ABSTRACT
Previous studies have shown that some lipoglycopeptide and lipopeptide antimicrobial agents may cause falsely elevated values for some phospholipid-dependent coagulation tests. The effect of oritavancin, a lipoglycopeptide antibiotic, on coagulation test results was explored using pooled human plasma samples spiked with drug and in a clinical study after an infusion of a single 1,200-mg intravenous dose of oritavancin in normal healthy volunteers. Pooled plasma with oritavancin added ex vivo showed concentration-dependent prolongation of prothrombin time/international normalized ratio (PT/INR), activated partial thromboplastin time (aPTT), and dilute Russell viper venom time (DRVVT) test results. In contrast, oritavancin had no effect on the activated protein C resistance assay, chromogenic anti-factor Xa assay (anti-FXa), thrombin time, and an immunoassay for the laboratory diagnosis of heparin-induced thrombocytopenia. In participants that received a single dose of oritavancin, elevations in PT/INR result, aPTT, DRVVT, activated clotting time, and silica clotting time occurred, with the maximum times to resolution of test interference determined to be 12, 120, 72, 24, and 18 h, respectively. The anti-FXa assay was unaffected, whereas transient elevations in D dimer levels were observed in 30% of participants, with a maximum time to resolution of 72 h. Although oritavancin has no impact on the coagulation system in vivo, a single dose of oritavancin can produce falsely elevated values of some coagulation tests used to monitor hemostasis. The interference of oritavancin on affected tests is transient, and the test results revert to normal ranges within specified times after dosing.
KEYWORDS: oritavancin, coagulation, hemostasis, ABSSSI, antibiotic, lipoglycopeptide
INTRODUCTION
Oritavancin is a lipoglycopeptide antibiotic approved as a single-dose treatment for adult patients with acute bacterial skin and skin structure infections (ABSSSI) caused or suspected to be caused by susceptible isolates of designated Gram-positive microorganisms (1–3). Oritavancin has been proven to be efficacious as a single-dose regimen due to its unique pharmacokinetic (PK) and pharmacodynamic (PD) properties, including a long terminal half-life in plasma of 245 h and potent concentration-dependent bactericidal activity.
Previous studies have shown that some lipoglycopeptide or lipopeptide antibiotics (e.g., telavancin and daptomycin) may artificially prolong phospholipid-dependent coagulation test results (4–7). Although these agents do not interfere with the coagulation system in vivo, their interference with coagulation assays can confound monitoring of hemostasis in the clinical setting. The potential for oritavancin to cause interference with phospholipid-based tests may therefore occur for a period following administration of a single dose. Thus, the effect of oritavancin on commonly used phospholipid-dependent and -independent coagulation assay results was determined using in vitro methodologies. This was followed by a phase 1 study to determine the maximum time to resolution of test interference in plasma obtained from normal healthy volunteers who received a single 1,200-mg dose of oritavancin.
(Part of this work was presented at IDWeek2016, New Orleans, LA [8].)
RESULTS
In vitro testing using pooled human citrated plasma spiked with oritavancin.
The assayed oritavancin concentrations spiked into pooled human citrated plasma were 2.1, 5.4, 8.3, 15.9, and 46.6 μg/ml, within 3.6% to 8% of the targeted nominal values. The effects of oritavancin at the assayed concentrations in pooled human citrated plasma on the coagulation assay results determined in vitro are summarized in Table 1.
TABLE 1.
Summary of the effects of oritavancin on coagulation test results determined in vitroa
| Coagulation test | Reagent | Assay measurement units | Normal reference range | Mean ± SD (% change relative to control) at the indicated oritavancin concn |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 2.1 μg/ml | 5.4 μg/ml | 8.3 μg/ml | 15.9 μg/ml | 46.6 μg/ml | ||||
| Phospholipid dependent | |||||||||
| PT/INR | STA-Neoplastine CI Plus | s | 11.9–14.1 | 13.2 ± 0.17 | 13.2 ± 0.16 (0) | 13.2 ± 0.13 (0) | 13.2 ± 0.11 (0) | 13.4 ± 0.13 (1.4) | 13.7 ± 0.11 (3.6) |
| HemosIL RecombiPlasTin 2G | s | 9.4–12.5 | 12.1 ± 0.11 | 12.1 ± 0.14 (0.2) | 12.2 ± 0.10 (1.1) | 12.3 ± 0.15 (1.9) | 12.4 ± 0.16 (2.3) | 13.2 ± 0.18 (8.9) | |
| Dade Innovin | s | 9.9–11.8 | 11.0 ± 0.06 | 11.0 ± 0.06 (0) | 11.1 ± 0.07 (0.6) | 11.1 ± 0.05 (1.0) | 11.3 ± 0.12 (2.5) | 11.8 ± 0.07 (7.0) | |
| aPTT | STA-PTTa | s | 23.4–36.4 | 31.8 ± 0.40 | 31.7 ± 0.35 (−0.2) | 32.5 ± 0.72 (2.2) | 33.3 ± 0.63 (5.0) | 36.3 ± 0.54 (14.4) | 59.8 ± 2.28 (88.3) |
| HemosIL SynthASil | s | 25.1–36.5 | 31.7 ± 0.28 | 32.0 ± 0.16 (0.9) | 32.2 ± 0.19 (1.5) | 32.7 ± 0.20 (3.3) | 34.1 ± 0.19 (7.6) | 42.5 ± 0.58 (34.2) | |
| Dade Actin FSL | s | 25.3–33.8 | 29.5 ± 0.61 | 29.5 ± 0.25 (−0.2) | 29.6 ± 0.28 (0.2) | 29.7 ± 0.30 (0.7) | 30.4 ± 0.50 (2.8) | 39.8 ± 0.85 (34.8) | |
| DRVVT | LA Check/LA Sure | s | <55.1 | 43.2 ± 1.07 | 43.6 ± 0.98 (0.9) | 43.4 ± 1.06 (0.5) | 44.3 ± 0.90 (2.6) | 44.8 ± 0.93 (3.7) | 53.6 ± 1.72 (24.0) |
| APCR | COATEST APC Resistance V | APC ratio | 2.2–4.0 | 2.3 ± 0.11 | 2.3 ± 0.11 (0) | 2.3 ± 0.10 (1.3) | 2.3 ± 0.11 (1.7) | 2.3 ± 0.09 (2.2) | 2.4 ± 0.13 (3.1) |
| Phospholipid independent | |||||||||
| Anti-FXa | STA-Rotachrom heparin | IU/ml | LLOQ = 0.10 IU/ml | <LLOQ | <LLOQ | <LLOQ | <LLOQ | <LLOQ | <LLOQ |
| TT | STA-Thrombin | s | <20.0 | 15.6 ± 0.21 | 15.6 ± 0.18 (0.3) | 15.8 ± 0.27 (1.2) | 15.7 ± 0.15 (1.0) | 15.7 ± 0.24 (0.9) | 15.7 ± 0.33 (0.9) |
| Anti-heparin-PF4 | Asserachrom HPIA | Normalized OD ratio | <0.8 | Negativeb | Negative | Negative | Negative | Negative | Negative |
| D dimer | STA-Liatest D-Di | μg/ml FEU | <0.5 μg/ml FEU | 0.21 ± 0.04 | 0.25 ± 0.04 (19.0) | 0.22 ± 0.05 (4.8) | 0.25 ± 0.05 (19.0) | 0.25 ± 0.04 (19.0) | 0.29 ± 0.04 (38.1) |
Anti-FXa, anti-factor Xa; APCR, activated protein C resistance; aPTT, activated partial thromboplastin time; DRVVT, dilute Russell's viper venom time; FEU, fibrinogen equivalent units; LLOQ, lower limit of quantitation; PT/INR, prothrombin time/international normalized ratio; TT, thrombin time.
Negative, a normalized optical density ratio of <0.8 is indicative of negativity for heparin-induced platelet antibodies.
The prothrombin time/international normalized ratio (PT/INR) result determined using the three tested reagents was unaffected (within 5% of the control sample values) at oritavancin concentrations ranging from 2.1 to 15.9 μg/ml (Table 1). At 46.6 μg/ml of oritavancin, the PT/INR result determined using both HemosIL RecombiPlasTin 2G and Dade Innovin reagents showed increases of between 5% and 10% compared with that of the control samples, whereas the PT/INR result determined with STA-Neoplastine CI Plus was within 5% of the control sample value; only the PT/INR result determined with HemosIL RecombiPlasTin 2G reagent exceeded the normal reference range of the assay.
Using the three reagents, the activated partial thromboplastin time (aPTT) was unaffected (within 5% of the control sample values) at oritavancin concentrations of 2.1 to 8.3 μg/ml (Table 1). At 15.9 μg/ml of oritavancin, the aPTT determined using the STA-PTTa reagent was prolonged by approximately 15% compared with that of the control sample, whereas the aPTT determined using both the HemosIL SynthASil and Dade Actin FSL reagents was within 8% of the values for the controls. With all three reagents, the aPTT was prolonged by more than 30% at 46.6 μg/ml of oritavancin compared with the aPTT of the control samples, with values also exceeding the normal reference ranges determined for each reagent.
Oritavancin concentrations ranging from 2.1 to 15.9 μg/ml had no effect (within 5% of the control sample value) on the dilute Russell viper venom time (DRVVT) test result. At 46.6 μg/ml of oritavancin, the DRVVT was increased by 24% compared with that of the control sample, but the value remained within the normal reference range of the assay. In contrast, the activated protein C resistance (APCR) assay result and the results for the phospholipid-independent assays of chromogenic anti-factor Xa (anti-FXa), thromboblastin time (TT), and heparin-induced thrombocytopenia (anti-heparin-PF4) were unaffected by oritavancin at all of the concentrations tested in pooled human citrated plasma (Table 1). In the D dimer assay, although elevations were observed relative to the value for the control sample, they did not exceed the lower limit of quantitation of the assay (<0.5 μg/ml fibrinogen equivalent units) (Table 1).
Phase 1 study.
A total of 20 participants were enrolled in and completed the phase 1 clinical study. Of the 20 participants, 55% were male and 45% were female. The majority of the participants were white (85.0%). The median age was 27.0 years (range, 19 to 58 years), and the median body mass index (BMI) was 24.6 kg/m2 (range, 20 to 30 kg/m2).
The mean oritavancin plasma concentration-time profile observed in the participants following administration of a single 1,200-mg dose of oritavancin as an intravenous (i.v.) infusion over 3 h is shown in Fig. 1 (dashed line). The single dose of oritavancin resulted in a mean maximum plasma concentration (Cmax) of 146.0 μg/ml (coefficient of variation [CV], 17.2%) and a mean total exposure (area under the concentration-time curve from 0 h to infinity [AUC0–∞]) of 2,788 μg · h/ml (CV, 23.4%). The mean clearance was 0.453 liters/h (CV, 22.7%).
FIG 1.
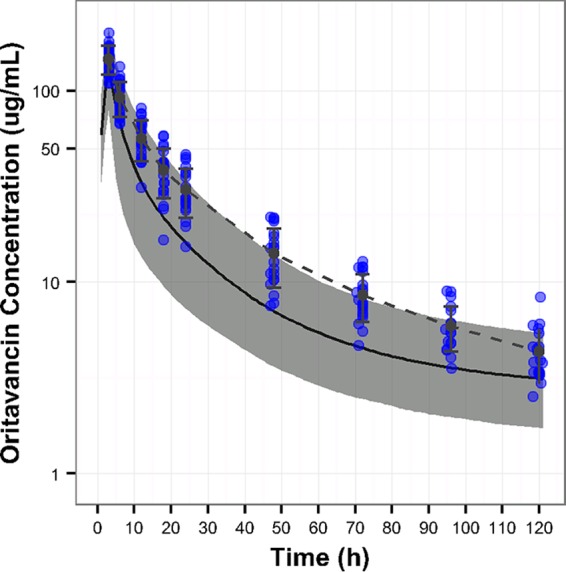
Mean (±standard deviation [SD]) plasma oritavancin concentration-time profile observed in participants administered a single 1,200-mg dose of oritavancin. The individual blue dots represent the observed oritavancin concentrations in plasma samples from participants administered a 3-h infusion of a single 1,200-mg dose of oritavancin, whereas the dashed line represents the mean (±SD) of those values. The solid black line and gray band represent the median oritavancin concentration-time profile and the 90% confidence interval, respectively, from the simulated distribution of concentrations using the population PK model from the SOLO studies (13).
As similarly observed in the in vitro testing, the PT/INR result determined using the STA-Neoplastine CI Plus reagent was unaffected by the presence of oritavancin (Table 2). However, oritavancin prolonged the PT/INR results when determined with the Dade Innovin and HemosIL RecombiPlasTin 2G reagents, with mean times to resolution of 6.2 and 1.5 h, respectively (Table 2). The maximum time to resolution of PT/INR test interference observed in the participants was 12 h (Table 3). As shown in Fig. 2, a relationship between plasma oritavancin concentrations and elevations in PT/INR results was observed (data shown using the Dade Innovin reagent).
TABLE 2.
Time to resolution of elevations in PT/INR and aPTT test results in normal healthy volunteers (n = 20) in the phase 1 clinical study
| Coagulation test | Reagent | Time to resolutiona (h) |
|
|---|---|---|---|
| Mean (SD) | Median (min, max) | ||
| PT/INR | STA-Neoplastine CI Plus | 0.00 (0.00) | 0.00 (0.00, 0.00) |
| HemosIL RecombiPlasTin 2G | 1.50 (2.67) | 0.00 (0.00, 6.00) | |
| Dade Innovin | 8.42 (3.58) | 6.18 (0.00, 12.00) | |
| aPTT | STA-PTTa | 89.75 (23.37) | 96.00 (48.0, 144.2b) |
| HemosIL SynthASil | 23.07 (13.82) | 18.02 (6.1, 48.0) | |
| Dade Actin FSL | 48.41 (28.86) | 48.00 (6.4, 119.8) | |
| TriniCLOT aPTT HS | 42.82 (23.73) | 48.00 (6.4, 96.0) | |
For PT/INR, time to resolution was defined as the first time postbaseline following the start of oritavancin infusion that a test value returned to the normal reference range after elevation. For aPTT, time to resolution was defined as the first time postbaseline following the start of oritavancin infusion that a test value returned to the maximum of baseline or the normal reference range after elevation. max, maximum; min, minimum; SD, standard deviation.
The STA-PTTa assay was not conducted for 1 subject at 120 h, but an unscheduled assessment at 144.2 h was performed: the aPTT was 36.6 s, within the normal reference range of the assay (39 s).
TABLE 3.
Maximum times to resolution of phospholipid-dependent and -independent coagulation test interference in participants administered a single 1,200-mg dose of oritavancin
| Coagulation test | Maximum time to resolution (h) |
|---|---|
| Phospholipid dependent | |
| PT/INR | 12 |
| aPTT | 120 |
| ACT | 24 |
| SCT | 18 |
| DRVVT | 72 |
| APCR | Unaffected |
| Phospholipid independent | |
| Anti-factor Xa | Unaffected |
| TT | Unaffected |
| Anti-heparin-PF4 | Unaffected |
| D dimer | 72 |
FIG 2.
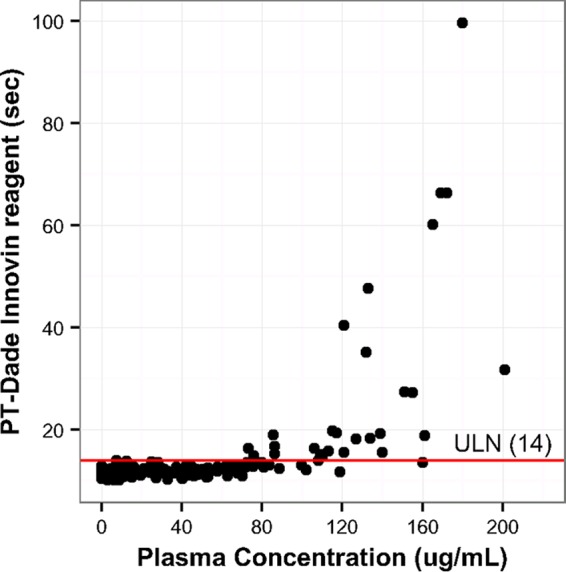
Relationship between oritavancin plasma concentrations and PT (using the Dade Innovin reagent) in participants (n = 20). The individual dots represent PT determinations observed at the corresponding oritavancin concentrations in subject plasma samples. The horizontal red line represents the upper limit of normal (ULN; 14 s) determined for the assay.
Oritavancin caused prolongation of aPTT determined using the four reagents (Table 2), with aPTT increasing as oritavancin concentrations increased (Fig. 3; shown using the STA-PTTa reagent). The mean time to resolution ranged from 23.1 h (HemosIL SynthASil) to 89.8 h (STA-PTTa), with a maximum time to resolution of 120 h (Table 3).
FIG 3.
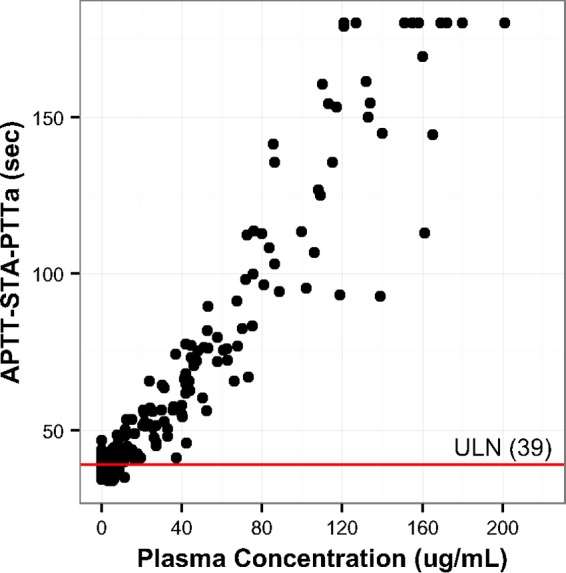
Relationship between oritavancin plasma concentrations and aPTT (using the STA-PTTa reagent) in participants (n = 20). The individual dots represent aPTT determinations observed at the corresponding oritavancin concentrations in subject plasma samples. The horizontal red line represents the upper limit of normal (ULN; 39 s) determined for the assay.
Other phospholipid-dependent tests were similarly affected by the presence of oritavancin in plasma samples obtained from participants in the phase 1 study. Increases in activated clotting time (ACT) were seen in 95.0% of participants following oritavancin administration, with a mean time to resolution of 13.2 h and a maximum time to resolution of 24 h (Table 3). All participants experienced increases in silica clotting time (SCT), with a mean time to resolution of 13.2 h and a maximum time to resolution of 18 h (Table 3). The mean time to resolution of the elevations in the DRVVT was 54.7 h, with a maximum time to resolution of 72 h. Relationships between increasing oritavancin plasma concentrations and elevations in ACT, SCT, and DRVVT were also observed (data not shown).
For the phospholipid-independent tests, no assay interference occurred in the chromogenic anti-FXa assay. Unexpected elevations in D dimer levels were observed in approximately 30% of participants, where maximal elevations occurred 12 to 24 h following the start of oritavancin administration with a mean time to resolution in affected participants of 10.5 h and a maximum time to resolution of 72 h (Table 3). There was no discernible relationship between oritavancin concentrations and D dimer elevations (data not shown).
DISCUSSION
These studies show that a single dose of oritavancin may cause elevations in certain test results used to assess the coagulation cascade. Similar to effects observed in other studies with telavancin and daptomycin (4, 6), the coagulation test elevations are likely a function of variable interactions of oritavancin with the proprietary phospholipid-containing reagents needed to initiate coagulation in the test systems. For example, the highest tested concentration of oritavancin (46.6 μg/ml) in vitro had no effect on the PT/INR result determined using the STA-Neoplastine CI Plus reagent, but increases of 7.0% to 8.9% in the PT/INR result were observed using the other two reagents. This differential effect was corroborated in the phase 1 study, as the PT/INR result determined with the STA-Neoplastine CI Plus reagent was unaffected whereas the mean time to resolution of PT/INR test interference determined using the other reagents ranged from 1.5 to 8.4 h. Furthermore, the test for APCR was unaffected at the tested concentrations of oritavancin in vitro, another indication that the nature and levels of phospholipid-containing reagents needed for clotting play an important role in this ex vivo phenomenon. Since clinical bleeding related to oritavancin use has not been reported in clinical studies (The Medicines Company, data on file), the elevations in phospholipid-dependent coagulation test results indicate an ex vivo test interference.
The prolongation of phospholipid-dependent coagulation test results by oritavancin occurred in a concentration-dependent manner, similar to that described for telavancin and daptomycin (4, 5, 7). In the phase 1 study, elevations in PT/INR results, aPTT, DRVVT, ACT, and SCT occurred in a transient manner following administration of a single 1,200-mg dose of oritavancin in participants. The relationship between the prolongation of PT/INR results and aPTT and the increasing oritavancin concentrations measured in the plasma from participants corroborates the concentration dependency of these effects.
As expected, oritavancin at the tested concentrations in vitro did not affect phospholipid-independent tests, such as anti-FXa, TT, or the anti-heparin-PF4 immunoassay. The anti-FXa test determined using plasma samples obtained from participants in the phase 1 study did not appear to be affected by oritavancin. However, it should be noted that the effects of oritavancin on anti-FXa levels were not determined in patients receiving therapeutic heparin (the usual indication for performing the anti-FXa assay) in this study. Unexpectedly, transient increases in D dimer levels were observed in approximately 30% of participants following oritavancin dosing. These elevations were not associated with either maximum plasma drug concentrations or with any relevant adverse event. In vitro, most oritavancin-spiked samples had elevated D dimer levels but remained below the lower limit of quantitation of the assay and did not approach the normal adult reference threshold (<0.5 μg/ml fibrinogen equivalent units [FEU]). It remains to be determined if these elevations result from nonspecific interactions between oritavancin and assay components. The D dimer elevations noted in some participants plausibly represent false-positive readings owing to their transient nature, their occurrence early in the course of therapy, and the observation that insertion of an intravenous catheter alone has been shown to be associated with false-positive D dimer results (9, 10). Nevertheless, a conservative approach was taken such that the maximum time to resolution of the elevations in D dimer levels (72 h) is indicated to inform clinicians of the potential for D dimer assay interference.
The mean oritavancin plasma concentration-time profile and associated mean (CV) pharmacokinetic parameters determined for the participants that received the single 1,200-mg dose of oritavancin in the current phase 1 study (Cmax = 148 μg/ml [CV, 17.2%], AUC0–∞ = 2,788 μg · h/ml [CV, 23.4%]) were comparable to those obtained from infected patients participating in the pivotal SOLO phase 3 studies (Cmax = 138 μg/ml [CV, 23.0%], AUC0–∞ = 2,800 μg · h/ml [CV, 28.6%]) (11). Therefore, the estimates for maximum time to resolution of test interference based on the phase 1 clinical study in healthy volunteers are expected to match resolution times in infected patients.
Since oritavancin is administered as a single-dose regimen to patients with acute bacterial skin and skin structure infection, the transient interference with the specified coagulation tests should not require further consideration beyond the specified maximum time to resolution of test interference. In contrast, both telavancin (12) and daptomycin (13, 14) require daily dosing, and consequently, monitoring of coagulation status in patients is recommended just prior to the administration of the next daily dose (at the time of plasma trough concentrations). The estimates of maximum time to resolution of test interference is intended to inform clinicians on how to manage patients who require coagulation monitoring in the setting of single-dose treatment with oritavancin.
MATERIALS AND METHODS
In vitro testing of coagulation assays.
Oritavancin powder (The Medicines Company, Parsippany, NJ) was dissolved into NERL reagent-grade water (ThermoFisher Scientific, Waltham, MA) and then diluted in pooled normal plasma (George King BioMedical Inc., Overland Park, KS) to achieve targeted nominal concentrations of 2, 5, 8, 15, and 45 μg/ml (reflecting the in vitro solubility limitations in human citrated plasma; data not shown). Control samples (no oritavancin) were prepared by spiking the same volume of water into pooled normal plasma. Samples were aliquoted and frozen at −70°C until testing. Oritavancin concentrations in each sample of citrated human plasma were determined using a qualified liquid chromatography method with tandem mass spectrometric (LC-MS/MS) detection (calibration range, 0.5 to 300 μg/ml; The Medicines Company, method on file). On each day of coagulation testing, the samples were thawed in a 37°C water bath for approximately 5 min and assayed within 4 h. Each sample was tested in triplicate in three independent assays (performed over a minimum of 2 days) in accordance with the manufacturers' protocols. Phospholipid-dependent coagulation tests included three of the most commonly used PT/INR (STA-Neoplastine CI Plus [Diagnostica Stago Inc., Parsippany, NJ], HemosIL RecombiPlasTin 2G [Instrumentation Laboratory, Bedford, MA], and Dade Innovin [Siemens Healthcare Diagnostics Inc., Marburg, Germany]) and aPTT (STA-PTTa [Diagnostica Stago Inc.], HemosIL SynthASil [Instrumentation Laboratory], and Dade Actin FSL [Siemens Healthcare Diagnostics]) commercial reagents/platforms in the United States (15). Other phospholipid-dependent coagulation tests included assays for the dilute Russell's venom viper time (DRVVT) (LA Check and LA Sure; Precision Biologic Inc., Dartmouth, Nova Scotia, Canada) and activated protein C resistance (APCR) (Chromogenix COATEST APC Resistance V; Instrumentation Laboratory). Phospholipid-independent coagulation tests included those for anti-factor Xa activity (anti-FXa) (STA-Rotachrom heparin; Diagnostica Stago Inc.), thrombin time (TT) (STA-Thrombin; Diagnostica Stago Inc.), and D dimer (STA-Liatest D-Di; Diagnostica Stago Inc.) and an assay to monitor heparin-induced thrombocytopenia (anti-heparin-PF4) (Asserachrom HPIA; Diagnostica Stago Inc.).
Coagulation test results determined in the presence of oritavancin were assessed as to whether they remained within the normal reference range determined for each assay. Where appropriate, the percent change at each oritavancin concentration relative to the control sample value is presented to assess whether coagulation test interference occurred in a concentration-dependent manner.
Phase 1 clinical study.
The phase 1, open-label, single-center, single-arm clinical study was conducted to examine the effects of a single 1,200-mg intravenous dose of oritavancin on multiple phospholipid-dependent and phospholipid-independent coagulation tests in normal healthy volunteers 18 to 65 years of age. The study was conducted in accordance and compliance with the ethical principles that have their origins in the Declaration of Helsinki, applicable U.S. Food and Drug Administration (FDA) regulations, ethics committee (EC) requirements, and good clinical practices (GCP) guidelines. The study protocol, informed consent form (ICF), and relevant supporting information were submitted to the EC by the investigator for review and approval before the study was initiated.
Participants in good health were required to have a body mass index of <45 kg/m2 and were excluded if PT/INR, aPTT, or ACT coagulation test results were outside the normal reference ranges at baseline (predose). Participants received a single oritavancin infusion of 1,200 mg over 3 h. Plasma samples were collected at 0, 3, 6, 12, 18, 24, 48, 72, 96, and 120 h after the start of oritavancin infusion and were stored at −20°C until coagulation testing and the pharmacokinetic analysis were performed. The primary objective of the study was to determine the time to resolution of test interference (mean, median, minimum, and maximum values), defined as the interval from the start of the oritavancin infusion to the time the result for all participants returned to the normal reference range or below the subject's baseline value for each coagulation test. The secondary objective of the study was to determine the relationship between oritavancin plasma levels and effects on coagulation tests. Oritavancin concentrations in each sample of citrated human plasma were determined using the qualified liquid chromatography method indicated above.
Phospholipid-dependent coagulation tests included the three PT/INR reagents STA-Neoplastine CI Plus, HemosIL RecombiPlasTin 2G, and Dade Innovin and the four aPTT reagents STA-PTTa, HemosIL SynthASil, Dade Actin FSL, and TriniCLOT aPTT HS (Trinity Biotech, Jamestown, NY). Other phospholipid-dependent coagulation tests performed included those for DRVVT (Instrumentation Laboratory, Bedford, MA), activated clotting time (ACT) (Activated Clotting Time Plus; Accriva Diagnostics, San Diego, CA), and silica clotting time (SCT) (HemosIL silica clotting time; Instrumentation Laboratory). HemosIL RecombiPlasTin 2G, TriniCLOT aPTT HS, and ACT assay reagents were used to assess baseline PT/INR, aPTT, and ACT values, respectively, of the normal healthy volunteers. Phospholipid-independent coagulation tests included those for anti-FXa (STA-Rotachrom heparin) and D dimer (HemosIL D-dimer HS 500; Instrumentation Laboratory). Coagulation tests were performed by following the protocols provided by the manufacturers.
For the statistical analysis, coagulation parameters were summarized using the pharmacodynamic-evaluable (PE) population to determine the time to resolution of elevations in the affected coagulation tests. The PE population was defined as all participants who received study drug, had a baseline coagulation test value, and had a postbaseline coagulation test value that was adequate to determine the time to resolution of elevations in PT and aPTT tests. The maximum time to resolution indicated represents the time point at which 100% of subjects reached resolution of the affected coagulation test result. For the secondary endpoint (relationship between oritavancin plasma levels and effects on coagulation tests), a logistic regression model was constructed to assess the risk of a clinically significant alteration in a coagulation parameter (i.e., values outside the normal reference range) using drug concentration as a continuous independent variable. Standard noncompartmental analysis (Phoenix WinNonlin 6.3) of the complete pharmacokinetic sampling profile from each participant was used to determine oritavancin pharmacokinetic parameters (mean maximum plasma concentration [Cmax], mean area under the plasma concentration-time curve from time zero extrapolated to time infinity [AUC0–∞], and mean plasma clearance [CL]). The mean oritavancin plasma concentration-time profile was plotted using R, Statistical Software (version 3.1.2), and is presented relative to the profile determined in patients treated with a single 1,200-mg dose of oritavancin for ABSSSI in the SOLO phase 3 studies (11).
ACKNOWLEDGMENTS
This study was funded by The Medicines Company, Parsippany, NJ.
Employees of The Medicines Company had a role as authors in the study design, interpretation, and the decision to submit the work for publication. Medical writing assistance was funded by The Medicines Company, Parsippany, NJ, under the direction of the authors by Latoya M. Mitchell of PharmaWrite, LLC, Princeton, NJ. A.B. is an employee of The Medicines Company; R.R.'s institution received clinical research funding from The Medicines Company; J.L.F. is a consultant for The Medicines Company; D.M.A.'s institution received research funding from The Medicines Company; S.T.'s institution received research funding from The Medicines Company; C.M.R. is a consultant for The Medicines Company; G.M. is an employee of The Medicines Company; D.S. is an employee of The Medicines Company; M.N.D. is an employee of The Medicines Company; J.L. is an employee of The Medicines Company.
The study design was done by A.B., J.L.F., D.M.A., S.T., G.M., C.M.R., J.L., and M.N.D. Study execution was done by J.L., R.R., D.S., D.M.A., and S.T. Analysis and interpretation of study data were done by all authors. Drafting, revising, and/or approving the initial submission and any subsequent version of the article were done by all authors. All authors agreed to the submission and claim responsibility for its content.
REFERENCES
- 1.Corey GR, Kabler H, Mehra P, Gupta S, Overcash JS, Porwal A, Giordano P, Lucasti C, Perez A, Good S, Jiang H, Moeck G, O'Riordan W, SOLO I Investigators. 2014. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med 370:2180–2190. doi: 10.1056/NEJMoa1310422. [DOI] [PubMed] [Google Scholar]
- 2.Corey GR, Good S, Jiang H, Moeck G, Wikler M, Green S, Manos P, Keech R, Singh R, Heller B, Bubnova N, O'Riordan W, SOLO II Investigators. 2015. Single-dose oritavancin versus 7-10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis 60:254–262. doi: 10.1093/cid/ciu778. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. Orbactiv® (oritavancin) for injection, for intravenous use. Prescribing information, January 2016 ed. The Medicines Company, Parsippany, NJ. [Google Scholar]
- 4.Barriere SL, Goldberg MR, Janc JW, Higgins DL, Macy PA, Adcock DM. 2011. Effects of telavancin on coagulation test results. Int J Clin Pract 65:784–789. doi: 10.1111/j.1742-1241.2011.02668.x. [DOI] [PubMed] [Google Scholar]
- 5.Gosselin R, Dager W, Roberts A, Freeman L, Gandy L, Gregg J, Dwyre D. 2011. Effect of telavancin (Vibativ) on routine coagulation test results. Am J Clin Pathol 136:848–854. doi: 10.1309/AJCPDPL4G7CERYJU. [DOI] [PubMed] [Google Scholar]
- 6.Webster PS, Oleson FB Jr, Paterson DL, Arkin CF, Mangili A, Craven DE, Adcock DM, Lindfield KC, Knapp AG, Martone WJ. 2008. Interaction of daptomycin with two recombinant thromboplastin reagents leads to falsely prolonged patient prothrombin time/international normalized ratio results. Blood Coagul Fibrinolysis 19:32–38. doi: 10.1097/MBC.0b013e3282f10275. [DOI] [PubMed] [Google Scholar]
- 7.van den Besselaar AM, Tripodi A. 2007. Effect of daptomycin on prothrombin time and the requirement for outlier exclusion in international sensitivity index calibration of thromboplastin. J Thromb Haemost 5:1975–1976. doi: 10.1111/j.1538-7836.2007.02679.x. [DOI] [PubMed] [Google Scholar]
- 8.Belley A, Robson R, Francis JL, Adcock DM, Tiefenbacher S, Rubino CM, Moeck G, Sylvester D, Dudley M, Loutit J. 2016. Interference of oritavancin on coagulation tests as assessed in vitro and in a phase 1 study of normal healthy volunteers, abstr 1807. IDWeek2016, New Orleans, LA, 26 to 30 October 2016. [Google Scholar]
- 9.Raimondi P, Bongard O, de Moerloose P, Reber G, Waldvogel F, Bounameaux H. 1993. D-dimer plasma concentration in various clinical conditions: implication for the use of this test in the diagnostic approach of venous thromboembolism. Thromb Res 69:125–130. doi: 10.1016/0049-3848(93)90009-D. [DOI] [PubMed] [Google Scholar]
- 10.Heffner A, Kline J. 2001. Role of the peripheral intravenous catheter in false-positive D-dimer testing. Acad Emerg Med 8:103–106. doi: 10.1111/j.1553-2712.2001.tb01272.x. [DOI] [PubMed] [Google Scholar]
- 11.Rubino CM, Bhavnani SM, Moeck G, Bellibas SE, Ambrose PG. 2015. Population pharmacokinetic analysis for a single 1,200-milligram dose of oritavancin using data from two pivotal phase 3 clinical trials. Antimicrob Agents Chemother 59:3365–3372. doi: 10.1128/AAC.00176-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anonymous. Vibativ® (televancin) for injection, for intravenous use. Prescribing information, May 2016 ed. Theravance Biopharma, South San Francisco, CA. [Google Scholar]
- 13.Anonymous. Cubicin® (daptomycin for injection) for intravenous use. Prescribing information, May 2016 ed. Merck Sharp & Dohme Corp., Kenilworth, NJ. [Google Scholar]
- 14.Anonymous. Cubicin® RF (daptomycin for injection), for intravenous use. Prescribing information, July 2016 ed. Merck Sharp & Dohme Corp., Kenilworth, NJ. [Google Scholar]
- 15.Anonymous. 2014. CGL-B coagulation (limited) survey, participant summary. In College of American Pathologists surveys and anatomic pathology education programs. http://www.cap.org/apps/docs/proficiency_testing/2014_surveys_catalog.pdf.


