ABSTRACT
The ANRS 12174 trial assessed the efficacy and tolerance of lopinavir (LPV)-ritonavir (LPV/r) prophylaxis versus those of lamivudine (3TC) prophylaxis administered to breastfed infants whose HIV-infected mothers were not on antiretroviral therapy. In this substudy, we assessed LPV/r and 3TC pharmacokinetics to evaluate the percentage of infants with therapeutic plasma concentrations and to discuss these data in the context of a prophylactic treatment. Infants from the South African trial site underwent blood sampling for pharmacokinetic study at weeks 6, 26, and 38 of life. We applied a Bayesian approach to derive the 3TC and LPV pharmacokinetic parameters on the basis of previously published pharmacokinetic models for HIV-infected children. We analyzed 114 LPV and 180 3TC plasma concentrations from 69 infants and 92 infants, respectively. A total of 30 LPV and 20 3TC observations were considered missing doses and discarded from the Bayesian analysis. The overall population analysis showed that 30 to 40% of the infants did not reach therapeutic targets, regardless of treatment group. The median LPV trough concentrations at weeks 6, 26, and 38 were 2.8 mg/liter (interquartile range [IQR], 1.7 to 4.4 mg/liter), 5.6 mg/liter (IQR, 3.2 to 7.7 mg/liter), and 3.4 mg/liter (IQR, 2.3 to 7.3 mg/liter), respectively. The median 3TC area under the curve from 0 to 12 h after the last drug intake were 5.6 mg · h/liter (IQR, 4.1 to 7.8 mg · h/liter), 5.9 mg · h/liter (IQR, 5.1 to 7.5 mg · h/liter), and 7.3 mg · h/liter (IQR, 4.9 to 8.5 mg · h/liter) at weeks 6, 26, and 38, respectively. Use of the therapeutic doses recommended by the WHO would have resulted in a higher proportion of infants achieving the targets. However, no HIV-1 infection was reported among these infants. These results suggest that the prophylactic targets for both 3TC and LPV may be lower than the therapeutic ones. For treatment, the WHO dosing guidelines should be suitable to maintain values above the therapeutic pharmacokinetic targets in most infants. (This study has been registered at ClinicalTrials.gov under identifier NCT00640263.)
KEYWORDS: breastfeeding, pharmacokinetics, preexposure prophylaxis
INTRODUCTION
According to the 2012 UNAIDS estimates, the postnatal transmission of HIV-1 from mother to infant through breastfeeding (BF) still represents a significant fraction of overall mother-to-child-transmission (MTCT) (1). Various antiretroviral prophylaxis options to prevent MTCT through breast milk have emerged during the last 15 years, such as (i) the use of antiretroviral therapy (ART) for the mothers during the BF period (WHO option B) or (ii) the initiation of antiretroviral prophylaxis solely in their infants (WHO option A) (2). The current WHO guidelines recommend option B+, which consists of treating all pregnant and breastfeeding women with antiretroviral therapy regardless of the WHO clinical stage or CD4 cell count, together with infant prophylaxis with nevirapine for the first 6 weeks of life (3). The ANRS 12174 trial (ClinicalTrials.gov identifier NCT00640263) was started in 2009 to assess the efficacy of new regimens to prevent postnatal HIV acquisition in breastfed infants born to HIV-infected mothers not eligible for combined ART (4). Randomized preexposure prophylaxis (PrEP) with lopinavir (LPV)-ritonavir (LPV/r) or lamivudine (3TC) was initiated in 1,273 uninfected infants from four African countries starting from the first week of life until 1 week after the cessation of BF. The results of the ANRS 12174 trial support an alternative strategy to option A or B for BF mothers, with a cumulative transmission rate of 1.5% (95% confidence interval, 0.8 to 2.2%) being reported at 12 months of age when this strategy is used (5).
In the present study, we aimed (i) to evaluate the proportion of children with therapeutic plasma concentrations, (ii) to simulate the concentrations that the 2016 WHO dosing guidelines would produce, and (iii) to discuss these data in the context of a prophylactic treatment.
RESULTS
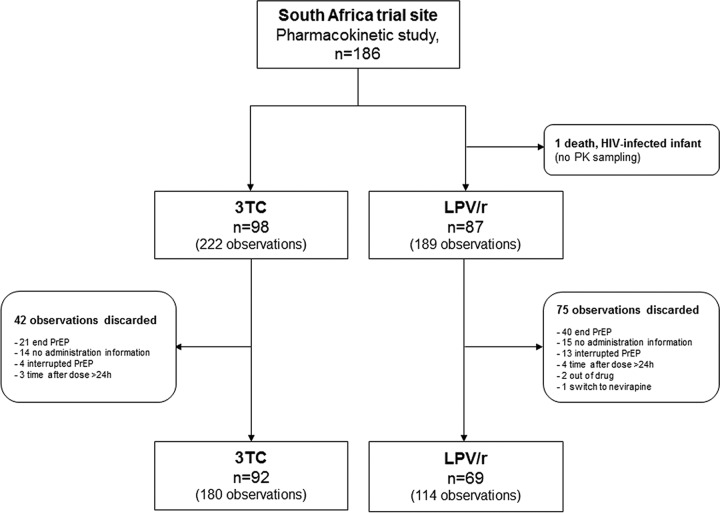
The pharmacokinetic analysis was performed on blood samples from 161 infants whose mothers were not on ART during breastfeeding. The flow chart of this subanalysis is presented in Fig. 1.
FIG 1.
Flowchart of the pharmacokinetic (PK) study.
Description of the population. (i) Population treated with lopinavir-ritonavir.
Sixty-nine infants (33 girls and 36 boys) and 114 plasma LPV concentrations were available for pharmacokinetic evaluation. The LPV/r regimen was initiated after a median postnatal age of 8 days (range, 5 to 15 days). Participant characteristics at the three visits for the collection of samples for pharmacokinetic analysis are summarized in Table 1. A total of 35 (51%), 23 (33%), and 11 (16%) infants had 1, 2, or 3 plasma samples available, respectively. The sample collection time relative to the time of dose administration ranged from 0.8 to 19.2 h with a median of 4.6 h (interquartile range [IQR], 3.8 to 6.0 h). No case of HIV-1 infection was reported among the 69 infants.
TABLE 1.
Summary of infant characteristics for the LPV/r and 3TC groupsa
| Treatment and characteristic | Median (IQR) value atb: |
||
|---|---|---|---|
| Wk 6 | Wk 26 | Wk 38 | |
| LPV/r | |||
| Infant age (wk) at visit | 6.0 (6.0–6.2) | 26.0 (25.8–26.1) | 38.0 (37.7–38.2) |
| Wt (kg) at blood sampling | 4.6 (4.2–5.0) | 7.3 (6.6–8.4) | 8.4 (7.3–9.2) |
| Length (cm) at blood sampling | 53.5 (52.4–54.5) | 64.5 (63.1–66.0) | 68.2 (65.9–69.5) |
| Serum creatinine concn (μmol/liter) | 23.0 (18.0–25.0) | 17.0 (15.0–20.5) | 19.0 (17.3–25.0) |
| ALAT concn (IU/liter) | 16.0 (13.0–19.0) | 14.0 (11.5–18.5) | 13.5 (13.0–15.0) |
| Hemoglobin concn (g/dl) | 10.9 (10.2–12.1) | 10.9 (9.9–11.5) | 11.2 (10.8–11.4) |
| Dose BID prescribed at preceding visit (mg) | 40/10 (40/10–40/10) | 80/20 (80/20–80/20) | 80/20 (80/20–80/20) |
| 3TC | |||
| Infant age (wk) at visit | 6.0 (6.0–6.1) | 26.0 (25.7–26.1) | 38.0 (37.7–38.6) |
| Wt (kg) at blood sampling | 4.7 (4.1–5.1) | 7.6 (7.1–8.2) | 8.7 (8.0–9.4) |
| Length (cm) at blood sampling | 54.0 (52.2–55.5) | 65.0 (63.9–66.7) | 69.6 (68.2–70.7) |
| Serum creatinine concn (μmol/liter) | 20.5 (18.0–23.0) | 18.0 (15.0–23.0) | 21.0 (18.3–25.0) |
| ALAT concn (IU/liter) | 20.0 (16.0–27.0) | 18.0 (15.0–21.0) | 17.5 (16.0–19.0) |
| Hemoglobin concn (g/dl) | 10.7 (9.8–11.7) | 10.7 (10.1–11.3) | 10.6 (10.1–11.3) |
| Dose BID prescribed at preceding visit (mg) | 7.5 (7.5–7.5) | 25.0 (25.0–37.5) | 50.0 (25.0–50.0) |
Abbreviations: IQR, interquartile range; BID, twice daily; ALAT, alanine aminotransferase.
For treatment with LPV/r, data are for 59, 32, and 23 infants at weeks 6, 26, and 38, respectively. For treatment with 3TC, data are for 82, 57, and 41 infants at weeks 6, 26, and 38, respectively.
(ii) Population treated with lamivudine.
Ninety-two infants (44 girls and 48 boys) and 180 plasma 3TC concentrations were available for pharmacokinetic evaluation. The 3TC regimen was initiated after a median postnatal age of 8 days (range, 5 to 12 days). Table 1 summarizes the participant characteristics. A total of 31 (34%), 34 (37%), and 27 (29%) infants had 1, 2, or 3 plasma samples available, respectively. The sample collection time relative to the time of dose administration ranged from 0.8 to 19.3 h with a median of 5.1 h (IQR, 3.7 to 6.3 h). No case of HIV-1 transmission was observed among the 92 children.
Missed medication doses. (i) Population treated with lopinavir-ritonavir.
Thirty LPV observations (26% of the overall observations) were classified as missing doses and removed from the simulated analyses: 25 observations had concentrations below the limit of quantification (LOQ), and 5 had particularly low plasma concentrations when the time between the time of drug administration and the time of blood sampling was considered. The median ratio of excluded observations to population predicted concentrations was 0.019 (range, 0.006 to 0.159).
(ii) Population treated with lamivudine.
A total of 20 3TC observations (11% of the overall observations) were discarded from the simulated analyses because of missing doses: 16 had concentrations below the LOQ and 4 had unexpectedly low plasma concentrations when the time between the time of drug administration and the time of blood sampling was considered. The median ratio of excluded observations to population predicted concentrations was 0.007 (range, 0.001 to 0.172).
Pharmacokinetics and drug dosage evaluations. (i) Population treated with lopinavir-ritonavir.
The observed LPV concentrations and the individual median predictions (6) are displayed as a function of time in Fig. S1 in the supplemental material. The LPV trough concentration (Ctrough), maximal concentration (Cmax), time to reach the maximal concentration (Tmax), area under the curve from 0 to 12 h after the last drug intake (AUC0–12), and apparent clearance (CL/F) per kilogram of body weight values for the 57 infants without missing doses were derived from samples collected at the visits scheduled for pharmacokinetic analysis (Table 2). The percentage of Ctrough values above the LPV pharmacokinetic target is reported in Table 3 for the different analysis sets.
TABLE 2.
Values of LPV and 3TC pharmacokinetic parametersa
| Treatment and parameter | Median (IQR) value atb: |
||
|---|---|---|---|
| Wk 6 | Wk 26 | Wk 38 | |
| LPV | |||
| Dose BID (mg) | 40 (40–40) | 80 (80–80) | 80 (80–80) |
| CL/F per kg (liter/h/kg) | 0.17 (0.12–0.29) | 0.09 (0.08–0.20) | 0.16 (0.08–0.22) |
| Ctrough (mg/liter) | 2.83 (1.73–4.43) | 5.57 (3.17–7.68) | 3.39 (2.28–7.26) |
| Cmax (mg/liter) | 5.82 (3.63–9.01) | 10.52 (6.33–14.75) | 6.49 (4.29 −12.86) |
| Tmax (h) | 3.0 (3.0–3.0) | 3.0 (3.0–3.1) | 3.1 (3.0–3.1) |
| AUC0–12 (mg · h/liter) | 54.5 (33.9–85.8) | 101.4 (60.3–141.4) | 62.1 (41.0–126.5) |
| 3TC | |||
| Dose BID (mg) | 7.5 (7.5–7.5) | 25 (25–25) | 50 (25–50) |
| CL/F per kg (liter/h/kg) | 0.32 (0.24–0.43) | 0.61 (0.53–0.76) | 0.70 (0.49–0.79) |
| Ctrough (mg/liter) | 0.15 (0.12–0.24) | 0.11 (0.09–0.16) | 0.13 (0.09–0.17) |
| Cmax (mg/liter) | 0.89 (0.66–1.19) | 1.10 (0.94–1.49) | 1.45 (0.97–1.68) |
| Tmax (h) | 2.0 (1.7–2.3) | 1.7 (1.4–2.0) | 1.6 (1.4–1.9) |
| AUC0–12 (mg · h/liter) | 5.6 (4.1–7.8) | 5.9 (5.1–7.5) | 7.3 (4.9–8.5) |
Abbreviations: LPV, lopinavir; 3TC, lamivudine; IQR, interquartile range; BID, twice daily; CL/F, apparent clearance; Ctrough, trough concentration, Cmax, maximal concentration, Tmax, time to reach the maximal concentration; AUC0–12, area under the curve from 0 to 12 h after the last drug intake.
For treatment with LPV, data are for 44, 26, and 14 infants at weeks 6, 26, and 38, respectively. For treatment with 3TC, data are for 77, 49, and 34 infants at weeks 6, 26, and 38, respectively.
TABLE 3.
Results for samples with Ctrough values of ≥1 mg/liter for the different population analysis sets
| Wt band (kg) | Overall population |
ANRS 12174 protocol dose populationa |
WHO simulated populationa,b |
|||
|---|---|---|---|---|---|---|
| n obs.c | No. (%) of infants with a Ctrough of ≥1 mg/liter | n obs. | No. (%) of infants with a Ctrough of ≥1 mg/liter | n obs. | No. (%) of infants with a Ctrough of ≥1 mg/liter | |
| <4 | 12 | 9 (75) | 11 | 9 (82) | 11 | 10 (91) |
| ≥4 | 102 | 71 (70) | 73 | 72 (99) | 73 | 73 (100) |
Data for samples from infants that may have missed a dose at the sampling time were excluded.
Data for the WHO simulated population are simulated concentrations that the 2016 WHO dosing guidelines would produce for HIV-1-infected children.
n obs., number of observations.
According to the ANRS 12174 trial weight bands, 70 to 75% of the LPV Ctrough values were above the 1-mg/liter target for the overall population. After exclusion of potential missed medication doses, 82 to 99% of Ctrough values would have been above the target (see the data for the ANRS 12174 protocol dose population in Table 3). On the basis of the simulation of the therapeutic WHO dosing guidelines for HIV-1-infected children (i.e., the weight-based doses in the WHO dosing guidelines are higher than those administered in this trial), LPV Ctrough values above the 1-mg/liter target would be achieved in 91 to 100% of cases.
(ii) Population treated with lamivudine.
The 3TC observed concentrations and the individual median predictions (7) are displayed as a function of time in Fig. S2. Table 2 summarizes the 3TC Ctrough, Cmax, Tmax, AUC0–12, and CL/F per kilogram values derived from samples from the 81 infants without missing doses collected at the visits scheduled for pharmacokinetic analysis. The percentage of AUC0–12 values ≥4.2 mg · h/liter by weight band for the different analysis sets is shown in Table 4.
TABLE 4.
Results for samples with 3TC AUC0–12 values of ≥4.2 mg · h/liter for the different population analysis sets
| Wt band (kg) | Overall population |
ANRS 12174 protocol dose populationa |
WHO simulated populationa,b |
|||
|---|---|---|---|---|---|---|
| n obs.c | No. (%) of infants with an AUC0–12 of ≥4.2 mg · h/liter | n obs. | No. (%) of infants with an AUC0–12 of ≥4.2 mg · h/liter | n obs. | No. (%) of infants with an AUC0–12 of ≥4.2 mg · h/liter | |
| <4 | 15 | 9 (60) | 13 | 9 (69) | 13 | 12 (92) |
| ≥4–8 | 117 | 82 (70) | 107 | 97 (91) | 107 | 103 (96) |
| ≥8 | 48 | 36 (75) | 40 | 36 (90) | 40 | 35 (88) |
Data for samples from infants that may have missed a dose at the sampling time were excluded.
Data for the WHO simulated population are simulated concentrations that the 2016 WHO dosing guidelines would produce for HIV-1-infected children.
n obs., number of observations.
For the different weight bands, 60 to 75% of AUC0–12 values were above the 4.2-mg · h/liter target for the overall population. A higher percentage of children in the ANRS 12174 protocol dose population than children in the overall population would have reached the threshold (i.e., 69 to 91% of children would have AUC0–12 values of ≥4.2 mg · h/liter). On the basis of the simulation of the therapeutic WHO dosing guidelines for HIV-1-infected children, 88 to 96% of the AUC0–12 values would be above the 4.2-mg · h/liter target.
DISCUSSION
The ANRS 12174 trial assessed the efficacy of infant LPV/r or 3TC prophylaxis to reduce the risk of HIV transmission during the breastfeeding period. According to an intention-to-treat analysis, an HIV infection rate of less than 1.6% was reported among the 1,273 infants born to mothers not eligible for ART (5). A total of eight and nine HIV-1 infections were observed in the LPV/r and 3TC arms, respectively (5). In this subanalysis, we studied LPV/r and 3TC pharmacokinetics to assess the consistency of the drug dosages used in the ANRS 12174 trial. A population approach was first tried, but the collected data were too sparse to estimate accurately the overall pharmacokinetic parameters. Thus, a Bayesian approach was preferred to the development of new pharmacokinetic models, given the robust population pharmacokinetic models for both 3TC and LPV/r that have previously been published for similar populations (6, 7). Interestingly, estimates of apparent clearances after other parameters were fixed were in agreement with the values reported by Nikanjam et al. (6) and Bouazza et al. (7) for LPV/r and 3TC, respectively. The model selected for 3TC was developed with data from 920 HIV-infected children (a total of 3,820 observations) using a meta-analysis of data from therapeutic drug monitoring and controlled clinical trials (7). The model selected for LPV was based on both sparse and intensive data coming from 30 HIV-infected infants who were started an LPV/r treatment at between 2 weeks and 6 months of life (a total of 607 concentration measurements) (6). These models take into account the developmental changes that occur during the first year of life by including weight- and age-related effects on the apparent clearance and volume of distribution parameters. We used the therapeutic pharmacokinetic thresholds previously associated with 3TC or LPV/r virological efficacy in children and adults (7–10) to evaluate the protocol drug dosages as well as the 2016 WHO dosing guidelines for HIV-infected infants. Regardless of the treatment group, 30 to 40% of LPV Ctrough or AUC0–12 values were below the corresponding therapeutic targets, due in part to missing doses. Several publications on PrEP in adults have shown that one of the major contributors to HIV-1 transmission is poor adherence to PrEP medications (11–13). The literature on adherence to PrEP in breastfeeding infants is scarce with a focus on nevirapine (14, 15). Of note, a higher percentage of missed medication doses was estimated in the LPV/r group than the 3TC group (26% for the LPV/r group versus 11% for the 3TC group). This may be explained, in part, by the fact that the LPV/r syrup formulation is often described to have an unpleasant taste (16). After exclusion of the data for samples from infants that may have missed a dose and according to the prophylaxis doses initially planned for use in the ANRS 12174 trial protocol, a lower percentage of infants would have been underdosed. This percentage would have been even lower if the current WHO dosing guidelines were used. Use of these guidelines would have resulted in less than 9% and 12% of the LPV Ctrough and 3TC AUC0–12 values, respectively, falling below the corresponding thresholds. Therefore, WHO dosing recommendations appear to be suitable for maintaining the values above the therapeutic pharmacokinetic targets for most infants aged 6 to 38 weeks.
Our study shows that none of the HIV-exposed infants whose values were below the therapeutic pharmacokinetic target acquired HIV infection during the trial period. These results suggest that the prophylactic targets for both 3TC and LPV may be lower than the therapeutic ones. The relationship between prophylactic and therapeutic targets remains an issue for other molecules used in the prevention of MTCT. A prophylactic target corresponding to 10 times the in vitro median inhibitory concentration has been proposed for nevirapine (17). This threshold is much lower than the Ctrough therapeutic target of 3 mg/liter for HIV-infected adults (10, 18). In contrast, the dose of zidovudine recommended for the prevention of MTCT results in exposure levels that are even higher than those obtained with the therapeutic adult dose (19). However, no formal dose-response analysis has been performed to determine if this higher initial exposure is needed.
This study has several limitations. The main limitation was the lack of pharmacokinetic and safety data for infants less than 6 weeks of age. Indeed, the LPV/r pharmacokinetic profile should be different in the first weeks of life, given the CYP3A ontogeny and the switch from CYP3A7 to CYP3A4/5 (20). Regarding safety data, to our knowledge, there is no evidence-based medicine to evaluate the tolerance of LPV/r at <6 weeks of age, but a few case reports on toxicity have been provided. Those reports mainly concerned premature babies and infants with cardiac dysfunction (and cases of overdosing) (21). A general warning from the FDA about the use of LPV/r in infants aged less than 14 days followed (22). The second limitation is that no prophylaxis doses were proposed in this study, as no HIV-1 transmission was observed in the 161 infants. Further larger studies designed to assess associations between the transmission rate and drug exposure are strongly needed in order to determine accurately the prophylaxis doses that ensure both drug safety and efficacy.
In conclusion, no case of HIV-1 infection was reported among the 161 infants evaluated in this study, even though a large proportion of children had 3TC and LPV levels below the therapeutic targets. This study raises the question of the optimal 3TC and LPV prophylactic targets needed to prevent MTCT during breastfeeding. This study also confirms that the WHO dosing guidelines provide 3TC and LPV exposure levels above the therapeutic targets in most infants.
MATERIALS AND METHODS
Study participants.
This pharmacokinetic study was part of the ANRS 12174 trial study, which was a large, randomized, multinational, double-blind, controlled trial conducted with 1,273 mother-infant pairs in Burkina Faso, South Africa, Uganda, and Zambia between December 2009 and February 2014. The study was approved by the National Ethics Committee on AIDS and was registered in the NIH international database (https://clinicaltrials.gov/) of clinical trials under ClinicalTrials.gov identifier NCT00640263. The study enrolled singleton infants and their mothers who were not eligible for ART according to WHO guidelines at the time of the study, i.e., because they had >350 CD4 cells/μl blood. Complete details on the study protocol have been published elsewhere (4). This pharmacokinetic analysis was performed on blood samples taken only from infants from the South African trial site in East London.
Study treatment.
All infants were on daily nevirapine until PCR excluded prenatal infection (in a blood sample taken within 7 days of life). Then, infants were randomly switched to receive either lamivudine or the lopinavir-ritonavir coformulation during the BF period. LPV/r and 3TC were administered using commercial syrup formulations. LPV/r was administered to infants twice daily (BID) according to the following dosing scheme: 40/10 mg BID for newborns weighing 2 to 3.9 kg and 80/20 mg BID for infants weighing more than 4 kg. Lamivudine was administered as follows: 7.5 mg BID for neonates weighing 2 to 3.9 kg, 25 mg BID for infants weighing 4 to 7.9 kg, and 50 mg BID for children weighing more than 8 kg. LPV/r and 3TC doses were prescribed at the visit preceding the blood sampling.
Sampling.
This study analyzed blood samples taken from infants from the South African trial site in East London. Samples for pharmacokinetic analysis were taken according to a random sampling scheme at three different visits: at the 6th, 26th, and 38th weeks of life. We recorded the following parameters for the infants: the dose of study drug prescribed; the date of prescription; the amount of time between the time of administration and the time of blood sampling; age; weight; length; and the alanine aminotransferase (ALAT), serum creatinine, and hemoglobin concentrations. The first sampling occurred after at least 4 weeks of treatment, guaranteeing that steady state was reached. Infant HIV-1 infection was assessed using an HIV-1 DNA real-time PCR on dried blood spots (Generic HIV DNA cell; Biocentric, France) at day 7 and at weeks 6, 14, 26, 38, and 50.
Analytical methods.
The plasma concentrations of LPV and 3TC were determined by the Department of Clinical Pharmacology (Hôpital Cochin, Paris, France) by means of validated liquid chromatography-tandem mass spectrometry methods (23). The limits of quantification (LOQs) were 100 ng/ml and 4 ng/ml for LPV and 3TC, respectively. Calibration curves were constructed with blank plasma spiked with concentrations ranging from 100 to 15,000 ng/ml for LPV and 4 to 2,000 ng/ml for 3TC. The inter- and intra-assay accuracies and precisions were between 0.23% and 11.37% for LPV concentrations. For 3TC, the mean interassay precision for controls containing small quantities of drug was 10%, and the inaccuracy at the LOQ was 4.5%.
Pharmacokinetic analyses.
The pharmacokinetic data collected in this trial were too sparse for use of a population approach. As several robust LPV/r and 3TC population pharmacokinetic models have been published for populations with an age range and ethnicity similar to those of the infants included in our study, a Bayesian approach was applied. The nonlinear mixed effect modeling program NONMEM was used to perform Bayesian estimations (the maximum number of function evaluations was set to 0 in the estimation step). Individual predicted concentrations (IPREDs) of lopinavir were derived from the model published by Nikanjam et al. (6). The authors reported that a one-compartment model with first-order absorption and elimination rate constants adequately described their data. Apparent clearance (CL/F) and volume of distribution (V/F) parameters were related to weight, and the relative bioavailability was shown to increase as a function of age. Individual 3TC pharmacokinetics were derived using the two-compartment model with linear absorption and elimination published by Bouazza et al. (7). The apparent clearance and volume of distribution parameters were allometrically scaled, and an additional effect of age on CL/F was determined. Infants from whom blood samples were obtained on multiple occasions were treated as separate subjects. IPREDs were determined for each blood sample from the available sampling times, according to the dosage history, weight at the time of blood sampling, and age covariate values. The trough concentration (Ctrough), maximal concentration (Cmax), time to reach the maximal concentration (Tmax), and area under the curve from 0 to 12 h after the last drug intake (AUC0–12) were derived for each blood sample. To account for missing doses, a total of 400 simulations of the models were performed using the infant's demographic data (i.e., body weight and postnatal age) and dosing characteristics. The observations below the 2.5th percentile were then discarded from the pharmacokinetic analyses, with nonadherence being assumed for these infants. The ratio of excluded observations to population predicted concentrations was also calculated (using values of half the LOQ for observations with levels below the LOQ).
ANRS 12174 trial and WHO drug dosage assessments.
We assessed the consistency of the LPV/r and 3TC ANRS 12174 trial weight-based drug dosages by calculating the percentage of (i) trough LPV concentrations above 1 mg/liter and (ii) 3TC AUC0–12 values above 4.2 mg · h/liter. These thresholds have been shown to be associated with virological efficacy for LPV/r and 3TC in HIV-infected children and adults (7–10).
Dosage assessment.
The ANRS 12174 trial and WHO dosages were assessed by the use of three different population analysis sets: the overall population, the ANRS 12174 protocol dose population, and the WHO simulated population.
(i) Overall population.
The analysis of the overall population was performed with blood samples available from the South African trial site according to the doses received by the children during the trial. Samples from infants that may have missed a dose at the sampling time were kept in this analysis.
(ii) ANRS 12174 protocol dose population.
Analysis of ANRS 12174 protocol dose population excluded samples from infants that may have missed a dose at the sampling time. Individual pharmacokinetic parameters for the other infants were used to assess the prophylaxis doses in accordance with the protocol.
(iii) WHO simulated population.
Analysis of the WHO simulated population excluded samples from infants that may have missed a dose at the sampling time. Individual pharmacokinetic parameters for all other participants were used to simulate the 2016 WHO dosing guidelines for HIV-infected children, which were issued after the start of the trial. For LPV/r, the dose recommendations were 80/20 mg BID for infants weighing 3 to 5.9 kg, 120/30 mg BID for infants weighing 6 to 9.9 kg, and 160/40 mg BID for children weighing 10 to 13.9 kg. For 3TC, the dosing guidelines were 30 mg BID for infants weighing 3 to 5.9 kg, 40 mg BID for infants weighing 6 to 9.9 kg, and 60 mg BID for children weighing 10 to 13.9 kg (3).
These analyses were performed according to the ANRS 12174 trial weight bands.
Supplementary Material
ACKNOWLEDGMENTS
We all report no disclosures or conflicts of interest.
The ANRS 12174 Trial Group included the following: at the University of Montpellier 1 (Montpellier, France), Philippe Van de Perre (chair of the protocol, principal investigator), Nicolas Nagot (project leader), Roselyne Vallo (central data manager and biostatistician), Valerie Marechal (central lab coordinator), Marianne Peries (biostatistician), Dorine Neveu (biostatistician), Vincent Foulongne (virologist), and Michel Segondy (virologist); at the University of Paris V (Paris, France), Stéphane Blanche (pediatrician), Jean-Marc Treluyer (pharmacologist), and Déborah Hirt (modeler); at Makerere University (Kampala, Uganda), James K. Tumwine (site principal investigator), Grace Ndeezi (investigator), Charles Karamagi (investigator), Philippa Musoke (investigator), Proscovia M. Mugaba and Mary Kwagala (site trial coordinators), Joan Murungi (study physician), Hawa Nabuuma Muweesi (lab coordinator), Evelyn Ninsiima (lab technologist), Simon Baryeija, Frederic Juma (pharmacists), and Caleb Bwengye Kata and Stuart Katushabe (data managers); at the University of Ouagadougou (Ouagadougou, Burkina Faso), Nicolas Meda (site principal investigator), Rasmata Ouédraogo (biologist), Diarra Yé (pediatrician), Eric Somé (site trial coordinator), Hugues A. Traoré (site clinical study monitor), Christelle Nadembega (site biological study monitor), Justin Konaté (assistant to the biological study monitor), Arsène Zongo (site pharmacist), Abass Ouédraogo (pharmacist assistant), Désiré Néboua (study physician), Aissatou Bélemviré (study physician), Armel Bambara (data manager), Justine Boncoungou (social worker), and Danielle Zoungrana (social worker); at the University of the Western Cape (Bellville, South Africa), Cheryl Nikodem (site principal investigator until February 2011), Justus Hofmeyr (site principal investigator from March 2011), Kim Harper (site co-principal investigator), Debra Jackson (coinvestigator), David Sanders (country principal investigator), Mandisa Singata-Madliki (project leader), Amwe Aku (study physician), Collins Okegbe-Eze (study physician), Xoliswa Williams (research clinician), Nolundi Mshweshwe (research clinician), Vatiswa Henge (pharmacist), Fikiswa Gomba (breastfeeding counselor), Tapiwa Gundu (data manager), and Oswell Khondowe (trainer); at the University of Zambia (Lusaka, Zambia), Chipepo Kankasa (site principal investigator), Mwiya Mwiya (site trial coordinator), Mildred Lusaka (UTH site coordinator), Mary Chizyuka (UTH site cocoordinator), Mary Phiri (Chawama site coordinator), Billies Imakando (Chawama site coordinator), Mwenechanya Musaku (study physician), Monica Kapasa (study physician), David Rutagwera (laboratory coordinator), Gondwe Clement (co-laboratory coordinator), Hilton Mwila Mwaba (lab scientist), Japhet Matoba (laboratory scientist), Chafye Siuluta (administrator), Katai Chola (data manager), and Patricia Mwamutanda (pharmacist); at the University of Bergen (Bergen, Norway), Thorkild Tylleskär (cochair of the protocol), Halvor Sommerfelt (investigator), Ingunn Engebretsen (investigator), Jørn Klungsøyr (investigator), Jan van den Broeck (investigator), and Jörn Blume (investigator); and at INSERM-ANRS (France), Claire Rekacewicz.
The French National Institute of Health and Medical Research-National Agency for Research on AIDS and Viral Hepatitis (INSERM-ANRS) sponsored the study. Funding was provided by INSERM-ANRS (ANRS 12174), including funds from the Total Foundation, the European Developing Countries Clinical Trials Partnership (EDCTP; grant number CT.2006.33020.004), and the Research Council of Norway (GlobVac grant number 183600).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01869-16.
REFERENCES
- 1.Joint United Nations Programme on HIV/ AIDS. 2013. UNAIDS report on the global AIDS epidemic 2013. Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland. [Google Scholar]
- 2.White AB, Mirjahangir JF, Horvath H, Anglemyer A, Read JS. 2014. Antiretroviral interventions for preventing breast milk transmission of HIV. Cochrane Database Syst Rev 10:CD011323. doi: 10.1002/14651858.CD011323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. 2016. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. WHO, Geneva, Switzerland. [PubMed] [Google Scholar]
- 4.Nagot N, Kankasa C, Meda N, Hofmeyr J, Nikodem C, Tumwine JK, Karamagi C, Sommerfelt H, Neveu D, Tylleskär T, Van de Perre P, PROMISE-PEP Group. 2012. Lopinavir/ritonavir versus lamivudine peri-exposure prophylaxis to prevent HIV-1 transmission by breastfeeding: the PROMISE-PEP trial protocol ANRS 12174. BMC Infect Dis 12:246. doi: 10.1186/1471-2334-12-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagot N, Kankasa C, Tumwine JK, Meda N, Hofmeyr GJ, Vallo R, Mwiya M, Kwagala M, Traore H, Sunday A, Singata M, Siuluta C, Some E, Rutagwera D, Neboua D, Ndeezi G, Jackson D, Maréchal V, Neveu D, Engebretsen IMS, Lombard C, Blanche S, Sommerfelt H, Rekacewicz C, Tylleskär T, Van de Perre P, ANRS 12174 Trial Group. 2016. Extended pre-exposure prophylaxis with lopinavir-ritonavir versus lamivudine to prevent HIV-1 transmission through breastfeeding up to 50 weeks in infants in Africa (ANRS 12174): a randomised controlled trial. Lancet 387:566–573. doi: 10.1016/S0140-6736(15)00984-8. [DOI] [PubMed] [Google Scholar]
- 6.Nikanjam M, Chadwick EG, Robbins B, Alvero C, Palumbo P, Yogev R, Pinto J, Hazra R, Hughes ML, Heckman BE. 2012. Assessment of lopinavir pharmacokinetics with respect to developmental changes in infants and the impact on weight band-based dosing. Clin Pharmacol Ther 91:243–249. doi: 10.1038/clpt.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouazza N, Foissac F, Fauchet F, Burger D, Kiechel J-R, Treluyer J-M, Capparelli EV, Lallemant M, Urien S. 2015. Lopinavir/ritonavir plus lamivudine and abacavir or zidovudine dose ratios for paediatric fixed-dose combinations. Antivir Ther 20:225–223. doi: 10.3851/IMP3016. [DOI] [PubMed] [Google Scholar]
- 8.Bouazza N, Tréluyer J-M, Msellati P, Van de Perre P, Diagbouga S, Nacro B, Hien H, Zoure E, Rouet F, Ouiminga A, Blanche S, Hirt D, Urien S. 2013. A novel pharmacokinetic approach to predict virologic failure in HIV-1-infected paediatric patients. AIDS 27:761–768. doi: 10.1097/QAD.0b013e32835caad1. [DOI] [PubMed] [Google Scholar]
- 9.Bouazza N, Urien S, Blanche S, Hirt D, Foissac F, Benaboud S, Tréluyer J-M, Frange P. 2014. Concentration-response model of lopinavir/ritonavir in HIV-1-infected pediatric patients. Pediatr Infect Dis J 33:e213–e218. doi: 10.1097/INF.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 10.Porte CJ, Back D, Blaschke T, Boucher CAB, Fletcher CV, Flexner C, Gerber J, Kashuba ADM, Schapiro JM, Burger DM. 2006. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Rev Antivir Ther 3:4–12. [Google Scholar]
- 11.Gengiah TN, Moosa A, Naidoo A, Mansoor LE. 2014. Adherence challenges with drugs for pre-exposure prophylaxis to prevent HIV infection. Int J Clin Pharm 36:70–85. doi: 10.1007/s11096-013-9861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blumenthal J, Haubrich R. 2013. Pre-exposure prophylaxis for HIV infection: how antiretroviral pharmacology helps to monitor and improve adherence. Expert Opin Pharmacother 14:1777–1785. doi: 10.1517/14656566.2013.812072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krakower DS, Mayer KH. 2015. Pre-exposure prophylaxis to prevent HIV infection: current status, future opportunities and challenges. Drugs 75:243–251. doi: 10.1007/s40265-015-0355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desmond AC, Moodley D, Conolly CA, Castel SA, Coovadia HM. 2015. Evaluation of adherence measures of antiretroviral prophylaxis in HIV exposed infants in the first 6 weeks of life. BMC Pediatr 15:23. doi: 10.1186/s12887-015-0340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shetty AK, Coovadia HM, Mirochnick MM, Maldonado Y, Mofenson LM, Eshleman SH, Fleming T, Emel L, George K, Katzenstein DA, Wells J, Maponga CC, Mwatha A, Jones SA, Abdool Karim SS, Bassett MT. 2003. Safety and trough concentrations of nevirapine prophylaxis given daily, twice weekly, or weekly in breast-feeding infants from birth to 6 months. J Acquir Immune Defic Syndr 34:482–490. doi: 10.1097/00126334-200312150-00006. [DOI] [PubMed] [Google Scholar]
- 16.Musiime V, Fillekes Q, Kekitiinwa A, Kendall L, Keishanyu R, Namuddu R, Young N, Opilo W, Lallemant M, Walker AS, Burger D, Gibb DM. 2014. The pharmacokinetics and acceptability of lopinavir/ritonavir minitab sprinkles, tablets, and syrups in African HIV-infected children. J Acquir Immune Defic Syndr 66:148–154. doi: 10.1097/QAI.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 17.Mirochnick M, Fenton T, Gagnier P, Pav J, Gwynne M, Siminski S, Sperling RS, Beckerman K, Jimenez E, Yogev R, Spector SA, Sullivan JL. 1998. Pharmacokinetics of nevirapine in human immunodeficiency virus type 1-infected pregnant women and their neonates. J Infect Dis 178:368–374. doi: 10.1086/515641. [DOI] [PubMed] [Google Scholar]
- 18.de Vries-Sluijs TEMS, Dieleman JP, Arts D, Huitema ADR, Beijnen JH, Schutten M, van der Ende ME. 2003. Low nevirapine plasma concentrations predict virological failure in an unselected HIV-1-infected population. Clin Pharmacokinet 42:599–605. doi: 10.2165/00003088-200342060-00009. [DOI] [PubMed] [Google Scholar]
- 19.Hirt D, Warszawski J, Firtion G, Giraud C, Chappuy H, Lechenadec J, Benaboud S, Urien S, Blanche S, Tréluyer J-M. 2013. High exposure to zidovudine during the first 2 weeks of life and concentration-toxicity relationships. J Acquir Immune Defic Syndr 63:555–562. doi: 10.1097/QAI.0b013e3182908c00. [DOI] [PubMed] [Google Scholar]
- 20.Hines RN. 2008. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther 118:250–267. doi: 10.1016/j.pharmthera.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Simon A, Warszawski J, Kariyawasam D, Le Chenadec J, Benhammou V, Czernichow P, Foissac F, Laborde K, Tréluyer J-M, Firtion G, Layouni I, Munzer M, Bavoux F, Polak M, Blanche S, ANRS French Perinatal Cohort Study Group. 2011. Association of prenatal and postnatal exposure to lopinavir-ritonavir and adrenal dysfunction among uninfected infants of HIV-infected mothers. JAMA 306:70–78. doi: 10.1001/jama.2011.915. [DOI] [PubMed] [Google Scholar]
- 22.Food and Drug Administration. 2011. FDA drug safety communication: serious health problems seen in premature babies given Kaletra (lopinavir/ritonavir) oral solution. Food and Drug Administration, Rockville, MD: http://www.fda.gov/Drugs/DrugSafety/ucm246002.htm#data_summary. [Google Scholar]
- 23.Illamola SM, Labat L, Benaboud S, Tubiana R, Warszawski J, Tréluyer JM, Hirt D. 2014. Determination of total and unbound concentrations of lopinavir in plasma using liquid chromatography-tandem mass spectrometry and ultrafiltration methods. J Chromatogr B Analyt Technol Biomed Life Sci 965:216–223. doi: 10.1016/j.jchromb.2014.06.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.