80:154–158. doi: 10.1016/j.diagmicrobio.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiedrowski LM, Guerrero DM, Perez F, Viau RA, Rojas LJ, Mojica MF, Rudin SD, Hujer AM, Marshall SH, Bonomo RA. 2014. Carbapenem-resistant Enterobacter cloacae isolates producing KPC-3, North Dakota, USA. Emerg Infect Dis 20:1583–1585. doi: 10.3201/eid2009.140344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hargreaves ML, Shaw KM, Dobbins G, Snippes Vagnone PM, Harper JE, Boxrud D, Lynfield R, Aziz M, Price LB, Silverstein KA, Danzeisen JL, Youmans B, Case K, Sreevatsan S, Johnson TJ. 2015. Clonal dissemination of Enterobacter cloacae harboring blaKPC-3 in the upper midwestern United States. Antimicrob Agents Chemother 59:7723–7734. doi: 10.1128/AAC.01291-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 2013. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep 62:165–170. [PMC free article] [PubMed] [Google Scholar]
- 11.Kanamori H, Parobek CM, Weber DJ, van Duin D, Rutala WA, Cairns BA, Juliano JJ. 2016. Next-generation sequencing and comparative analysis of sequential outbreaks caused by multidrug-resistant Acinetobacter baumannii at a large academic burn center. Antimicrob Agents Chemother 60:1249–1257. doi: 10.1128/AAC.02014-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salipante SJ, SenGupta DJ, Cummings LA, Land TA, Hoogestraat DR, Cookson BT. 2015. Application of whole-genome sequencing for bacterial strain typing in molecular epidemiology. J Clin Microbiol 53:1072–1079. doi: 10.1128/JCM.03385-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Group NCSP, Henderson DK, Palmore TN, Segre JA. 2012. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med 4:148ra116. doi: 10.1126/scitranslmed.3004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezaei E, Safari H, Naderinasab M, Aliakbarian H. 2011. Common pathogens in burn wound and changes in their drug sensitivity. Burns 37:805–807. doi: 10.1016/j.burns.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Yali G, Jing C, Chunjiang L, Cheng Z, Xiaoqiang L, Yizhi P. 2014. Comparison of pathogens and antibiotic resistance of burn patients in the burn ICU or in the common burn ward. Burns 40:402–407. doi: 10.1016/j.burns.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Bahar MA, Jamali S, Samadikuchaksaraei A. 2010. Imipenem-resistant Pseudomonas aeruginosa strains carry metallo-beta-lactamase gene blaVIM in a level I Iranian burn hospital. Burns 36:826–830. doi: 10.1016/j.burns.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Rastegar Lari A, Azimi L, Rahbar M, Fallah F, Alaghehbandan R. 2013. Phenotypic detection of Klebsiella pneumoniae carbapenemase among burns patients: first report from Iran. Burns 39:174–176. doi: 10.1016/j.burns.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 18.Markowitz SM, Smith SM, Williams DS. 1983. Retrospective analysis of plasmid patterns in a study of burn unit outbreaks of infection due to Enterobacter cloacae. J Infect Dis 148:18–23. doi: 10.1093/infdis/148.1.18. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Camacho E, Gomez-Gil R, Tobes R, Manrique M, Lorenzo M, Galvan B, Salvarelli E, Moatassim Y, Salanueva IJ, Pareja E, Codoner FM, Alvarez-Tejado M, Garcillan-Barcia MP, De la Cruz F, Mingorance J. 2014. Genomic analysis of the emergence and evolution of multidrug resistance during a Klebsiella pneumoniae outbreak including carbapenem and colistin resistance. J Antimicrob Chemother 69:632–636. doi: 10.1093/jac/dkt419. [DOI] [PubMed] [Google Scholar]
- 20.Kanamori H, Parobek CM, Juliano JJ, van Duin D, Weber DJ, Rutala WA. 2016. Genomic analysis of transmission of KPC-3-producing Enterobacter cloacae and Klebsiella pneumoniae, abstr 7987 Society for Healthcare Epidemiology of America Spring 2016 Conference, Atlanta, GA, May 2016. [Google Scholar]
- 21.Sheppard AE, Stoesser N, Wilson DJ, Sebra R, Kasarskis A, Anson LW, Giess A, Pankhurst LJ, Vaughan A, Grim CJ, Cox HL, Yeh AJ, Modernising Medical Microbiology Informatics Group, Sifri CD, Walker AS, Peto TE, Crook DW, Mathers AJ. 2016. Nested Russian doll-like genetic mobility drives rapid dissemination of the carbapenem resistance gene blaKPC. Antimicrob Agents Chemother 60:3767–3778. doi: 10.1128/AAC.00464-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalben M, Varkulja G, Basso M, Krebs VL, Gibelli MA, van der Heijden I, Rossi F, Duboc G, Levin AS, Costa SF. 2008. Investigation of an outbreak of Enterobacter cloacae in a neonatal unit and review of the literature. J Hosp Infect 70:7–14. doi: 10.1016/j.jhin.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Marchaim D, Navon-Venezia S, Schwaber MJ, Carmeli Y. 2008. Isolation of imipenem-resistant Enterobacter species: emergence of KPC-2 carbapenemase, molecular characterization, epidemiology, and outcomes. Antimicrob Agents Chemother 52:1413–1418. doi: 10.1128/AAC.01103-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoesser N, Sheppard AE, Shakya M, Sthapit B, Thorson S, Giess A, Kelly D, Pollard AJ, Peto TE, Walker AS, Crook DW. 2015. Dynamics of MDR Enterobacter cloacae outbreaks in a neonatal unit in Nepal: insights using wider sampling frames and next-generation sequencing. J Antimicrob Chemother 70:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazarovitch T, Amity K, Coyle JR, Ackerman B, Tal-Jasper R, Ofer-Friedman H, Hayakawa K, Bogan C, Lephart PR, Kaplansky T, Maskit M, Azouri T, Zaidenstein R, Perez F, Bonomo RA, Kaye KS, Marchaim D. 2015. The complex epidemiology of carbapenem-resistant Enterobacter infections: a multicenter descriptive analysis. Infect Control Hosp Epidemiol 36:1283–1291. doi: 10.1017/ice.2015.186. [DOI] [PubMed] [Google Scholar]
- 26.Lerner A, Adler A, Abu-Hanna J, Meitus I, Navon-Venezia S, Carmeli Y. 2013. Environmental contamination by carbapenem-resistant Enterobacteriaceae. J Clin Microbiol 51:177–181. doi: 10.1128/JCM.01992-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber DJ, Rutala WA, Kanamori H, Gergen MF, Sickbert-Bennett EE. 2015. Carbapenem-resistant Enterobacteriaceae: frequency of hospital room contamination and survival on various inoculated surfaces. Infect Control Hosp Epidemiol 36:590–593. doi: 10.1017/ice.2015.17. [DOI] [PubMed] [Google Scholar]
- 28.Kanamori H, Weber DJ, Rutala WA. 2016. Healthcare outbreaks associated with a water reservoir and infection prevention strategies. Clin Infect Dis 62:1423–1435. doi: 10.1093/cid/ciw122. [DOI] [PubMed] [Google Scholar]
- 29.Tofteland S, Naseer U, Lislevand JH, Sundsfjord A, Samuelsen O. 2013. A long-term low-frequency hospital outbreak of KPC-producing Klebsiella pneumoniae involving intergenus plasmid diffusion and a persisting environmental reservoir. PLoS One 8:e59015. doi: 10.1371/journal.pone.0059015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leitner E, Zarfel G, Luxner J, Herzog K, Pekard-Amenitsch S, Hoenigl M, Valentin T, Feierl G, Grisold AJ, Hogenauer C, Sill H, Krause R, Zollner-Schwetz I. 2015. Contaminated handwashing sinks as the source of a clonal outbreak of KPC-2-producing Klebsiella oxytoca on a hematology ward. Antimicrob Agents Chemother 59:714–716. doi: 10.1128/AAC.04306-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Lu X, Zong Z. 2012. Enterobacteriaceae producing the KPC-2 carbapenemase from hospital sewage. Diagn Microbiol Infect Dis 73:204–206. doi: 10.1016/j.diagmicrobio.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Vergara-Lopez S, Dominguez MC, Conejo MC, Pascual A, Rodriguez-Bano J. 2013. Wastewater drainage system as an occult reservoir in a protracted clonal outbreak due to metallo-β-lactamase-producing Klebsiella oxytoca. Clin Microbiol Infect 19:E490–E498. doi: 10.1111/1469-0691.12288. [DOI] [PubMed] [Google Scholar]
- 33.Izdebski R, Baraniak A, Herda M, Fiett J, Bonten MJ, Carmeli Y, Goossens H, Hryniewicz W, Brun-Buisson C, Gniadkowski M. 2015. MLST reveals potentially high-risk international clones of Enterobacter cloacae. J Antimicrob Chemother 70:48–56. doi: 10.1093/jac/dku359. [DOI] [PubMed] [Google Scholar]
- 34.Girlich D, Poirel L, Nordmann P. 2015. Clonal distribution of multidrug-resistant Enterobacter cloacae. Diagn Microbiol Infect Dis 81:264–268. doi: 10.1016/j.diagmicrobio.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Stoesser N, Sheppard AE, Moore CE, Golubchik T, Parry CM, Nget P, Saroeun M, Day NP, Giess A, Johnson JR, Peto TE, Crook DW, Walker AS, Modernizing Medical Microbiology Informatics Group. 2015. Extensive within-host diversity in fecally carried extended-spectrum-beta-lactamase-producing Escherichia coli isolates: implications for transmission analyses. J Clin Microbiol 53:2122–2131. doi: 10.1128/JCM.00378-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathers AJ, Stoesser N, Sheppard AE, Pankhurst L, Giess A, Yeh AJ, Didelot X, Turner SD, Sebra R, Kasarskis A, Peto T, Crook D, Sifri CD. 2015. Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae at a single institution: insights into endemicity from whole-genome sequencing. Antimicrob Agents Chemother 59:1656–1663. doi: 10.1128/AAC.04292-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. 2012. CRE toolkit—guidance for control of carbapenem-resistant Enterobacteriaceae (CRE). Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 38.Centers for Disease Control and Prevention National Healthcare Safety Network. 2016. CDC/NHSN surveillance definitions for specific types of infections. CDC, Atlanta, GA. [Google Scholar]
- 39.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 20th informational supplement. M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 40.Coil D, Jospin G, Darling AE. 2015. A5-miseq: an updated pipeline to assemble microbial genomes from Illumina MiSeq data. Bioinformatics 31:587–589. doi: 10.1093/bioinformatics/btu661. [DOI] [PubMed] [Google Scholar]
- 41.Parobek CM, Bailey JA, Hathaway NJ, Socheat D, Rogers WO, Juliano JJ. 2014. Differing patterns of selection and geospatial genetic diversity within two leading Plasmodium vivax candidate vaccine antigens. PLoS Negl Trop Dis 8:e2796. doi: 10.1371/journal.pntd.0002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holt J, McMillan L. 2014. Constructing Burrows-Wheeler transforms of large string collections via merging, p 464–471. Proceedings of the 5th ACM Conference on Bioinformatics, Computational Biology, and Health Informatics. Association for Computing Machinery, New York, NY. [Google Scholar]
- 43.Holt J, McMillan L. 2014. Merging of multi-string BWTs with applications. Bioinformatics 30:3524–3531. doi: 10.1093/bioinformatics/btu584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H. 26 May 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv arXiv:1303.3997v2 [q-bio.GN] http://arXiv.org/abs/1207.3907v2.
- 45.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. 2010. The Genome Analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wood DE, Salzberg SL. 2014. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 15:R46. doi: 10.1186/gb-2014-15-3-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, Jelsbak L, Sicheritz-Ponten T, Ussery DW, Aarestrup FM, Lund O. 2012. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 50:1355–1361. doi: 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyoshi-Akiyama T, Hayakawa K, Ohmagari N, Shimojima M, Kirikae T. 2013. Multilocus sequence typing (MLST) for characterization of Enterobacter cloacae. PLoS One 8:e66358. doi: 10.1371/journal.pone.0066358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol 43:4178–4182. doi: 10.1128/JCM.43.8.4178-4182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carattoli AZE, García-Fernández A, Voldby Larsen M, Lund O, Villa L, Møller Aarestrup F, Hasman H. 2014. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naas T, Cuzon G, Truong HV, Nordmann P. 2012. Role of ISKpn7 and deletions in blaKPC gene expression. Antimicrob Agents Chemother 56:4753–4759. doi: 10.1128/AAC.00334-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Croucher NJ, Page AJ, Connor TR, Delaney AJ, Keane JA, Bentley SD, Parkhill J, Harris SR. 2015. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res 43:e15. doi: 10.1093/nar/gku1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.