ABSTRACT
We compared the efficacy of telavancin (TLV) and daptomycin (DAP) in an experimental rabbit endocarditis model caused by two clinically derived daptomycin-resistant (DAPr) methicillin-resistant Staphylococcus aureus (MRSA) strains. TLV treatment significantly reduced MRSA densities in all target tissues and increased the percentage of these organs rendered culture negative compared to those with the untreated control or DAP-treated animals. These results demonstrate that TLV has potent in vivo efficacy against DAPr MRSA isolates in this invasive endovascular infection model.
KEYWORDS: TLV, DAPr MRSA, endocarditis, daptomycin resistance, MRSA, telavancin, infective endocarditis
TEXT
Staphylococcus aureus is the most common cause of endovascular infections, including infective endocarditis (IE) (1). Despite the use of new antibiotics, such as daptomycin (DAP), the morbidity and mortality associated with S. aureus infections remain unacceptably high (2). DAP is a lipopeptide antibiotic with activity against a wide range of Gram-positive bacteria, including methicillin-resistant S. aureus (MRSA) (3). However, there have been increasing reports in which initially DAP-susceptible (DAPs) MRSA strains have developed DAP resistance (DAPr) both in vitro and in vivo as a result of exposure to DAP (4, 5).
Telavancin (TLV) is a lipoglycopeptide agent with a dual mechanism of action: cell wall synthesis inhibition and depolarization of the bacterial cell membrane (6). Recently, we studied five clinically derived DAPs/DAPr MRSA isogenic strain pairs isolated from patients who failed DAP therapy for TLV in vitro susceptibility (7). We found that all five MRSA strain sets were susceptible to TLV (MICs, ≤0.38 μg/ml), using the original MIC testing methods (8). Importantly, TLV therapy produced a significant MRSA density reduction in target tissues versus untreated or DAP-treated animals in experimental IE due to a single DAPr strain, REF2145 (7). These results demonstrated that TLV has potent bactericidal activity both in vitro and in vivo against DAPr MRSA strains (7). However, only one DAPr MRSA strain was tested in the IE model (7). In addition, the Clinical and Laboratory Standards Institute revised the antimicrobial susceptibility testing method for TLV in 2014 (9). The revised method provides more precise and reproducible TLV MICs and demonstrates that the previous technique underestimated the in vitro TLV potency (9, 10). Moreover, the revised CLSI methods decreased the TLV MIC interpretive breakpoint criterion for susceptibility for S. aureus from ≤1.0 μg/ml to ≤0.12 μg/ml (9). Therefore, in the current studies, we (i) retested the TLV MICs of the five clinically derived DAPs/DAPr MRSA strain pairs by using the revised CLSI method (10), (ii) performed in vitro time-kill assays based on the new TLV MICs, and (iii) investigated the therapeutic efficacy of TLV versus DAP in the IE model due to two additional clinically derived DAPr MRSA strains (B2.0 and SA684).
(This work was presented in part at the ASM Microbe, Boston, MA, 16 to 20 June 2016, abstract 4928 [11].)
Using the revised broth microdilution method, which includes dimethyl sulfoxide (DMSO) for panel production and polysorbate 80 (P-80), we found that TLV MICs were 3- to 6-fold lower than those in the previous results (Table 1) (7). These results were consistent with recent reports indicating that the previous method underestimated the in vitro TLV potency (10).
TABLE 1.
Susceptibilities of TLV and DAP on DAPs/DAPr MRSA strain pairs
| Strain | Clinical source of isolated DAPs/DAPr strain pairs | MIC (μg/ml)a |
|
|---|---|---|---|
| TLV (O/R) | DAP | ||
| A0.5 | Bloodstream | 0.25/0.06 | 0.5 |
| B2.0b | Bloodstream | 0.38/0.12 | 2.0 |
| SA675 | Endocarditis | 0.38/0.06 | 0.25 |
| SA684b | Endocarditis | 0.38/0.12 | 2.0 |
| MRSA11/11 | Endocarditis | 0.25/0.06 | 0.38 |
| REF2145 | Endocarditis | 0.38/0.12 | 4.0 |
| A214 | Bloodstream | 0.25/0.06 | 0.5 |
| A215 | Bloodstream | 0.38/0.12 | 3.0 |
| L282 | Bloodstream | 0.25/0.06 | 0.38 |
| L283 | Bloodstream | 0.38/0.12 | 2.0 |
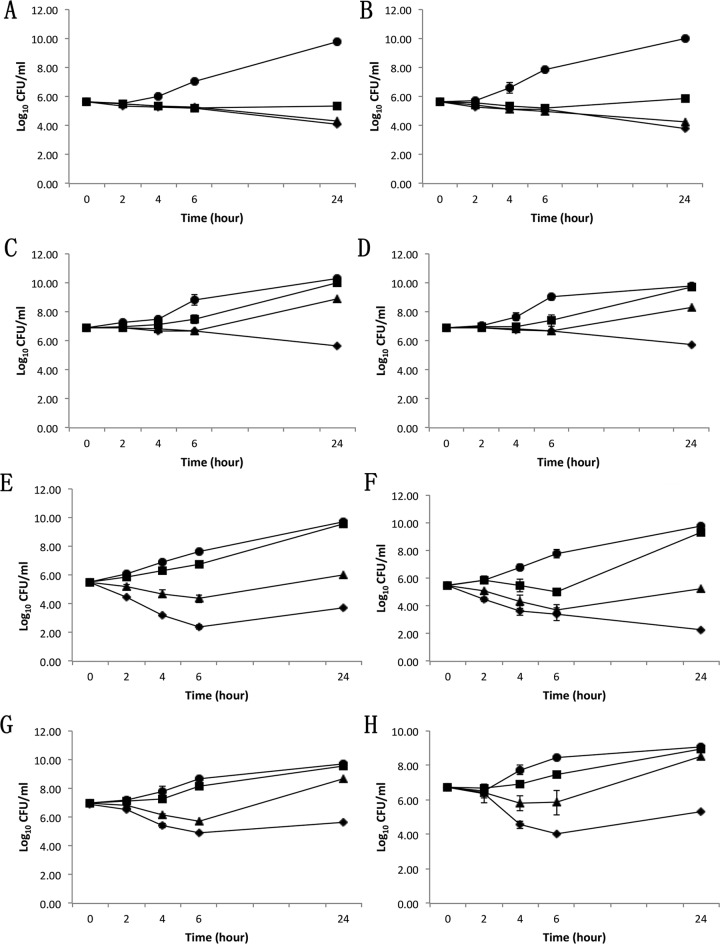
The in vitro time-kill curves were performed based on the new TLV MICs observed in this study, with an initial inoculum of 105 or 107 CFU/ml of study strain (to encompass bacterial counts commonly achieved in target tissues of animals with experimental IE) (12–14). For these assays, we prioritized two DAPr MRSA strains (B2.0 and SA684), initially isolated from patients who experienced DAP therapy failure (15, 16). At 105 CFU/ml initial inoculum, TLV at 2 times the MIC and 5 times the MIC prevented regrowth of both DAPr MRSA strains (Fig. 1A and B), but DAP at only 5 times the MIC prevented regrowth of one DAPr MRSA strain (Fig. 1F). At 107 CFU/ml initial inoculum, only TLV at 5 times the MIC (Fig. 1C and D), but no DAP concentration (Fig. 1G and H), was effective at preventing the regrowth of both DAPr MRSA strains. These in vitro time-kill analyses revealed that TLV was more active than DAP against the two DAPr MRSA strains.
FIG 1.
In vitro TLV and DAP time-kill curves against DAPr MRSA strains B2.0 (panels A and C for TLV, and panels E and G for DAP) and SA684 (panels B and D for TLV, and panels F and H for DAP) at initial inocula of 105 CFU/ml and 107 CFU/ml. Time-kill experiments were performed using Mueller-Hinton broth in the presence of 0 (●), 1× (■), 2× (▲), and 5× (◆) the MICs of TLV.
For the in vivo experiments, we demonstrated that the infective dose required to infect 95% of animals (ID95) of our two DAP MRSA strains in the IE model was 104 CFU/animal (data not shown). For the efficacy studies, animals were infected with this ID95 inoculum. At 24 h after infection, animals were randomly assigned to one of the four groups (10 animals/group): (i) untreated controls; (ii) TLV at 30 mg/kg of body weight, intravenously (i.v.), twice daily, which simulates the pharmacokinetic (PK) profile of the recommended human clinical dose (10 mg/kg, i.v. once daily) (7, 17, 18); (iii) DAP at 12 mg/kg, i.v. once daily, which mimics the human-like PK of the human standard dose (6 mg/kg, i.v., once daily) (15, 18); or (iv) DAP at 18 mg/kg, i.v. once daily, which mimics the human-like PK of high-dose DAP (10 mg/kg, i.v., once daily) (15). Treatment lasted for 3 days. At 24 h after the last therapeutic doses, antibiotic-treated animals were sacrificed. Control animals were euthanized at 24 h postinfection to determine the bacterial density in target tissue before antibiotic therapy. At sacrifice, the target tissues were removed and quantitatively cultured. Target tissue counts were expressed as mean log10 CFU/g of tissue ± standard deviation (SD). To compare tissue MRSA counts among the regimens, a nonparametric Kruskal-Wallis test was used. P values of <0.05 were considered statistically significant.
We observed that only TLV treatment significantly reduced MRSA densities in all three target tissues in the IE model due to two DAPr MRSA strains versus untreated controls and DAP-treated groups (Table 2). Importantly, TLV-treated rabbits had 71 to 100% culture-negative target tissues (71% and 100% in animals infected with the B2.0 and SA684 strains, respectively), while DAP therapy did not sterilize any tissue cultures (data not shown). In addition, 29% mortality was observed in the DAP 12 mg/kg treatment group and 0% mortality in the TLV treatment groups (data not shown).
TABLE 2.
MRSA density in target tissue in IE model caused by DAPr MRSA strain B2.0 or SA684
| Treatment group (no. of animals)a | Log10 CFU/g of tissue (mean ± SD) |
||
|---|---|---|---|
| Vegetation | Kidney | Spleen | |
| Strain B2.0 | |||
| Control (9) | 8.17 ± 0.41 | 6.32 ± 0.86 | 6.13 ± 0.72 |
| DAP, 12, once daily (7) | 8.39 ± 0.67 | 6.38 ± 0.80 | 5.86 ± 0.60 |
| DAP, 18, once daily (8) | 7.54 ± 0.94 | 4.10 ± 0.50b | 4.35 ± 0.79b |
| TLV, 30, b.i.d. (8) | 0.66 ± 0.11c | 0.49 ± 0.16c | 0.53 ± 0.25c |
| Strain SA684 | |||
| Control (8) | 8.57 ± 0.34 | 6.94 ± 0.77 | 6.40 ± 0.85 |
| DAP, 12, once daily (7) | 8.72 ± 0.55 | 7.01 ± 0.65 | 6.66 ± 0.77 |
| DAP, 18, once daily (7) | 8.49 ± 0.47 | 6.91 ± 0.80 | 5.81 ± 0.68 |
| TLV, 30, b.i.d. (7) | 1.31 ± 0.96b | 0.84 ± 0.66b | 1.00 ± 0.76b |
All drug dosages are given in milligrams per kilogram of body weight, and all drugs were administered intravenously. b.i.d., twice daily.
P < 0.005 versus untreated controls.
P < 0.001 versus untreated controls and DAP-treated groups.
These results were consistent with our prior single-strain investigation of TLV's excellent activity in experimental IE due to DAPr MRSA strains (7). It may not be suitable to directly compare TLV versus DAP efficacy in the IE model due to DAPr MRSA strains because of their inherent reduced susceptibility to DAP. However, it is important in demonstrating that TLV does have great activity against infections caused by these strains, while the use of DAP would not be an appropriate option. In addition to DAPr MRSA strains, other studies also demonstrated that TLV had significantly better efficacy than with vancomycin and DAP in the IE models due to a broad range of MRSA strains, including vancomycin-intermediate S. aureus (VISA) and glycopeptide-intermediate S. aureus (GISA) (17–19). Taken together, these results suggest that TLV may be a viable alternative for the treatment of IE caused by MRSA strains resistant to other glycopeptide or lipopeptide antibiotics.
ACKNOWLEDGMENT
This work was supported by a research grant from Theravance Biopharma Antibiotics, Inc (to Y.Q.X.).
REFERENCES
- 1.Fowler VG Jr, Justice A, Moore C, Benjamin DK Jr, Woods CW, Campbell S, Reller LB, Corey GR, Day NP, Peacock SJ. 2005. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis 40:695–703. doi: 10.1086/427806. [DOI] [PubMed] [Google Scholar]
- 2.Chambers HF, DeLeo FR. 2009. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakoulas G, Eliopoulos GM, Alder J, Eliopoulos CT. 2003. Efficacy of daptomycin in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 47:1714–1718. doi: 10.1128/AAC.47.5.1714-1718.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boucher HW, Sakoulas G. 2007. Perspectives on daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin Infect Dis 45:601–608. doi: 10.1086/520655. [DOI] [PubMed] [Google Scholar]
- 5.Jones T, Yeaman MR, Sakoulas G, Yang SJ, Proctor RA, Sahl HG, Schrenzel J, Xiong YQ, Bayer AS. 2008. Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob Agents Chemother 52:269–278. doi: 10.1128/AAC.00719-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nannini EC, Stryjewski ME. 2008. A new lipoglycopeptide: telavancin. Expert Opin Pharmacother 9:2197–2207. doi: 10.1517/14656566.9.12.2197. [DOI] [PubMed] [Google Scholar]
- 7.Xiong YQ, Hady WA, Bayer AS, Chen L, Kreiswirth BN, Yang SJ. 2012. Telavancin in therapy of experimental aortic valve endocarditis in rabbits due to daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 56:5528–5533. doi: 10.1128/AAC.00922-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CLSI. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. M02-A11, M07-A9, and M11-A8. [Google Scholar]
- 9.Karlowsky JA, Nichol K, Zhanel GG. 2015. Telavancin: mechanisms of action, in vitro activity, and mechanisms of resistance Clin Infect Dis 61(Suppl 2):S58–S68. [DOI] [PubMed] [Google Scholar]
- 10.Farrell DJ, Mendes RE, Rhomberg PR, Jones RN. 2014. Revised reference broth microdilution method for testing telavancin: effect on MIC results and correlation with other testing methodologies. Antimicrob Agents Chemother 58:5547–5551. doi: 10.1128/AAC.03172-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdelhady W, Bayer AS, Gonzales R, Li L, Xiong YQ. 2016. Efficacy of telavancin (TLV) and daptomycin (DAP) in an experimental endocarditis (IE) model due to DAP-resistant methicillin-resistant Staphylococcus aureus (MRSA), abstr A-4928. ASM Microbe, Boston, MA, 16 to 20 June 2016. [Google Scholar]
- 12.Abdelhady W, Bayer AS, Seidl K, Moormeier DE, Bayles KW, Cheung A, Yeaman MR, Xiong YQ. 2014. Impact of vancomycin on sarA-mediated biofilm formation: role in persistent endovascular infections due to methicillin-resistant Staphylococcus aureus. J Infect Dis 209:1231–1240. doi: 10.1093/infdis/jiu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seidl K, Chen L, Bayer AS, Hady WA, Kreiswirth BN, Xiong YQ. 2011. Relationship of agr expression and function with virulence and vancomycin treatment outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 55:5631–5639. doi: 10.1128/AAC.05251-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong YQ, Fowler VG Jr, Yeaman MR, Perdreau-Remington F, Kreiswirth BN, Bayer AS. 2009. Phenotypic and genotypic characteristics of persistent methicillin-resistant Staphylococcus aureus bacteremia in vitro and in an experimental endocarditis model. J Infect Dis 199:201–208. doi: 10.1086/595738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers HF, Basuino L, Diep BA, Steenbergen J, Zhang S, Tattevin P, Alder J. 2009. Relationship between susceptibility to daptomycin in vitro and activity in vivo in a rabbit model of aortic valve endocarditis. Antimicrob Agents Chemother 53:1463–1467. doi: 10.1128/AAC.01307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaatz GW, Lundstrom TS, Seo SM. 2006. Mechanisms of daptomycin resistance in Staphylococcus aureus. Int J Antimicrob Agents 28:280–287. doi: 10.1016/j.ijantimicag.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 17.Madrigal AG, Basuino L, Chambers HF. 2005. Efficacy of telavancin in a rabbit model of aortic valve endocarditis due to methicillin-resistant Staphylococcus aureus or vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 49:3163–3165. doi: 10.1128/AAC.49.8.3163-3165.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong YQ, Abdelhady W, Tang C, Bayer AS. 2016. Comparative efficacy of telavancin and daptomycin in experimental endocarditis due to multi-clonotype MRSA strains. J Antimicrob Chemother 71:2890–2894. doi: 10.1093/jac/dkw249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miro JM, Garcia-de-la-Maria C, Armero Y, de-Lazzari E, Soy D, Moreno A, del Rio A, Almela M, Mestres CA, Gatell JM, Jimenez-de-Anta MT, Marco F, Hospital Clinic Experimental Endocarditis Study Group. 2007. Efficacy of telavancin in the treatment of experimental endocarditis due to glycopeptide-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 51:2373–2377. doi: 10.1128/AAC.01266-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrell DJ, Krause KM, Benton BM. 2011. In vitro activity of telavancin and comparator antimicrobial agents against a panel of genetically defined staphylococci. Diagn Microbiol Infect Dis 69:275–279. doi: 10.1016/j.diagmicrobio.2010.09.017. [DOI] [PubMed] [Google Scholar]