ABSTRACT
In critically ill patients, drug exposure may be influenced by altered drug distribution and clearance. Earlier studies showed that the variability in caspofungin exposure was high in intensive care unit (ICU) patients. The primary objective of this study was to determine if the standard dose of caspofungin resulted in adequate exposure in critically ill patients. A multicenter prospective study in ICU patients with (suspected) invasive candidiasis was conducted in the Netherlands from November 2013 to October 2015. Patients received standard caspofungin treatment, and the exposure was determined on day 3 of treatment. An area under the concentration-time curve from 0 to 24 h (AUC0–24) of 98 mg · h/liter was considered adequate exposure. In case of low exposure (i.e., <79 mg · h/liter, a ≥20% lower AUC0–24), the caspofungin dose was increased and the exposure reevaluated. Twenty patients were included in the study, of whom 5 had a positive blood culture. The median caspofungin AUC0–24 at day 3 was 78 mg · h/liter (interquartile range [IQR], 69 to 97 mg · h/liter). A low AUC0–24 (<79 mg · h/liter) was seen in 10 patients. The AUC0–24 was significantly and positively correlated with the caspofungin dose in mg/kg/day (P = 0.011). The median AUC0–24 with a caspofungin dose of 1 mg/kg was estimated using a pharmacokinetic model and was 114.9 mg · h/liter (IQR, 103.2 to 143.5 mg · h/liter). In conclusion, the caspofungin exposure in ICU patients in this study was low compared with that in healthy volunteers and other (non)critically ill patients, most likely due to a larger volume of distribution. A weight-based dose regimen is probably more suitable for patients with substantially altered drug distribution. (This study has been registered at ClinicalTrials.gov under registration no. NCT01994096.)
KEYWORDS: antifungal therapy, intensive care, pharmacokinetics, therapeutic drug monitoring
INTRODUCTION
Invasive candidiasis is an important cause of morbidity and mortality in immunocompromised and critically ill patients. Patients in intensive care units (ICUs) are especially at risk for invasive candidiasis due to the presence of risk factors such as the use of a central venous catheter, parenteral nutrition, renal replacement therapy, mechanical ventilation, previous broad-spectrum antibiotic therapy, immunosuppression, neutropenia, or recent major surgery (1, 2). Candidemia is the fourth most common cause of health care-associated bloodstream infections in ICU patients, and Candida species are the most commonly isolated health care-associated bloodstream pathogens (3). A mortality of 40% has been reported in patients with invasive candidiasis. Moreover, invasive candidiasis is associated with a prolonged hospital stay and increased costs (4–9).
Prompt initiation of effective antifungal therapy in the appropriate dosage is required to improve the outcome in patients with invasive candidiasis (10, 11). The Infectious Diseases Society of America (IDSA) and the European Society for Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the management of candidiasis recommend the use of an echinocandin, such as caspofungin, as primary therapy for (suspected) invasive candidiasis in critically ill patients (12, 13). In healthy volunteers receiving the standard dose of caspofungin, the mean area under the concentration-time curve from 0 to 24 h (AUC0–24) was 98 mg · h/liter (14–16).
In critically ill patients, drug exposure may be influenced by altered drug distribution and clearance (17–21), and this may require drug concentration-guided dosing (22). For ICU patients the inter- and intraindividual variabilities in caspofungin trough concentrations appeared to be high (23). Factors that were associated with low caspofungin plasma concentrations were body weight above 75 kg and hypoalbuminemia, which is present in up to 60% of ICU patients (23, 24). Capillary permeability, third spacing, and multiorgan failure may also influence the caspofungin concentration in ICU patients (23). Furthermore, caspofungin exposure might be influenced by disease severity (10, 11).
The primary objective of this study was to determine if the standard dose of caspofungin resulted in adequate exposure in critically ill patients. In addition, the exposure was reevaluated after a dose escalation in patients with low drug exposure. Finally, we used a population pharmacokinetic model to estimate at which dose the target exposure was reached.
RESULTS
Twenty patients were included in the study, and their medical records were reviewed. The median age of the patients was 56 years (range, 25 to 83 years), and 16 patients (80.0%) were Caucasian. The patients' characteristics are shown in Table 1. Patients had suspected invasive candidiasis based on culture results (Table 1) and clinical symptoms. Five patients had a positive blood culture. The median Candida score was 3.5 out of 5 (interquartile range [IQR], 2.0 to 4.0). Patients received caspofungin for a median period of 7 days (range, 3 to 25 days). Treatment with caspofungin was continued in 8 patients (40.0%), and antifungal treatment was switched to fluconazole in 6 patients (30.0%) with a Candida albicans strain that was susceptible to fluconazole. Treatment was switched to liposomal amphotericin B in 2 patients (10.0%) (in 1 patient because of lack of efficacy and in 1 patient because of possible retinal involvement). In total, 4 patients (20.0%) died during treatment with caspofungin, of whom 1 patient had a positive blood culture for Candida glabrata (MIC, 0.125 mg/liter). Liver enzymes increased in 3 patients (15.0%) during treatment with caspofungin; all 3 patients received the standard dose of caspofungin. According to the Naranjo adverse drug reaction probability scale (25), there was a possible relationship with the use of caspofungin (score of 2 out of 13 for all 3 patients).
TABLE 1.
Patient characteristics
| Characteristica | Value for patients (n = 20) |
|---|---|
| No. (%) male | 11 (55) |
| Median (range) age, yr | 56 (25–83) |
| Median (range) wt, kg | 78 (48–139) |
| No. (%) with underlying condition: | |
| Abdominal | 8 (40) |
| Cardiovascular | 2 (10) |
| Renal | 1 (5) |
| Respiratory | 4 (20) |
| Otherb | 5 (25) |
| No. (%) with reason for ICU admission: | |
| Sepsis | 10 (50) |
| Abdominal | 6 (30) |
| Renal | 2 (10) |
| Other | 2 (10) |
| Renal insufficiency | 2 (10) |
| Respiratory insufficiency | 6 (30) |
| Postoperative | 2 (10) |
| Median (range) stay in ICU, days | 16 (4–45) |
| No. (%) with CVVH | 8 (40) |
| Median (range) score | |
| APACHE II | 19 (8–34) |
| APACHE IV | 95 (47–169) |
| SAPS3 | 59 (31–104) |
| SOFA | 8 (2–20) |
| No. (%) with Candida species: | |
| C. albicans | 10 (50) |
| C. glabrata | 7 (35) |
| C. tropicalis | 1 (5) |
| Not specified | 2 (10) |
| No. (%) with culture matrix: | |
| Blood | 5 (25) |
| Drain fluidc | 6 (30) |
| CVC tip | 2 (10) |
| Pus in a closed spaced | 4 (20) |
| Throat | 3 (15) |
Abbreviations: APACHE, acute physiology and chronic health evaluation; CVC, central venous catheter; CVVH, continuous venovenous hemofiltration; ICU, intensive care unit; SAPS3, simplified acute physiology score; SOFA, sepsis-related organ failure assessment.
One patient each with HIV, lymphoma, Mizuho disease, spondylodiscitis, and pressure ulcers.
Three patients with abdominal fluid and three patients with pleural fluid.
Two abdominal, 1 hip, and 1 cardiovascular.
Caspofungin pharmacokinetics and exposure.
The population pharmacokinetic parameters of caspofungin are described using a two-compartment model, and the results are shown in Table 2. The median AUC0–24 of caspofungin was 78.1 mg · h/liter (range, 61.4 to 129.4 mg · h/liter). The mean plasma concentration-time curve is shown in Fig. 1. The AUC0–24 was below 79 mg · h/liter (i.e., ≥20% reduction) in 10 patients (50.0%). The caspofungin exposure and dose adjustments for the individual patients are shown in Table 3. The AUC0–24 of caspofungin showed no significant correlation with the caspofungin dose (not corrected for the weight of the patient) (correlation coefficient, 0.164; P = 0.490). The AUC0–24 of caspofungin showed a significant correlation with the caspofungin dose, corrected for the weight of the patient (mg/kg/day) (correlation coefficient, 0.557; P = 0.011). In addition, the weight of the patients was significantly correlated with the volume of distribution in the central compartment (V1) (correlation coefficient, 0.602; P = 0.005) and clearance (CL) (correlation coefficient, 0.604; P = 0.005) of caspofungin. No significant correlation of the caspofungin AUC0–24 with patient age, liver enzymes, albumin concentration, or disease severity scores was found (Table 4). Furthermore, the caspofungin AUC0–24 was not significantly different in patients with different gender, race, presence of continuous venovenous hemofiltration (CVVH), presence of sepsis, or interacting comedication (Table 5). Caspofungin trough concentrations were determined in 9 patients when patients were on an adequate dose regimen. The median trough concentration was 2.6 mg/liter (range, 1.0 to 3.4 mg/liter). The individual trough concentrations for each patient are shown in Table 3.
TABLE 2.
Population pharmacokinetic parameters of caspofungin (n = 20)
| Parametera | Median (range) |
|---|---|
| AUC0–24 (mg · h/liter) | 78.1 (61.4–129.4) |
| Cmin (mg/liter) | 1.7 (1.1–3.9) |
| Cmax (mg/liter) | 7.4 (4.7–14.7) |
| CL (liters/h) | 0.66 (0.37–1.26) |
| V1 (liters) | 9.1 (5.5–13.2) |
| V2 (liters) | 8.6 (5.2–59.0) |
| t1/2α (h) | 2.5 (2.2–4.0) |
| t1/2β (h) | 19.8 (12.3–66.0) |
Abbreviations: AUC0–24, area under the concentration-time curve from 0 to 24 h; Cmax, maximal concentration; Cmin, minimal concentration; CL, clearance; V1, volume of distribution in the central compartment; V2, volume of distribution in the peripheral compartment; t1/2α, half-life at α phase; t1/2β, half-life at β phase.
FIG 1.
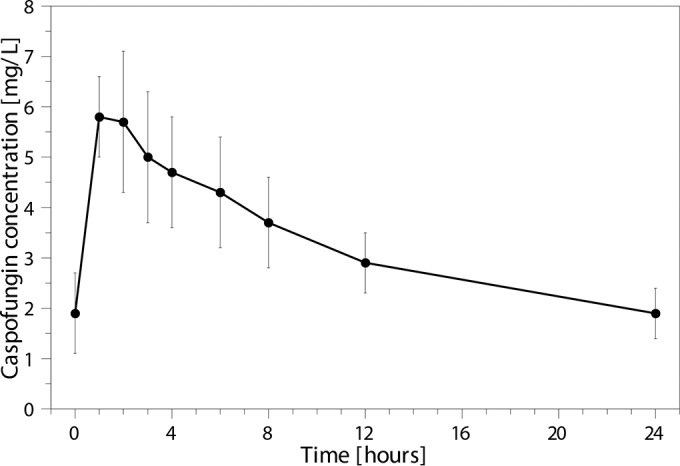
Caspofungin concentration-time curve on day 3 of treatment, showing mean with standard deviation for each time point.
TABLE 3.
Caspofungin exposure and dose adjustments for individual patients
| Patient | Wt (kg) | Caspofungin dose 1 |
Caspofungin dose 2 |
Trough concn (mg/liter)b | ||
|---|---|---|---|---|---|---|
| Dose (mg) | AUC0–24 (mg · h/liter)a | Dose (mg) | AUC0–24 (mg · h/liter) | |||
| 1 | 60 | 35c | 67.0 | 50 | 113.6 | |
| 2 | 100 | 70 | 101.9 | 2.6 | ||
| 3 | 95 | 70 | 81.8 | |||
| 4 | 48 | 50 | 109.3 | 3.4 | ||
| 5 | 79 | 50 | 71.6 | —d | ||
| 6 | 80 | 50 | 62.6 | — | ||
| 7 | 70 | 35c | 74.2 | 50 | 109.3 | 2.2 (1.0–2.8) |
| 8 | 94 | 70 | 68.0 | 100 | 127.8 | 3.0 |
| 9 | 74 | 50 | 82.1 | 2.2 | ||
| 10 | 76 | 50 | 64.3 | — | ||
| 11 | 74 | 50 | 73.2 | 70 | 91.6 | 2.0 (1.5–2.7) |
| 12 | 99 | 70 | 128.1 | 3.4 (3.4–3.4) | ||
| 13 | 83 | 70 | 82.1 | 2.8 (2.1–2.8) | ||
| 14 | 80 | 50 | 98.9 | |||
| 15 | 85 | 70 | 80.8 | |||
| 16 | 52 | 50 | 91.0 | |||
| 17 | 75 | 50 | 73.1 | — | ||
| 18 | 139 | 70 | 61.4 | 100 | 74.8 | |
| 19 | 75 | 50 | 129.4 | 2.2 (1.6–3.1) | ||
| 20 | 76 | 50 | 75.4 | — | ||
AUC0–24, area under the concentration-time curve over 24 h.
Caspofungin trough concentration expressed as median with range.
Reduced caspofungin dose due to severe liver failure/cirrhosis (Child-Pugh score C).
—, caspofungin was discontinued.
TABLE 4.
Spearman correlations with the caspofungin AUC0–24a
| Characteristic | Correlation coefficient | P value |
|---|---|---|
| Age (years) | 0.093 | 0.698 |
| Caspofungin dose (mg/kg/day) | 0.057 | 0.011 |
| Liver enzymes | ||
| ALT (U/liter) | −0.267 | 0.255 |
| AST (U/liter) | −0.259 | 0.270 |
| Bilirubin (μmol/liter) | −0.109 | 0.657 |
| Alkaline phosphatase (U/liter) | −0.085 | 0.729 |
| γ-Glutamyl transpeptidase (U/liter) | −0.060 | 0.801 |
| Albumin (g/liter) | 0.213 | 0.367 |
| Disease severity scores | ||
| APACHE II | 0.220 | 0.352 |
| APACHE IV | 0.194 | 0.426 |
| SAPS3 | 0.341 | 0.141 |
| SOFA | 0.216 | 0.361 |
Abbreviations: AUC0–24, area under the concentration-time curve from 0 to 24 h; ALT, alanine aminotransaminase; APACHE, acute physiology and chronic health evaluation; AST, aspartate aminotransaminase; SAPS, simplified acute physiology score; SOFA, sepsis-related organ failure assessment.
TABLE 5.
Caspofungin AUC0–24 in different patient groupsa
| Characteristic | Caspofungin AUC0–24h (mg · h/liter)b in patients: |
P valuec | |
|---|---|---|---|
| With characteristic | Without characteristic | ||
| Gender (male) | 74.2 (64.3–82.1) | 91.0 (72.4–105.6) | 0.175 |
| Race (Caucasian) | 78.6 (72.0–96.9) | 74.4 (67.3–96.6) | 0.682 |
| CVVH | 74.8 (67.3–102.2) | 82.0 (72.0–96.9) | 0.734 |
| Sepsis | 77.5 (70.5–103.8) | 78.6 (67.8–84.3) | 0.796 |
| Corticosteroid use | 81.8 (68.0–101.9) | 77.0 (72.0–94.7) | 0.600 |
Abbreviations: AUC0–24, area under the concentration-time curve from 0 to 24 h; CVVH, continuous venovenous hemofiltration.
Caspofungin AUC0–24h expressed as median with interquartile range.
Determined by using the Mann-Whitney U test.
Bayesian adaptive dose selection to reach the target exposure of 98 mg · h/liter resulted in a median weight-based dose regimen of caspofungin of 0.9 mg/kg (IQR, 0.7 to 1.0 mg/kg). Selecting a caspofungin dose of 1 mg/kg resulted in a median AUC0–24 of 114.9 mg · h/liter (IQR, 103.2 to 143.5 mg · h/liter). When a fixed-dose regimen of 50 mg for patients with a weight of <70 kg, 70 mg for patients weighing between 70 and 100 kg, and 100 mg for patients with a weight of ≥100 kg was selected, the median AUC0–24 was 104.3 mg · h/liter (IQR, 88.6 to 123.6 mg · h/liter).
DISCUSSION
The median AUC0–24 of caspofungin was 78 mg · h/liter and the caspofungin exposure was low in 50% of the patients receiving the standard dose of caspofungin. The caspofungin AUC0–24 was significantly correlated with the caspofungin dose in mg/kg/day, and the weight of the patients was significantly correlated with the central V1 and CL of caspofungin. Based on the results of our study, the current dose regimen, of 50 mg for patients weighing ≤80 kg and 70 mg for patients weighing >80 kg, may not be the most suitable dose regimen for ICU patients to reach the target exposure of caspofungin. Based on the population pharmacokinetic model we developed, a weight-based dose regimen of 1 mg/kg once daily would probably be a more appropriate dose regimen for ICU patients. Simulation of this dose in the model resulted in an AUC0–24 between 98 mg · h/liter, the limit set for efficacy (14–16), and 210 mg · h/liter, the limit set by the manufacturer of caspofungin for which a dose reduction is recommended (15), in all patients. The association with body weight was in agreement with an earlier study of caspofungin trough concentrations in ICU patients (23) and with a study in overweight and obese people where lower caspofungin AUCs were observed in obese persons than in thinner persons (26). Caspofungin has shown good tolerability at higher doses. The use of caspofungin in a dose of 70 to 200 mg per day was well tolerated, and the incidence of drug-related adverse events was similar for the standard and high-dose regimens (27–31). One clinical trial studied the efficacy of a higher caspofungin dose (150 mg daily). This study showed no clear benefits of this dosing strategy; however, the study was not performed particularly in ICU patients, and the caspofungin exposure was not measured (28). Since our model was built with data from only 20 patients and most patients had suspected invasive candidiasis, a weight-based dose regimen of 1 mg/kg needs to be confirmed in a larger data set with regard to the efficacy of the treatment.
The median AUC0–24 of caspofungin found in this study was 78 mg · h/liter. The AUC0–24 was low compared to the AUC0–24 of 98 mg · h/liter established in healthy volunteers (14–16), an AUC0–24 of 110 to 117 mg · h/liter found in non-critically ill patients (32, 33), and an AUC0–24 of 89 to 116 mg · h/liter established in ICU patients (34, 35). The volume of distribution in the peripheral compartment (V2) was larger than that in healthy volunteers and patients in other studies (15, 16, 32–35). The half-life (t1/2) was comparable to the t1/2 in other ICU patients (34) and was longer than the t1/2 in healthy volunteers (15, 16). The low AUC0–24 of caspofungin in our study is therefore most likely the result of a larger V. Fluid extravasation as a result of endothelial dysfunction and capillary leak, edema in sepsis, ascites, and other third spaces, fluid resuscitation, and hypoalbuminemia are factors that are frequently present in ICU patients and can all lead to an increased V and a lower drug concentration (18–20). Low AUC values and increased V in ICU patients are also seen with other antifungal drugs, such as fluconazole and anidulafungin (36, 37). Although one study showed a lower response rate among patients with a higher acute physiology and chronic health evaluation II (APACHE II) score (38), the AUC0–24 of caspofungin was not associated with disease severity scores, which was in agreement with recent findings (34, 36). Hence, the use of disease severity scores does not seem appropriate to predict pharmacokinetic parameters.
Two patients in our study suffered from severe liver damage or cirrhosis (Child-Pugh score C), and they therefore received a daily caspofungin dose of 35 mg according to the summary of product characteristics of caspofungin. The AUC0–24 was low in both patients, and the dose was increased to 50 mg, after which the AUC0–24 was adequate and liver enzymes remained stable. These results are in accordance with two case reports where 50 mg and 70 mg were given to patients with moderate hepatic dysfunction and where the exposure was similar to the exposure in healthy volunteers (39, 40). These findings illustrate that liver drug metabolism may be underestimated in the presence of liver test abnormalities or evidence of cirrhosis (41). Furthermore, a recent study in ICU patients with Child-Pugh score B showed that the recommended caspofungin dose of 35 mg resulted in low caspofungin exposure, and a simulation of several dosing regimens showed that adequate expose was reached with a daily caspofungin dose of 50 to 70 mg (42). Since the Child-Pugh score is highly driven by the albumin level, ICU patients with hypoalbuminemia are often incorrectly classified with a Child-Pugh B score. Based on the results of that study, the authors state that the caspofungin maintenance dose should not be reduced in noncirrhotic ICU patients based on the Child-Pugh score if the classification is driven by hypoalbuminemia, as the decreased maintenance dose results in a significantly lower caspofungin exposure (42). Combined with the case reports and recent study (39, 40, 42), our findings suggest that patients with moderate hepatic dysfunction should perhaps initially receive a higher empirical maintenance dose, with close follow-up with therapeutic drug monitoring (TDM) and monitoring of liver enzymes. Further research in this patient group is needed to provide evidence for the development of new dosing recommendations.
Since caspofungin was used as empirical treatment for suspected invasive candidiasis, the MIC of the Candida species was determined in only 4 patients. In vivo studies have demonstrated that the AUC/MIC ratio is a good descriptor of the echinocandin exposure-response relationship. In these in vivo studies, an AUC0–24/MIC ratio of 865 was established for a 1-log kill of C. albicans for caspofungin, an AUC0–24/MIC ratio of 450 for a 1-log kill of C. glabrata, and an AUC0–24/MIC ratio of 1,185 for a 1-log kill of C. parapsilosis (MIC values determined according to CLSI criteria) (14). Considering an AUC0–24 of approximately 100 mg · h/liter, the AUC0–24/MIC ratio target can be reached when C. albicans strains have MIC values of <0.125, when C. glabrata strains have MIC values of <0.25, and when C. parapsilosis strains have MIC values of <0.10. However, C. parapsilosis strains often have MIC values of >0.10, and therefore the AUC0–24 of approximately 100 mg · h/liter is not sufficient to reach the AUC0–24 target ratio (43). The median AUC0–24 of the patients in this study was lower; however, in case of an infection with a Candida species with a low MIC value, the AUC0–24/MIC ratio can still be sufficient with a lower AUC0–24 value. Meanwhile, a large variability in caspofungin MIC values among testing laboratories is seen, and routine in vitro susceptibility testing of caspofungin against Candida by using the CLSI or EUCAST method is currently not recommended until this reproducibility problem in susceptibility testing is resolved (44). Besides, in case of empirical treatment, the MIC of the Candida species is not known at the start of the treatment. Then, the strategy would be to acquire at least adequate exposure to cover susceptible Candida species, since a delay in appropriate antifungal therapy affects mortality (10).
In conclusion, the AUC0–24 of caspofungin in ICU patients in this study was low compared with that in healthy volunteers and other (non)critically ill patients, most likely due to a larger volume of distribution. A weight-based dose regimen of 1 mg/kg/day for patients with an increased volume of distribution and clearance, and TDM in patients with liver failure, is probably a more suitable approach to achieve optimal exposure of caspofungin, although this approach needs to be confirmed with a larger data set.
MATERIALS AND METHODS
Study design.
This multicenter prospective study was conducted at the 46-bed ICU department of the University Medical Center Groningen and the 18-bed ICU department of the Medisch Spectrum Twente, the Netherlands, from November 2013 to October 2015. Patients were eligible for inclusion if the following criteria were met: (1) age ≥18 years, (2) admission to the ICU department, (3) (suspected) invasive candidiasis according to the 2008 definition of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group (45), and (4) treatment with caspofungin. In both centers caspofungin was the standard treatment for (suspected) invasive candidiasis. Patients were excluded if they did not have an arterial line for blood sampling. The local ethics committee of both hospitals approved the study (Institutional Review Board 2012-371), and the study was registered at ClinicalTrials.gov under registration no. NCT01994096. Written informed consent was obtained from the patient or the legal representative of the patient.
Caspofungin was administered once daily by intravenous infusion over 1 h. The recommended dose regimen of caspofungin consists of a loading dose of 70 mg on day one, followed by a daily maintenance dose of 50 mg for patients weighing ≤80 kg or a daily maintenance dose of 70 mg for patients weighing >80 kg. In patients with moderate hepatic insufficiency (Child-Pugh score B), a dose reduction to 35 mg per day is recommended by the manufacturer of caspofungin. The manufacturer has no clinical experience with adult patients with severe hepatic insufficiency (Child-Pugh score C) (46). In this study, patients with severe hepatic insufficiency received a daily dose of 35 mg. As a steady state of caspofungin is reached on the first day after the loading dose (16), blood samples were taken on day 3 (±1 day) just before administration of caspofungin and 1, 2, 3, 4, 6, 8, 12, and 24 h after the start of the infusion. Caspofungin plasma concentrations were determined within 24 h, using a validated liquid chromatography-tandem mass spectrometry assay (47). An AUC0–24 of 98 mg · h/liter or higher was considered adequate exposure. C. albicans is the most common species seen in candidemia, and in vivo studies showed an AUC0–24/MIC ratio of 865 for a 1-log kill of C. albicans for caspofungin. The epidemiological cutoff value for C. albicans is 0.125 mg/liter. Therefore, an AUC of approximately 100 mg · h/liter is sufficient for the treatment of susceptible C. albicans species. Furthermore, this AUC value is in line with the AUC of 98 mg · h/liter that was seen in healthy volunteers receiving the standard dose of caspofungin (14–16). A reduction of at least 20% relative to the target AUC0–24 (i.e., AUC0–24 of <79 mg · h/liter) was considered a clinically relevant reduction (46, 48). In patients with an AUC0–24 of <79 mg · h/liter, the caspofungin dose was increased. In case of a 20 to 40% lower AUC0–24, the dose was increased by 40%, and in case of a >40% lower AUC0–24, the caspofungin dose was doubled. If the caspofungin dose was adjusted, the AUC0–24 was reevaluated 3 days (± 1day) after dose adjustment. When target exposure was attained, trough levels were measured every 3 days during treatment on the ICU, with a maximum of 28 days. This enabled the evaluation of potential fluctuations in caspofungin concentration over time. In case of an infection with a fluconazole-susceptible Candida strain, antifungal treatment could be switched to fluconazole (step-down therapy), as judged by the attending physician. In case an echinocandin was indicated, treatment with caspofungin was continued. Mortality was assessed at day 28 after the start of the caspofungin treatment.
Data collection.
Patient data were collected through review of the medical records using a standardized case report form. Demographic and clinical data were collected, including age, race, gender, weight, underlying condition, reason for ICU admission, whether or not receiving continuous venovenous hemofiltration (CVVH), Candida species and localization, and the Candida score (49). Vital signs (temperature, blood pressure, heart rate, respiratory rate, and oxygenation) and laboratory parameters (leukocyte count, C-reactive protein, albumin, bilirubin, alkaline phosphatase, aspartate aminotransaminase, alanine aminotransaminase, γ-glutamyl transpeptidase, serum electrolytes, serum urea, and serum creatinine concentration) were routinely measured on the ICU. These parameters were used to calculate disease severity scores on the day that the first AUC0–24 of caspofungin was obtained, including the acute physiology and chronic health evaluation II (APACHE II) (50) and APACHE IV (51) scores, the simplified acute physiology score (SAPS3) (52), and the sepsis-related organ failure assessment (SOFA) (53). Furthermore, data on caspofungin dose adjustments, duration of treatment with caspofungin, and relevant comedication were recorded. The potential causal relationship of adverse events with the use of caspofungin was analyzed by the attending physician and the local investigator using the Naranjo adverse drug reaction probability scale (25).
Pharmacokinetic and statistical analyses.
The caspofungin AUC0–24 was calculated using the log-linear trapezoidal rule with KINFIT, MWPharm 3.80 (Mediware, the Netherlands) (54). A two-compartment model based on the observed caspofungin concentrations was created in MWPharm using the KINPOP module with an iterative 2-stage Bayesian procedure. As a starting point for the model, pharmacokinetic parameters, including the clearance (CL), volume of distribution in the central compartment (V1), volume of distribution in the peripheral compartment (V2), and half-lives (t1/2) for distribution and elimination, were derived from those in the literature (46). Based on the initial model and the patient data, a population pharmacokinetic model for our population was parameterized. Subsequently, individual pharmacokinetic parameters were determined for each included patient from fitting the model to the patient data. Interindividual variability of the pharmacokinetic parameters was assumed to be log-normally distributed, and an assay error of 0.1 mg/liter (lower limit of quantification) plus 0.15 times the measured concentration (mg/liter) was taken into account. Individual pharmacokinetic parameters were used to describe the medians and ranges for the pharmacokinetic parameters in this population. Continuous covariates included age, weight, length, and creatinine clearance of the patient and the caspofungin dose. Categorical covariates included the gender of the patient. Finally, we used the individual pharmacokinetic data in combination with the population pharmacokinetic model to derive at which dose the target exposure of ≥98 mg · h/liter was reached.
We assessed the correlation of the caspofungin AUC0–24 with the caspofungin dose, corrected for the weight of the patient (mg/kg/day, total body weight of the patient at hospital admission), with factors that can influence the pharmacokinetics of caspofungin, such as the patient's age, liver enzymes, albumin concentration, and disease severity score. Furthermore, we compared the caspofungin AUC0–24 between patient groups with different gender, different race, whether or not receiving CVVH, the presence of sepsis, and interacting comedication. A Spearman correlation coefficient was calculated to determine correlations between two continuous variables. For comparing two groups, the Mann-Whitney U test was used. All statistical analyses were performed using SPSS Statistics for Windows, version 22.0 (IBM SPSS, Armonk, NY). A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENT
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
REFERENCES
- 1.Blumberg HM, Jarvis WR, Soucie JM, Edwards JE, Patterson JE, Pfaller MA, Rangel Frausto MS, Rinaldi MG, Saiman L, Wiblin RT, Wenzel RP. 2001. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. Clin Infect Dis 33:177–186. doi: 10.1086/321811. [DOI] [PubMed] [Google Scholar]
- 2.Kullberg BJ, Arendrup MC. 2015. Invasive candidiasis. N Engl J Med 373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 3.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK. 2014. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfaller M, Neofytos D, Diekema D, Azie N, Meier-Kriesche HU, Quan SP, Horn D. 2012. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance®) registry, 2004-2008. Diagn Microbiol Infect Dis 74:323–323. doi: 10.1016/j.diagmicrobio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Bassetti M, Taramasso L, Nicco E, Molinari MP, Mussap M, Viscoli C. 2011. Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS One 6:e24198. doi: 10.1371/journal.pone.0024198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, Herwaldt L, Pfaller M, Diekema D. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis 37:1172–1177. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 7.Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM. 2009. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 48:1695–1703. doi: 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- 8.Pappas PG, Rex JH, Lee J, Hamill RJ, Larsen RA, Powderly W, Kauffman CA, Hyslop N, Mangino JE, Chapman S, Horowitz HW, Edwards JE, Dismukes WE. 2003. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin Infect Dis 37:634–643. doi: 10.1086/376906. [DOI] [PubMed] [Google Scholar]
- 9.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. 2005. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis 41:1232–1239. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 10.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 11.Morrell M, Fraser VJ, Kollef MH. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 49:3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Flörl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ. 2012. ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18:19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 14.Andes D, Diekema DJ, Pfaller MA, Bohrmuller J, Marchillo K, Lepak A. 2010. In Vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob Agents Chemother 54:2497–2506. doi: 10.1128/AAC.01584-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mistry GC, Migoya E, Deutsch PJ, Winchell G, Hesney M, Li S, Bi S, Dilzer S, Lasseter KC, Stone JA. 2007. Single- and multiple-dose administration of caspofungin in patients with hepatic insufficiency: implications for safety and dosing recommendations. J Clin Pharmacol 47:951–961. doi: 10.1177/0091270007303764. [DOI] [PubMed] [Google Scholar]
- 16.Stone JA, Holland SD, Wickersham PJ, Sterrett A, Schwartz M, Bonfiglio C, Hesney M, Winchell GA, Deutsch PJ, Greenberg H, Hunt TL, Waldman SA. 2002. Single- and multiple-dose pharmacokinetics of caspofungin in healthy men. Antimicrob Agents Chemother 46:739–745. doi: 10.1128/AAC.46.3.739-745.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felton TW, Hope WW, Roberts JA. 2014. How severe is antibiotic pharmacokinetic variability in critically ill patients and what can be done about it? Diagn Microbiol Infect Dis 79:441–447. doi: 10.1016/j.diagmicrobio.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Pea F, Viale P, Furlanut M. 2005. Antimicrobial therapy in critically ill patients: a review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin Pharmacokinet 44:1009–1034. doi: 10.2165/00003088-200544100-00002. [DOI] [PubMed] [Google Scholar]
- 19.Power BM, Forbes AM, van Heerden PV. 1998. Pharmacokinetics of drugs used in critically ill adults. Clin Pharmacokinet 34:25–56. doi: 10.2165/00003088-199834010-00002. [DOI] [PubMed] [Google Scholar]
- 20.Smith BS, Yogaratnam D, Levasseur-Franklin KE, Forni A, Fong J. 2012. Introduction to drug pharmacokinetics in the critically ill patient. Chest 141:1327–1336. doi: 10.1378/chest.11-1396. [DOI] [PubMed] [Google Scholar]
- 21.Udy AA, Roberts JA, Lipman J. 2013. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med 39:2070–2082. doi: 10.1007/s00134-013-3088-4. [DOI] [PubMed] [Google Scholar]
- 22.Veringa A, Sturkenboom MG, Dekkers BG, Koster RA, Roberts JA, Peloquin CA, Touw DJ, Alffenaar JW. 2016. LC-MS/MS for therapeutic drug monitoring of anti-infective drugs. Trends Anal Chemistry doi: 10.1016/j.trac.2015.11.026. [DOI] [Google Scholar]
- 23.Nguyen TH, Hoppe-Tichy T, Geiss HK, Rastall AC, Swoboda S, Schmidt J, Weigand MA. 2007. Factors influencing caspofungin plasma concentrations in patients of a surgical intensive care unit. J Antimicrob Chemother 60:100–106. doi: 10.1093/jac/dkm125. [DOI] [PubMed] [Google Scholar]
- 24.Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J. 2011. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinets 50:99–110. doi: 10.2165/11539220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. 1981. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 26.Hall RG, Swancutt MA, Meek C, Leff R, Gumbo T. 2013. Weight drives caspofungin pharmacokinetic variability in overweight and obese people: fractal power signatures beyond two-thirds or three-fourths. Antimicrob Agents Chemother 57:2259–2264. doi: 10.1128/AAC.01490-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maertens J, Glasmacher A, Herbrecht R, Thiebaut A, Cordonnier C, Segal BH, Killar J, Taylor A, Kartsonis N, Patterson TF, Aoun M, Caillot D, Sable C. 2006. Multicenter, noncomparative study of caspofungin in combination with other antifungals as salvage therapy in adults with invasive aspergillosis. Cancer 107:2888–2897. doi: 10.1002/cncr.22348. [DOI] [PubMed] [Google Scholar]
- 28.Betts RF, Nucci M, Talwar D, Gareca M, Queiroz-Telles F, Bedimo RJ, Herbrecht R, Ruiz-Palacios G, Young JA, Baddley JW, Strohmaier KM, Tucker KA, Taylor AF, Kartsonis NA. 2009. A multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin Infect Dis 48:1676–1684. doi: 10.1086/598933. [DOI] [PubMed] [Google Scholar]
- 29.Cornely OA, Lasso M, Betts R, Klimko N, Vazquez J, Dobb G, Velez J, Williams-Diaz A, Lipka J, Taylor A, Sable C, Kartsonis N. 2007. Caspofungin for the treatment of less common forms of invasive candidiasis. J Antimicrob Chemother 60:363–369. doi: 10.1093/jac/dkm169. [DOI] [PubMed] [Google Scholar]
- 30.Cornely OA, Vehreschild JJ, Vehreschild MJ, Würthwein G, Arenz D, Schwartz S, Heussel CP, Silling G, Mahne M, Franklin J, Harnischmacher U, Wilkens A, Farowski F, Karthaus M, Lehrnbecher T, Ullmann AJ, Hallek M, Groll AH. 2011. Phase II dose escalation study of caspofungin for invasive aspergillosis. Antimicrob Agents Chemother 55:5798–5803. doi: 10.1128/AAC.05134-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Safdar A, Rodriguez G, Rolston KV, O'Brien S, Khouri IF, Shpall EJ, Keating MJ, Kantarjian HM, Champlin RE, Raad II, Kontoyiannis DP. 2007. High-dose caspofungin combination antifungal therapy in patients with hematologic malignancies and hematopoietic stem cell transplantation. Bone Marrow Transplant 39:157–164. doi: 10.1038/sj.bmt.1705559. [DOI] [PubMed] [Google Scholar]
- 32.Groll AH, Silling G, Young C, Schwerdtfeger R, Ostermann H, Heinz WJ, Gerss J, Kolve H, Lanvers-Kaminsky C, Vieira Pinheiro JP, Gammelin S, Cornely OA, Wuerthwein G. 2010. Randomized comparison of safety and pharmacokinetics of caspofungin, liposomal amphotericin B, and the combination of both in allogeneic hematopoietic stem cell recipients. Antimicrob Agents Chemother 54:4143–4149. doi: 10.1128/AAC.00425-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Würthwein G, Young C, Lanvers-Kaminsky C, Hempel G, Trame MN, Schwerdtfeger R, Ostermann H, Heinz WJ, Cornely OA, Kolve H, Boos J, Silling G, Groll AH. 2012. Population pharmacokinetics of liposomal amphotericin B and caspofungin in allogeneic hematopoietic stem cell recipients. Antimicrob Agents Chemother 56:536–543. doi: 10.1128/AAC.00265-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muilwijk EW, Schouten JA, van Leeuwen HJ, van Zanten AR, de Lange DW, Colbers A, Verweij PE, Burger DM, Pickkers P, Brüggemann RJ. 2014. Pharmacokinetics of caspofungin in ICU patients. J Antimicrob Chemother 69:3294–3299. doi: 10.1093/jac/dku313. [DOI] [PubMed] [Google Scholar]
- 35.Weiler S, Seger C, Pfisterer H, Stienecke E, Stippler F, Welte R, Joannidis M, Griesmacher A, Bellmann R. 2013. Pharmacokinetics of caspofungin in critically ill patients on continuous renal replacement therapy. Antimicrob Agents Chemother 57:4053–4057. doi: 10.1128/AAC.00335-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Wanrooy MJ, Rodgers MG, Uges DR, Arends JP, Zijlstra JG, van der Werf TS, Kosterink JG, Alffenaar JW. 2014. Low but sufficient anidulafungin exposure in critically ill patients. Antimicrob Agents Chemother 58:304–308. doi: 10.1128/AAC.01607-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinnollareddy MG, Roberts JA, Lipman J, Akova M, Bassetti M, De Waele JJ, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Dimopoulos G. 2015. Pharmacokinetic variability and exposures of fluconazole, anidulafungin, and caspofungin in intensive care unit patients: Data from multinational Defining Antibiotic Levels in Intensive Care Unit (DALI) Patients Study. Crit Care 19:33. doi: 10.1186/s13054-015-0758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mora-Duarte J, Betts R, Rotstein C, Colombo AL, Thompson-Moya L, Smietana J, Lupinacci R, Sable C, Kartsonis N, Perfect J. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 347:2020–2029. doi: 10.1056/NEJMoa021585. [DOI] [PubMed] [Google Scholar]
- 39.van der Elst KC, Brüggemann RJ, Rodgers MG, Alffenaar JW. 2012. Plasma concentrations of caspofungin at two different dosage regimens in a patient with hepatic dysfunction. Transpl Infect Dis 14:440–443. doi: 10.1111/j.1399-3062.2011.00716.x. [DOI] [PubMed] [Google Scholar]
- 40.Spriet I, Meersseman W, Annaert P, de Hoon J, Willems L. 2011. Pharmacokinetics of caspofungin in a critically ill patient with liver cirrhosis. Eur J Clin Pharmacol 67:753–755. doi: 10.1007/s00228-011-1066-8. [DOI] [PubMed] [Google Scholar]
- 41.Elbekai RH, Korashy HM, El-Kadi AO. 2004. The effect of liver cirrhosis on the regulation and expression of drug metabolizing enzymes. Curr Drug Metab 5:157–167. doi: 10.2174/1389200043489054. [DOI] [PubMed] [Google Scholar]
- 42.Martial LC, Brüggemann RJ, Schouten JA, van Leeuwen HJ, van Zanten AR, de Lange DW, Muilwijk EW, Verweij PE, Burger DM, Aarnoutse RE, Pickkers P, Dorlo TP. 2016. Dose reduction of caspofungin in intensive care unit patients with Child Pugh B will result in suboptimal exposure. Clin Pharmacokinet 55:723–733. doi: 10.1007/s40262-015-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfaller MA, Boyken L, Hollis RJ, Messer SA, Tendolkar S, Diekema DJ. 2006. In vitro susceptibilities of Candida spp. to caspofungin: four years of global surveillance. J Clin Microbiol 44:760–763. doi: 10.1128/JCM.44.3.760-763.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante B, Canton E, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Fothergill A, Fuller J, Gaustad P, Gonzalez GM, Guarro J, Lass-Flörl C, Lockhart SR, Meis JF, Moore CB, Ostrosky-Zeichner L, Pelaez T, Pukinskas SR, St-Germain G, Szeszs MW, Turnidge J. 2013. Interlaboratory variability of caspofungin MICs for Candida spp. using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother 57:5836–5842. doi: 10.1128/AAC.01519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merck Sharp & Dohme. 2009. Cancidas. Summary of product characteristics; Merck Sharp & Dohme, Readington, NJ. [Google Scholar]
- 47.van Wanrooy MJ, Santoe RN, van der Elst KC, Wilmer CM, van Hateren K, Wessels AM, Greijdanus B, Alffenaar JW, Uges DR. 2013. Simultaneous quantification of anidulafungin and caspofungin in plasma by an accurate and simple liquid chromatography tandem mass-spectrometric method. Ther Drug Monit 35:778–784. doi: 10.1097/FTD.0b013e31829591a7. [DOI] [PubMed] [Google Scholar]
- 48.McCormack PL, Perry CM. 2005. Caspofungin, a review of its use in the treatment of fungal infections. Drugs 65:2049–2068. doi: 10.2165/00003495-200565140-00009. [DOI] [PubMed] [Google Scholar]
- 49.León C, Ruiz-Santana S, Saavedra P, Almirante B, Nolla-Salas J, Alvarez-Lerma F, Garnacho-Montero J, León MA. 2006. A bedside scoring system (“candida score”) for early antifungal treatment in nonneutropenic critically ill patients with candida colonization. Crit Care Med 34:730–737. doi: 10.1097/01.CCM.0000202208.37364.7D. [DOI] [PubMed] [Google Scholar]
- 50.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. Apache II: a severity of disease classification system. Crit Care Med 13:818–829. [PubMed] [Google Scholar]
- 51.Zimmerman JE, Kramer AA, McNair DS, Malila FM. 2006. Acute physiology and chronic health evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med 34:1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 52.Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR. 2005. SAPS 3—from evaluation of the patient to evaluation of the intensive care unit. 2. Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med 31:1345–1355. doi: 10.1007/s00134-005-2763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. 1996. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med 22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 54.Proost JH, Meijer DK. 1992. MW/Pharm, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput Biol Med 22:155–163. doi: 10.1016/0010-4825(92)90011-B. [DOI] [PubMed] [Google Scholar]


