ABSTRACT
The activity of plazomicin and clinically relevant aminoglycosides was tested against 346 extended-spectrum-β-lactamase/AmpC-producing Escherichia coli urinary isolates, and the results were correlated with the presence of aminoglycoside-modifying enzymes (AMEs). Data showed that plazomicin was very active against all ESBL/AmpC-producing E. coli urinary isolates. Its activity was not related to the AME genes studied.
KEYWORDS: AMEs, ESBL, Escherichia coli, plazomicin, aminoglycosides
TEXT
Escherichia coli strains producing extended-spectrum β-lactamases (ESBLs) have emerged as major global pathogens, primarily associated with urinary tract infections (1). These strains possess plasmids that carry genes conferring resistance to multiple antibiotic classes (2). As a result, therapeutic options against these β-lactam-resistant E. coli infections are extremely limited.
Aminoglycoside resistance in Gram-negatives is mainly conferred by production of aminoglycoside-modifying enzymes (AMEs) (3). Genes encoding AMEs are located on mobile genetic elements along with other resistance determinants, resulting in multidrug-resistant (MDR) isolates (3). Plazomicin is a next-generation aminoglycoside modified to evade AMEs. The compound is currently under clinical development for the treatment of complicated urinary tract infections (cUTIs) and acute pyelonephritis as a single agent (4, 5).
In this study, we evaluated the activity of plazomicin and clinically relevant aminoglycosides against 346 ESBL/AmpC-producing E. coli urinary isolates. The presence of four AME genes was also investigated, and the relationship between the AME genes detected and the resistance phenotype found was determined.
The isolates were obtained prospectively during 2013 at the Hospital Clínico San Carlos (Madrid, Spain). Only one isolate per patient was included. PCR characterization (6, 7) showed 302 ESBL producers and 44 AmpC producers.
MICs of gentamicin, tobramycin, amikacin, and plazomicin were determined by the agar dilution method. MICs of plazomicin were also determined by the broth microdilution method (8). Antimicrobial agents were obtained from their respective manufacturers. Plazomicin was supplied from Achaogen (South San Francisco, CA). The results were interpreted according the guidelines of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (9).
All isolates resistant to at least one of the aminoglycosides studied were tested by PCR for the presence of AME genes. Sets of primers for the following genes were included in the PCR assay: aac(3)-IIa (10); aac(6′)-Ib (11); ant(2″)-Ia (12); and aph(3′)-Ia (11).
Comparisons of MICs for each antibiotic between groups were performed by Mann-Whitney U test. Correlations between pairs of variables were calculated by Spearman's rank test. The significance level was considered a P value of ≤0.05. Statistical analysis was performed using IBM SPSS 20 (SPSS Inc., Chicago, IL).
Overall, the highest resistance rate was observed for tobramycin (38.3%), followed by gentamicin (27.7%). Amikacin showed good activity, with a 1.5% resistance rate and a MIC90 of 8 mg/liter; similar results were previously reported in ESBL-producing E. coli (13, 14).
Plazomicin showed the best activity, with a MIC range of 0.25 to 4 mg/liter and MIC50 and MIC90 values of 1 mg/liter against all ESBL- and AmpC-producing E. coli isolates (data obtained by agar method). No significant differences were found between agar dilution MICs and microdilution MICs (MIC50, 0.5 mg/liter; MIC90, 1 mg/liter; MIC range, 0.125 to 4 mg/liter). Our findings are consistent with previous studies that showed plazomicin to be highly active against MDR clinical isolates of Enterobacteriaceae, including ESBL-producing E. coli isolates (15, 16).
The levels of susceptibility to aminoglycosides in relation to the β-lactamase type produced by the isolates are summarized in Table 1. All the AmpC isolates were amikacin susceptible, with MICs of ≤8 mg/liter.
TABLE 1.
In vitro susceptibility to different aminoglycosides of 346 Escherichia coli isolates in relation to the β-lactamase type produceda
| Aminoglycoside | ESBL (n = 302 isolates) |
AmpC (n = 44 isolates) |
||||||
|---|---|---|---|---|---|---|---|---|
| MIC50 (mg/liter) | MIC90 (mg/liter) | Range | No. (%) of resistant isolates | MIC50 (mg/liter) | MIC90 (mg/liter) | Range | No. (%) of resistant isolates | |
| Gentamicin | 1 | 64 | ≤0.125 to >64 | 86 (28.6) | 1 | 64 | 0.5 to >64 | 11 (25) |
| Tobramycin | 1 | 32 | ≤0.125 to >64 | 123 (40.9) | 1 | 8 | 0.5 to 32 | 12 (27.3) |
| Amikacin | 4 | 8 | 0.5 to >64 | 5 (1.7) | 2 | 4 | 2 to 8 | 0 |
| Plazomicin | 1 | 1 | 0.25 to 4 | NA | 1 | 1 | 0.5 to 2 | NA |
Resistance data were determined on the basis of EUCAST susceptibility breakpoints. NA, not applicable.
Of the 346 E. coli isolates, 144 (41.5%) were resistant to at least one of the aminoglycosides studied. Five different resistance phenotypes were observed among these 144 resistant isolates. Resistance to gentamicin/tobramycin was observed for 86 strains; resistance to tobramycin for 45 strains; resistance to gentamicin for 8 strains; resistance to amikacin/gentamicin/tobramycin for 3 strains; and resistance to amikacin/tobramycin for 2 strains.
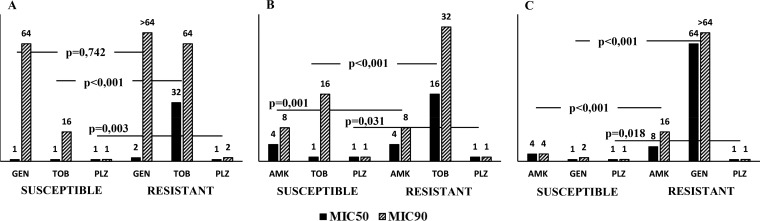
As can be seen in Fig. 1, the median MICs of the respective aminoglycosides were significantly higher for the tobramycin- and gentamicin-susceptible and -resistant isolates. Amikacin comparisons showed results that were analogous to those obtained with tobramycin and plazomicin but not to those obtained with gentamicin. The level of correlation between the MICs for plazomicin and amikacin by Spearman's rank test was higher (r = 0.600; P < 0.001) than the level of correlation between the MICs for plazomicin and tobramycin (r = 0.271; P < 0.001) and gentamicin (r = 0.112; P = 0.038), respectively. This is the first description of a correlation between the MICs of plazomicin and those of amikacin, tobramycin, and gentamicin in E. coli isolates, although a correlation between the plazomicin MICs and those of gentamicin has been reported in carbapenemase-producing Klebsiella pneumoniae (17).
FIG 1.
In vitro susceptibility to various aminoglycosides stratified by (A) amikacin (AMK) susceptibility, (B) gentamicin (GEN) susceptibility, and (C) tobramycin (TOB) susceptibility. P values denote differences in median MIC values of the respective aminoglycosides between the amikacin-, gentamicin-, and tobramycin-susceptible and -resistant isolates. The epidemiological cutoff was considered for amikacin resistance (MIC > 8 mg/liter). The MIC50 and MIC90 values are shown above the respective bars. PLZ, plazomicin.
The prevalence of combinations of AME genes among 144 E. coli isolates resistant to aminoglycosides is shown in Table 2. At least one AME gene was detected in 94.5% of strains. The most common AME gene was aac(6′)-Ib (90 strains; 62.5%), followed by aac(3)-IIa (37 strains; 25.7%), aph(3′)-Ia (30 strains; 20.8%), and ant(2″)-Ia (19 strains; 13.2%). In total, 98 isolates (68%) contained only one of the evaluated AME genes, 36 (25%) contained two of them, and 2 (1.4%) harbored three. Using EUCAST breakpoints (9), the AME presence was not always correlated with aminoglycoside resistance. Similar results were previously reported both in E. coli isolates and in K. pneumoniae isolates (17, 18).
TABLE 2.
Prevalence of aminoglycoside-modifying enzyme gene combinations in 144 Escherichia coli isolates resistant to aminoglycosides in relation to aminoglycoside MICsa
| AME gene(s) | No. (%) of isolates | MIC50/MIC90 (mg/liter) |
Range |
No. (%) of resistant isolates |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMK | GEN | TOB | PLZ | AMK | GEN | TOB | PLZ | AMK | GEN | TOB | PLZ | ||
| aac(6′)Ib | 64 (44.4) | 8/16 | 2/>64 | 16/32 | 1/1 | 2 to 64 | 0.5 to >64 | 8 to 64 | 0.25 to 4 | 2 (3.1) | 24 (37.5) | 64 (100) | NA |
| aac(3)IIa | 21 (14.5) | 4/4 | >64/>64 | 8/8 | 1/1 | 2 to 8 | 64 to >64 | 4 to 32 | 0.5 to 2 | 0 (0) | 21 (100) | 19 (90.5) | NA |
| aph(3′)Ia | 9 (6.3) | 4/8 | 64/64 | 8/32 | 0.5/2 | 2 to 8 | 2 to 64 | 4 to 32 | 0.5 to 2 | 0 (0) | 8 (88.9) | 7 (77.8) | NA |
| ant(2″)Ia | 4 (2.8) | 2/4 | 64/64 | 8/32 | 0.5/1 | 2 to 4 | 64 to 64 | 8 to 32 | 0.5 to 1 | 0 (0) | 4 (100) | 4 (100) | NA |
| aa(6′)Ib + aph(3′)Ia | 13 (9.02) | 8/16 | 64/64 | 32/32 | 1/1 | 4 to 64 | 0.5 to >64 | 16 to 32 | 0.5 to 1 | 1 (7.7) | 8 (61.5) | 13 (100) | NA |
| aac(6′)Ib + ant(2″)Ia | 7 (4.9) | 8/32 | 32/64 | 32/64 | 1/4 | 2 to 32 | 8 to 64 | 8 to 64 | 0.5 to 4 | 1 (14.3) | 7 (100) | 7 (100) | NA |
| aac(6′)Ib + aac(3)IIa | 5 (3.5) | 8/>64 | >64/>64 | 32/>64 | 0.5/1 | 2 to >64 | 64 to >64 | 16 to >64 | 0.5 to 1 | 1 (20) | 5 (100) | 5 (100) | NA |
| aac(3)IIa + ant(2″)Ia | 5 (3.5) | 4/4 | 64/64 | 8/8 | 1/1 | 2 to 4 | 64 to 64 | 4 to 8 | 0.5 to 1 | 0 (0) | 5 (100) | 4 (80) | NA |
| aac(3)IIa + aph(3′)Ia | 4 (2.8) | 2/4 | 64/>64 | 8/8 | 0.5/1 | 2 to 4 | 64 to >64 | 8 to 8 | 0.5 to 1 | 0 (0) | 4 (100) | 4 (100) | NA |
| ant(2″)Ia + aph(3′)Ia | 2 (1.4) | 2/4 | 64/>64 | 16/16 | 0.5/1 | 2 to 4 | 64 to >64 | 16 to 16 | 0.5 to 1 | 0 (0) | 2 (100) | 2 (100) | NA |
| aac(6′)Ib + aac(3)Iia + aph(3′)Ia | 1 (0.7) | 8/8 | 32/32 | 16/16 | 1/1 | 8 to 8 | 32 to 32 | 16 to 16 | 1 to 1 | 0 (0) | 1 (100) | 1 (100) | NA |
| aac(3)IIa + ant(2″)Ia + aph(3′)Ia | 1 (0.7) | 8/8 | >64/>64 | 16/16 | 2/2 | 8 to 8 | >64 to >64 | 16 to 16 | 2 to 2 | 0 (0) | 1 (100) | 1 (100) | NA |
| None | 8 (5.5) | 4/8 | 32/64 | 8/32 | 0.5/2 | 2 to 8 | 1 to 64 | 1 to 32 | 0.5 to 2 | 0 (0) | 7 (87.5) | 5 (62.5) | NA |
Resistance data were determined on the basis of EUCAST susceptibility breakpoints. AME, aminoglycoside-modifying enzyme; AMK, amikacin; GEN, gentamicin; TOB, tobramycin; PLZ, plazomicin; NA, not applicable.
PCR screening showed that aac(6′)-Ib was the most prevalent gene. Similar results have been reported by other authors (17, 18). All strains with aac(6′)-Ib were tobramycin resistant. Only 2 of the 58 isolates that harbored the aac(6′)-Ib gene alone expressed phenotypic resistance to amikacin, although it has been reported that aac(6′)-Ib confers resistance to both antibiotics (3). These observations were consistent with previous studies where, despite the possession of aac(6′)-Ib, low amikacin MICs have been reported for E. coli and K. pneumoniae strains (14, 17). In our study, moreover, there was a statistically significant relationship (P < 0.01) between the presence of an aac(6′)-Ib gene and an amikacin MIC of >8 mg/liter. These data showed that the presence of aac(6′)-Ib is required for expression of amikacin MICs above the epidemiological cutoff value (ECOFF).
The second-most-common gene in our study was aac(3)-IIa, although in other studies, this was the most prevalent gene, followed by aac(6′)-Ib (14, 19). The 21 isolates with this resistance gene alone were resistant to gentamicin; the presence of aac(3)-IIa in any combination of genes was associated with gentamicin resistance (P < 0.01). The resistance to gentamicin observed in our study was predominantly caused by the presence of the aac(3)-IIa gene; similar findings have previously been reported (18).
The presence of the ant(2″)-Ia gene was found to be related to gentamicin resistance, as previously described (14, 19). The presence of aph(3′)-Ia was not associated with resistance to any of the studied aminoglycosides.
In conclusion, in this study, the strains exhibited a remarkable diversity of AMEs; the AME genes involved in clinical aminoglycoside resistance were aac(3)-IIa, aac(6′)-Ib, and ant(2″)-Ia. High amikacin MICs, above the ECOFF established by EUCAST, have been shown to be related to the presence of aac(6′)-Ib. The activity of plazomicin was excellent regardless of the AME pattern; it may become a welcomed addition for the treatment of cUTIs, but the real position of this antibiotic will be revealed once pending phase III studies are completed.
ACKNOWLEDGMENTS
We declare that we have no conflicts of interest.
This work has been funded by grant PI13/01471 as part of the National R&D Plan, AES 2013, and cofunded by the ISCIII and the European Regional Development Fund (ERDF).
REFERENCES
- 1.D'Andrea MM, Arena F, Pallecchi L, Rossolini GM. 2013. CTX-M-type beta-lactamases: a successful story of antibiotic resistance. Int J Med Microbiol 303:305–317. doi: 10.1016/j.ijmm.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Woodford N, Carattoli A, Karisik E, Underwood A, Ellington MJ, Livermore DM. 2009. Complete nucleotide sequences of plasmids pEK204, pEK499, and pEK516, encoding CTX-M enzymes in three major Escherichia coli lineages from the United Kingdom, all belonging to the international O25:H4-ST131 clone. Antimicrob Agents Chemother 53:4472–4482. doi: 10.1128/AAC.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramirez MS, Tolmasky ME. 2010. Aminoglycoside modifying enzymes. Drug Resist Updat 13:151–171. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karaiskos I, Souli M, Giamarellou H. 2015. Plazomicin: an investigational therapy for the treatment of urinary tract infections. Expert Opin Investig Drugs 24:1501–1511. doi: 10.1517/13543784.2015.1095180. [DOI] [PubMed] [Google Scholar]
- 5.U.S. National Institutes of Health. http://clinicaltrials.gov. [Google Scholar]
- 6.Coque TM, Oliver A, Perez-Diaz JC, Baquero F, Canton R. 2002. Genes encoding TEM-4, SHV-2, and CTX-M-10 extended-spectrum beta-lactamases are carried by multiple Klebsiella pneumoniae clones in a single hospital (Madrid, 1989 to 2000). Antimicrob Agents Chemother 46:500–510. doi: 10.1128/AAC.46.2.500-510.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez-Pérez FJ, Hanson ND. 2002. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol 40:2153–2162. doi: 10.1128/JCM.40.6.2153-2162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CLSI. 2009. Performance standards for antimicrobial susceptibility testing. Nineteenth informational supplement. CLSI document M100-S19 CLSI, Wayne, PA. [Google Scholar]
- 9.The European Committee on Antimicrobial Susceptibility Testing. 2013. Break-point tables for interpretation of MICs and zone diameters. Version 3.1 http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_3.1.pdf.
- 10.Jensen VF, Jakobsen L, Emborg HD, Seyfarth AM, Hammerum AM. 2006. Correlation between apramycin and gentamicin use in pigs and an increasing reservoir of gentamicin-resistant Escherichia coli. J Antimicrob Chemother 58:101–107. doi: 10.1093/jac/dkl201. [DOI] [PubMed] [Google Scholar]
- 11.Akers KS, Chaney C, Barsoumian A, Beckius M, Zera W, Yu X, Guymon C, Keen EF III, Robinson BJ, Mende K, Murray CK. 2010. Aminoglycoside resistance and susceptibility testing errors in Acinetobacter baumannii-calcoaceticus complex. J Clin Microbiol 48:1132–1138. doi: 10.1128/JCM.02006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hujer KM, Hujer AM, Hulten EA, Bajaksouzian S, Adams JM, Donskey CJ, Ecker DJ, Massire C, Eshoo MW, Sampath R, Thomson JM, Rather PN, Craft DW, Fishbain JT, Ewell AJ, Jacobs MR, Paterson DL, Bonomo RA. 2006. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob Agents Chemother 50:4114–4123. doi: 10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Díaz MA, Hernández-Bello JR, Rodríguez-Baño J, Martínez-Martínez L, Calvo J, Blanco J, Pascual A; Spanish Group for Nosocomial Infections (GEIH) . 2010. Diversity of Escherichia coli strains producing extended-spectrum beta-lactamases in Spain: second nationwide study. J Clin Microbiol 48:2840–2845. doi: 10.1128/JCM.02147-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haldorsen BC, Simonsen GS, Sundsfjord A, Samuelsen O, Norwegian Study Group on Aminoglycoside Resistance. 2014. Increased prevalence of aminoglycoside resistance in clinical isolates of Escherichia coli and Klebsiella spp. in Norway is associated with the acquisition of AAC(3)-II and AAC(6′)-Ib. Diagn Microbiol Infect Dis 78:66–69. doi: 10.1016/j.diagmicrobio.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Galani I, Souli M, Daikos GL, Chrysouli Z, Poulakou G, Psichogiou M, Panagea T, Argyropoulou A, Stefanou I, Plakias G, Giamarellou H, Petrikkos G. 2012. Activity of plazomicin (ACHN-490) against MDR clinical isolates of Klebsiella pneumoniae, Escherichia coli, and Enterobacter spp. from Athens, Greece. J Chemother 24:191–194. doi: 10.1179/1973947812Y.0000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walkty A, Adam H, Baxter M, Denisuik A, Lagace-Wiens P, Karlowsky JA, Hoban DJ, Zhanel GG. 2014. In vitro activity of plazomicin against 5,015 gram-negative and gram-positive clinical isolates obtained from patients in Canadian hospitals as part of the CANWARD study, 2011–2012. Antimicrob Agents Chemother 58:2554–2563. doi: 10.1128/AAC.02744-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almaghrabi R, Clancy CJ, Doi Y, Hao B, Chen L, Shields RK, Press EG, Iovine NM, Townsend BM, Wagener MM, Kreiswirth B, Nguyen MH. 2014. Carbapenem-resistant Klebsiella pneumoniae strains exhibit diversity in aminoglycoside-modifying enzymes, which exert differing effects on plazomicin and other agents. Antimicrob Agents Chemother 58:4443-4451. doi: 10.1128/AAC.00099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernández-Martínez M, Miró E, Ortega A, Bou G, González-López JJ, Oliver A, Pascual A, Cercenado E, Oteo J, Martínez-Martínez L, Navarro F; Spanish Network for the Research in Infectious Diseases (REIPI). 2015. Molecular identification of aminoglycoside-modifying enzymes in clinical isolates of Escherichia coli resistant to amoxicillin/clavulanic acid isolated in Spain. Int J Antimicrob Agents 46:157–163. doi: 10.1016/j.ijantimicag.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Xiao Y, Hu Y. 2012. The major aminoglycoside-modifying enzyme AAC(3)-II found in Escherichia coli determines a significant disparity in its resistance to gentamicin and amikacin in China. Microb Drug Resist 18:42–46. doi: 10.1089/mdr.2010.0190. [DOI] [PubMed] [Google Scholar]