ABSTRACT
Echinocandins target the fungal cell wall by inhibiting biosynthesis of the cell wall carbohydrate β-1,3-glucan. This antifungal drug class exhibits a paradoxical effect that is characterized by the resumption of growth of otherwise susceptible strains at higher drug concentrations (approximately 4 to 32 μg/ml). The nature of this phenomenon is still unknown. In this study, we analyzed the paradoxical effect of the echinocandin caspofungin on the pathogenic mold Aspergillus fumigatus. Using a conditional fks1 mutant, we show that very high caspofungin concentrations exert an additional antifungal activity besides inhibition of the β-1,3-glucan synthase. This activity could explain the suppression of paradoxical growth at very high caspofungin concentrations. Additionally, we found that exposure to inhibitory caspofungin concentrations always causes initial growth deprivation independently of the capability of the drug concentration to induce the paradoxical effect. Paradoxically growing hyphae emerge from microcolonies essentially devoid of β-1,3-glucan. However, these hyphae expose β-1,3-glucan again, suggesting that β-1,3-glucan synthesis is restored. In agreement with this hypothesis, we found that expression of the β-1,3-glucan synthase Fks1 is an essential requirement for the paradoxical effect. Surprisingly, overexpression of fks1 renders A. fumigatus more susceptible, whereas reduced expression leads to hyphae that are more resistant to the growth-inhibitory and limited fungicidal activity of caspofungin. Upregulation of chitin synthesis appears to be of minor importance for the paradoxical effect, since paradoxically growing hyphae exhibit significantly less chitin than the growth-deprived parental microcolonies. Our results argue for a model where the paradoxical effect primarily relies on recovery of β-1,3-glucan synthase activity.
KEYWORDS: Aspergillus fumigatus, caspofungin, echinocandin, fks1, glucan synthase, paradoxical effect
INTRODUCTION
Echinocandin antifungals have fungicidal activity against most Candida spp. but only fungistatic activity against Aspergillus spp. (1, 2). Due to their broad activity and favorable safety profile, echinocandins have emerged as a major armament against invasive fungal infections. The mode of action of echinocandins relies on the noncompetitive inhibition of glucan synthase, an enzyme that is required for biosynthesis of β-1,3-glucan, a primary fungal cell wall carbohydrate.
Three semisynthetic derivatives, namely, caspofungin, anidulafungin, and micafungin, were approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for clinical use. Each of these echinocandins inhibits the growth of susceptible Candida and Aspergillus spp. under in vitro conditions at concentrations below 1 μg ml−1 (approximately 0.03 to 0.5 μg ml−1, depending on the agent, species, and isolate). However, in a certain range of higher concentrations (approximately 4 to 32 μg ml−1), a significant number of susceptible strains of Candida and Aspergillus paradoxically resume growth. This echinocandin-specific phenomenon was termed the “paradoxical effect” and initially described in 1988 for the nonclinical echinocandin derivative cilofungin (3). However, its mechanistic nature and clinical relevance are still matters of debate (2, 4–6).
Over the years, multiple hypotheses were conceived in order to explain the paradoxical effect of the echinocandins (reviewed in references 4, 5, and 7). They included mutations in the gene encoding the β-1,3-glucan synthase, upregulation of β-1,3-glucan synthase activity, and inactivation of the drug by either degradation or change of its physical state. However, none of the three models held true. In a dedicated study, neither mutations in the β-1,3-glucan synthase gene that correlated with the phenotype nor upregulation of the enzymatic activity was observed (8). Analysis of the culture media after growth indicated that the drug was not degraded by strains that showed the paradoxical effect (9). In addition, the absence of the paradoxical effect in a significant number of echinocandin-susceptible strains, as well as its suppression at very high concentrations, argues against a physical state change (e.g., precipitation) (8–10).
A number of studies support an alternative model that is based on the following observations. First, a compensatory increase of cell wall chitin was repeatedly observed after exposure of Aspergillus fumigatus and Candida albicans to echinocandins (11–14). Second, disruption of calcineurin signaling or cell wall integrity signaling in C. albicans or A. fumigatus, either by pharmacologic inhibition or genetic modification, suppresses the paradoxical effect (15, 16). Third, transcriptional upregulation of chitin synthesis relies on calcineurin signaling in C. albicans and A. fumigatus (12, 16). Similarly, at least in C. albicans, chitin synthesis additionally relies on cell wall integrity signaling (12). Fourth, reduced expression, as well as pharmacologic inhibition, of Hsp90 abolishes the paradoxical effect (17, 18). Based on these observations, the alternative model proposes that echinocandins activate the calcineurin and cell wall integrity signaling pathways, which in turn induce a compensatory increase of cell wall chitin and Hsp90 that enables the paradoxical effect (reviewed in references 5 and 7).
In this work, we exploited the ability of A. fumigatus to grow in the absence of the β-1,3-glucan synthase (Fks1) and cell wall β-1,3-glucan (14) to study the paradoxical effect. We show that expression of the β-1,3-glucan synthase is required for the paradoxical effect and that increased expression of the β-1,3-glucan synthase renders A. fumigatus more susceptible to caspofungin. Finally, we propose a refined model for the paradoxical effect of echinocandins.
RESULTS
Echinocandin-induced growth deprivation precedes the paradoxical effect.
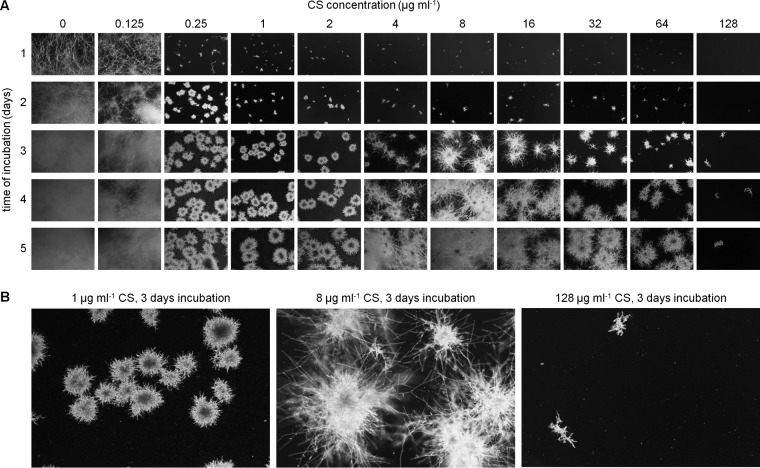
Not all echinocandin-susceptible fungal strains are capable of paradoxical growth. To define experimental conditions to investigate the paradoxical effect, we analyzed the caspofungin susceptibility of A. fumigatus strain AfS35 (19). Growth in Aspergillus minimal medium (AMM) supplemented with 2-fold dilutions of caspofungin was followed and documented over time in a 24-well plate at 37°C (Fig. 1A). After 1 day of incubation, growth of Aspergillus was drastically suppressed at caspofungin concentrations of ≥0.25 μg ml−1. In contrast, caspofungin concentrations of ≤0.125 μg ml−1 did not inhibit or only moderately inhibited (0.125 μg ml−1) growth compared to the control. Notably, conidia at caspofungin concentrations of ≥0.25 μg ml−1 were still able to germinate, but the emerging microcolonies grew very slowly and were characterized by irregular, short, hyperbranching hyphae. After 3 days of incubation, the morphology and growth of the microcolonies incubated with 4 to 64 μg ml−1 caspofungin began to differ from those cultured in the presence of 0.25 to 2 μg ml−1 caspofungin, thereby defining the onset of the paradoxical effect. At concentrations of >64 μg ml−1, paradoxical growth apparently did not occur. Closer inspection of paradoxical growth revealed that long hyphae reminiscent of wild-type growth in the absence of echinocandins emerged from the slowly growing microcolonies (Fig. 1B). This effect became most prominent after 4 days (Fig. 1A). Our data demonstrate that a common echinocandin-induced growth deprivation characterized by microcolonies with irregular, short, hyperbranching hyphae precedes the onset of the paradoxical effect on day 3.
FIG 1.
Growth of A. fumigatus in the presence of different caspofungin concentrations followed over time. (A and B) Wild-type conidia were inoculated in AMM in a 24-well plate (5 × 103 conidia per well). The medium was supplemented with the indicated amounts of caspofungin (CS). Representative dark-field images were taken after 1, 2, 3, 4, and 5 days of incubation at 37°C. (B) Magnifications of selected dark-field images depicted in panel A.
Only echinocandin concentrations that invoke the paradoxical effect yield progenitor hyphae devoid of β-1,3-glucan.
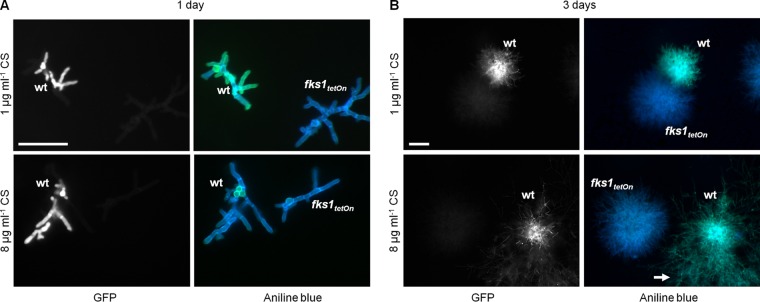
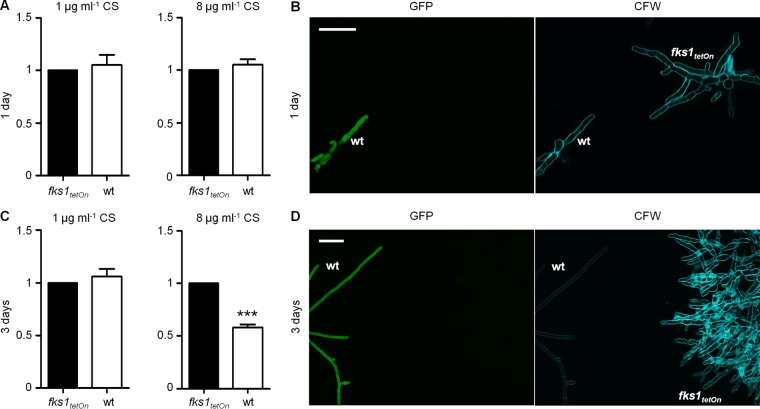
Echinocandins directly inhibit β-1,3-glucan synthesis. We speculated that the extent of β-1,3-glucan synthesis inhibition correlates with the echinocandin concentration. We used aniline blue, a β-1,3-glucan-specific dye, to determine the presence of β-1,3-glucan in A. fumigatus hyphae growing in the presence of caspofungin. As an internal staining control, we used a conditional A. fumigatus β-1,3-glucan synthase mutant. This fks1tetOn mutant was constructed in the same genetic background by replacing the endogenous promoter with a doxycycline-inducible Tet-On promoter. Under repressive conditions, the mutant mimics the phenotype of a Δfks1 deletion mutant and yields slowly growing hyperbranching hyphae very similar to those of the caspofungin-treated wild type (14). To allow discrimination of the wild type and the conditional fks1tetOn control strain, experiments were performed with a wild-type strain that expresses a cytosolic variant of green fluorescent protein (GFP). As shown in Fig. 2A, wild-type hyphae growing in the presence of 1 μg ml−1 caspofungin contained a significant amount of aniline blue-stainable β-1,3-glucan after 1 day of incubation compared to the control hyphae of the repressed fks1tetOn strain. In contrast, wild-type hyphae growing for 1 day in the presence of 8 μg ml−1 caspofungin, a concentration that invokes the paradoxical effect, were not specifically stained by aniline blue and in this respect were indistinguishable from the hyphae of the repressed fks1tetOn strain (Fig. 2A). Notably, despite the different staining patterns, the growth rates and morphologies of the wild type raised in the presence of 1 or 8 μg ml−1 caspofungin did not significantly differ. This demonstrates that higher caspofungin concentrations can block β-1,3-glucan synthesis after 1 day of incubation.
FIG 2.
Paradoxically growing hyphae expose β-1,3-glucan. (A and B) Conidia of the wild type expressing GFP and fks1tetOn were inoculated in AMM on coverslips. The medium was supplemented with the indicated amounts of CS. After 1 day of incubation (A) or 3 days of incubation (B), hyphae were fixed, stained with aniline blue, and immediately analyzed with a fluorescence microscope. (Left) GFP fluorescence. (Right) Glucan-specific (green) and nonspecific (blue) aniline blue fluorescence. Scale bars, 50 μm (A) and 200 μm (B) for all images. The arrow in panel B indicates paradoxically growing hyphae.
Paradoxically growing hyphae expose β-1,3-glucan.
It was previously reported that paradoxically growing C. albicans hyphae expose β-1,3-glucan (10). We therefore analyzed A. fumigatus microcolonies raised under conditions that induce the paradoxical effect for the presence of β-1,3-glucan. As expected, paradoxical growth became evident after 3 days of incubation in medium supplemented with 8 μg ml−1 caspofungin. Aniline blue staining of the colonies revealed the presence of a significant amount of β-1,3-glucan (Fig. 2B). Not surprisingly, the wild type cultured in the presence of 1 μg ml−1 caspofungin also exposed β-1,3-glucan after 3 days of incubation. However, under these conditions, no morphological switch to paradoxical growth was observed (Fig. 2B). Taken together, our results support the previous observation that paradoxically growing hyphae expose β-1,3-glucan (10).
Expression of the glucan synthase Fks1 is a prerequisite for the paradoxical effect.
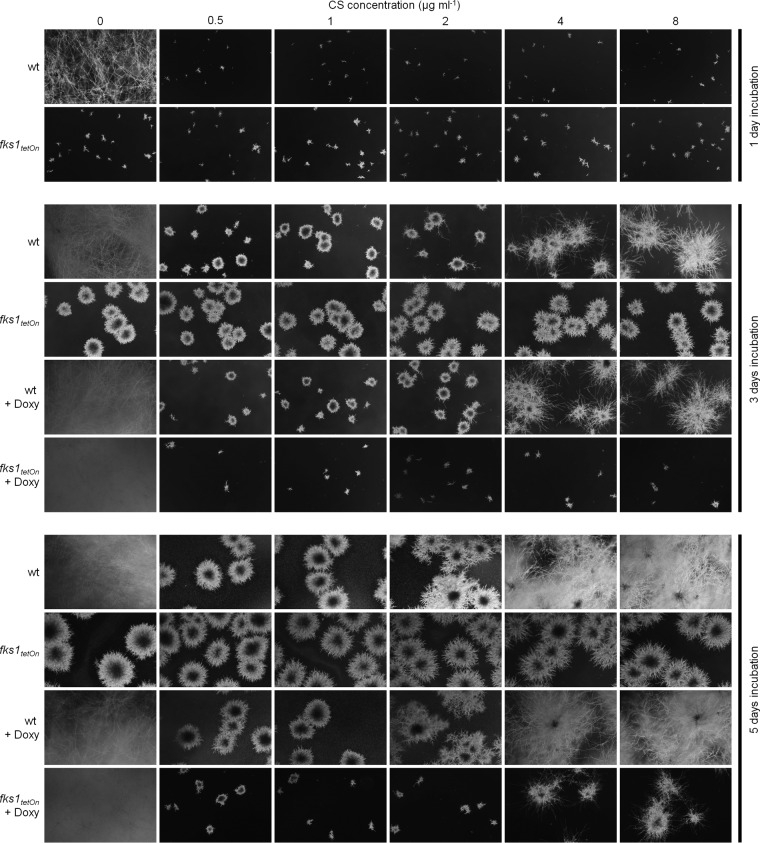
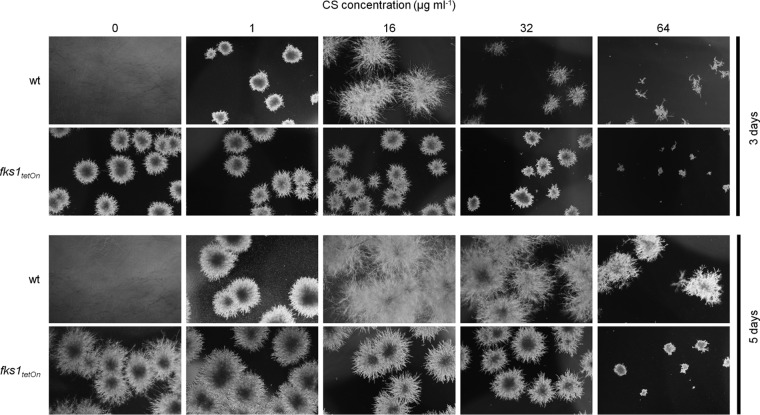
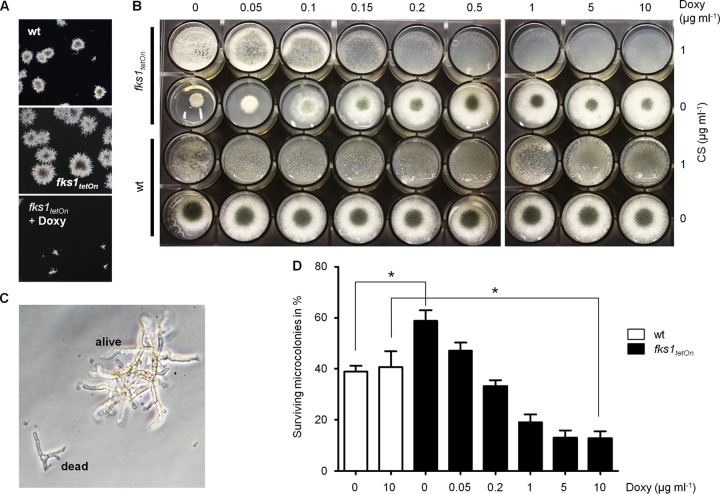
Surprisingly, the repressed fks1tetOn mutant formed no paradoxically growing hyphae under conditions that induce the paradoxical effect in the wild type (Fig. 2B). To further investigate this observation, we analyzed and compared the growth of the wild type and the conditional fks1tetOn strain in the presence of caspofungin. In agreement with our previous results (14), growth of the fks1tetOn strain under repressive conditions was not affected at any time, even in the presence of caspofungin concentrations as high as 32 μg ml−1 (Fig. 3). Unexpectedly, incubation in the presence of very high caspofungin concentrations (>32 μg ml−1) significantly impaired the emergence of fks1tetOn microcolonies under repressive conditions (Fig. 4). Similarly, and in agreement with previous studies (4), paradoxical growth of the wild type was also diminished (64 μg ml−1) or even virtually abolished (128 μg ml−1) in the presence of very high caspofungin concentrations (Fig. 4; see Fig. S1 in the supplemental material).
FIG 3.
Expression of β-1,3-glucan synthase is required for paradoxical growth. Conidia of the wild type and the fks1tetOn strain were inoculated in AMM in a 24-well plate (5 × 103 conidia per well). The medium was supplemented with the indicated amounts of CS. Where indicated (+ Doxy), The medium was supplemented with 10 μg ml−1 doxycycline. Representative dark-field images were taken after 1, 3, and 5 days of incubation at 37°C.
FIG 4.
Very high caspofungin concentrations exert additional antifungal activity beyond inhibition of the β-1,3-glucan synthase. Conidia of the indicated strains were inoculated in AMM in a 24-well plate (5 × 103 conidia per well). The medium was supplemented with the indicated amounts of CS. Representative dark-field images were taken after 3 and 5 days of incubation at 37°C.
Even after prolonged incubation in the presence of high caspofungin concentrations, the fks1tetOn strain under repressive conditions never switched to paradoxical growth. Under induced conditions, the fks1tetOn strain showed caspofungin susceptibility that was similar to that of the wild type (14) (Fig. 3) and the ability for paradoxical growth (Fig. 3). However, the onset of the paradoxical effect was delayed for approximately 1 to 2 days compared to the wild type (Fig. 3). In conclusion, this demonstrates that expression of fks1 is an essential requirement for the paradoxical effect of echinocandins.
Expression of the glucan synthase Fks1 contributes to the antifungal activity of echinocandins.
The growth characteristics of the conditional fks1tetOn hyphae growing under repressed conditions is very similar to that of the wild type growing in the presence of inhibitory concentrations of caspofungin (14). However, we repeatedly observed that the sizes of emerging microcolonies of the mutant lacking fks1 expression were significantly larger than those of the wild type inhibited by caspofungin (Fig. 3 and 5A). On the other hand, microcolonies of the fks1tetOn strain under induced conditions were significantly smaller than those of the wild type cultured in the presence of inhibitory caspofungin concentrations (Fig. 3 and 5A). To further dissect this phenomenon, we analyzed the growth phenotype of the fks1tetOn strain under induced and repressed conditions on solid medium. As shown in Fig. 5B, the fks1tetOn strain spread on AMM agar supplemented with 1 μg ml−1 caspofungin and increasing concentrations of doxycycline grew much better in the absence of doxycycline (no induction) or in the presence of low doxycycline concentrations (weak induction) than in the presence of high doxycycline concentrations (strong induction) (Fig. 5B). This effect nicely correlated with the striking growth reduction of the mutant spotted on solid agar without doxycycline and the apparent overexpression phenotype represented by reduced sporulation in the presence of high doxycycline concentrations (Fig. 5B). In contrast, growth of the wild type spread on AMM agar or AMM agar supplemented with 1 μg ml−1 caspofungin was not affected by doxycycline (Fig. 5B).
FIG 5.
Expression of fks1 increases the fungicidal and growth-inhibitory activity of caspofungin. (A) Conidia of the indicated strains were inoculated in AMM supplemented with 1 μg ml−1 caspofungin. Where indicated (+ Doxy), the medium was supplemented with 10 μg ml−1 doxycycline. After 3 days of incubation at 37°C, representative dark-field images were taken. (B) Conidia of the indicated strains were spread (4 × 104 conidia per well) (top row) or spotted (1.5 × 103 conidia per well) (bottom row) on AMM agar. The medium was supplemented with the indicated amounts of CS and doxycycline (Doxy). The images were taken after 2 days of incubation at 37°C. (C) Representative bright-field image of a dead and a viable wild-type microcolony raised in AMM in the presence of 1 μg ml−1 caspofungin for 2 days at 37°C. The primary criterion for viability was light refraction; viable hyphae were bright, and dead hyphae were dark. (D) Conidia of the wild type and the fks1tetOn strain were inoculated in AMM supplemented with 1 μg ml−1 caspofungin in a 24-well plate (4 × 103 conidia per well). The medium was supplemented with the indicated amounts of Doxy. After 48 h of incubation at 37°C, the percentage of surviving microcolonies was determined. Experiments were performed in triplicate. Statistical significance (*, P ≤ 0.05) was calculated with a two-tailed unpaired (assuming equal variances) Student t test. The error bars indicate standard deviations.
We next analyzed the survival rates of germinating conidia in the presence of echinocandins. To this end, conidia of the wild type and the conditional fks1tetOn mutant were inoculated in medium supplemented with 1 μg ml−1 caspofungin. After 2 days of incubation, the survival and continued growth of germinating conidia were microscopically evaluated (Fig. 5C). As shown in Fig. 5D, approximately 40% of the wild-type conidia survived the germination process and formed viable microcolonies independently of the presence or absence of doxycycline. The survival rates of the fks1tetOn mutant under repressive conditions were significantly higher (approximately 60%) (Fig. 5D). In contrast, induction of fks1 expression significantly reduced the survival of the fks1tetOn strain in a concentration-dependent manner (approximately 10% in the presence of 10 μg ml−1 doxycycline) (Fig. 5D). These findings indicate that decreased expression of fks1 renders A. fumigatus more resistant to echinocandins and overexpression of fks1 results in enhanced susceptibility.
Transition to paradoxical growth is accompanied by normalization of cell wall chitin.
We and others previously reported that inhibition of β-1,3-glucan synthesis, either by pharmacologic inhibition with echinocandins or by decreased expression or deletion of the β-1,3-glucan synthase (Fks1), causes a compensatory increase of cell wall chitin (11–14). It was proposed that this increase of cell wall chitin causes the paradoxical effect (4, 5). To address this question, we analyzed the cell wall chitin contents of Aspergillus hyphae of paradoxically and nonparadoxically growing hyphae. The conditional fks1tetOn strain under repressed conditions was used as a reference, as its growth is virtually unaffected by caspofungin. As shown in Fig. 6A and B, staining with calcofluor white, a chitin-specific dye, revealed no significant differences in the amounts of cell wall chitin for the wild-type and repressed fks1tetOn hyphae raised in the presence of 1 or 8 μg ml−1 caspofungin for 1 day. Similarly, we observed no difference in the amounts of cell wall chitin of the wild-type and the repressed fks1tetOn hyphae after prolonged incubation for 3 days in the presence of 1 μg ml−1 caspofungin, a condition that did not induce the paradoxical effect (Fig. 6C). Incubation for 3 days in the presence of 8 μg ml−1 caspofungin induced the paradoxical effect in the wild type. Analysis and comparison of the paradoxically growing wild-type hyphae with hyphae of the repressed fks1tetOn strain revealed a significant decrease of cell wall chitin (Fig. 6C and D). This demonstrates that the recurrence of β-1,3-glucan in paradoxically growing hyphae is accompanied by normalization of the cell wall chitin content.
FIG 6.
Paradoxically growing hyphae exhibit significantly less chitin than their echinocandin-inhibited progenitor microcolonies. Conidia of the wild type expressing GFP and fks1tetOn were inoculated in AMM on coverslips. The medium was supplemented with 1 μg ml−1 or 8 μg ml−1 CS. After 1 day (A and B) and 3 days (C and D) of incubation at 37°C, hyphae were fixed, stained with calcofluor white, and analyzed with a confocal laser scanning microscope. (A and C) The chitin content was quantified by comparing the calcofluor white fluorescence signal intensities of at least 7 and up to 17 (8 μg ml−1 caspofungin; 3 days) hyphal sections per strain and condition. The signal intensities of the wild type were normalized to those of the fks1tetOn strain under repressive conditions (baseline correction; the fluorescence intensity of fks1tetOn was set to 100%). The statistical significance (***, P ≤ 0.001) was calculated with a two-tailed unpaired (assuming equal variances) Student t test. The error bars indicate standard deviations. (B and D) Representative images of GFP fluorescence and calcofluor white (CFW) fluorescence after the indicated times of incubation. Scale bars, 25 μm.
DISCUSSION
Although the paradoxical effect of echinocandins has been known for decades, its nature has remained obscure until now. We analyzed this phenomenon in A. fumigatus in more detail. It was speculated that upregulation of chitin synthesis produces the paradoxical effect (5, 7), but our observation that paradoxically growing hyphae exhibit significantly less chitin than their parental growth-inhibited microcolonies clearly argues against a direct mechanistic role of upregulated chitin synthesis. On the other hand, an indirect contribution of upregulated chitin synthesis to the paradoxical effect is apparent. We and others have previously shown that inhibition of β-1,3-glucan synthesis causes a significant and immediate increase of cell wall chitin (11–14), and in agreement with a compensatory role of cell wall chitin, pharmacologic inhibitors of chitin synthesis, e.g., nikkomycin Z, were reported to act synergistically with echinocandins (12, 20). In our opinion, it is conceivable that upregulation of chitin synthesis is essential for survival in the initial growth phase in the presence of echinocandins (<3 days), which clearly is a requirement for the paradoxical growth that occurs after 3 days.
Aniline blue staining revealed that paradoxically growing A. fumigatus hyphae possess β-1,3-glucan. This is in very good agreement with similar findings in C. albicans reported recently (10). Notably, the paradoxically growing Aspergillus hyphae emerge from colonies that were initially devoid of β-1,3-glucan. We could also show that expression of the β-1,3-glucan synthase Fks1 is an essential requirement for occurrence of the paradoxical effect. Based on these observations, we propose that the paradoxical effect results from the restoration of β-1,3-glucan synthesis by recovery or de novo synthesis of functional Fks1. The fact that higher caspofungin concentrations are required for paradoxical growth suggests that β-1,3-glucan synthesis has to be substantially inhibited before the paradoxical growth effect can be triggered.
How Aspergillus and Candida restore β-1,3-glucan synthase activity in the presence of high echinocandin concentrations remains to be shown. One possibility could be the activation of a chaperone that protects or stabilizes the β-1,3-glucan synthase. Interestingly, it was previously shown that the chaperone Hsp90 is required for the paradoxical effect and that it localizes to the cell wall upon echinocandin exposure (17, 21–23). Although highly speculative, this could provide a possible mechanism for the restoration of β-1,3-glucan synthase activity.
Interestingly, we found that growth of the conditional fks1tetOn strain under repressed conditions is strongly inhibited by very high caspofungin concentrations (>64 μg ml−1). Similarly, and in good agreement with previous reports (4), the paradoxical effect of the wild type almost disappeared at such very high caspofungin concentrations. Since the fks1tetOn strain under repressed conditions already suffers from an extensive genetic depletion of the echinocandin target Fks1, our result demonstrates that echinocandins must have additional antifungal activity at very high concentrations that goes beyond inhibition of the β-1,3-glucan synthase. Possible additional antifungal activities of caspofungin, such as inhibition of β-1,6-glucan synthesis or induction of apoptosis, were previously suggested (24–26) but unfortunately not further investigated. The decrease of the paradoxical growth of the wild type at such high concentrations might therefore not exclusively indicate disappearance of the paradoxical effect. Instead, it might simply reflect the additional antifungal activity of the echinocandin at very high concentrations.
Finally, we have shown that the expression of the β-1,3-glucan synthase negatively influences Aspergillus survival and growth in the presence of caspofungin. One could argue that the upregulation of cell wall chitin following downregulation of fks1 expression (14) partially protects Aspergillus from the cell wall-damaging activity of echinocandins. However, chitin levels of nonparadoxically growing hyphae raised in the presence of caspofungin do not depend on fks1 expression (Fig. 6). This and the fact that the β-1,3-glucan synthase expression dependency of caspofungin efficacy persists for days argue against a key role of cell wall chitin in this context. We therefore suggest that the expression of fks1 or the accumulation of inactive, echinocandin-bound Fks1 in the cell membrane negatively affects other cell wall-remodeling mechanisms.
In summary, we have shown that (i) the paradoxical effect relies on the reactivation of the β-1,3-glucan synthase Fks1; (ii) upregulated chitin synthesis stabilizes the cell wall early during caspofungin exposure but does not mediate the paradoxical effect; (iii) echinocandins must have an additional antifungal activity at very high concentrations, which explains the virtual absence of a paradoxical effect at such concentrations; and (iv) the expression rate of fks1 paradoxically contributes to the fungicidal and growth-inhibitory activity of echinocandins on A. fumigatus.
MATERIALS AND METHODS
Strains, culture conditions, and chemicals.
The nonhomologous end joining-deficient A. fumigatus strain AfS35, a derivative of D141, was used as the wild type in this study (19). The wild-type strain that expresses a cytosolic variant of GFP was constructed by transforming AfS35 with pJW103 (27). The conditional β-1,3-glucan synthase fks1tetOn mutant was described recently (14). Briefly, the endogenous promoter of fks1 (AFUA_6G12400) was replaced with a conditional doxycycline-inducible Tet-On promoter (28). All the strains were maintained on Aspergillus minimal medium at 37°C (29). When indicated, the medium was supplemented with doxycycline (631311; Clontech, Mountain View, CA, USA) or caspofungin diacetate (SML0425; Sigma-Aldrich, St. Louis, MO, USA). Solid medium was supplemented with 2% (wt/vol) agar (214030; BD, Franklin Lakes, NJ, USA). Conidia of the conditional fks1tetOn strain were raised under induced conditions on medium supplemented with 0.5 μg ml−1 doxycycline.
Calcofluor white and aniline blue staining procedures.
Conidia were inoculated on coverslips in 24-well plates. After the indicated incubation period, hyphae were fixed in 3.7% formaldehyde in Dulbecco's phosphate-buffered saline (PBS) for 3 min and washed with double-distilled H2O (ddH2O). For calcofluor white staining, samples were stained for approximately 1 min with 10 mg ml−1 calcofluor white dissolved in ddH2O. For aniline blue staining, samples were stained for 60 min with a freshly prepared staining solution (0.05% [wt/vol] aniline blue diammonium salt [415049; Sigma-Aldrich, St. Louis, MO, USA] dissolved in Dulbecco's PBS and pH adjusted to 9.5). The calcofluor white-stained samples were subsequently washed with ddH2O and mounted with Vectashield mounting medium (H-1000; Vector, Burlingame, CA, USA). The aniline blue-stained samples were mounted with the staining solution and immediately analyzed with a fluorescence microscope.
Microscopy.
The growth over time of A. fumigatus cultured in 24-well plates was followed and documented with an Axiovert 25 inverted microscope (Carl Zeiss MicroImaging, Göttingen, Germany) and an EOS 550D digital camera (Canon, Tokyo, Japan). Fluorescence microscopy of aniline blue-stained hyphae was performed and documented with a BX61 microscope (Olympus, Tokyo, Japan) and a modified U-MNUA2 filter cube (extinction filter, 360 to 370 nm; dichromatic mirror, 400 nm; Olympus, Tokyo, Japan). To this end, the emission filter of the U-MNUA2 cube (420 to 460 nm) was replaced with the emission filter (510 to 550 nm) of the U-MWIBA cube (Olympus, Tokyo, Japan). Cell wall calcofluor white fluorescence was analyzed and quantified with an SP5 confocal laser scanning microscope (Leica Microsystems, Wetzlar, Germany) similarly to the method described previously (14). Briefly, the signal intensity along a vector vertically piercing a hypha was plotted and analyzed with the Leica LAS AF software (Leica Microsystems, Wetzlar, Germany). The maximum fluorescence intensity was determined for at least 7 and up to 17 vector pairs (one of the wild type and one of the fks1tetOn control strain under repressive conditions) obtained from different images per condition. The fluorescence intensity was subsequently analyzed relative to the maximum intensity of the fks1tetOn control strain (baseline correction; the fluorescence intensity of the fks1tetOn strain was set to 100%) with GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). Statistical significance was calculated with a two-tailed unpaired (assuming equal variances) Student t test.
Supplementary Material
ACKNOWLEDGMENTS
We thank Frank Ebel and Karl Dichtl for critically reading the manuscript.
This work was supported by the Förderprogramm für Forschung und Lehre (FöFoLe) of the Medical Faculty of the Ludwig-Maximilians-Universität München.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01690-16.
REFERENCES
- 1.Chen SC-A, Slavin MA, Sorrell TC. 2011. Echinocandin antifungal drugs in fungal infections: a comparison. Drugs 71:11–41. doi: 10.2165/11585270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Walker LA, Gow NAR, Munro CA. 2010. Fungal echinocandin resistance. Fungal Genet Biol 47:117–126. doi: 10.1016/j.fgb.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall GS, Myles C, Pratt KJ, Washington JA. 1988. Cilofungin (LY121019), an antifungal agent with specific activity against Candida albicans and Candida tropicalis. Antimicrob Agents Chemother 32:1331–1335. doi: 10.1128/AAC.32.9.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiederhold NP. 2009. Paradoxical echinocandin activity: a limited in vitro phenomenon? Med Mycol 47(Suppl 1):S369–S375. doi: 10.1080/13693780802428542. [DOI] [PubMed] [Google Scholar]
- 5.Steinbach WJ, Lamoth F, Juvvadi PR. 2015. Potential microbiological effects of higher dosing of echinocandins. Clin Infect Dis 61(Suppl 6):S669–S677. doi: 10.1093/cid/civ725. [DOI] [PubMed] [Google Scholar]
- 6.Vanstraelen K, Lagrou K, Maertens J, Wauters J, Willems L, Spriet I. 2013. The Eagle-like effect of echinocandins: what's in a name? Expert Rev Anti Infect Ther 11:1179–1191. doi: 10.1586/14787210.2013.841543. [DOI] [PubMed] [Google Scholar]
- 7.Wiederhold NP. 2007. Attenuation of echinocandin activity at elevated concentrations: a review of the paradoxical effect. Curr Opin Infect Dis 20:574–578. doi: 10.1097/QCO.0b013e3282f1be7f. [DOI] [PubMed] [Google Scholar]
- 8.Stevens DA, White TC, Perlin DS, Selitrennikoff CP. 2005. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn Microbiol Infect Dis 51:173–178. doi: 10.1016/j.diagmicrobio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Stevens DA, Espiritu M, Parmar R. 2004. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob Agents Chemother 48:3407–3411. doi: 10.1128/AAC.48.9.3407-3411.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rueda C, Cuenca-Estrella M, Zaragoza O. 2014. Paradoxical growth of Candida albicans in the presence of caspofungin is associated with multiple cell wall rearrangements and decreased virulence. Antimicrob Agents Chemother 58:1071–1083. doi: 10.1128/AAC.00946-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens DA, Ichinomiya M, Koshi Y, Horiuchi H. 2006. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for beta-1,6-glucan synthesis inhibition by caspofungin. Antimicrob Agents Chemother 50:3160–3161. doi: 10.1128/AAC.00563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker LA, Munro CA, de Bruijn I, Lenardon MD, McKinnon A, Gow NAR. 2008. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog 4:e1000040. doi: 10.1371/journal.ppat.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortwendel JR, Juvvadi PR, Pinchai N, Perfect BZ, Alspaugh JA, Perfect JR, Steinbach WJ. 2009. Differential effects of inhibiting chitin and 1,3-{beta}-d-glucan synthesis in ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob Agents Chemother 53:476–482. doi: 10.1128/AAC.01154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dichtl K, Samantaray S, Aimanianda V, Zhu Z, Prévost M-C, Latgé J-P, Ebel F, Wagener J. 2015. Aspergillus fumigatus devoid of cell wall β-1,3-glucan is viable, massively sheds galactomannan and is killed by septum formation inhibitors. Mol Microbiol 95:458–471. doi: 10.1111/mmi.12877. [DOI] [PubMed] [Google Scholar]
- 15.Wiederhold NP, Kontoyiannis DP, Prince RA, Lewis RE. 2005. Attenuation of the activity of caspofungin at high concentrations against Candida albicans: possible role of cell wall integrity and calcineurin pathways. Antimicrob Agents Chemother 49:5146–5148. doi: 10.1128/AAC.49.12.5146-5148.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fortwendel JR, Juvvadi PR, Perfect BZ, Rogg LE, Perfect JR, Steinbach WJ. 2010. Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin. Antimicrob Agents Chemother 54:1555–1563. doi: 10.1128/AAC.00854-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamoth F, Juvvadi PR, Fortwendel JR, Steinbach WJ. 2012. Heat shock protein 90 is required for conidiation and cell wall integrity in Aspergillus fumigatus. Eukaryot Cell 11:1324–1332. doi: 10.1128/EC.00032-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamoth F, Juvvadi PR, Gehrke C, Asfaw YG, Steinbach WJ. 2014. Transcriptional activation of heat shock protein 90 mediated via a proximal promoter region as trigger of caspofungin resistance in Aspergillus fumigatus. J Infect Dis 209:473–481. doi: 10.1093/infdis/jit530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krappmann S, Sasse C, Braus GH. 2006. Gene targeting in Aspergillus fumigatus by homologous recombination is facilitated in a nonhomologous end-joining-deficient genetic background. Eukaryot Cell 5:212–215. doi: 10.1128/EC.5.1.212-215.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganesan LT, Manavathu EK, Cutright JL, Alangaden GJ, Chandrasekar PH. 2004. In-vitro activity of nikkomycin Z alone and in combination with polyenes, triazoles or echinocandins against Aspergillus fumigatus. Clin Microbiol Infect 10:961–966. doi: 10.1111/j.1469-0691.2004.00996.x. [DOI] [PubMed] [Google Scholar]
- 21.Lamoth F, Juvvadi PR, Soderblom EJ, Moseley MA, Asfaw YG, Steinbach WJ. 2014. Identification of a key lysine residue in heat shock protein 90 required for azole and echinocandin resistance in Aspergillus fumigatus. Antimicrob Agents Chemother 58:1889–1896. doi: 10.1128/AAC.02286-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh SD, Robbins N, Zaas AK, Schell WA, Perfect JR, Cowen LE. 2009. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog 5:e1000532. doi: 10.1371/journal.ppat.1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaneko Y, Ohno H, Imamura Y, Kohno S, Miyazaki Y. 2009. The effects of an hsp90 inhibitor on the paradoxical effect. Jpn J Infect Dis 62:392–393. [PubMed] [Google Scholar]
- 24.Bartlett MS, Current WL, Goheen MP, Boylan CJ, Lee CH, Shaw MM, Queener SF, Smith JW. 1996. Semisynthetic echinocandins affect cell wall deposition of Pneumocystis carinii in vitro and in vivo. Antimicrob Agents Chemother 40:1811–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldmesser M, Kress Y, Mednick A, Casadevall A. 2000. The effect of the echinocandin analogue caspofungin on cell wall glucan synthesis by Cryptococcus neoformans. J Infect Dis 182:1791–1795. doi: 10.1086/317614. [DOI] [PubMed] [Google Scholar]
- 26.Hao B, Cheng S, Clancy CJ, Nguyen MH. 2013. Caspofungin kills Candida albicans by causing both cellular apoptosis and necrosis. Antimicrob Agents Chemother 57:326–332. doi: 10.1128/AAC.01366-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dichtl K, Ebel F, Dirr F, Routier FH, Heesemann J, Wagener J. 2010. Farnesol misplaces tip-localized Rho proteins and inhibits cell wall integrity signalling in Aspergillus fumigatus. Mol Microbiol 76:1191–1204. doi: 10.1111/j.1365-2958.2010.07170.x. [DOI] [PubMed] [Google Scholar]
- 28.Helmschrott C, Sasse A, Samantaray S, Krappmann S, Wagener J. 2013. Upgrading fungal gene expression on demand: improved systems for doxycycline-dependent silencing in Aspergillus fumigatus. Appl Environ Microbiol 79:1751–1754. doi: 10.1128/AEM.03626-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill TW, Kafer E. 2001. Improved protocols for Aspergillus salt stock solutions. Fungal Genet Newsl 48:20–21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.