ABSTRACT
Bacteriophages (phages) are known to effectively kill extracellular multiplying bacteria. The present study demonstrated that phages penetrated bovine mammary epithelial cells and cleared intracellular Staphylococcus aureus in a time-dependent manner. In particular, phage vB_SauM_JS25 reached the nucleus within 3 h postincubation. The phages had an endocytotic efficiency of 12%. This ability to kill intracellular host bacteria suggests the utility of phage-based therapies and may protect patients from recurrent infection and treatment failure.
KEYWORDS: MAC-T cells, Staphylococcus aureus, bacteriophages
TEXT
Although traditionally considered an extracellular pathogen, Staphylococcus aureus is able to internalize into host cells, including professional (i.e., macrophages and neutrophils) and nonprofessional (i.e., bovine mammary epithelial cells) phagocytic cells, which is associated with chronic and recurrent infections (1, 2). Phage therapy appears to be a potent and safe alternative tool for treating such bacterial infections. Recent studies have investigated the effect of experimental phage therapy on intracellular killing of bacteria in patients' peripheral blood monocytes, polymorphonuclear neutrophils, and murine macrophages (3–6). We set out to study the intracellular killing potential of such lytic phages in nonprofessional phagocytic cells (7, 8). We investigated this question using phage vB_SauM_JS25, a broad-spectrum virulent phage belonging to the family Myoviridae (9).
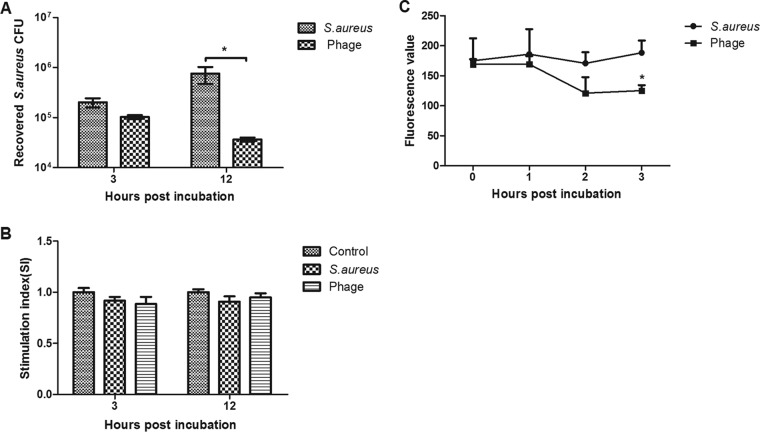
Mammary epithelial cells play an essential role in the surveillance of mammary tissue during infection by assisting immune cell recruitment and bacterial recognition (10). Can phages penetrate within nonprofessional phagocytic cells and eliminate intracellular S. aureus? To answer this, bovine mammary epithelial (MAC-T) cells were cultured in 24-well plates (105 cells/well), incubated overnight (37°C, 5% CO2) in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (Sigma, Milan, Italy) and then infected with S. aureus JYG2 (106 bacteria/well). One hour after infection at 37°C in 5% CO2, cells were washed three times with phosphate-buffered saline (PBS), and extracellular bacteria were killed with lysostaphin (20 μg/ml). Two hours later, cells were washed and treated with phage vB_SauM_JS25 (108 PFU/well) and incubated for another 3 or 12 h. Then cells were washed three times with PBS and treated with citric acid buffer (40 mM citric acid, 10 mM KCl, 135 mM NaCl, pH 3.0) for 2 min to inactivate any phage particles that remained on the surface (11). The cells were washed two times with medium to remove the acid buffer, followed by digestion with 0.25% trypsogen and lysis with Triton X-100 (final concentration, 0.1%) to recover intracellular bacteria. Each lysate was then serially diluted in saline and plated on Baird-Parker agar (12). After 3 h of incubation, we observed a modest reduction in intracellular bacteria in phage-treated cells (Fig. 1A), although this was not statistically significant (P > 0.05). However, after 12 h of incubation, phage treatment resulted in a significant reduction in intracellular bacteria (P < 0.05). The longer the cells were inoculated with phage vB_SauM_JS25, the higher the observed inhibitory effect.
FIG 1.
Bacteriophage vB_SauM_JS25 eliminates intracellular Staphylococcus aureus JYG2 in a time-dependent manner. (A) MAC-T cells were distributed in 24-well plates (105 cells/well), incubated overnight, and then infected with S. aureus JYG2 (106 bacteria/well). After incubation for 1 h at 37°C in 5% CO2, extracellular bacteria were killed with lysostaphin (20 μg/ml). Cells were washed with DMEM. Two hours later, cells were treated with phage vB_SauM_JS25 (108 PFU/well) and incubated for another 3 or 12 h. Then, cells were washed with PBS and treated with citric acid buffer for 2 min to inactivate any phage particles that remained on the surface, followed by lysis and dilution. (B) Cytotoxic damage caused by S. aureus JYG2 and phage vB_SauM_JS25 on MAC-T cells was determined by MTT assay. Stimulation index (SI) was calculated as SI = optical density at 570 nm (OD570) of treatment cells/OD570 of control cells. (C) MAC-T cells were infected with S. aureus JYG2 labeled with WGA 488 conjugate. Cells were washed with DMEM. After 2 h of treatment, cells were incubated with phage vB_SauM_JS25 (106 PFU/well). At the indicated time, the fluorescence in each well was measured at 495 nm for excitation and 519 nm for emission with a fluorescence plate reader. *, statistical analysis using one-way or two-way analysis of variance at P < 0.05.
To determine whether this reduction in bacteria depended on cytotoxic damage, MAC-T cell viability was measured using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) (Sigma-Aldrich, St. Louis, MO) as previously described (4). The cytotoxic damage did not differ among the three groups at any time (Fig. 1B). To confirm this result by fluorescence assay, S. aureus JYG2 was fluorescently labeled with Alexa Fluor 488 conjugate of wheat germ agglutinin (WGA) (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. WGA binds to N-acetylglucosamine residues. MAC-T cells plated in 96-well plates were infected with S. aureus JYG2 labeled with WGA conjugate (104 bacteria/well). After incubation for 1 h at 37°C in 5% CO2, extracellular bacteria were killed with lysostaphin (20 μg/ml). Cells were washed with DMEM. After 2 h of treatment, cells were incubated with phage vB_SauM_JS25 (106 PFU/well). At the indicated time, fluorescence in a whole well was measured with a fluorescence plate reader (Tecan Infinite M200). Consistently, phage vB_SauM_JS25 significantly (P < 0.05) inhibited intracellular bacteria at 3 h postincubation (Fig. 1C).
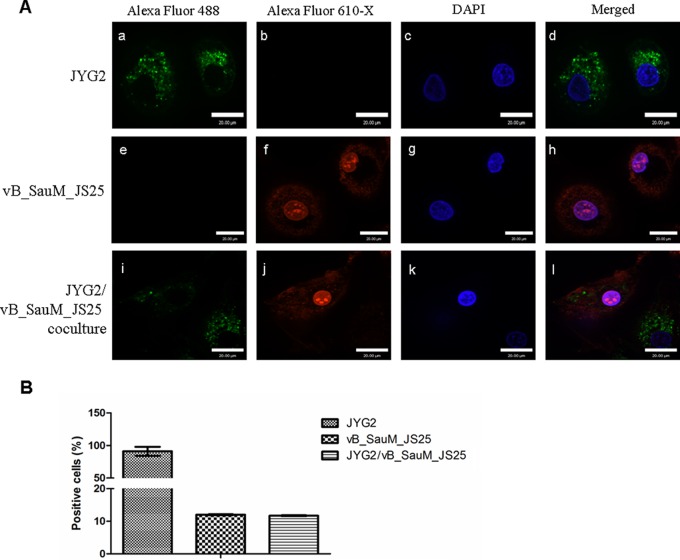
Phages can interact with mucus-producing cells via immunoglobulin-like protein domains and reduce microbial colonization (13). To ensure that phage vB_SauM_JS25 penetrated bovine mammary epithelial cells and eliminated intracellular S. aureus, phage vB_SauM_JS25 and S. aureus JYG2 were fluorescently labeled with Alexa Fluor 488 conjugate and 610-X N-hydroxysuccinimide (NHS) ester (Invitrogen), respectively. The NHS ester of 610-X can be used to label the primary amines (R-NH2) of proteins. The phages were ultrafiltrated through a cellulose membrane (Millipore, Billerica, MA) with a nominal molecular mass limit of 100 kDa to remove excess Alexa Fluor 610-X. S. aureus JYG2 was washed three times before infection. MAC-T cells were plated in a 12-mm glass-based dish (Nunc, Thermo Fisher Scientific, Waltham, MA). The cells were challenged with S. aureus JYG2 labeled with WGA conjugate (106 bacteria/well). One hour later, cells were washed with DMEM, and extracellular bacteria were killed with lysostaphin (20 μg/ml). After 2 h of treatment, cells were washed and incubated with Alexa Fluor 610-X conjugate-labeled phage vB_SauM_JS25 (108 PFU/well). Three hours later, cells were treated with citric acid buffer (40 mM citric acid, 10 mM KCl, 135 mM NaCl, pH 3.0). Then washed cells were labeled with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen) in PBS for 5 min and subsequently washed again for 10 min. Glass-based dishes were mounted onto microscope slides, and the samples were examined using an UltraView VoX confocal microscope (PerkinElmer) (14). The percentage of Alexa Fluor 488- or 610-X-labeled positive cells was determined by counting 200 cells from 10 randomly selected fields at ×400 magnification on a confocal microscope as previously described (15). Briefly, the phage positive rate was recorded as (number of 610-X-labeled positive cells/200 cells) × 100%. Results indicated that phage vB_SauM_JS25 crossed MAC-T cell membranes and cleared intracellular S. aureus (Fig. 2A). In particular, phage vB_SauM_JS25 reached the nucleus within 3 h postincubation (Fig. 2Af, h, j, and l). The reason for entrance of labeled phage into the nucleus is unclear and needs further investigation. The endocytotic efficiency of phage vB_SauM_JS25 was ∼12% (Fig. 2B). Previous studies showed that the phage particles transferred to the interior of macrophages and significantly reduced the damage caused by the cytotoxic effects of bacteria on phagocytes (4, 16). Moreover, Barr et al. (13) reported that interaction of phages and mucus-producing cells does occur in the elimination of bacteria, and this interaction is based on immunoglobulin-like domains. The interaction via immunoglobulin-like domains may help phages cross MAC-T cell membranes. The mechanism by which phages are internalized in MAC-T cells will be further studied.
FIG 2.
Confocal microscopy of internalized phage vB_SauM_JS25 and intracellular S. aureus JYG2. (A) Nucleus (blue, DAPI, c, g, k), JYG2 (green, Alex Fluor 488 conjugated WGA, a, e, i), and vB_SauM_JS25 (red, Alex Fluor 610-X, b, f, j) are shown in the same focal plane. vB_SauM_JS25 (red) is colocalized with JYG2 (green) in the merged image (d, h, l). The maximum intensity projection shows all Z-stacks simultaneously. Scale bar, 20 μm. (B) Percentage of MAC-T cells positively labeled after being infected with stained JYG2 and vB_SauM_JS25, individually or combined. A total of 200 cells from 10 randomly selected fields at ×400 magnification were analyzed by confocal microscopy.
Our in vitro results with MAC-T bovine mammary epithelial cells suggest that phage vB_SauM_JS25 may be a potential therapeutic candidate for treatment of S. aureus infection of bovine mammary epithelial cells in vivo.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant nos. 31602078 and 31302009), Jiangsu Provincial Natural Science Foundation of China (BK20140458), the Jiangsu Agricultural Science and Technology Foundation [CX (14)5062], and the National Agricultural Product Quality and Safety Risk Assessment (GJFP201601203).
We are grateful to Honglin Jiang for providing MAC-T cells.
We thank Jiangsu Collaborative Innovation Center of Meat Production and Processing, Quality and Safety Control.
REFERENCES
- 1.Bardiau M, Detilleux J, Farnir F, Mainil JG, Ote I. 2014. Associations between properties linked with persistence in a collection of Staphylococcus aureus isolates from bovine mastitis. Vet Microbiol 169:74–79. doi: 10.1016/j.vetmic.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Alva-Murillo N, Medina-Estrada I, Baez-Magana M, Ochoa-Zarzosa A, Lopez-Meza JE. 2015. The activation of the TLR2/p38 pathway by sodium butyrate in bovine mammary epithelial cells is involved in the reduction of Staphylococcus aureus internalization. Mol Immunol 68:445–455. doi: 10.1016/j.molimm.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Jonczyk-Matysiak E, Lusiak-Szelachowska M, Klak M, Bubak B, Miedzybrodzki R, Weber-Dabrowska B, Zaczek M, Fortuna W, Rogoz P, Letkiewicz S, Szufnarowski K, Gorski A. 2015. The effect of bacteriophage preparations on intracellular killing of bacteria by phagocytes. J Immunol Res 2015:482863. doi: 10.1155/2015/482863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur S, Harjai K, Chhibber S. 2014. Bacteriophage-aided intracellular killing of engulfed methicillin-resistant Staphylococcus aureus (MRSA) by murine macrophages. Appl Microbiol Biotechnol 98:4653–4661. doi: 10.1007/s00253-014-5643-5. [DOI] [PubMed] [Google Scholar]
- 5.Capparelli R, Parlato M, Borriello G, Salvatore P, Iannelli D. 2007. Experimental phage therapy against Staphylococcus aureus in mice. Antimicrob Agents Chemother 51:2765–2773. doi: 10.1128/AAC.01513-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broxmeyer L, Sosnowska D, Miltner E, Chacon O, Wagner D, McGarvey J, Barletta RG, Bermudez LE. 2002. Killing of Mycobacterium avium and Mycobacterium tuberculosis by a mycobacteriophage delivered by a nonvirulent mycobacterium: a model for phage therapy of intracellular bacterial pathogens. J Infect Dis 186:1155–1160. doi: 10.1086/343812. [DOI] [PubMed] [Google Scholar]
- 7.Nieth A, Verseux C, Barnert S, Suss R, Romer W. 2015. A first step toward liposome-mediated intracellular bacteriophage therapy. Expert Opin Drug Deliv 12:1411–1424. doi: 10.1517/17425247.2015.1043125. [DOI] [PubMed] [Google Scholar]
- 8.Peng L, Chen BW, Luo YA, Wang GZ. 2006. Effect of mycobacteriophage to intracellular mycobacteria in vitro. Chin Med J (Engl) 119:692–695. [PubMed] [Google Scholar]
- 9.Zhang L, Bao H, Wei C, Zhang H, Zhou Y, Wang R. 2015. Characterization and partial genomic analysis of a lytic Myoviridae bacteriophage against Staphylococcus aureus isolated from dairy cows with mastitis in Mid-east of China. Virus Genes 50:111–117. doi: 10.1007/s11262-014-1130-4. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert FB, Cunha P, Jensen K, Glass EJ, Foucras G, Robert-Granie C, Rupp R, Rainard P. 2013. Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system. Vet Res 44:40. doi: 10.1186/1297-9716-44-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brindley MA, Maury W. 2008. Equine infectious anemia virus entry occurs through clathrin-mediated endocytosis. J Virol 82:1628–1637. doi: 10.1128/JVI.01754-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slinko S, Caspersen C, Ratner V, Kim JJ, Alexandrov P, Polin R, Ten VS. 2007. Systemic hyperthermia induces ischemic brain injury in neonatal mice with ligated carotid artery and jugular vein. Pediatr Res 62:65–70. doi: 10.1203/PDR.0b013e3180676cad. [DOI] [PubMed] [Google Scholar]
- 13.Barr JJ, Auro R, Furlan M, Whiteson KL, Erb ML, Pogliano J, Stotland A, Wolkowicz R, Cutting AS, Doran KS, Salamon P, Youle M, Rohwer F. 2013. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc Natl Acad Sci U S A 110:10771–10776. doi: 10.1073/pnas.1305923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Y, Barros M, Vennemann T, Gallagher DT, Yin Y, Linden SB, Heselpoth RD, Spencer DJ, Donovan DM, Moult J, Fischetti VA, Heinrich F, Losche M, Nelson DC. 2016. A bacteriophage endolysin that eliminates intracellular streptococci. eLife 5:e13152. doi: 10.7554/eLife.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai YC, Chang HW, Jeng CR, Lin TL, Lin CM, Wan CH, Pang VF. 2012. The effect of infection order of porcine circovirus type 2 and porcine reproductive and respiratory syndrome virus on dually infected swine alveolar macrophages. BMC Vet Res 8:174. doi: 10.1186/1746-6148-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kazmierczak Z, Piotrowicz A, Owczarek B, Hodyra K, Miernikiewicz P, Lecion D, Harhala M, Gorski A, Dabrowska K. 2014. Molecular imaging of T4 phage in mammalian tissues and cells. Bacteriophage 4:e28364. doi: 10.4161/bact.28364. [DOI] [PMC free article] [PubMed] [Google Scholar]