ABSTRACT
Ceftazidime-avibactam (CAZ-AVI) is a recently approved β-lactam–β-lactamase inhibitor combination with the potential to treat serious infections caused by carbapenem-resistant organisms. Few patients with such infections were included in the CAZ-AVI clinical trials, and clinical experience is lacking. We present a case series of patients with infections caused by carbapenem-resistant Enterobacteriaceae (CRE) or Pseudomonas aeruginosa (CRPa) who were treated with CAZ-AVI salvage therapy on a compassionate-use basis. Physicians who had prescribed CAZ-AVI completed a case report form. We used descriptive statistics to summarize patient characteristics and treatment outcomes. We used the Wilcoxon rank sum test and Fisher's exact test to compare patients by treatment outcome. The sample included 36 patients infected with CRE and two with CRPa. The most common infections were intra-abdominal. Physicians categorized 60.5% of patients as having life-threatening infections. All but two patients received other antibiotics before CAZ-AVI, for a median of 13 days. The median duration of CAZ-AVI treatment was 16 days. Twenty-five patients (65.8%) concurrently received other antibiotics to which their pathogen was nonresistant in vitro. Twenty-eight patients (73.7%, 95% confidence interval [CI], 56.9 to 86.6%) experienced clinical and/or microbiological cure. Five patients (20.8%) with documented microbiological cure died, whereas 10 patients (71.4%) with no documented microbiological cure died (P = 0.01). In three-quarters of cases, CAZ-AVI (alone or combined with other antibiotics) cured infections caused by carbapenem-resistant organisms, 95% of which had failed previous therapy. Microbiological cure was associated with improved survival. CAZ-AVI shows promising clinical results for infections for which treatment options are limited.
KEYWORDS: carbapenem resistance, case series, ceftazidime-avibactam
INTRODUCTION
Ceftazidime-avibactam (CAZ-AVI), a β-lactam–β-lactamase inhibitor combination, was approved by the U.S. Food and Drug Administration (FDA) in February 2015 (1). It is indicated for the treatment of complicated urinary tract infection (cUTI) and complicated intra-abdominal infection (cIAI) (in combination with metronidazole) in adults with limited or no other therapeutic options. A promising characteristic of CAZ-AVI is its potential to treat infections caused by carbapenem-resistant Enterobacteriaceae (CRE) or carbapenem-resistant Pseudomonas aeruginosa (CRPa). Avibactam recovers the activity of ceftazidime by inhibiting Ambler class A, class C, and some class D beta-lactamases, including the KPC and OXA-48 carbapenemases; CAZ-AVI is not active against metallo-β-lactamases (MBLs), such as NDM, IMP, and VIM (2, 3). In an in vitro study that included 276 meropenem-nonsusceptible Klebsiella species isolates, 98.9% were susceptible to CAZ-AVI (4). In a second study, 67.4% of 396 meropenem-nonsusceptible P. aeruginosa isolates were susceptible to CAZ-AVI (5). A third study reported 100% susceptibility to CAZ-AVI among 133 non-carbapenemase-producing Enterobacteriaceae isolates in which the mechanism of carbapenem resistance was extended-spectrum beta-lactamase (ESBL) and/or AmpC production together with porin deficiency (6).
Clinical data on the efficacy of CAZ-AVI against carbapenem-resistant organisms in humans are scarce. In phase II trials (7, 8) and recently completed phase III trials (9, 10) of CAZ-AVI to treat cUTI and cIAI, the comparator drug was a carbapenem; therefore, patients with infections caused by carbapenem-resistant organisms were excluded. In a pathogen-directed open-label phase III trial (the REPRISE trial) comparing CAZ-AVI to the best available therapy for treatment of cUTI and cIAI caused by ceftazidime-resistant Gram-negative organisms (11), few patients with carbapenem-resistant infections met the trial's inclusion criteria. Among the 292 Enterobacteriaceae isolates recovered from 288 patients in the REPRISE trial, only nine harbored non-MBL carbapenemases: six KPC-producing Klebsiella pneumoniae isolates and three OXA-48-producing K. pneumoniae isolates (12). In an effort to amass data about the effectiveness of CAZ-AVI against carbapenem-resistant organisms, we present a case series of patients with infections caused by CRE or CRPa who were treated with CAZ-AVI salvage therapy on a compassionate-use basis. Our primary aim was to evaluate three outcomes: clinical cure at the end of treatment, microbiological cure at the end of treatment, and all-cause in-hospital mortality. Our secondary aim was to identify predictors of cure and of survival.
RESULTS
Sample.
Of the 25 physicians contacted, 17 responded and 15 contributed data for at least one patient with an infection caused by CRE or CRPa that was sensitive to CAZ-AVI, for a total of 38 patients. Included patients had been treated in Europe and Australia in the years 2013 to 2016. Fifteen patients came from a single hospital in Spain that had an outbreak of KPC-producing K. pneumoniae; the characteristics of this strain have been previously described (13). Thirty-six patients (94.7%) received CAZ-AVI as salvage therapy after treatment with other antibiotics had failed; in the other two patients, CAZ-AVI was the first antibiotic chosen because no other appropriate treatment was available. Three patients were treated at institutions where CAZ-AVI clinical trials were conducted, but they were treated on a compassionate-use basis because they met the trial's exclusion criteria.
Organisms.
Thirty-four patients were infected with Klebsiella pneumoniae, one with Klebsiella oxytoca, one with Escherichia coli, and two with P. aeruginosa. Antimicrobial susceptibilities are presented in Table 1. All isolates but one were classified by the local laboratories as resistant to imipenem. In the patient with an imipenem-susceptible carbapenemase-producing organism (OXA-48-producing E. coli), treatment with imipenem had resulted in microbiological failure. All isolates were resistant to ceftazidime alone, with MICs ranging from 8 to ≥64 μg/ml. Only 14 of 34 isolates tested (41.2%) were susceptible to colistin. Table 2 summarizes the MICs of carbapenems in the 33 isolates for which carbapenem susceptibility was reported quantitatively. One patient with KPC-producing K. pneumoniae with an imipenem MIC of <2 and a meropenem MIC of 2 had failed treatment with a multidrug regimen that included meropenem; carbapenem treatment was not tried in the other two patients with imipenem MICs of 2. Exact results of disk diffusion testing for CAZ-AVI susceptibility were available for all but five isolates, with zone diameters ranging from 21 to 32 mm for Enterobacteriaceae and from 20 to 23 mm for P. aeruginosa. (FDA breakpoints are 21 mm and 18 mm, respectively [14].)
TABLE 1.
Antimicrobial susceptibility of isolates from patients with carbapenem-resistant infections treated with compassionate-use CAZ-AVI
| Antibiotic | No. of isolates testeda | % Susceptible |
|---|---|---|
| Imipenem | 36 | 2.8b |
| Meropenem | 33 | 0.0 |
| Ceftazidime | 38 | 0.0 |
| Colistin | 34 | 41.2 |
| Gentamicin | 37 | 51.4 |
| Amikacin | 38 | 31.6 |
| Tigecycline | 32 | 62.5 |
| Fosfomycin | 29 | 55.2 |
Sample included 34 K. pneumoniae, 1 K. oxytoca, 1 E. coli, and 2 P. aeruginosa isolates.
Patient with OXA-48-producing E. coli who had failed imipenem treatment (MIC not reported).
TABLE 2.
MICs (in µg/ml) of carbapenems in 33 isolates for which susceptibility was reported quantitatively
| Organism and antibiotica | No. of isolates with MIC of: |
|||
|---|---|---|---|---|
| <2 | 2 | 4 to 8 | >8 | |
| Enterobacteriaceae | ||||
| Imipenem (n = 29) | 1 | 2 | 2 | 24 |
| Meropenem (n = 27) | 0 | 1 | 2 | 24 |
| P. aeruginosa | ||||
| Imipenem (n = 2) | 0 | 0 | 0 | 2 |
| Meropenem (n = 1) | 0 | 0 | 0 | 1 |
n, number of isolates.
Patient characteristics and prior treatment.
Patients' demographic and clinical characteristics are shown in Table 3. All but two patients had received antibiotics before CAZ-AVI (median, 3 drugs) and had failed treatment. Among those who received prior antibiotics, the median duration of treatment before CAZ-AVI was started was 13 days. The most commonly prescribed agents were tigecycline (n = 26), meropenem (n = 18), gentamicin (n = 16), fosfomycin (n = 14), and colistin (n = 11).
TABLE 3.
Characteristics of patients with carbapenem-resistant infections treated with compassionate-use CAZ-AVI
| Characteristic | Value (n = 38)a |
|---|---|
| Demographic characteristics | |
| Age in yr, median (IQRb) | 61 (47–67) |
| Male sex | 25 (65.8) |
| Location before hospitalization | |
| Home | 33 (86.8) |
| Transferred from another hospital | 5 (13.2) |
| Comorbidities | |
| Transplant recipient | 5 (13.2) |
| Diabetes mellitus | 8 (21.1) |
| Immunosuppressionc | 10 (26.3) |
| Renal disease | 7 (18.4) |
| Cardiovascular disease | 11 (28.9) |
| McCabe score of >1 | 19 (50.0) |
| Infection characteristics | |
| Organism and carbapenemase | |
| Klebsiella pneumoniae | |
| KPC | 22 |
| OXA-48 | 12 |
| Klebsiella oxytoca (KPC) | 1 |
| Escherichia coli (OXA-48) | 1 |
| Pseudomonas aeruginosa | 2 |
| Hospital-acquired infection | 34 (89.5) |
| Bacteremia | 26 (68.4) |
| Polymicrobial infection | 11 (29.0) |
| Life-threatening infection (high risk of death within 30 days) | 23 (60.5) |
| Antibiotics before CAZ-AVI | |
| Received antibiotics before CAZ-AVI for this infection | 36 (94.7) |
| Days of antibiotic treatment before CAZ-AVI, median (IQR) | 13 (7–31) |
| No. of antibiotics before CAZ-AVI, median (IQR) | 3 (3–4) |
| Other treatments before CAZ-AVI | |
| Surgery to remove the source of infection | 16 (42.1) |
| Removal of foreign body involved in infection | 9 (23.7) |
| Clinical status at start of CAZ-AVI treatment | |
| Mechanical ventilation | 14 (36.8) |
| Vasopressor support | 17 (44.7) |
| Unconscious | 12 (31.6) |
| CAZ-AVI treatment | |
| Days of treatment, median (IQR) | 16 (14–21) |
| Extended infusion | 36 (94.7) |
| Concurrent antibiotic treatmentd | 25 (65.8) |
| Received standard CAZ-AVI dose | 24 (63.2) |
Values are number (%) of patients unless indicated otherwise.
IQR, interquartile range.
Immunosuppression was defined as posttransplant, chemotherapy in past 6 weeks, systemic steroids (>20 mg of prednisone) or other immunosuppressive agents in past 2 weeks, absolute neutrophil count of <500/μl, or HIV/AIDS.
During CAZ-AVI treatment, patient received another antibiotic to which organism was nonresistant in vitro.
CAZ-AVI treatment.
Characteristics of CAZ-AVI treatment are presented in Table 3. The minimum length of treatment was 3 days. Twenty-four patients (63.2%) were given the standard dose of CAZ-AVI throughout their treatment (2 g ceftazidime–0.5 g avibactam every 8 h). Fourteen patients with renal impairment received adjusted doses. Twenty-five patients (65.8%) were treated concurrently with at least one other antibacterial to which their organism was nonresistant in vitro; the most common agents were tigecycline (n = 11), amikacin (n = 9), and fosfomycin (n = 4).
Outcomes.
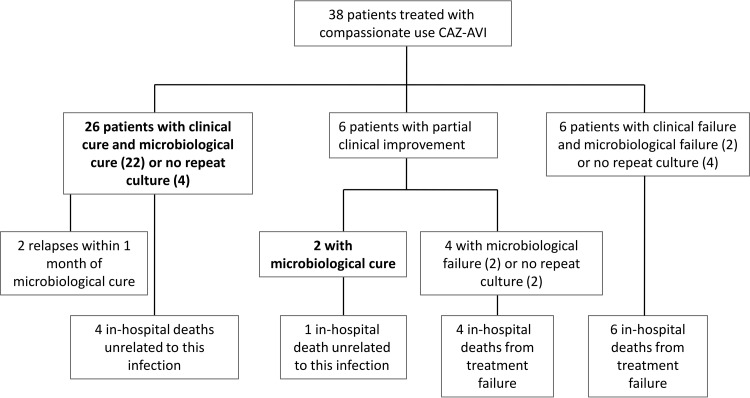
Treatment outcomes are presented in Fig. 1. Twenty-eight patients (73.7%; 95% confidence interval [CI], 56.9 to 86.6%) experienced clinical and/or documented microbiological cure at the end of treatment. All-cause in-hospital mortality was 39.5% (95% CI, 24.0 to 56.6%). Ten patients died during their hospitalization because of treatment failure, such that infection-related mortality was 26.3% (95% CI, 13.4 to 43.1%). Among these 10 patients, the median time from the start of CAZ-AVI treatment to death was 19 days (interquartile range [IQR], 7 to 31 days). Six of them were still receiving CAZ-AVI within 1 day of their death. Nine of the 13 patients (69.2%) who received CAZ-AVI as monotherapy achieved clinical and/or microbiological cure, compared to 19 out of 25 patients (76.0%) given a concurrent antibiotic with in vitro activity against their pathogen (P = 0.71).
FIG 1.
Outcomes of patients with carbapenem-resistant infections treated with compassionate-use CAZ-AVI.
For three of the four patients with documented microbiological failure, the repeat positive isolates were tested for susceptibility to CAZ-AVI; none had developed CAZ-AVI resistance. Two patients with documented microbiological cure of infections caused by KPC-producing K. pneumoniae experienced a relapse, one at 16 days and the other at 30 days after the end of both CAZ-AVI treatment and discharge from the hospital. In the first case, the isolate remained susceptible to CAZ-AVI by disk diffusion testing and the patient experienced clinical and microbiological cure of the relapse infection following dual therapy with CAZ-AVI and gentamicin. In the second case, the relapse isolate was not tested for CAZ-AVI susceptibility; repeat CAZ-AVI treatment was not considered because of the patient's poor prognosis, and the patient died 73 days after infection onset of causes not directly related to the infection.
Table 4 presents treatment outcomes according to infection site. Among the 38 patients, there were 46 infections, not including secondary bacteremia. Eighteen of the 46 infections (39.1%) were either cIAI or cUTI, indications for which CAZ-AVI was approved; 13 patients had only cIAI or cUTI, with or without bacteremia. All-cause mortality was 14.3% in patients with primary or central-line associated bacteremia and 42.3% in patients with any bacteremia. Five out of 24 patients (20.8%) with documented microbiological cure died (of causes unrelated to the infection), whereas 10 of 14 patients (71.4%) with no documented microbiological cure died (P = 0.01).
TABLE 4.
Outcomes of patients with carbapenem-resistant infections treated with compassionate-use CAZ-AVI, by infection site
| Infection sitea | Total no. of cases | No. (%) of cases with: |
Patients with: |
Mortality among patients with microbiological cure |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bacteremia | Life-threatening infection | Documented microbiological cure | Clinical cure |
In-hospital death |
||||||
| No. (%) | 95% CI | No. (%) | 95% CI | No. (%) | 95% CI | |||||
| All patients | 38 | 26 (68.4) | 23 (60.5) | 24 (63.2) | 26 (68.4) | 51.3–82.5 | 15 (39.5) | 24.0–56.6 | 5 (20.8) | 7.1–42.2 |
| Intra-abdominal | 15 | 11 (73.3) | 8 (53.3) | 6 (40.0) | 10 (66.7) | 38.4–88.2 | 6 (40.0) | 16.3–67.7 | 1 (16.7) | 0.4–64.1 |
| Pneumoniab | 7 | 6 (85.7) | 5 (71.4) | 3 (42.9) | 3 (42.9) | 9.9–81.6 | 5 (71.4) | 29.0–96.3 | 1 (33.3) | 0.8–90.6 |
| Skin and soft tissue | 4 | 3 (75.0) | 1 (25.0) | 1 (25.0) | 1 (25.0) | 0.6–80.6 | 2 (50.0) | 6.8–93.2 | 0 (0.0) | 0.0–97.5 |
| Urinary tract | 3 | 2 (66.7) | 1 (33.3) | 2 (66.7) | 2 (66.7) | 9.4–99.2 | 2 (66.7) | 9.4–99.2 | 1 (50) | 1.3–98.7 |
| Primary or catheter-associated bacteremia | 7 | 7 (100) | 7 (100) | 7 (100.0) | 7 (100) | 59.0–100 | 1 (14.3) | 0.4–57.9 | 1 (14.3) | 0.4–57.9 |
| Any bacteremia | 26 | 26 (100) | 20 (76.9) | 18 (69.2) | 18 (69.2) | 48.2–85.7 | 11 (42.3) | 23.4–63.1 | 4 (22.2) | 6.4–47.6 |
| Endocarditis | 2 | 1 (50.0) | 1 (50.0) | 2 (100.0) | 2 (100.0) | 15.8–100 | 1 (50.0) | 1.3–98.7 | 1 (50) | 1.3–98.7 |
| Osteomyelitis | 3 | 0 (0.0) | 0 (0.0) | 2 (66.7) | 2 (66.7) | 9.4–99.2 | 1 (33.3) | 0.8–90.6 | 0 (0.0) | 0.0–84.2 |
| Surgical site infection | 2 | 1 (50.0) | 2 (100) | 1 (50.0) | 1 (50.0) | 1.3–98.7 | 1 (50.0) | 1.3–98.7 | 0 (0.0) | 0.0–97.5 |
| Otherc | 3 | 1 (33.3) | 2 (66.7) | 3 (100) | 2 (66.7) | 9.4–99.2 | 1 (33.3) | 0.8–90.6 | 1 (33.3) | 0.8–90.6 |
Patients may have multiple infection sites.
Pneumonia cases included 6 cases of ventilator-associated pneumonia and 1 case of hospital-acquired pneumonia.
Other infection types (1 patient each) were ventriculitis/subdural abscess, prosthetic joint infection, and mucositis.
Table 5 compares characteristics of patients by treatment outcome. Patients treated for a longer time with other antibiotics prior to CAZ-AVI administration were less likely to experience clinical cure (P = 0.06) and microbiological cure (P = 0.01). Among patients infected with Enterobacteriaceae, survival was higher in patients with KPC carbapenemase than in those with OXA-48: 17 of 23 patients (73.9%) with KPC-producing organisms survived until discharge, compared to 5 of 13 patients (38.5%) with OXA-48 producers (P = 0.07). These 2 groups of patients did not differ by the proportion with life-threatening infection (P = 0.73) or high McCabe score (P = 0.50). Neither of the two cases of relapse occurred in patients with OXA-48; one of the four cases of documented microbiological failure occurred in a patient with OXA-48.
TABLE 5.
Comparison of characteristics of patients with carbapenem-resistant infections treated with compassionate-use CAZ-AVI, by treatment outcomea
| Characteristic | Clinical cure |
P value | Documented microbiological cure |
P value | Survival to hospital discharge |
P value | |||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 26) | No (n = 12) | Yes (n = 24) | No (n = 14) | Yes (n = 23) | No (n = 15) | ||||
| Age in yr, median (IQR) | 54 (46–65) | 62 (58–69) | 0.25 | 63 (48–70) | 58 (46–63) | 0.41 | 54 (47–66) | 61 (44–68) | 0.68 |
| Male sex | 17 (65.4) | 8 (66.7) | 1.00 | 14 (58.3) | 11 (78.6) | 0.29 | 16 (69.6) | 9 (60.0) | 0.73 |
| McCabe score of >1 | 14 (53.9) | 5 (41.7) | 0.73 | 14 (58.3) | 5 (35.7) | 0.31 | 12 (52.2) | 7 (46.7) | 1.00 |
| Days of antibiotic treatment before CAZ-AVI, median (IQR) | 9 (5–17) | 21 (10–50) | 0.06 | 8 (3–16) | 29 (11–45) | 0.01 | 10 (5–20) | 15 (6–31) | 0.38 |
| No. of antibiotics before CAZ-AVI, median (IQR) | 3 (2–3) | 3 (3–6) | 0.19 | 3 (2–3) | 3 (3–6) | 0.15 | 3 (2–4) | 3 (2–4) | 0.75 |
| Life-threatening infection | 15 (57.7) | 8 (66.7) | 0.73 | 14 (58.3) | 9 (64.3) | 1.00 | 12 (52.2) | 11 (73.3) | 0.31 |
| Carbapenemaseb | |||||||||
| KPC | 17 (68.0) | 6 (54.6) | 0.48 | 18 (75.0) | 5 (41.7) | 0.07 | 17 (77.3) | 6 (42.9) | 0.07 |
| OXA-48 | 8 (32.0) | 5 (45.5) | 6 (25.0) | 7 (58.3) | 5 (22.7) | 8 (57.1) | |||
| Polymicrobial infection | 6 (23.1) | 5 (41.7) | 0.27 | 4 (16.7) | 7 (50.0) | 0.06 | 6 (26.1) | 5 (33.3) | 0.72 |
| cIAI or CUTI as sole infection site (with or without bacteremia) | 10 (38.5) | 3 (25.0) | 0.49 | 7 (29.2) | 6 (42.9) | 0.49 | 9 (39.1) | 4 (26.7) | 0.50 |
| Bacteremia | 18 (69.2) | 8 (66.7) | 1.00 | 18 (75.0) | 8 (57.1) | 0.30 | 15 (65.2) | 11 (73.3) | 0.73 |
| Surgery to remove the source of infection before or during CAZ-AVI | 13 (50.0) | 6 (50.0) | 1.00 | 11 (45.8) | 8 (57.1) | 0.74 | 12 (52.2) | 7 (46.7) | 1.00 |
| Mechanical ventilation at start of CAZ-AVI | 8 (30.8) | 6 (50.0) | 0.30 | 8 (33.3) | 6 (42.9) | 0.73 | 5 (21.7) | 9 (60.0) | 0.04 |
| On vasopressors at start of CAZ-AVI | 11 (42.3) | 6 (50.0) | 0.73 | 10 (41.7) | 7 (50.0) | 0.74 | 8 (34.8) | 9 (60.0) | 0.19 |
| Unconscious at start of CAZ-AVI | 6 (23.1) | 6 (50.0) | 0.14 | 6 (25.0) | 6 (42.9) | 0.30 | 4 (17.4) | 8 (53.3) | 0.03 |
| Concurrent active antibiotic treatmentc | 18 (69.2) | 7 (58.3) | 0.71 | 16 (66.7) | 9 (64.3) | 1.00 | 14 (60.9) | 11 (73.3) | 0.50 |
| Received standard CAZ-AVI dose | 17 (65.4) | 7 (58.3) | 0.73 | 15 (62.5) | 9 (64.3) | 1.00 | 15 (65.2) | 9 (60.0) | 1.00 |
Values are number (%) of patients unless indicated otherwise.
Among 36 patients with Enterobacteriaceae infections.
During CAZ-AVI treatment, patient received another antibiotic to which the organism was nonresistant in vitro.
Six patients (15.8%) developed adverse events that were attributed to CAZ-AVI. Blood alkaline phosphatase increased in two patients; nausea/vomiting, Clostridium difficile-associated diarrhea, convulsions, and disorientation progressing to stupor occurred in one patient each.
DISCUSSION
The proportion of Gram-negative infections caused by carbapenem-resistant strains is increasing. According to U.S. surveillance systems, carbapenem resistance in nosocomial infections caused by Klebsiella spp. rose from 1.6% in 2001 to 10.4% in 2011 (15). Regional differences in resistance are striking: in the 2014 report of the EARS-Net European surveillance system, the proportion of Klebsiella spp. isolated from blood or cerebrospinal fluid that was carbapenem resistant ranged from 0.0% in Norway, Sweden, Finland, and Estonia to 62.3% in Greece (16).
Treatment options for carbapenem-resistant organisms are limited; they include primarily colistin, aminoglycosides, tigecycline (for CRE only), fosfomycin, and double-carbapenem therapy. A recent systematic review by Falagas et al. of 20 nonrandomized studies compared mortality following different antibiotic regimens for CRE infections (17). Mortality was variously defined as 28- or 30-day mortality, in-hospital mortality, infection-related mortality, or unspecified. Mortality associated with the most common treatment regimens was up to 57% for colistin alone, up to 80% for tigecycline alone, up to 64% for colistin-tigecycline, up to 50% for gentamicin-tigecycline, and up to 67% for combined therapy with colistin and a carbapenem. While colistin has been considered the mainstay of therapy for carbapenem-resistant infections (18), colistin resistance among CRE isolates has increased (19). In our study, 59% of tested isolates were colistin resistant. Although we lack information about the laboratory methods used at each study site, and some methods for colistin susceptibility testing are flawed (with inaccurate results more likely to be false-susceptible than false-resistant) (20), these test results are relevant because they influenced the decision to use CAZ-AVI. The rationale behind double-carbapenem therapy for carbapenem-resistant organisms is that ertapenem, which is more easily hydrolyzed by carbapenemases, saturates these enzymes so that higher concentrations of the second carbapenem are available to treat the infection. Two recent articles summarized the outcomes of 29 patients who received double-carbapenem therapy for infections caused by carbapenem-resistant K. pneumoniae; clinical success was achieved in 16 cases (55%) (21, 22).
In our study, 73.7% of patients with infections caused by CRE or CRPa who received salvage therapy with CAZ-AVI experienced clinical and/or microbiological cure, and all-cause in-hospital mortality was 39.5%. Among those cured were patients with infections at difficult-to-treat sites, such as endocarditis and osteomyelitis, for which CAZ-AVI has not been studied. Mortality among patients with bacteremia was 42%. In previous studies, all-cause mortality from CRE bacteremia ranged from 19% (among patients given combination therapy that included a carbapenem) to 94% (23–28). In our study, microbiological cure was a predictor of survival: 79% of patients with negative cultures at the end of treatment survived until discharge. Among the 21% of patients who achieved microbiological cure but did not survive, mortality was attributed to other, non-infection-related causes. The delayed onset of CAZ-AVI treatment was associated with worse clinical and microbiological outcomes. Minimizing CAZ-AVI use in order to prevent the emergence of resistance is critical; however, waiting to exhaust all other (and potentially more toxic) treatment options before resorting to CAZ-AVI may reduce a patient's likelihood of being cured.
We found that having an infection caused by an OXA-48-producing pathogen, as opposed to a KPC-producing pathogen, was a predictor of mortality; the association did not reach statistical significance (P = 0.07), likely because of the small sample size. OXA-48 does not hydrolyze ceftazidime efficiently and does not usually cause ceftazidime resistance. However, most OXA-48-producing isolates are resistant to ceftazidime due to coproduction of ESBL enzymes (29). Because ESBLs are well inhibited by avibactam and OXA-48 is partly inhibited by avibactam (30), the higher mortality observed in patients infected with OXA-48 producers is unexpected and intriguing. We do not have further details on the strains that can shed light on this finding, and further studies are warranted.
Shields et al. recently published a single-center case series of 37 patients with CRE infections treated with CAZ-AVI for at least 3 days (31). In contrast to our study, in which 95% of patients received CAZ-AVI as salvage therapy, CAZ-AVI was the first drug used to treat CRE infections in Shields' study. CAZ-AVI was administered as monotherapy in 70% of patients. Fifty-nine percent of patients experienced clinical success, defined as survival without recurrence at 30 days, clinical improvement, and negative cultures within 7 days of the start of treatment. Thirty-day all-cause mortality was 24%. Because of differences in how outcomes were defined, the results of Shields' study and ours are not directly comparable. Notably, three patients in Shields' study developed CAZ-AVI resistance following 10 to 19 days of treatment. A study of KPC-producing Enterobacteriaceae demonstrated the in vitro selection of CAZ-AVI-resistant mutants, primarily via alterations to the blaKPC Ω loop (32). In our study, when CAZ-AVI susceptibility testing was repeated in 1 of 2 patients with relapse of infection and 3 of 4 patients with documented microbiological failure, no resistance was detected.
We found two additional clinical studies of CAZ-AVI treatment for carbapenem-resistant infections. The first was a case report of a 64-year-old woman who received compassionate-use CAZ-AVI for bacteremia caused by KPC-producing K. pneumoniae that did not respond to colistin and dual-carbapenem therapy. She was treated successfully with CAZ-AVI and ertapenem (22). The second was a case series of three patients (aged 72 to 89 years) with CRE bacteremia that had not responded to previous antibiotics. All three achieved clinical and microbiological cure following monotherapy with CAZ-AVI (33).
In our case series, there were six adverse events that were attributed to CAZ-AVI, three of which were severe. One patient who received CAZ-AVI in combination with two other antibiotics developed C. difficile infection during treatment. Like nearly all antibiotics, CAZ-AVI alters the normal flora of the colon, predisposing patients to C. difficile infection, as has been shown in healthy volunteers given CAZ-AVI (34). The risk of C. difficile infection following CAZ-AVI treatment appears to be low: only three of 1,204 (0.2%) patients treated with CAZ-AVI in three phase III clinical trials developed C. difficile infection (9–11). There were two neurological adverse events in our case series: convulsions and disorientation with progression to stupor. While it is not clear if these events were related to CAZ-AVI use or to other factors, the neurotoxic effects of cephalosporins are described mostly in the elderly and in patients with renal impairment or prior neurologic disease (35). Both patients in our study who developed neurotoxicity were aged 70 years or over, with normal renal function.
The main limitation of this study, as with any case series, is the lack of a concurrent control group; comparison is to the published experience summarized above. Comparison to a concurrent control group is problematic because of the potential for selection bias, confounding by indication, and confounding by time to treatment. A second limitation is that the experience with CAZ-AVI in the context of compassionate use may not be generalizable to the population of patients who may be candidates for CAZ-AVI treatment. Patients in compassionate-use programs often have severe acute illness or comorbidities that make them ineligible for clinical trials, increasing their risk of adverse outcomes (36). On the other hand, a sample of such patients may be biased toward clinical success: physicians may be more likely to request compassionate-use drugs for patients with a favorable underlying diagnosis, and immortal time bias may be present, as patients need to survive long enough for the drug to arrive. A third limitation is that two-thirds of patients received CAZ-AVI concurrently with other antibiotics with in vitro activity against their pathogen. In these patients, it is difficult to ascertain whether the clinical success was associated with CAZ-AVI, the concurrent antibiotic, or the combination of both. However, when we compared patients who received CAZ-AVI monotherapy to those who were given additional antibiotics, the proportions cured were similar; thus, we believe CAZ-AVI had a major role in the cure. Finally, the small sample size may have precluded us from identifying significant predictors of clinical cure, microbiological cure, and mortality.
In summary, we have presented a case series of patients treated with CAZ-AVI for carbapenem-resistant infections on a compassionate-use basis. The majority of patients had life-threatening infections, some in difficult-to-cure sites, and 95% of them had failed previous antibiotic treatments. Three-quarters of the patients experienced clinical and/or microbiological cure following CAZ-AVI treatment. CAZ-AVI shows promising clinical results for infections for which treatment options are extremely limited.
MATERIALS AND METHODS
Data collection.
When data for this paper were collected, CAZ-AVI was not approved for use in the European Union. Upon our request, AstraZeneca (a codeveloper of CAZ-AVI) contacted physicians to whom they had provided compassionate-access CAZ-AVI to treat carbapenem-resistant infections and invited them to participate in the study. AstraZeneca had no further involvement. Twenty-five physicians agreed to participate and were sent case report forms. To be eligible for inclusion, patients must have received at least one dose of CAZ-AVI in the context of compassionate use. Data collected included patient demographic characteristics, comorbidities and McCabe score (37), a description of the infection treated by CAZ-AVI (including an antibiogram), treatment with other antibiotics before or concurrently with CAZ-AVI, reasons for using CAZ-AVI, details of CAZ-AVI treatment, adverse events, the clinical and microbiological response, and relapse of infection.
Microbiological methods.
Each hospital conducted antibiotic susceptibility testing according to its own protocols. We report susceptibility as it was interpreted by the local laboratories. All sites tested for susceptibility to CAZ-AVI by disk diffusion; results were interpreted according to the breakpoints set by the FDA (14). Plasma concentrations of ceftazidime and avibactam were not assessed during the course of treatment.
Statistical methods.
The main outcome variables were clinical response at the end of treatment, microbiological response at the end of treatment, and all-cause in-hospital mortality. Clinical response was classified as cure, partial improvement, or treatment failure resulting in death and was analyzed as cure versus partial improvement or treatment failure. Microbiological response was classified as a negative culture, positive culture, or culture not repeated and was analyzed as documented cure (negative culture) versus positive culture or culture not repeated. Patient characteristics measured as continuous variables were summarized by median and interquartile range; categorical variables were summarized as proportions. The McCabe score was treated as a dichotomous variable, with a score of >1 indicating underlying disease with death expected within 5 years. Patient characteristics were compared by outcome using the Wilcoxon rank sum test for continuous variables and Fisher's exact test for categorical variables. Analyses were performed using Stata version 13 (Stata Corporation, College Station, TX).
ACKNOWLEDGMENTS
E.T. and Y.C. were responsible for the study design, literature search, data analysis, and writing of the article. All other authors contributed cases to the case series, assisted with data analysis and writing, and reviewed the manuscript.
AstraZeneca provided the list of physicians who contributed cases to this study; AstraZeneca did not initiate, design, or fund this study, nor was it involved in data analysis or writing of the article. J.T.-C. has received unrestricted grants for educational activities from AstraZeneca. N.B. has received honoraria from AstraZeneca for development of educational presentations, consultancy tasks, and/or for the payment of travel/accommodations for scientific purposes. Y.C. has received funds from AstraZeneca in the form of consulting fees, research grants, and speaker's honoraria. All other authors have no interests to declare.
This work was not supported by any external funding agency. Coauthors J.T.-C. and N.B. are funded by the Ministerio de Economía y Competitividad, Instituto de Salud Carlos III, cofinanced by the European Development Regional Fund (ERDF) “A way to achieve Europe,” Spanish Network for Research in Infectious Diseases (REIPI RD12/0015).
REFERENCES
- 1.U.S. Food and Drug Administration. 26 February 2015. FDA approves new antibacterial drug Avycaz. FDA news release; U.S. FDA, Silver Spring, MD: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm435629.htm. Accessed 13 September 2016. [Google Scholar]
- 2.Zhanel GG, Lawson CD, Adam H, Schweizer F, Zelenitsky S, Lagacé-Wiens PR, Denisuik A, Rubinstein E, Gin AS, Hoban DJ, Lynch JP III, Karlowsky JA. 2013. Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination. Drugs 73:159–177. doi: 10.1007/s40265-013-0013-7. [DOI] [PubMed] [Google Scholar]
- 3.van Duin D, Bonomo RA. 2016. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis 63:234–241. doi: 10.1093/cid/ciw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castanheira M, Mills JC, Costello SE, Jones RN, Sader HS. 2015. Ceftazidime-avibactam activity tested against Enterobacteriaceae isolates from U.S. hospitals (2011 to 2013) and characterization of β-lactamase-producing strains. Antimicrob Agents Chemother 59:3509–3517. doi: 10.1128/AAC.00163-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sader HS, Castanheira M, Farrell DJ, Flamm RK, Jones RN. 2015. Ceftazidime-avibactam activity when tested against ceftazidime-nonsusceptible Citrobacter spp., Enterobacter spp., Serratia marcescens, and Pseudomonas aeruginosa from United States medical centers (2011-2014). Diagn Microbiol Infect Dis 83:389–394. doi: 10.1016/j.diagmicrobio.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Dupont H, Gaillot O, Goetgheluck AS, Plassart C, Emond JP, Lecuru M, Gaillard N, Derdouri S, Lemaire B, Girard de Courtilles M, Cattoir V, Mammeri H. 2015. Molecular characterization of carbapenem-nonsusceptible enterobacterial isolates collected during a prospective interregional survey in France and susceptibility to the novel ceftazidime-avibactam and aztreonam-avibactam combinations. Antimicrob Agents Chemother 60:215–221. doi: 10.1128/AAC.01559-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vazquez JA, González Patzán LD, Stricklin D, Duttaroy DD, Kreidly Z, Lipka J, Sable C. 2012. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr Med Res Opin 28:1921–1931. doi: 10.1185/03007995.2012.748653. [DOI] [PubMed] [Google Scholar]
- 8.Lucasti C, Popescu I, Ramesh MK, Lipka J, Sable C. 2013. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind, phase II trial. J Antimicrob Chemother 68:1183–1192. doi: 10.1093/jac/dks523. [DOI] [PubMed] [Google Scholar]
- 9.Mazuski JE, Gasink LB, Armstrong J, Broadhurst H, Stone GG, Rank D, Llorens L, Newell P, Pachl J. 2016. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection—results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis 62:1380–1389. doi: 10.1093/cid/ciw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagenlehner FM, Sobel JD, Newell P, Armstrong J, Huang X, Stone GG, Yates K, Gasink LB. 2016. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis 63:754–762. doi: 10.1093/cid/ciw378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmeli Y, Armstrong J, Laud PJ, Newell P, Stone G, Wardman A, Gasink LB. 2016. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis 16:661–673. doi: 10.1016/S1473-3099(16)30004-4. [DOI] [PubMed] [Google Scholar]
- 12.Mendes RE, Castanheira M, Woosley LN, Costello SE, Stone GG, Flamm RK, Jones RN. 2015. β-Lactamase characterization of baseline Enterobacteriaceae (ENT) from a phase 3 trial of ceftazidime-avibactam (CAZ-AVI) for the treatment of infections caused by CAZ-nonsusceptible (NS) pathogens, abstr 1176. Abstr IDWeek 2015, , San Diego, CA: https://idsa.confex.com/idsa/2015/webprogram/Paper53048.html Accessed 13 September 2016. [Google Scholar]
- 13.Gonzalez-Padilla M, Torre-Cisneros J, Rivera-Espinar F, Pontes-Moreno A, López-Cerero L, Pascual A, Natera C, Rodríguez M, Salcedo I, Rodríguez-López F, Rivero A, Rodríguez-Baño J. 2015. Gentamicin therapy for sepsis due to carbapenem-resistant and colistin-resistant Klebsiella pneumoniae. J Antimicrob Chemother 70:905–913. doi: 10.1093/jac/dku432. [DOI] [PubMed] [Google Scholar]
- 14.Actavis. 2015. Avycaz package insert. Actavis, Parsippany, NJ: http://www.allergan.com/assets/pdf/avycaz_pi Accessed 13 September 2016. [Google Scholar]
- 15.Centers for Disease Control and Prevention. 2013. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep 62:165–170. [PMC free article] [PubMed] [Google Scholar]
- 16.European Centre for Disease Prevention and Control. 2016. Antimicrobial resistance interactive database (EARS-Net). http://ecdc.europa.eu/en/healthtopics/antimicrobial-resistance-and-consumption/antimicrobial_resistance/database/Pages/database.aspx Accessed 13 September 2016.
- 17.Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. 2014. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother 58:654–663. doi: 10.1128/AAC.01222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pogue JM, Ortwine JK, Kaye KS. 2015. Optimal usage of colistin: are we any closer? Clin Infect Dis 61:1778–1780. doi: 10.1093/cid/civ723. [DOI] [PubMed] [Google Scholar]
- 19.van Duin D, Doi Y. 2015. Outbreak of colistin-resistant, carbapenemase-producing Klebsiella pneumoniae: are we at the end of the road? J Clin Microbiol 53:3116–3117. doi: 10.1128/JCM.01399-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dafopoulou K, Zarkotou O, Dimitroulia E, Hadjichristodoulou C, Gennimata V, Pournaras S, Tsakris A. 2015. Comparative evaluation of colistin susceptibility testing methods among carbapenem-nonsusceptible Klebsiella pneumoniae and Acinetobacter baumannii clinical isolates. Antimicrob Agents Chemother 59:4625–4630. doi: 10.1128/AAC.00868-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cprek JB, Gallagher JC. 2015. Ertapenem-containing double-carbapenem therapy for treatment of infections caused by carbapenem-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother 60:669–673. doi: 10.1128/AAC.01569-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camargo JF, Simkins J, Beduschi T, Tekin A, Aragon L, Pérez-Cardona A, Prado CE, Morris MI, Abbo LM, Cantón R. 2015. Successful treatment of carbapenemase-producing pandrug-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 59:5903–5908. doi: 10.1128/AAC.00655-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villegas MV, Pallares CJ, Escandón-Vargas K, Hernández-Gómez C, Correa A, Álvarez C, Rosso F, Matta L, Luna C, Zurita J, Mejía-Villatoro C, Rodríguez-Noriega E, Seas C, Cortesía M, Guzmán-Suárez A, Guzmán-Blanco M. 2016. Characterization and clinical impact of bloodstream infection caused by carbapenemase-producing Enterobacteriaceae in seven Latin American countries. PLoS One 11:e0154092. doi: 10.1371/journal.pone.0154092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balkan II, Aygün G, Aydın S, Mutcalı SI, Kara Z, Kuşkucu M, Midilli K, Şemen V, Aras S, Yemişen M, Mete B, Özaras R, Saltoğlu N, Tabak F, Öztürk R. 2014. Blood stream infections due to OXA-48-like carbapenemase-producing Enterobacteriaceae: treatment and survival. Int J Infect Dis 26:51–56. doi: 10.1016/j.ijid.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Ben-David D, Kordevani R, Keller N, Tal I, Marzel A, Gal-Mor O, Maor Y, Rahav G. 2012. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect 18:54–60. doi: 10.1111/j.1469-0691.2011.03478.x. [DOI] [PubMed] [Google Scholar]
- 26.Chang HJ, Hsu PC, Yang CC, Kuo AJ, Chia JH, Wu TL, Lee MH. 2011. Risk factors and outcomes of carbapenem-nonsusceptible Escherichia coli bacteremia: a matched case-control study. J Microbiol Immunol Infect 44:125–130. doi: 10.1016/j.jmii.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, Maiuro G, Tedeschi S, Celani L, Cardellino CS, Spanu T, Marchese A, Ambretti S, Cauda R, Viscoli C, Viale P, ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva). 2015. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 70:2133–2143. doi: 10.1093/jac/dkv086. [DOI] [PubMed] [Google Scholar]
- 28.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, Georgiadou S, Markogiannakis A, Goukos D, Skoutelis A. 2014. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 58:2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poirel L, Potron A, Nordmann P. 2012. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 67:1597–1606. doi: 10.1093/jac/dks121. [DOI] [PubMed] [Google Scholar]
- 30.Lagacé-Wiens P, Walkty A, Karlowsky JA. 2014. Ceftazidime-avibactam: an evidence-based review of its pharmacology and potential use in the treatment of Gram-negative bacterial infections. Core Evid 9:13–25. doi: 10.2147/CE.S40698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, Press EG, Kreiswirth BN, Clancy CJ, Nguyen MH. 13 September 2016. Clinical outcomes, drug toxicity and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis pii: ciw636 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livermore DM, Warner M, Jamrozy D, Mushtaq S, Nichols WW, Mustafa N, Woodford N. 2015. In vitro selection of ceftazidime-avibactam resistance in Enterobacteriaceae with KPC-3 carbapenemase. Antimicrob Agents Chemother 59:5324–5330. doi: 10.1128/AAC.00678-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu G, Abraham T, Lee S. 2016. S. Ceftazidime-avibactam for treatment of carbapenem-resistant Enterobacteriaceae bacteremia. Clin Infect Dis 63:1147–1148. doi: 10.1093/cid/ciw491. [DOI] [PubMed] [Google Scholar]
- 34.Rashid MU, Rosenborg S, Panagiotidis G, Löfdal KS, Weintraub A, Nord CE. 2015. Ecological effect of ceftazidime/avibactam on the normal human intestinal microbiota. Int J Antimicrob Agents 46:60–65. doi: 10.1016/j.ijantimicag.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 35.Grill MF, Maganti R. 2008. Cephalosporin-induced neurotoxicity: clinical manifestations, potential pathogenic mechanisms, and the role of electroencephalographic monitoring. Ann Pharmacother 42:1843–1850. doi: 10.1345/aph.1L307. [DOI] [PubMed] [Google Scholar]
- 36.Rosenblatt M, Kuhlik B. 2015. Principles and challenges in access to experimental medicines. JAMA 313:2023–2024. doi: 10.1001/jama.2015.4135. [DOI] [PubMed] [Google Scholar]
- 37.McCabe WR, Jackson GG. 1962. Gram negative bacteremia. I. Etiology and ecology. Arch Intern Med 110:845–847. [Google Scholar]