ABSTRACT
The three-direct-acting antiviral (3D) regimen containing ombitasvir, paritaprevir, ritonavir, and dasabuvir with or without ribavirin (RBV) is approved for treatment of hepatitis C virus (HCV) genotype 1 (GT1)/human immunodeficiency virus type 1 (HIV-1) coinfection. Results of a pharmacokinetic substudy of 3D and darunavir are presented. HCV/HIV-1-coinfected subjects were randomized to maintain an antiretroviral regimen with darunavir at 800 mg once daily (QD) or switched to a regimen with darunavir at 600 mg twice daily (BID). On study day 1, subjects received 3D and RBV plus darunavir for 12 weeks. Pharmacokinetic parameters were compared for darunavir and ritonavir with and without 3D (week 4 and day −1). Pharmacokinetic parameters of 3D were compared to historical data. Ten subjects received darunavir QD, and 12 subjects received darunavir BID. The central value ratios (90% confidence interval [CI]) for maximum concentrations (Cmax), area under the plasma concentration-time curve between 0 and 24 h postdose (AUC24), and trough plasma concentration at 24 h postdose (C24) of darunavir administered QD with 3D versus administration of darunavir alone were 0.92 (0.72, 1.18), 0.83 (0.71, 0.98), and 0.64 (0.44, 0.93), respectively. The ratios (90% CI) for darunavir Cmax, AUC12, and C12 administered BID with 3D were 0.92 (0.76, 1.12), 0.88 (0.73, 1.05), and 0.73 (0.58, 0.92), respectively. Exposures of 3D were similar to or slightly lower than those in historical data. All darunavir trough concentrations (Ctrough) associated with an HIV-1 RNA level of >40 copies/ml were above the darunavir 50% effective concentration (EC50) of 550 ng/ml for resistant virus. In conclusion, the 3D regimen with darunavir QD or BID did not affect darunavir Cmax and AUC, whereas the darunavir Ctrough decreased. Changes in pharmacokinetic parameters of 3D were not considered clinically significant. Episodes of intermittent HIV-1 viremia were infrequent and were not associated with darunavir Ctrough values below 550 ng/ml. (This study has been registered at ClinicalTrials.gov under identifier NCT01939197.)
KEYWORDS: DAA, darunavir, HCV, HIV, paritaprevir, dasabuvir, ombitasvir
INTRODUCTION
Approximately 2.3 million people infected with the human immunodeficiency virus (HIV) are coinfected with hepatitis C virus (HCV) (1). Historically, the use of pegylated interferon (pegIFN) plus ribavirin (RBV) for the treatment of HCV has been associated with very low rates of sustained virological response (SVR) and significant toxicities in this patient population (2). The introduction of safe and highly effective direct-acting antivirals (DAA) for the treatment of HCV, however, changed the landscape of treatment in HCV/HIV-coinfected patients. Comparable SVR rates have been reported between HCV/HIV-coinfected patients and monoinfected HCV patients receiving all-oral DAA regimens (3–5). Although newer DAAs are associated with fewer drug interactions than older treatment options, the potential for interactions exists, especially in HCV/HIV-coinfected patients taking multidrug antiretroviral therapy.
The all-oral three-DAA (3D) regimen of ombitasvir (OBV)/paritaprevir (PTV)/ritonavir (RTV or r) and dasabuvir (DSV) for the treatment of chronic HCV genotype 1 (GT1) infection is approved for treatment in HCV patients coinfected with human immunodeficiency virus type 1 (HIV-1). PTV is a nonstructural protein 3/nonstructural protein 4A (NS3/NS4A) protease inhibitor (identified as a lead compound by AbbVie and Enanta) coadministered with the pharmacokinetic (PK) enhancer RTV. OBV is a nonstructural protein 5A (NS5A) inhibitor, and DSV is a NS5B nonnucleoside polymerase inhibitor. OBV and PTV/r have been coformulated into a single fixed-dose combination tablet (PTV at 75 mg, ritonavir at 50 mg, and OBV at 12.5 mg) given as two tablets once daily (QD). DSV is available as a 250-mg tablet dosed twice daily (BID).
The major concern for drug interactions with the 3D regimen is the significant cytochrome P450 inhibition by RTV. Initial pharmacokinetic studies in healthy volunteers evaluated the combination of darunavir (DRV) at 800 mg QD or 600 mg BID with the 3D regimen (6). In the presence of 3D, DRV maximum concentrations (Cmax) and values for area under the plasma concentration-time curve (AUC) when DRV was administered QD or BID were comparable to DRV Cmax and AUC values when DRV was administered QD or BID with 100 mg of RTV. However, in the presence of 3D, DRV trough concentrations (Ctrough) decreased 48% with the QD regimen and 43% with the BID regimen. DRV 800 mg QD is recommended in Europe if administered at the same time as the 3D regimen and in the absence of extensive protease inhibitor resistance. DRV, however, is not recommended in combination with the 3D regimen in the United States. A phase 2 pilot cohort study was designed to evaluate the safety and efficacy of several antiretroviral regimens in HCV/HIV-coinfected patients receiving the 3D regimen before evaluation of the regimens in a larger phase 3 study. A substudy compared the pharmacokinetics of DRV administered with RTV QD or BID to the pharmacokinetics of DRV administered with the 3D regimen in patients coinfected with HCV/HIV.
RESULTS
Demographics.
Due to subject availability, only 22 subjects were enrolled in the pharmacokinetic substudy: 10 subjects in arm C and 12 subjects in arm D. Ritonavir data for one subject in arm C at day −1 were excluded from the analyses since PK parameters could not be determined due to missing data at several time points. Baseline patient demographics are summarized in Table 1.
TABLE 1.
Demographic summary for all subjectsa
| Arm (no. of subjects) | DRV dose | Mean ± SD (minimum, maximum) |
No. (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | Wt (kg) | Ht (cm) | BMI (kg/m2) | Sex (male/female) | Race (White/black/Asian) | HCV genotype (1a/1b) | Cirrhosis diagnosis | ||
| C (10) | QD | 55.0 ± 6.62 (44, 65) | 78.9 ± 13.6 (61, 103) | 174 ± 12.6 (155, 188) | 26.1 ± 3.1 (21, 31) | 8 (80)/2 (20) | 7 (70)/3 (30) | 9 (90)/1 (10) | 0 (0) |
| D (12) | BID | 52.4 ± 11.3 (34, 68) | 82.4 ± 17.3 (59, 111) | 172 ± 8.9 (160, 188) | 27.9 ± 6.1 (22, 38) | 9 (75)/3 (25) | 5 (42)/6 (50)/1 (8) | 6 (50)/6 (50) | 3 (25) |
| Total (22) | 53.6 ± 9.35 (34, 68) | 80.8 ± 15.5 (59, 111) | 173 ± 10.5 (155, 188) | 27.1 ± 5.0 (21, 38) | 17 (77)/5 (23) | 12 (54.5)/9 (41)/1 (4.5) | 15 (68)/7 (32) | 3 (13.6) | |
SD, standard deviation; BMI, body mass index.
Plasma HIV RNA.
Two (20%) subjects receiving DRV QD (arm C) and three (25%) subjects receiving DRV BID (arm D) had at least one plasma HIV-1 RNA value that was greater than or equal to the lower limit of quantitation (LLOQ) during the treatment period. In arm C, one subject was observed to have a viral load of >40 copies/ml at day 1 (before initiation of the 3D regimen), week 4, week 6, week 8, and week 10 of therapy; the highest viral load was ≤79 copies/ml. The other subject in arm C had a viral load of 50 copies/ml at week 10 but reached viral suppression by week 12. In arm D, one subject was observed to have a viral load of >40 copies/ml at day 1 (before initiation of the 3D regimen), week 1, week 4, and week 6 of therapy but viral load suppression was achieved by week 8. One subject had a viral load of 42 copies/ml at week 8 but returned to viral load suppression by week 10. One subject had a viral load of 64 copies/ml at the end of study treatment that was resuppressed at the subsequent visit. No subjects in either treatment arm required a switch of their HIV-1 antiretroviral therapy (ART) regimen due to loss of plasma HIV-1 RNA suppression.
Pharmacokinetics.
The geometric mean (arithmetic mean, %CV) DRV and RTV pharmacokinetic parameters after QD or BID dosing with (week 4) and without (day −1) the 3D regimen are summarized in Table 2. DRV Cmax, AUC12 or AUC24, and C12 or C24 ratios of central values and 90% confidence intervals for the comparisons of all subjects receiving DRV QD or BID are presented in Table 3. Geometric mean (%CV) PK parameters for OBV, PTV, RTV, DSV, and RBV are summarized in Table 4. DRV concentration-time profiles are shown in Fig. 1.
TABLE 2.
Pharmacokinetic parameters of darunavir and ritonavir QD (arm C) and BID (arm D)
| Parameter | Geometric mean (arithmetic mean, % CV)h |
|
|---|---|---|
| Study day −1a | Wk 4b | |
| Arm C: darunavir 800 mg QD | ||
| No. of subjects | 10 | 10 |
| Cmax (ng/ml) | 7,380 (7,570, 24) | 6,820 (7,210, 30) |
| Tmax (h)c | 3.0 (2.0–6.0) | 3.0 (2.0–6.0) |
| AUC24 (ng · h/ml) | 86,800 (90,000, 25) | 72,300 (75,000, 27) |
| t½ (h)d,e | 11.7 (12.5, 37) | 7.9 (8.7, 48) |
| C24 (ng/ml) | 1,630 (1,770, 45) | 1,050 (1,240, 63) |
| Arm C: ritonavir 100 mg QD | ||
| No. of subjects | 9f | 10 |
| Cmax (ng/ml) | 496 (539, 38) | 881 (992, 46) |
| Tmax (h)c | 5.0 (2.0–8.0) | 4.0 (2.0–5.0) |
| AUC24 (ng · h/ml) | 5,570 (5,880, 33) | 6,910 (7,300, 32) |
| C24 (ng/ml) | 71.9 (74.0, 30) | 48.1 (62.0, 76) |
| Arm D: darunavir 600 mg BID | ||
| No. of subjects | 12 | 12 |
| Cmax (ng/ml) | 8,120 (8,610, 35) | 7,470 (8,040, 41) |
| Tmax (h)c | 4.0 (0.0–5.0) | 3.0 (1.0–5.0) |
| AUC12 (ng · h/ml) | 66,100 (73,700, 48) | 57,900 (65,500, 49) |
| t½ (h)d,g | 8.9 (10.7, 69) | 5.8 (6.3, 68) |
| C12 (ng/ml) | 3,550 (4,530, 62) | 2,590 (3,470, 67) |
| Arm D: ritonavir 100 mg BID | ||
| No. of subjects | 12 | 12 |
| Cmax (ng/ml) | 941 (1,100, 59) | 1,210 (1,440, 64) |
| Tmax (h)c | 4.0 (0.0–5.0) | 4.0 (0.0–6.0) |
| AUC12 (ng · h/ml) | 6,530 (7,430, 54) | 7,520 (8,820, 67) |
| C12 (ng/ml) | 313 (353, 50) | 304 (386, 73) |
Study day −1, darunavir/ritonavir at 800/100 mg QD or 600/100 mg BID.
Study day 28, darunavir at 800 mg QD or 600 mg BID (plus ritonavir at 100 mg with evening dose) plus ombitasvir/paritaprevir/r at 25/150/100 mg QD plus dasabuvir at 250 mg BID.
Median (minimum to maximum).
Harmonic mean (median, percent pseudo-CV).
n = 9.
One subject scheduled for RTV administration was excluded from the summary at day −1 since PK parameters could not be determined due to missing data at several time points.
n = 11.
CV, coefficient of variation. Data represent geometric means, arithmetic means, and percent CV except where otherwise indicated.
TABLE 3.
DRV Cmax, AUC, and Ctrough ratios of central values and 90% confidence intervals for darunavir at 800 mg QD (arm C) or darunavir at 600 mg BID (arm D)
| Pharmacokinetic parameter | Central valueb |
Ratio of central valuesa |
||
|---|---|---|---|---|
| Study day −1c | Wk 4d | Point estimatee | 90% confidence interval | |
| Darunavir 800 mg QD | ||||
| Cmax (ng/ml) | 7,380 | 6,820 | 0.924 | 0.723–1.18 |
| AUC24 (ng · h/ml) | 86,800 | 72,300 | 0.833 | 0.711–0.975 |
| C24 (ng/ml) | 1,630 | 1,050 | 0.643 | 0.443–0.934 |
| Darunavir 600 mg BID | ||||
| Cmax (ng/ml) | 8,120 | 7,470 | 0.921 | 0.755–1.12 |
| AUC12 (ng · h/ml) | 66,100 | 58,000 | 0.876 | 0.732–1.05 |
| C12 (ng/ml) | 3,550 | 2,590 | 0.730 | 0.578–0.921 |
Week 4/day −1 ratio.
Antilogarithm of the least-squares means for logarithms.
Study day −1, darunavir/ritonavir at 800/100 mg QD or 600 mg/100 mg BID.
Week 4, darunavir at 800 mg QD or darunavir at 600 mg BID (plus ritonavir at 100 mg with the evening dose) plus ombitasvir/paritaprevir/r at 25/150/100 mg QD plus dasabuvir at 250 mg BID.
Antilogarithm of the difference of the least-squares means for logarithms.
TABLE 4.
Pharmacokinetic parameters of DAAs, ritonavir, and ribavirin in HCV/HIV-coinfected subjects at week 4
| Antiviral and parameter | Geometric mean (% coefficient of variation) |
|
|---|---|---|
| Darunavir 800 mg QD (n = 10) | Darunavir 600 mg BID (n = 12) | |
| Ombitasvir | ||
| Cmax (ng/ml) | 92.6 (35) | 71.7 (38) |
| AUC24 (ng · h/ml) | 1,120 (36) | 944 (40) |
| C24 (ng/ml) | 20 (70) | 21 (45) |
| Paritaprevir | ||
| Cmax (ng/ml) | 1,440 (106) | 1,240 (109) |
| AUC24 (ng · h/ml) | 7,650 (139) | 6,710 (124) |
| C24 (ng/ml) | 19 (218) | 44 (133) |
| Ritonavir | ||
| Cmax (ng/ml) | 881 (46) | 1,209 (64) |
| AUCt (ng · h/ml) | 6,910 (32) | 7,517 (67)a |
| C24 (ng/ml) | 48 (76) | NAb |
| Dasabuvir | ||
| Cmax (ng/ml) | 725 (45) | 572 (39) |
| AUC12 (ng · h/ml) | 4,400 (48) | 3,510 (40) |
| C12 (ng/ml) | 198 (33) | 118 (46) |
| Ribavirin | ||
| Cmax (ng/ml) | 2,210 (24) | 2,810 (31) |
| AUC12 (ng · h/ml) | 21,500 (27) | 27,400 (36) |
| C12 (ng/ml) | 1,610 (34) | 1,970 (44) |
Value is AUC12.
NA, not applicable since RTV was dosed BID.
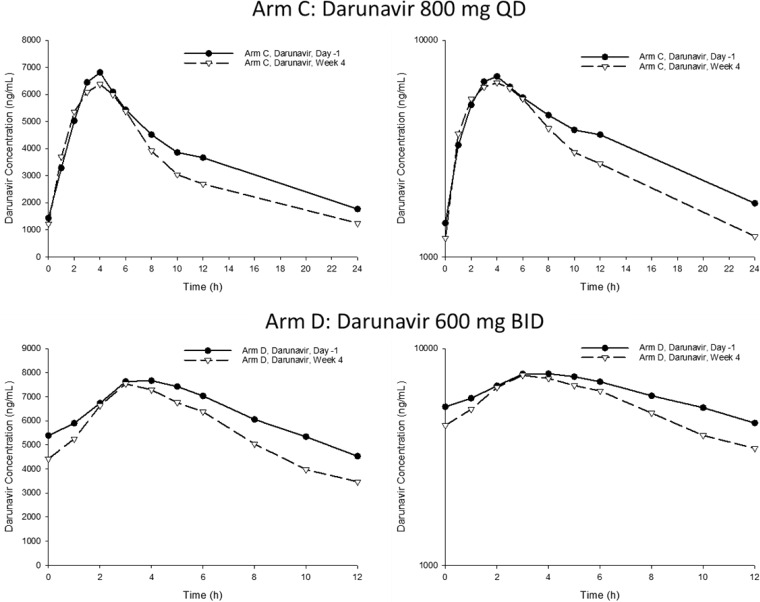
FIG 1.
Mean darunavir QD and BID plasma concentration-time profiles on linear (left) and log-linear (right) scales. Darunavir/ritonavir was administered at 800/100 mg QD or 600/100 mg BID on day 1; 3D/darunavir was administered at 800 mg QD or 600 mg BID (plus 100 mg ritonavir with evening dose).
Sparse plasma samples for quantitation of DRV, ritonavir, and DAAs were collected at various times throughout the treatment period. Not all sparse samples were collected 22 to 26 h postdose for the QD arm and 10 to 14 h postdose for the BID arm. Thus, 123 DRV trough concentrations (60 in arm C and 63 in arm D) were available from 22 subjects over the treatment period.
In subjects taking DRV QD, 52 of 60 (87%) Ctrough values were above 550 ng/ml, the 50% effective concentration (EC50) (adjusted for protein binding) for resistant virus (7–9). The lowest measured DRV exposure for the one subject who failed to maintain viral load suppression was 618 ng/ml at week 4. The DRV Ctrough value in the subject who had a viral load of >40 copies/ml at week 10 was 595 ng/ml. However, 2 weeks later, the Ctrough value was 3,890 ng/ml and the plasma HIV-1 RNA level was suppressed. In subjects taking DRV BID, 60 of 63 (95%) Ctrough values were above 550 ng/ml. In both arms, all DRV trough concentrations associated with an HIV-1 RNA level of >40 copies/ml were above the DRV EC50 of 550 ng/ml for resistant virus.
DISCUSSION
These are the first intensive pharmacokinetic data of DRV QD or BID in combination with the 3D regimen in HCV/HIV-coinfected adults. The DRV Cmax and AUC values after QD or BID dosing were comparable between coadministration with the 3D regimen and coadministration with 100 mg of ritonavir. However, in the presence of 3D, the DRV C24 with QD dosing decreased by 36% and the DRV C12 with BID dosing decreased by 27% compared with DRV/r without the 3D regimen.
Darunavir concentrations in HCV/HIV-coinfected subjects receiving the 3D regimen were similar to what was previously observed in HIV-monoinfected adults receiving DRV/r at 800/100 mg QD or 600/100 mg BID. The ARTEMIS trial evaluated DRV pharmacokinetics using noncompartmental analyses from intensive sampling, similar to analyses in the current study, at 4, 24, and 48 weeks of DRV/r at 800/100 mg QD in 9, 13, and 10 HIV-monoinfected subjects, respectively (10). The DRV AUC24 mean ± standard deviation (SD) at week 4 was 64,230 ± 18,210, at week 24 was 66,950 ± 18,610, and at week 48 was 75,620 ± 26,440 ng · h/ml. The DRV C24 mean ± SD at week 4 was 1,440 ± 513.9, at week 24 was 1,644 ± 726.9, and at week 48 was 1,447 ± 705.7 ng/ml. The DRV AUC24 and C24 mean ± SD in HCV/HIV-coinfected subjects receiving DRV at 800 mg with the 3D regimen were 74,916 ± 20,029 ng · h/ml and 1,244 ± 782 ng/ml, respectively. The GRACE trial evaluated 32 HIV-monoinfected subjects receiving DRV/r at 600/100 mg BID (11). After 4 weeks of treatment, DRV AUC12 and C12 mean ± SD were 62,360 ± 25,020 ng · h/ml and 3,559 ± 2,385 ng/ml, respectively, values similar to the DRV AUC12 and C12 mean ± SD of 65,494 ± 32,256 ng · h/ml and 3,470 ± 2,339 ng/ml seen in HCV/HIV-coinfected subjects receiving 3D in combination with DRV at 600 mg BID.
Results from our study did not suggest a relationship between the plasma HIV-1 RNA level of >40 copies/ml and DRV Ctrough values below 550 ng/ml, the mean protein binding adjusted EC50 for DRV-resistant virus. Simulated data of DRV/r at 800/100 mg QD and 600/100 mg BID in HIV-monoinfected subjects calculated using data from Kakuda et al. (10) suggest that 7.2% of Ctrough values after QD dosing and 10.3% of Ctrough values after BID dosing would fall below 550 ng/ml. These predictions are similar to our findings, where 11% of Ctrough values in subjects taking DRV QD and 5% of Ctrough values in subjects taking DRV BID were below 550 ng/ml. Again, none of the Ctrough values below 550 ng/ml were associated with a plasma HIV-1 RNA level of >40 copies/ml.
Exposures of OBV, PTV, RTV, DSV, and RBV in HCV/HIV-1-coinfected subjects receiving a DRV-based regimen were generally similar to (or slightly lower than) the exposures in phase 1 studies and HCV monoinfected subjects (12). The ranges of geometric mean OBV, PTV, and RTV AUC24 values from phase I studies were 1,050 to 1,600, 3,760 to 16,500, and 7,270 to 12,700 ng · h/ml, respectively. Geometric mean OBV, PTV, and RTV AUC24 values with DRV QD were 1,118, 7,653, and 6,909 ng · h/ml, respectively, and with DRV BID were 944, 6,714, and 15,034 ng · h/ml. The ranges of geometric mean DSV and RBV AUC12 values were 5,530 to 7,740 and 2,929 to 6,200 ng · h/ml, respectively. Geometric mean DSV and RBV AUC12 values with DRV QD were 4,403 and 21,506 ng · h/ml, respectively, and with DRV BID were 3,509 and 27,373 ng · h/ml, respectively. The DSV AUC12 value in HCV/HIV-coinfected patients was approximately 37% lower than the DSV range from phase I studies. A decrease in DSV AUC12 of up to 50% is not considered clinically significant (13). Furthermore, the clinical impact of decreased DSV exposures may have been diminished by the low number of cirrhotic subjects enrolled into the study and the addition of RBV to the 3D regimen regardless of subgenotype (1b versus 1a).
Over 1,200 DAA Ctrough values have been collected in phase 3 studies. The geometric mean (%CV) Ctrough values [geometric mean range from phase 3 studies] in subjects receiving DRV at 800 QD and 600 mg BID for OBV were 20 (70) and 21 (45) ng/ml [24 to 33 ng/ml], for PTV were 19 (218) and 44 (133) ng/ml [16 to 33 ng/ml], and for DSV were 198 (33) and 118 (46) ng/ml [175 to 270 ng/ml], respectively. The geometric mean Ctrough value for RTV from the DRV QD arm was 48 (70) ng/ml [40 to 50 ng/ml]. Since an extra dose of RTV was administered in the evening in the DRV BID arm, the RTV Ctrough value was not compared to phase 3 data. Geometric mean (%CV) RBV Ctrough values were 1,605 (34) for the DRV QD arm and 1,969 (44) [2,060 to 2,330 ng/ml] for the DRV BID arm. Exposures of DAAs and RBV in combination with DRV did not appear to compromise efficacy, as the intention-to-treat SVR12 rate of 100% (22/22 subjects) demonstrated robust efficacy of the 3D regimen in this population (14).
A recently published case report stated that two HCV/HIV-coinfected patients receiving DRV at 800 mg QD in combination with the 3D regimen failed antiviral therapy due to decreased PTV exposures (15). The PTV Ctrough values after 1 and 2 months of the 3D regimen with DRV were 36 and 52 ng/ml, respectively, for one patient and 114 and 75 ng/ml, respectively, for the second patient. These PTV Ctrough values are higher than the geometric mean PTV Ctrough value of 19 ng/ml observed in the current study when administered with DRV QD, and no subjects in the current study experienced HCV virological failure (12). Furthermore, the patient-reported Ctrough values are higher than the PTV Ctrough range of 16 to 33 ng/ml observed in phase 3 studies without DRV. The case report references a PTV Ctrough range of 150 to 300 ng/ml, which the authors appear to have estimated from PTV Cmax values reported in the literature. One of the referenced studies dosed PTV at 200 mg instead of the approved dose of 150 mg, which may explain why the authors estimated a higher PTV Ctrough range than that observed in phase 3 studies of the 3D regimen. Thus, patients in the case report were unlikely to have failed the 3D regimen due to low PTV exposures when it was administered with DRV QD.
There are limitations to the current study. Subjects receiving DRV with the 3D regimen had intensive pharmacokinetic sampling out to 12 h postdose at week 4. For those receiving DRV QD, the concentration at time 0 was used as the C24 to estimate the DRV AUC24. Thus, 50% of the DRV AUC was estimated rather than calculated based on actual concentrations. Although DRV was at steady state and the concentration at time 0 should be similar to the C24, estimating AUC24 introduces additional variability into these DRV pharmacokinetic parameters. Furthermore, wide confidence intervals of some pharmacokinetic estimates were a result of the small sample size of the study.
In conclusion, pharmacokinetic and clinical data on DRV at 800 mg QD or 600 mg BID in combination with the 3D regimen support its use in HCV/HIV-1-coinfected patients when administered at the same time as the 3D regimen. These results, however, should not be extrapolated to HIV treatment-experienced adult patients who harbor DRV resistance mutations and are receiving DRV/ritonavir at 600/100 mg BID. When coadministered with the 3D regimen, DRV should be taken without ritonavir, since ritonavir is provided at 100 mg QD as part of the 3D regimen. Patients taking DRV at 600 mg BID would receive an additional 100 mg of ritonavir with the evening DRV dose.
MATERIALS AND METHODS
Study M14-004, TURQUOISE I, is a phase 2/3, randomized, open-label, multicenter study evaluating the safety and efficacy of OBV/PTV/r and DSV coadministered with RBV for 12 or 24 weeks in adults with HCV GT1/HIV-1 coinfection who are HCV treatment naive or pegIFN/RBV experienced, including those with compensated cirrhosis. Part 1b of the study consisted of a phase 2 pilot cohort that included a pharmacokinetic substudy. The study was designed to randomize approximately 30 cirrhotic and noncirrhotic eligible subjects on a stable DRV QD-containing HIV antiretroviral regimen in a 1:1 ratio to maintain DRV/r at 800/100 mg QD (arm C) or switch to DRV/r at 600/100 mg BID (arm D). Beginning at study day 1, all subjects in part 1b received OBV/PTV/r at 25/150/100 mg QD plus DSV at 250 mg BID plus RBV for 12 weeks during the treatment period. RBV was administered based on weight at 1,000 or 1,200 mg divided twice daily. For subjects in arm C, ritonavir at 100 mg from the 3D regimen boosted the DRV at 800 mg. For subjects in arm D, ritonavir at 100 mg from the 3D regimen boosted the morning dose of DRV at 600 mg. An additional dose of ritonavir at 100 mg was administered with the evening dose of DRV at 600 mg. Subjects with evidence of past virological failure of two or more HIV-1 antiretroviral regimens or DRV resistance-associated mutations per the DRV United States product insert were excluded from the study.
All study medications were administered with a standardized meal, providing approximately 40% of the daily calories from fat and up to 45% of the daily calories from carbohydrates (approximately 2,200 calories/day). Blood samples for PK analyses were collected prior to morning dosing of DRV/r and at 1, 2, 3, 4, 5, 6, 8, 10, and 12 h after morning dosing on study day −1, prior to the morning dose on day 0, and after morning dosing at week 4. Sparse sampling occurred at day 1 and weeks 1, 2, 6, 8, 10, and 12. Samples for HIV-1 RNA were obtained at day 1 and at weeks 1, 2, 4, 6, 8, 10, and 12 during treatment with the HCV DAAs. If a subject's HIV-1 RNA level was <40 copies/ml at the previous time point and was ≥40 copies/ml at the next assessment, the subject's HIV-1 RNA test was repeated. If the subject's repeat HIV-1 RNA test value was ≥500 copies/ml, a sample was obtained for HIV-1 genotypic testing.
Bioanalytical methods.
Plasma concentrations of OBV, PTV, RTV, DSV, and RBV were determined, using validated liquid chromatography methods with tandem mass spectrometric detection, by the Drug Analysis Department at AbbVie (16). The lower limits of quantitation (LLOQ) for OBV, PTV, RTV, DSV, and RBV were established at 0.464 ng/ml (inter-run accuracy [% bias], −0.8% to 5.0%; inter-run precision [%CV], 1.7% to 8.8%), 0.601 ng/ml (% bias, −1.9% to 6.5%; %CV, 1.0% to 8.2%), 5.10 ng/ml (% bias, 0.7% to 9.8%; %CV, 2.2% to 7.5%), 4.39 ng/ml (% bias, −0.6% to 6.7%; %CV, 1.2% to 8.1%), and 18.7 ng/ml (% bias, −3.6% to 7.9%; %CV, 1.3% to 12.8%), respectively. Samples quantified below the lowest standard were reported as zero. Plasma concentrations of DRV were determined using validated liquid chromatography methods with tandem mass spectrometric detection by PPD Laboratories (Middleton, WI). The LLOQ for DRV was established at 10.0 ng/ml (accuracy, −0.0433% to 4.22%; %CV, 3.8% to 7.04%). Samples quantified below the lowest standard were reported as zero.
Pharmacokinetic assessment and analysis.
The maximum observed plasma concentration (Cmax), the time to reach maximum concentration (Tmax), the trough plasma concentration (C24 for QD drugs and C12 for BID drugs), and the area under the concentration-time curve (AUC12 or AUC24) were determined by noncompartmental methods for each drug at the steady state. DRV and RTV PK parameters were determined at day −1 and at week 4. PK parameters for the DAAs and RBV were determined at week 4. For QD drugs (DRV QD, OBV, PTV, and RTV) at week 4, the concentration at time zero was used as the C24 and to determine the AUC24. Trough plasma concentrations (Ctrough), based on binning the sparse samples by time interval, were determined for all subjects for whom data were available. The time interval of >22 to 26 h was considered the Ctrough for drugs administered QD, and the time interval of >10 to 14 h was considered the Ctrough for drugs administered BID.
Statistical analysis.
Statistical analyses were performed using SAS software, version 9.2 (SAS Institute, Inc., Cary, NC). The effect of the 3D regimen on DRV was evaluated by comparing DRV exposures at week 4 when DRV was coadministered with the 3D regimen to those seen at study day −1 when DRV with RTV was administered alone. The effect of the 3D regimen on DRV was estimated by analyzing natural log-transformed Cmax, AUC, and Ctrough values using a repeated measures analysis framework. The model had the visit (study day −1 and week 4) as a fixed effect. The within-subject variability was accounted for utilizing the repeated statement for the effect of the visit. Central value ratios and 90% confidence intervals (CIs) for DRV Cmax, AUC, and Ctrough were calculated to quantify the magnitude of the drug interaction.
ACKNOWLEDGMENTS
The study was sponsored by AbbVie. AbbVie contributed to the study design, research, and interpretation of data and the writing, reviewing, and approving of the publication. All authors are employees of AbbVie and may hold AbbVie stock or stock options.
REFERENCES
- 1.Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, Yanny I, Razavi H, Vickerman P. 2016. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 16:797–808. doi: 10.1016/S1473-3099(15)00485-5. [DOI] [PubMed] [Google Scholar]
- 2.Munteanu DI, Rockstroh JK. 2013. New agents for the treatment of hepatitis C in patients co-infected with HIV. Ther Adv Infect Dis 1:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sulkowski MS, Eron JJ, Wyles D, Trinh R, Lalezari J, Wang C, Slim J, Bhatti L, Gathe J, Ruane PJ, Elion R, Bredeek F, Brennan R, Blick G, Khatri A, Gibbons K, Hu YB, Fredrick L, Schnell G, Pilot-Matias T, Tripathi R, Da Silva-Tillmann B, McGovern B, Campbell AL, Podsadecki T. 2015. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA 313:1223–1231. doi: 10.1001/jama.2015.1328. [DOI] [PubMed] [Google Scholar]
- 4.Molina JM, Orkin C, Iser DM, Zamora FX, Nelson M, Stephan C, Massetto B, Gaggar A, Ni L, Svarovskaia E, Brainard D, Subramanian GM, McHutchison JG, Puoti M, Rockstroh JK; PHOTON-2 study team. 2015. Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): a multicentre, open-label, non-randomised, phase 3 study. Lancet 385:1098–1106. doi: 10.1016/S0140-6736(14)62483-1. [DOI] [PubMed] [Google Scholar]
- 5.Rockstroh JK, Nelson M, Katlama C, Lalezari J, Mallolas J, Bloch M, Matthews GV, Saag MS, Zamor PJ, Orkin C, Gress J, Klopfer S, Shaughnessy M, Wahl J, Nguyen BY, Barr E, Platt HL, Robertson MN, Sulkowski M. 2015. Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. Lancet HIV 2:e319–e327. doi: 10.1016/S2352-3018(15)00114-9. [DOI] [PubMed] [Google Scholar]
- 6.Khatri A, Dutta S, Wang H, Podsadecki T, Trinh R, Awni W, Menon R. 2016. Evaluation of drug-drug interactions between hepatitis C antiviral agents ombitasvir, paritaprevir/ritonavir, and dasabuvir and HIV-1 protease inhibitors. Clin Infect Dis 62:972–979. doi: 10.1093/cid/civ1213. [DOI] [PubMed] [Google Scholar]
- 7.Janssen Pharmaceuticals, Inc. 2015. Prezista (darunavir) (package insert). Janssen Pharmaceuticals, Inc., Titusville, NJ. [Google Scholar]
- 8.Boffito M, Miralles D, Hill A. 2008. Pharmacokinetics, efficacy and safety of darunavir/ritonavir 800/100 mg once-daily in treatment-naïve and -experienced patients. HIV Clin Trials 9:418–427. doi: 10.1310/hct0906-418. [DOI] [PubMed] [Google Scholar]
- 9.Kakuda T, Sekar VJ, Vis P, et al. 2010. Intrinsic/extrinsic covariates and darunavir pharmacokinetics in treatment-experienced patients in GRACE (Gender, Race And Clinical Experience), abstr 16. Abstr 11th Int Wksp Clin Pharmacol HIV Ther, Sorrento, Italy, 7 to 9 April 2010. [Google Scholar]
- 10.Kakuda TN, Brochot A, Tomaka FL, Vangeneugden T, Van De Casteele T, Hoetelmans RM. 2014. Pharmacokinetics and pharmacodynamics of boosted once-daily darunavir. J Antimicrob Chemother 69:2591–2605. doi: 10.1093/jac/dku193. [DOI] [PubMed] [Google Scholar]
- 11.Kakuda T, Sekar V, Vis P, Coate B, Ryan R, Anderson D, De La Rosa G, Mrus J. 2012. Pharmacokinetics and pharmacodynamics of darunavir and etravirine in HIV-1-infected, treatment-experienced patients in the Gender, Race, and Clinical Experience (GRACE) trial. AIDS Res Treat 2012:186987. doi: 10.1155/2012/186987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Center for Drug Evaluation and Research. July 2016, accession date. Clinical pharmacology and biopharmaceutics review(s). http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206619Orig1s000ClinPharmR.pdf. [Google Scholar]
- 13.Center for Drug Evaluation and Research. July 2016, accession date. Clinical pharmacology and biopharmaceutics review(s). http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206619Orig1s000SumR.pdf. [Google Scholar]
- 14.Wyles D, Saag D, Trinh R, Lalezari J, Adeyemi O, Bhatti L, Khatri A, Hu YB, Shulman NS, Ruane P. 2016. TURQUOISE-I part 1b: ombitasvir/paritaprevir/r + dasabuvir + RBV for HCV/HIV coinfection. Abstr 23rd Conf Retroviruses Opportunistic Infect, abstr 574. [Google Scholar]
- 15.Sollima S, D'Avolio A, Cattaneo D, Micheli V, Milazzo L, Gervasoni C. 2016. Darunavir-based antiretroviral therapy may affect the efficacy of ombitasvir/paritaprevir/ritonavir and dasabuvir in HCV/HIV-1-coinfected patients. Clin Infect Dis 63:285–286. doi: 10.1093/cid/ciw292. [DOI] [PubMed] [Google Scholar]
- 16.Polepally AR, Dutta S, Hu B, Podsadecki TJ, Awni WM, Menon RM. 2016. Drug-drug interaction of omeprazole with the HCV direct-acting antiviral agents paritaprevir/ritonavir and ombitasvir with and without dasabuvir. Clin Pharmacol Drug Dev 5:269–277. doi: 10.1002/cpdd.246. [DOI] [PubMed] [Google Scholar]