ABSTRACT
Although human immunodeficiency virus (HIV) coinfection is the most important risk factor for a poor antituberculosis (anti-TB) treatment response, its effect on the pharmacokinetics of the first-line drugs in children is understudied. This study examined the pharmacokinetics of the four first-line anti-TB drugs in children with TB with and without HIV coinfection. Ghanaian children with TB on isoniazid, rifampin, pyrazinamide, and ethambutol for at least 4 weeks had blood samples collected predose and at 1, 2, 4, and 8 hours postdose. Drug concentrations were determined by validated liquid chromatography-mass spectrometry methods and pharmacokinetic parameters calculated using noncompartmental analysis. The area under the concentration-time curve from 0 to 8 h (AUC0–8), maximum concentration (Cmax), and apparent oral clearance divided by bioavailability (CL/F) for each drug were compared between children with and without HIV coinfection. Of 113 participants, 59 (52.2%) had HIV coinfection. The baseline characteristics were similar except that the coinfected patients were more likely to have lower weight-for-age and height-for-age Z scores (P < 0.05). Rifampin, pyrazinamide, and ethambutol median body weight-normalized CL/F values were significantly higher, whereas the plasma AUC0–8 values were lower, in the coinfected children than in those with TB alone. In the multivariate analysis, drug dose and HIV coinfection jointly influenced the apparent oral clearance and AUC0–8 for rifampin, pyrazinamide, and ethambutol. Isoniazid pharmacokinetics were not different by HIV coinfection status. HIV coinfection was associated with lower plasma exposure of three of the four first-line anti-TB drugs in children. Whether TB/HIV-coinfected children need higher dosages of rifampin, pyrazinamide, and ethambutol requires further investigation. (This study has been registered at ClinicalTrials.gov under identifier NCT01687504.)
KEYWORDS: children, first-line anti-TB drugs, human immunodeficiency virus, pharmacokinetics, tuberculosis
INTRODUCTION
Human immunodeficiency virus (HIV) infection in children increases the risk of developing active tuberculosis (TB) (1–4) and is associated with a 2- to 6-fold-greater risk of dying from TB (3, 5–8). The poor TB treatment outcome in HIV-infected children is in part due to ongoing immunosuppression and delay in initiation of antiretroviral therapy (9). However, ineffective drug therapy due to suboptimal drug dosages likely contributes to the high risk of death (6, 7, 10) or relapse after treatment (5, 11). Clinical studies in adults with TB have shown that low plasma antituberculosis (anti-TB) drug concentrations are associated with a delayed treatment response (12, 13) or risk of treatment failure (14–16). In particular, low plasma exposure to the rifamycins in the setting of intermittent therapy in HIV-infected patients with TB has been associated with treatment failure and emergence of rifampin resistance (16–18). While there are limited data on the relationship between pharmacokinetic (PK) parameters and pharmacodynamics in children, one study from India reported an association between low plasma maximum concentrations (Cmax) of rifampin and pyrazinamide and unfavorable clinical outcomes in HIV-infected children (19).
Whether HIV infection adversely affects the pharmacokinetics of anti-TB drugs in children is not well studied. Several studies in adults suggest that HIV infection is associated with low plasma concentrations of rifampin and ethambutol (20–23), while others have reported no significant effect of HIV infection (24–26). Studies in children, which were limited by small sample sizes, have reported no significant effect of HIV infection on plasma concentrations of the first-line anti-TB drugs studied (27, 28). A recent comparative study that included 24 HIV-infected and 32 non-HIV-infected children given previously recommended lower drug dosages for children found significantly lower areas under the concentration-time curve from 0 to 4 h (AUC0–4) of ethambutol but no differences in the pharmacokinetics of the other first-line drugs between the two groups (29). Given concerns about inadequate anti-TB drug exposures in children and poor treatment response, particularly in children with HIV coinfection (30), the World Health Organization (WHO) recommended revised higher dosages of the anti-TB drugs in all children in 2010 (31). This study examined whether the pharmacokinetics of isoniazid, rifampin, pyrazinamide, and ethambutol in children with TB/HIV coinfection were different from those in children with TB alone.
RESULTS
Study population.
Between October 2012 and August 2015, a total of 168 children with a clinical diagnosis of TB were screened, of whom 131 (78.0%) were enrolled. Of the 37 excluded patients, 27 lived too far from the study site or could not return for study visits, 8 were in the continuation phase of anti-TB treatment, and 2 were older than 14 years old. Of the 131 enrolled patients, 113 patients had plasma drug assay data available; 5 had samples collected but did not meet the shipping deadline to be included in the analysis. Thirteen patients did not complete sampling (6 withdrew or discontinued the study, 4 were lost to follow-up, 2 died, and 1 was found to be ineligible).
Of the 113 study participants, 59 (52.2%) had HIV coinfection, 63 (55.8%) were male, 24 (21.2%) were <2 years old, and 54 (47.8%) were <5 years old (Table 1). Except for pyrazinamide, the median drug dosages were within the revised WHO-recommended ranges. The median dosage of pyrazinamide of 24.8 mg/kg was below the new recommended range of 30 to 40 mg/kg because of the low ratio of the drug in the fixed-dose combination (FDC) tablet. There were no significant differences in the demographic and clinical characteristics of the patients with TB alone and those with TB/HIV coinfection except that the coinfected patients had lower median weight-for-age Z score (WAZ) (−2.7 versus −2.1; P = 0.023) and height-for-age Z score (HAZ) (−2.8 versus −1.6; P < 0.001) and were more likely to have pulmonary TB (94.9% versus 52.8%; P < 0.001). In addition, the median values for platelet count, serum sodium, alkaline phosphatase, and albumin were lower but that for aspartate transaminase (AST) was higher in the coinfected patients than in those with TB alone (Table 2).
TABLE 1.
Baseline characteristics of children with TB with and without HIV coinfection
| Characteristic | Value for group |
P value | ||
|---|---|---|---|---|
| All (n = 113) | TB (n = 54) | TB/HIV (n = 59) | ||
| Median (IQR) age, yr | 5.0 (2.2–8.3) | 4.8 (2.0–8.3) | 5.0 (2.9–8.9) | 0.642 |
| No. (%) with age, yr | 0.822 | |||
| <2 | 24 (21.2) | 12 (22.2) | 12 (20.3) | |
| ≥2 | 89 (78.8) | 42 (77.8) | 47 (79.7) | |
| No. (%) with age, yr | 0.708 | |||
| <5 | 54 (47.8) | 27 (50.0) | 27 (45.8) | |
| ≥5 | 59 (52.2) | 27 (50.0) | 32 (54.2) | |
| No. (%) male | 63 (55.8) | 32 (59.3) | 31 (52.5) | 0.570 |
| Median (IQR) body wt, kg | 14.0 (8.8–19.5) | 14.1 (8.8–21.8) | 14.0 (8.5–19.0) | 0.406 |
| Median (IQR) height, cm | 99.0 (82.0–119.0) | 99.0 (82.0–122.0) | 98.0 (82.0–117.0) | 0.553 |
| Nutritional status | ||||
| Median (IQR) wt-for-age Z scorea | −2.5 (−3.8 to −1.4) | −2.1 (−3.1 to −0.9) | −2.7 (−4.0 to −2.0) | 0.023 |
| Median (IQR) height-for-age Z score | −2.0 (−3.2 to −1.1) | −1.6 (−2.4 to −0.6) | −2.8 (−3.9 to −1.8) | <0.001 |
| Median (IQR) BMI-for-age Z score | −1.8 (−2.9 to −0.5) | −1.4 (−2.9 to −0.6) | −2.0 (−3.4 to −0.4) | 0.547 |
| Median (IQR) midarm circumference, cm | 14.0 (12.0–16.0) | 14 (13.0–16.5) | 13 (11.2–15.5) | 0.131 |
| Median (IQR) head circumference, cm | 48.0 (46.0–50.0) | 48.0 (46.0–51.0) | 48 (45.0–49.0) | 0.143 |
| Median (IQR) drug dose, mg/kg | ||||
| Isoniazid | 11.2 (9.1–12.8) | 11.4 (9.5–12.8) | 10.4 (9.0–12.9) | 0.314 |
| Rifampin | 15.8 (13.6–18.8) | 16.7 (14.2–18.8) | 15.2 (13.5–18.8) | 0.262 |
| Pyrazinamide | 24.8 (22.6–30.0) | 26.5 (23.5–32.3) | 24.7 (22.3–29.0) | 0.228 |
| Ethambutol | 16.9 (15.0–20.6) | 18.0 (15.8–22.7) | 16.5 (14.9–19.4) | 0.167 |
| No. (%) with TB disease classification: | <0.001 | |||
| Pulmonary | 85 (75.2) | 29 (52.8) | 56 (94.9) | |
| Extrapulmonary | 28 (24.8) | 25 (47.2) | 3 (5.1) | |
| No. (%) with outcome: | 0.513 | |||
| Completed treatment | 99 (87.6) | 47 (85.2) | 52 (88.1) | |
| Lost to follow-up | 8 (7.1) | 4 (7.4) | 4 (6.8) | |
| Discontinued study | 4 (3.5) | 3 (5.6) | 1 (1.7) | |
| Died | 2 (1.8) | 0 (0.0) | 2 (3.4) | |
The software could not calculate weight-for-age Z score for participants who were over 10 years old.
TABLE 2.
Baseline laboratory test results for children with TB with and without HIV coinfection
| Characteristic (n) | Median value (IQR) for group |
P value | ||
|---|---|---|---|---|
| All (n = 113) | TB (n = 54) | TB/HIV (n = 59) | ||
| White blood cell count, 109/liter (72) | 8.6 (6.0–11.9) | 9.6 (6.4–11.9) | 7.7 (5.2–11.7) | 0.215 |
| Absolute neutrophil count, 1,000 (65) | 3.2 (1.9–4.8) | 3.4 (2.2–5.7) | 2.8 (1.9–3.6) | 0.101 |
| Hemoglobin, g/dl (73) | 9.5 (8.7–10.7) | 9.8 (9.3–11.0) | 9.3 (8.4–10.4) | 0.073 |
| Hematocrit, % (70) | 29.2 (26.6–32.9) | 30.3 (28.4–34.0) | 28.2 (25.5–32.9) | 0.052 |
| Platelets, 109/liter (72) | 316 (206.5–508.0) | 450.0 (275.0–587.0) | 276.0 (190.0–350.0) | 0.001 |
| Sodium, mmol/liter (48) | 132 (127.5–135.5) | 133.0 (128.5–136.0) | 130.0 (127.0–133.5) | 0.007 |
| Potassium, mmol/liter (46) | 4.1 (3.8–4.5) | 4.2 (3.9–4.5) | 4.1 (3.8–4.5) | 0.491 |
| Blood urea nitrogen, mmol/liter 79 | 2.8 (1.9–3.7) | 2.6 (1.9–3.7) | 2.9 (1.9–3.6) | 0.632 |
| Serum creatinine, μmol/liter (79) | 36.0 (27.0–50.0) | 35.3 (28.0–51.0) | 37.0 (23.0–47.0) | 0.199 |
| Glomerular filtration rate, ml/min/1.73 m2 (77) | 102.4 (85.9–126.7) | 98.7 (85.9–115.3) | 103.4 (84.1–138.4) | 0.659 |
| AST, U/liter (790 | 37.0 (29.0–51.0) | 31.0 (25.0–40.0) | 43.6 (36.0–56.1) | <0.001 |
| ALT, U/liter (75) | 22.0 (15.2–36.0) | 19.0 (14.0–40.0) | 22.6 (18.0–34.0) | 0.062 |
| Alkaline phosphatase, U/liter (77) | 386.0 (292.0–563.0) | 510.0 (340.0–637.0) | 370.0 (160.8–474.0) | <0.001 |
| Total bilirubin, μmol/liter (76) | 6.0 (4.3–9.0) | 7.0 (5.0–9.0) | 5.0 (4.0–8.0) | 0.412 |
| Albumin, g/liter (79) | 38.0 (32.0–43.0) | 42.0 (38.0–43.0) | 34.0 (30.8–38.0) | <0.001 |
| HIV-related laboratory tests | ||||
| CD4 cells | ||||
| Count, cells/μl (46) | 460.0 (77.0–925.0) | |||
| % (42) | 10.0 (6.0–18.0) | |||
| HIV-1 RNA, copies/ml (34) | 267,912 (70,300–886,059) | |||
| Log10 HIV-1 RNA (34) | 5.4 (4.8–5.9) | |||
| HIV-1 RNA >100,000 copies/ml | 25 (73.5%) | |||
Among the HIV-infected patients, the median (interquartile range [IQR]) baseline CD4 count was 460.0 (77.0 to 925.0) cells/μl (n = 46), percentage of CD4 cells was 10.0% (6.0 to 18.0%) (n = 42), and viral load was 267,912 (70,300 to 886,059) copies/ml (n = 34). Overall, 25 (73.5%) of the 34 patients with viral load data available had HIV RNA levels of >100,000 copies/ml.
PK of the anti-TB drugs.
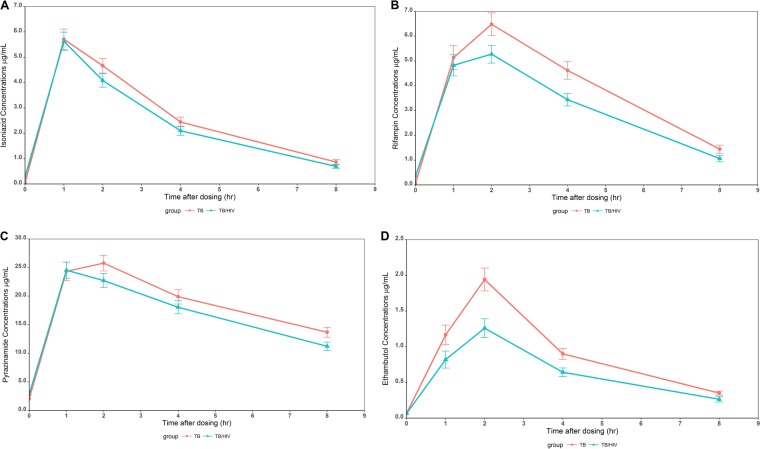
The plasma concentration-time profiles of each drug by HIV infection status are shown in Fig. 1. The concentrations of isoniazid at all the sampling points appeared to be similar in the children with and without HIV coinfection (Fig. 1A). The concentrations of rifampin, pyrazinamide, and ethambutol after the 1-hour postdose time point were numerically lower in the patients with TB/HIV coinfection than in those with TB alone (Fig. 1B, C, and D). In fact, the median rifampin and ethambutol Cmax were significantly lower in the HIV/TB-coinfected patients than in those with TB alone (P < 0.05).
FIG 1.
Plasma concentration-time profiles of isoniazid (A), rifampin (B), pyrazinamide (C), and ethambutol (D) in children with TB and HIV/TB coinfection.
The pharmacokinetic (PK) parameters of the drugs in all participants and by HIV infection status are shown in Table 3. Except for time to Cmax (Tmax), there were no significant differences in the isoniazid pharmacokinetic parameters between the two groups (P > 0.05). Rifampin, pyrazinamide, and ethambutol median AUC0–8 were significantly lower, while median body weight-normalized predicted apparent oral clearance as a function of bioavailability (CL/F) was significantly higher, in the children with TB/HIV coinfection than in those with TB alone (Table 3). For rifampin and ethambutol, the median Cmax were significantly lower in the HIV/TB-coinfected patients than in those with TB alone (Table 3). The proportion of patients with median Cmax of ethambutol of <2 μg/ml (the lower limit of the therapeutic target range) was significantly higher in the TB/HIV-coinfected patients than in those with TB alone (75.4% versus 42.3%; P = 0.0017). There was a trend toward a higher proportion of TB/HIV-coinfected patients than those with TB alone having median rifampin Cmax of <8 μg/ml (72.9% versus 55.6%; P = 0.076).
TABLE 3.
Steady-state pharmacokinetic parameter estimates for antituberculosis drugs by HIV coinfection status
| Drug and parameter | Value for group |
P value | ||
|---|---|---|---|---|
| All (n = 113) | TB (n = 54) | TB/HIV (n = 59) | ||
| Isoniazid | ||||
| Cmax, μg/ml | 5.75 (4.27–7.47) | 5.85 (4.27–7.47) | 5.32 (4.03–7.61) | 0.794 |
| Tmax, h | 1.05 (1.00–1.22) | 1.07 (1.00–1.45) | 1.02 (1.00–1.15) | 0.015 |
| AUC0–8, μg · h/ml | 19.73 (13.24–26.43) | 21.15 (16.48–25.92) | 18.37 (12.20–26.90) | 0.231 |
| Normalized Vz/F, liters/kg | 1.51 (1.20–1.90) | 1.55 (1.27–1.84) | 1.47 (1.15–2.00) | 0.957 |
| Normalized CL/F, liters/h/kg | 0.47 (0.33–0.71) | 0.44 (0.32–0.70) | 0.50 (0.33–0.78) | 0.405 |
| Rifampin | ||||
| Cmax, μg/ml | 6.39 (4.92–8.77) | 7.65 (5.20–9.10) | 5.83 (3.71–8.26) | 0.025 |
| Tmax, h | 2.00 (1.07–2.17) | 2.01 (1.13–2.28) | 1.83 (1.02–2.08) | 0.090 |
| AUC0–8, μg · h/ml | 27.25 (20.62–36.32) | 30.49 (21.93–38.44) | 24.88 (15.95–35.27) | 0.030 |
| Normalized Vz/F, liters/kg | 1.46 (1.11–2.10) | 1.37 (1.08–2.05) | 1.63 (1.21–2.22) | 0.142 |
| Normalized CL/F, liters/h/kg | 0.50 (0.37–0.74) | 0. 45 (0.35–0.66) | 0.53 (0.41–0.86) | 0.044 |
| Pyrazinamide (n = 110) | ||||
| Cmax, μg/ml | 26.05 (21.70–35.10) | 26.90 (23.15–34.60) | 24.60 (20.60–36.50) | 0.259 |
| Tmax, h | 1.16 (1.00–2.07) | 1.93 (1.05–2.10) | 1.04 (1.00–1.77) | 0.006 |
| AUC0–8, μg · h/ml | 140.50 (114.18–186.19) | 151.04 (124.64–188.81) | 126.53 (105.41–182.34) | 0.034 |
| Normalized Vz/F, liters/kg | 0.87 (0.74–1.08) | 0.88 (0.75–1.08) | 0.85 (0.73–1.19) | 0.661 |
| Normalized CL/F, liters/h/kg | 0.11 (0.08–0.14) | 0.10 (0.07–0.12) | 0.12 (0.09–0.16) | 0.010 |
| Ethambutol (n = 110) | ||||
| Cmax, μg/ml | 1.67 (0.87–2.68) | 2.28 (1.48–3.05) | 1.33 (0.75–1.92) | <0.001 |
| Tmax, h | 2.04 (1.70–2.23) | 2.03 (1.40–2.20) | 2.05 (1.83–2.33) | 0.641 |
| AUC0–8, μg · h/ml | 6.17 (3.71–9.15) | 7.57 (5.16–10.68) | 4.76 (2.98–6.76) | <0.001 |
| Normalized Vz/F, liters/kg | 8.98 (6.68–18.09) | 8.12 (5.97–11.04) | 11.25 (7.70–18.72) | 0.008 |
| Normalized CL/F, liters/h/kg | 2.38 (1.76–3.38) | 1.96 (1.51–2.73) | 2.95 (2.20–3.81) | 0.001 |
In the multivariate analysis of factors associated with the anti-TB drug pharmacokinetics (Table 4), we assessed model fittings by r2, proportion of the variance in pharmacokinetic parameters explained by selected models, and model significance. Dose and N-acetyl transferase 2 (NAT2) slow metabolizer genotype status influenced isoniazid Cmax (r2 = 0.09; P = 0.005), AUC0–8 (r2 = 0.21; P < 0.001), and CL/F (r2 = 0.41; P < 0.001). For rifampin, dose, sex, and HIV coinfection status jointly influenced Cmax (r2 = 0.12; P = 0.001), AUC0–8 (r2 = 0.17; P < 0.001), and CL/F (r2 = 0.15; P < 0.001). For pyrazinamide, dose and HIV coinfection jointly influenced AUC0–8 (r2 = 0.17; P < 0.001), and CL/F (r2 = 0.26; P < 0.001), while dose only influenced Cmax (r2 = 0.10; P < 0.001). For ethambutol, dose, sex, and HIV coinfection status were joint predictors of Cmax (r2 = 0.32; P < 0.001), AUC0–8 (r2 = 0.32; P < 0.001), and CL/F (r2 = 0.29; P < 0.001).
TABLE 4.
Coefficient and standardized estimates of joint predictors of drug plasma pharmacokinetics from multivariate analysis using MCP modeling
| Drug | Parameter | Predictor | r2a | P value for model significance | Parameter estimate (SE) | P value | Standardized estimateb |
|---|---|---|---|---|---|---|---|
| Isoniazid | Cmax | Dose (mg) | 0.09 | 0.005 | 0.002 (0.001) | 0.007 | 0.249 |
| NAT2 non-slow vs slow | 0.177 (0.095) | 0.063 | 0.171 | ||||
| AUC0–8 | Dose (mg) | 0.21 | <0.001 | 0.030 (0.013) | 0.020 | 0.201 | |
| NAT2 non-slow vs slow | 8.351 (1.739) | <0.001 | 0.408 | ||||
| CL/F | Dose (mg) | 0.41 | <0.001 | 0.005 (0.001) | <0.001 | 0.451 | |
| NAT2 non-slow vs slow | −0.650 (0.107) | <0.001 | −0.447 | ||||
| Rifampin | Cmax | Dose (mg) | 0.12 | <0.001 | 0.002 (0.001) | 0.001 | 0.300 |
| TB/HIV vs TB | −0.182 (0.107) | 0.092 | −0.153 | ||||
| AUC0–8 | Dose (mg) | 0.17 | <0.001 | 0.042 (0.012) | 0.001 | 0.312 | |
| Male vs female | −4.234 (2.473) | 0.090 | −0.150 | ||||
| TB/HIV vs TB | −5.364 (2.474) | 0.032 | −0.191 | ||||
| CL/F | Dose (mg) | 0.15 | <0.001 | 0.002 (0.001) | <0.001 | 0.367 | |
| Male vs female | 0.178 (0.120) | 0.141 | 0.131 | ||||
| TB/HIV vs TB | 0.195 (0.120) | 0.108 | 0.144 | ||||
| Pyrazinamide | Cmax | Dose (mg) | 0.10 | <0.001 | 0.001 (<0.001) | 0.001 | 0.312 |
| AUC0–8 | Dose (mg) | 0.17 | <0.001 | 0.099 (0.022) | <0.001 | 0.391 | |
| TB/HIV vs TB | −10.638 (9.369) | 0.259 | −0.101 | ||||
| CL/F | Dose (mg) | 0.26 | <0.001 | 0.002 (<0.001) | <0.001 | 0.509 | |
| TB/HIV vs TB | 0.195 (0.133) | 0.144 | 0.124 | ||||
| Ethambutol | Cmax | Dose (mg) | 0.32 | <0.001 | 0.002 (<0.001) | <0.001 | 0.439 |
| Male vs female | −0.154 (0.115) | 0.181 | −0.109 | ||||
| TB/HIV vs TB | −0.396 (0.115) | 0.001 | −0.280 | ||||
| AUC0–8 | Dose (mg) | 0.32 | <0.001 | 0.010 (0.002) | <0.001 | 0.439 | |
| Male vs female | −1.009 (0.576) | 0.083 | −0.141 | ||||
| TB/HIV vs TB | −2.005 (0.579) | 0.001 | −0.280 | ||||
| CL/F | Dose (mg) | 0.29 | <0.001 | 0.002 (<0.001) | <0.001 | 0.491 | |
| Male vs female | 0.201 (0.106) | 0.061 | 0.156 | ||||
| TB/HIV vs TB | 0.349 (0.106) | 0.001 | 0.272 |
Proportion of the variance in PK parameters explained by selected models.
The standardized estimates are all in the same standardized units, so that the relative strength in predicting outcomes can be assessed by comparing them. For example, the standardized coefficient estimate of 0.312 for dose in predicting rifampin AUC0–8 means that a one-standard-deviation increase in dose leads to a 0.312-standard-deviation increase in predicted rifampin AUC0–8, with the other variables held constant. The predicting power of the predictors for rifampin AUC0–8 can be ranked as dose > HIV infection status > sex.
Safety and tolerability.
Of the 113 patients included in this analysis, 99 (87.6%) completed therapy, 8 patients were lost to follow-up, 4 discontinued the study, and 2 died. The two patients who died were both HIV infected and severely malnourished. Of the patients who had liver function tests (LFTs) done at baseline and 4 weeks on anti-TB therapy, only one HIV-infected patient developed grade 3 elevation of AST. No patients developed grade 4 elevation of AST or alanine aminotransferase (ALT) at 4 weeks of anti-TB treatment or required treatment discontinuation to medication side effects.
DISCUSSION
The main finding of this study is that children with TB/HIV coinfection had significantly lower plasma exposure and a higher apparent oral clearance of rifampin, pyrazinamide, and ethambutol. In addition, the median Cmax of rifampin and ethambutol were significantly lower in the children with TB/HIV coinfection than in those with TB alone. While our study was not designed to investigate the relationship between pharmacokinetics and TB treatment outcome, the lower median plasma exposure and Cmax of rifampin and ethambutol in the HIV-coinfected patients is concerning and raises the question of whether inadequate drug pharmacokinetics is a contributor to the higher risk of an unfavorable treatment response. At least one group reported a relationship between lower rifampin and pyrazinamide Cmax and unfavorable TB treatment outcome (death, failure, and default) in HIV-infected children (19) and in a combined group of HIV-infected and uninfected children in India treated with a thrice-weekly regimen (32). Thus, strategies to improve TB treatment outcomes in children, especially with HIV coinfection, should include optimized dosages of rifampin, pyrazinamide, and ethambutol. For rifampin and ethambutol, dosages higher than those currently recommended may be needed in TB/HIV-coinfected children, but for pyrazinamide, increasing the ratio of pyrazinamide in the available FDC tablet to allow for the higher recommended dosages for all children may be adequate.
Prior studies that examined the effect of HIV infection on the pharmacokinetics of anti-TB drugs in children were inconclusive, likely because of small sample size. Among Malawian children, Cmax and AUC0–24 of pyrazinamide (n = 27) and Cmax of ethambutol (n = 18) were similar in the children with and without HIV infection (27). Another study among 44 South African children (21 HIV infected) reported similar rifampin Cmax and AUC0–6 irrespective of HIV infection status (28). In contrast, a recent study that enrolled 66 children with TB (24 HIV coinfected) in India who were given previously recommended daily dosages of the drugs reported significantly lower ethambutol Cmax and AUC0–4 in the TB/HIV-coinfected children than in the children with TB alone, but rifampin, pyrazinamide, and isoniazid Cmax and AUC0–4 were similar between the two groups (29). Another Indian study that included 84 HIV-uninfected and 77 HIV-infected children given three-times-weekly dosages based on the old treatment guidelines found significantly lower rifampin Cmax and AUC0–8 in the HIV-infected children than in those with TB alone (32). The current study found significantly lower rifampin and ethambutol Cmax and AUC0–8, as well as a lower pyrazinamide AUC0–8, in the TB/HIV-coinfected children than in the children with TB alone. The strength of the current study includes a sample size adequate to detect a significant effect of HIV infection, use of the revised WHO recommended dosages for children in over 90% of the participants, and use of the available dispersible FDC tablets, which taken together make our findings relevant to current childhood TB treatment recommendations.
The underlying mechanism for the effect of HIV coinfection on the drug pharmacokinetics could be different for each drug. The lower plasma exposure of the three drugs in the TB/HIV-coinfected patients could be due to impaired absorption and/or enhanced metabolism or excretion, since the two groups were given similar dosages. With noncompartmental analysis and oral dosage forms, the two main PK parameters, CL/F and volume of distribution in the terminal phase divided by bioavailability (Vz/F), are both tied to bioavailability F. While it is possible for a dramatic increase or decrease in either CL or Vz to occur, a more likely explanation in the case of oral dosage forms is a change in F. This is especially true when both CL and Vz are seen to be similarly affected; that is, both CL and Vz get larger because F is smaller. Finally, there are several other studies in adults pointing to the same problem, i.e., malabsorption in HIV-infected patients (20–23). The HIV-infected children were not yet started on antiretroviral drugs at the time of pharmacokinetic sampling, so drug-drug interactions with antiretroviral drugs was not factor in this study. Given that we did not measure metabolite concentrations or perform intestinal absorption tests, we are not able to attribute the lower drug exposure to impaired absorption or enhance metabolism or elimination.
In the multivariate analysis, drug dose was a joint factor with HIV infection in influencing the pharmacokinetics of all three affected drugs. Sex was the other joint factor that affected rifampin and ethambutol pharmacokinetics. However, based on the model fitting results, dose is the major influential factor for pharmacokinetic parameters of all the first-line anti-TB drugs, suggesting that dose adjustment in children, especially those with HIV coinfection, will help achieve the necessary pharmacokinetic profile associated with efficacy in adults.
There were some limitations of our study. The pharmacokinetic sampling was done at five time points (time zero and 1, 2, 4, and 8 hours postdose) but not over the entire 24-hour dosing interval, and so we could not determine the trough concentrations of the drugs. However, such a sampling scheme has been used in other pediatric studies, when sampling at steady state is considered sufficient to estimate key pharmacokinetic parameters such as Cmax and AUC up to the time of the last sample (33, 34). The factors selected in the multivariate analysis do not explain all the interindividual variability in the pharmacokinetics. While in our multivariate analysis the selected models were statistically significant, the small r2 values suggest that there might be other significant effects for pharmacokinetics, which deserves further study.
In conclusion, our study provides evidence that HIV coinfection in children with TB adversely affects the pharmacokinetic profiles of three of the four first-line drugs. Whether this is the underlying reason for the higher risk of unfavorable TB treatment outcome in children with TB/HIV coinfection than in those with only TB requires further evaluation. Drug formulations that will allow for the upper end of the currently recommended dosage range for pyrazinamide to be administered could help optimize the pharmacokinetic profile of the drug in TB/HIV-coinfected patients. However, for rifampin and ethambutol, higher dosages than currently recommended may be needed, especially in HIV-infected children, as dose was the main factor associated with AUC and Cmax after correcting for HIV infection status.
MATERIALS AND METHODS
Study population and design.
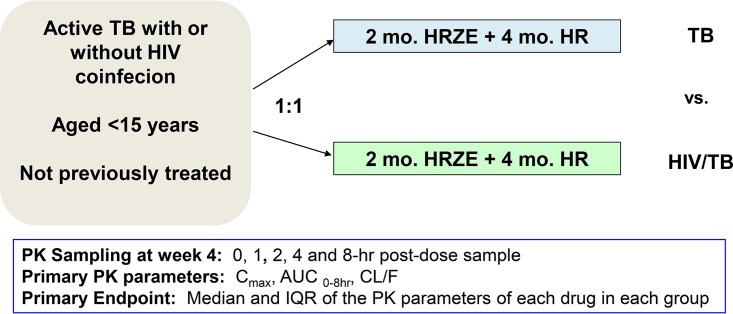
A two-arm, parallel-assignment pharmacokinetic study in children with active TB with or without HIV coinfection was performed at Komfo Anokye Teaching Hospital (KATH), Kumasi, Ghana (Fig. 2). Children aged 3 months to 14 years old, for whom informed consent was provided by a parent or guardian, were recruited from outpatient clinics and inpatient wards at KATH. Children whose parents refused to provide consent or could not return for scheduled visits were excluded.
FIG 2.
Study design and overview of endpoints. H, isoniazid; R, rifampin; Z, pyrazinamide; E, ethambutol.
A complete medical history, physical examination, and nutritional status assessment were performed at enrollment and relevant data collected using standardized forms. Baseline measurements prior to initiation of anti-TB treatment included complete blood count (CBC), blood urea nitrogen, serum creatinine, and liver function tests (LFTs), as well as CD4 cell count determination and plasma HIV-1 RNA levels in the HIV-infected children. LFTs were repeated at week 4 of therapy or when clinically indicated to evaluate for drug toxicity. Study participants were evaluated at 2 weeks of anti-TB treatment and then monthly to assess for adverse events and clinical response to therapy. The Institutional Review Boards (IRBs) of KATH, Ghana, and Lifespan Hospitals, Providence, RI, reviewed and approved the study. All parents or guardians of study participants provided signed informed consent. The study was registered with ClinicalTrials.gov under identifier NCT01687504.
Anti-TB treatment regimen and drug dosages.
The treatment regimen, using revised dosage recommendations (31), consisted of isoniazid at 7 to 15 mg/kg, rifampin at 10 to 20 mg/kg, pyrazinamide at 30 to 40 mg/kg, and ethambutol at 15 to 25 mg/kg daily for 2 months and then isoniazid at 7 to 15 mg/kg and rifampin at 10 to 20 mg/kg daily for 4 months. The medications were dosed according to WHO guidelines for using available dispersible fixed-dose combination (FDC) TB medicines for children (35). At the beginning of the study and prior to the adoption of the WHO recommended elevated dosages of the drugs in Ghana, 11 children received the old recommended dosages of isoniazid and rifampin. The medications were either swallowed or dispersed in water in a plastic cup and ingested, and administration was observed by a health care worker during hospitalization and by a family member at home.
PK sampling.
Pharmacokinetic (PK) sampling was performed at or after 4 weeks of anti-TB treatment as previously described (36). At the time of sampling, none of the HIV-coinfected patients were on antiretroviral therapy. On the day of sampling, medications were administered after an overnight fast in nonbreastfed children. Children on exclusive breastfeeding were allowed to breastfeed as needed throughout the study. A light standard breakfast was provided 30 min after dosing. Once the 2-hour sample was obtained, children were allowed to eat without restrictions. Blood samples were collected at time zero (predose) and at 1, 2, 4, and 8 hours postdose. This sampling scheme, when conducted at steady state, was considered sufficient to estimate key pharmacokinetic parameters such as maximum concentration (Cmax) and area under the concentration-time curve (AUC) (33). The samples, collected in EDTA-coated tubes, were placed immediately on ice and centrifuged within 30 min at 3,000 × g for 10 min. Plasma was stored at −80°C until shipment on dry ice to the University of Cape Town, Cape Town, South Africa, for drug concentrations assays.
PK analysis.
Drug concentrations were determined using validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods as we previously described (36). The observed Cmax and time to Cmax (Tmax) were determined by inspection of the serum concentration-time graphs for each drug. The calculation of AUC from time zero to 8 h (AUC0–8), predicted apparent oral clearance as a function of bioavailability (CL/F), and volume of distribution in the terminal phase as a function of bioavailability (Vz/F) was performed using noncompartmental analysis (Phoenix software; Pharsight Corporation, Mountain View, CA).
Sample size and power justification.
The primary endpoints were Cmax and AUC0–8 in children with active TB with or without HIV coinfection. The power analysis was based on comparison of Cmax in children with TB and in those with TB/HIV coinfection. The literature showed that Cmax has a coefficient of variation (CV) of 27 to 68% (37). With 58 children per group (116 in total) and a 15% dropout rate, the study had 80% power to detect at least a 17% to 37% difference in pharmacokinetic parameters between the two groups. The power analysis was based on a 2-sample t test with a two-sided significance level of 0.05.
Statistical analysis.
Statistical analyses were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC). The weight-for-age Z score (WAZ), height-for-age Z score (HAZ), and body mass index (BMI)-for-age Z score were calculated based on the U.S. National Center for Health Statistics (NCHS) reference median values using statistical macros for children ages <5 years old and 5 to 19 years old provided by the WHO (38). Bivariate analyses of association between patient factors and anti-TB drug pharmacokinetic parameters were assessed with the Wilcoxon rank sum test for continuous variables or the Fisher exact test for categorical variables. Multivariate regression was used to explore the joint effect of demographics and clinical variables on the PK parameters. The minimax concave penalty (MCP) (39, 40) variable selection method was used to select the predictors that were influential on pharmacokinetic parameters (Cmax, AUC0–8, and CL/F). The MCP variable selection has the properties of unbiasedness, sparsity, and continuity, meaning that coefficient estimators are nearly unbiased for informative covariates, automatically set to zero for noninformative covariates, and continuous in data to avoid instability in model prediction. Inference and model fitting statistics of r2 were obtained through model refitting using the selected variables to explore the magnitude of effects and the proportion of the variance in PK parameters explained by selected models. For all analyses, a P value of <0.05 was considered significant.
ACKNOWLEDGMENTS
We thank the study participants and the supportive staff of the TB and HIV clinics at KATH who helped with patient enrollment. We also thank Maxwell Owusu and Eugene Adu Awhireng for their assistance in specimen handling and processing.
All authors report no conflicts of interest.
This work was supported primarily by the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health (grant HD071779). Fizza S. Gillani was supported in part by the Life Span/Tufts/Brown Center for AIDS Research (P30 AI042853). The pharmacokinetic laboratory at the University of Cape Town is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (under award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701) under the auspices of the Adult Clinical Trial Group. Hongmei Yang utilized core services and support from the University of Rochester Center for AIDS Research (CFAR), an NIH-funded program (P30 AI078498).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Elenga N, Kouakoussui KA, Bonard D, Fassinou P, Anaky MF, Wemin ML, Dick-Amon-Tanoh F, Rouet F, Vincent V, Msellati P. 2005. Diagnosed tuberculosis during the follow-up of a cohort of human immunodeficiency virus-infected children in Abidjan, Cote d'Ivoire: ANRS 1278 study. Pediatr Infect Dis J 24:1077–1082. doi: 10.1097/01.inf.0000190008.91534.b7. [DOI] [PubMed] [Google Scholar]
- 2.Madhi SA, Petersen K, Madhi A, Khoosal M, Klugman KP. 2000. Increased disease burden and antibiotic resistance of bacteria causing severe community-acquired lower respiratory tract infections in human immunodeficiency virus type 1-infected children. Clin Infect Dis 31:170–176. doi: 10.1086/313925. [DOI] [PubMed] [Google Scholar]
- 3.Jeena PM, Pillay P, Pillay T, Coovadia HM. 2002. Impact of HIV-1 co-infection on presentation and hospital-related mortality in children with culture proven pulmonary tuberculosis in Durban, South Africa. Int J Tuberc Lung Dis 6:672–678. [PubMed] [Google Scholar]
- 4.Mukadi YD, Wiktor SZ, Coulibaly IM, Coulibaly D, Mbengue A, Folquet AM, Ackah A, Sassan-Morokro M, Bonnard D, Maurice C, Nolan C, Kreiss JK, Greenberg AE. 1997. Impact of HIV infection on the development, clinical presentation, and outcome of tuberculosis among children in Abidjan, Cote d'Ivoire. AIDS 11:1151–1158. doi: 10.1097/00002030-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 5.dos Santos Dias E, do Prado TN, da Silva Guimaraes AL, Ramos MC, Sales CM, de Fatima Almeida Lima E, Sant'Anna CC, Sanchez M, Maciel EL. 2015. Childhood tuberculosis and human immunodeficiency virus status in Brazil: a hierarchical analysis. Int J Tuberc Lung Dis 19:1305–1311. doi: 10.5588/ijtld.14.0619. [DOI] [PubMed] [Google Scholar]
- 6.Hesseling AC, Westra AE, Werschkull H, Donald PR, Beyers N, Hussey GD, El-Sadr W, Schaaf HS. 2005. Outcome of HIV infected children with culture confirmed tuberculosis. Arch Dis Child 90:1171–1174. doi: 10.1136/adc.2004.070466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palme IB, Gudetta B, Bruchfeld J, Muhe L, Giesecke J. 2002. Impact of human immunodeficiency virus 1 infection on clinical presentation, treatment outcome and survival in a cohort of Ethiopian children with tuberculosis. Pediatr Infect Dis J 21:1053–1061. doi: 10.1097/00006454-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Schaaf HS, Marais BJ, Whitelaw A, Hesseling AC, Eley B, Hussey GD, Donald PR. 2007. Culture-confirmed childhood tuberculosis in Cape Town, South Africa: a review of 596 cases. BMC Infect Dis 7:140. doi: 10.1186/1471-2334-7-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yotebieng M, Van Rie A, Moultrie H, Cole SR, Adimora A, Behets F, Meyers T. 2010. Effect on mortality and virological response of delaying antiretroviral therapy initiation in children receiving tuberculosis treatment. AIDS 24:1341–1349. doi: 10.1097/QAD.0b013e328339e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marais BJ, Graham SM, Cotton MF, Beyers N. 2007. Diagnostic and management challenges for childhood tuberculosis in the era of HIV. J Infect Dis 196(Suppl 1):S76–S85. doi: 10.1086/518659. [DOI] [PubMed] [Google Scholar]
- 11.Schaaf HS, Krook S, Hollemans DW, Warren RM, Donald PR, Hesseling AC. 2005. Recurrent culture-confirmed tuberculosis in human immunodeficiency virus-infected children. Pediatr Infect Dis J 24:685–691. doi: 10.1097/01.inf.0000172933.22481.36. [DOI] [PubMed] [Google Scholar]
- 12.Magis-Escurra C, van den Boogaard J, Ijdema D, Boeree M, Aarnoutse R. 2012. Therapeutic drug monitoring in the treatment of tuberculosis patients. Pulm Pharmacol Ther 25:83–86. doi: 10.1016/j.pupt.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Mehta JB, Shantaveerapa H, Byrd RP Jr, Morton SE, Fountain F, Roy TM. 2001. Utility of rifampin blood levels in the treatment and follow-up of active pulmonary tuberculosis in patients who were slow to respond to routine directly observed therapy. Chest 120:1520–1524. doi: 10.1378/chest.120.5.1520. [DOI] [PubMed] [Google Scholar]
- 14.Patel KB, Belmonte R, Crowe HM. 1995. Drug malabsorption and resistant tuberculosis in HIV-infected patients. N Engl J Med 332:336–337. [DOI] [PubMed] [Google Scholar]
- 15.Van Tongeren L, Nolan S, Cook VJ, FitzGerald JM, Johnston JC. 2013. Therapeutic drug monitoring in the treatment of tuberculosis: a retrospective analysis. Int J Tuberc Lung Dis 17:221–224. doi: 10.5588/ijtld.12.0279. [DOI] [PubMed] [Google Scholar]
- 16.Weiner M, Benator D, Burman W, Peloquin CA, Khan A, Vernon A, Jones B, Silva-Trigo C, Zhao Z, Hodge T, Tuberculosis Trials Consortium. 2005. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin Infect Dis 40:1481–1491. doi: 10.1086/429321. [DOI] [PubMed] [Google Scholar]
- 17.Chang KC, Leung CC, Yew WW, Chan SL, Tam CM. 2006. Dosing schedules of 6-month regimens and relapse for pulmonary tuberculosis. Am J Respir Crit Care Med 174:1153–1158. doi: 10.1164/rccm.200605-637OC. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Munsiff SS, Driver CR, Sackoff J. 2005. Relapse and acquired rifampin resistance in HIV-infected patients with tuberculosis treated with rifampin- or rifabutin-based regimens in New York City, 1997-2000. Clin Infect Dis 41:83–91. doi: 10.1086/430377. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran G, Kumar AK, Bhavani PK, Kannan T, Kumar SR, Gangadevi NP, Banurekha VV, Sekar L, Ravichandran N, Mathevan G, Sanjeeva GN, Dayal R, Swaminathan S. 2015. Pharmacokinetics of first-line antituberculosis drugs in HIV-infected children with tuberculosis treated with intermittent regimens in India. Antimicrob Agents Chemother 59:1162–1167. doi: 10.1128/AAC.04338-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peloquin CA, Nitta AT, Burman WJ, Brudney KF, Miranda-Massari JR, McGuinness ME, Berning SE, Gerena GT. 1996. Low antituberculosis drug concentrations in patients with AIDS. Ann Pharmacother 30:919–925. [DOI] [PubMed] [Google Scholar]
- 21.Perlman DC, Segal Y, Rosenkranz S, Rainey PM, Remmel RP, Salomon N, Hafner R, Peloquin CA. 2005. The clinical pharmacokinetics of rifampin and ethambutol in HIV-infected persons with tuberculosis. Clin Infect Dis 41:1638–1647. doi: 10.1086/498024. [DOI] [PubMed] [Google Scholar]
- 22.Sahai J, Gallicano K, Swick L, Tailor S, Garber G, Seguin I, Oliveras L, Walker S, Rachlis A, Cameron DW. 1997. Reduced plasma concentrations of antituberculosis drugs in patients with HIV infection. Ann Intern Med 127:289–293. doi: 10.7326/0003-4819-127-4-199708150-00006. [DOI] [PubMed] [Google Scholar]
- 23.Tappero JW, Bradford WZ, Agerton TB, Hopewell P, Reingold AL, Lockman S, Oyewo A, Talbot EA, Kenyon TA, Moeti TL, Moffat HJ, Peloquin CA. 2005. Serum concentrations of antimycobacterial drugs in patients with pulmonary tuberculosis in Botswana. Clin Infect Dis 41:461–469. doi: 10.1086/431984. [DOI] [PubMed] [Google Scholar]
- 24.Choudhri SH, Hawken M, Gathua S, Minyiri GO, Watkins W, Sahai J, Sitar DS, Aoki FY, Long R. 1997. Pharmacokinetics of antimycobacterial drugs in patients with tuberculosis, AIDS, and diarrhea. Clin Infect Dis 25:104–111. doi: 10.1086/514513. [DOI] [PubMed] [Google Scholar]
- 25.Taylor B, Smith PJ. 1998. Does AIDS impair the absorption of antituberculosis agents? Int J Tuberc Lung Dis 2:670–675. [PubMed] [Google Scholar]
- 26.van Oosterhout JJ, Dzinjalamala FK, Dimba A, Waterhouse D, Davies G, Zijlstra EE, Molyneux ME, Molyneux EM, Ward S. 2015. Pharmacokinetics of antituberculosis drugs in HIV-positive and HIV-negative adults in Malawi. Antimicrob Agents Chemother 59:6175–6180. doi: 10.1128/AAC.01193-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham SM, Bell DJ, Nyirongo S, Hartkoorn R, Ward SA, Molyneux EM. 2006. Low levels of pyrazinamide and ethambutol in children with tuberculosis and impact of age, nutritional status, and human immunodeficiency virus infection. Antimicrob Agents Chemother 50:407–413. doi: 10.1128/AAC.50.2.407-413.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaaf HS, Willemse M, Cilliers K, Labadarios D, Maritz JS, Hussey GD, McIlleron H, Smith P, Donald PR. 2009. Rifampin pharmacokinetics in children, with and without human immunodeficiency virus infection, hospitalized for the management of severe forms of tuberculosis. BMC Med 7:19. doi: 10.1186/1741-7015-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukherjee A, Velpandian T, Singla M, Kanhiya K, Kabra SK, Lodha R. 2016. Pharmacokinetics of isoniazid, rifampicin, pyrazinamide and ethambutol in HIV-infected Indian children. Int J Tuberc Lung Dis 20:666–672. doi: 10.5588/ijtld.15.0288. [DOI] [PubMed] [Google Scholar]
- 30.Graham SM. 2011. Treatment of paediatric TB: revised WHO guidelines. Paediatr Respir Rev 12:22–26. doi: 10.1016/j.prrv.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 31.WHO. 2010. Rapid advice: treatment of tuberculosis in children. http://apps.who.int/iris/bitstream/10665/44444/1/9789241500449_eng.pdf Accessed 29 September 2016. [PubMed]
- 32.Ramachandran G, Kumar AK, Kannan T, Bhavani PK, Kumar SR, Gangadevi NP, Banurekha VV, Sudha V, Venkatesh S, Ravichandran N, Kalpana S, Mathevan G, Sanjeeva GN, Agarwal D, Swaminathan S. 2016. Low serum concentrations of rifampicin and pyrazinamide associated with poor treatment outcomes in children with tuberculosis related to HIV status. Pediatr Infect Dis J 35:530–534. doi: 10.1097/INF.0000000000001069. [DOI] [PubMed] [Google Scholar]
- 33.Thee S, Seddon JA, Donald PR, Seifar HI, Werely CJ, Hesseling AC, Rosenkranz B, Roll S, Magdorf K, Schaaf HS. 2011. Pharmacokinetics of isoniazid, rifampicin, and pyrazinamide in children younger than two years of age with tuberculosis: evidence for implementation of revised World Health Organization recommendations. Antimicrob Agents Chemother 55:5560–5567. doi: 10.1128/AAC.05429-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bekker A, Schaaf HS, Draper HR, van der Laan L, Murray S, Wiesner L, Donald PR, McIlleron HM, Hesseling AC. 2016. Pharmacokinetics of rifampin, isoniazid, pyrazinamide, and ethambutol in infants dosed according to revised WHO-recommended treatment guidelines. Antimicrob Agents Chemother 60:2171–2179. doi: 10.1128/AAC.02600-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO. 2009. Dosing instructions for the use of currently available fixed-dose combination TB medicines for children. http://www.who.int/tb/challenges/interim_paediatric_fdc_dosing_instructions_sept09.pdf Accessed on 29 September 2016.
- 36.Kwara A, Enimil A, Gillani FS, Yang H, Sarfo AM, Dompreh A, Ortsin A, Osei-Tutu L, Kwarteng Owusu S, Wiesner L, Norman J, Kurpewski J, Peloquin CA, Ansong D, Antwi S. 2015. Pharmacokinetics of first-line antituberculosis drugs using WHO revised dosage in children with tuberculosis with and without HIV coinfection. J Pediatric Infect Dis Soc doi: 10.1093/jpids/piv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chideya S, Winston CA, Peloquin CA, Bradford WZ, Hopewell PC, Wells CD, Reingold AL, Kenyon TA, Moeti TL, Tappero JW. 2009. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis 48:1685–1694. doi: 10.1086/599040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO. 2011. Physical status: the use and interpretation of anthropometry. Report of a WHO expert committee. Technical report series no. 854 World Health Organization, Geneva, Swizerland. [PubMed] [Google Scholar]
- 39.Breheny P, Huang J. 2011. Coordinate descent algorithms for nonconvex penalized regression, with applications to biological feature selection. Ann Appl Stat 5:232–253. doi: 10.1214/10-AOAS388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang C-H. 2010. Nearly unbiased variable selection under minimax concave penalty. Ann Stat 38:894–942. doi: 10.1214/09-AOS729. [DOI] [Google Scholar]