ABSTRACT
We developed a rat model of methicillin-resistant Staphylococcus epidermidis (MRSE) foreign body-associated osteomyelitis and used it to compare tedizolid alone and in combination with rifampin against rifampin alone, vancomycin plus rifampin, and vancomycin alone. A clinical strain of MRSE was inoculated into the proximal tibia, and a stainless steel wire with a precolonized MRSE biofilm was implanted. Following a 1-week infection period, 92 rats received either no treatment (n = 17) or 14 days of intraperitoneal tedizolid (n = 15), tedizolid plus rifampin (n = 15), rifampin (n = 15), vancomycin plus rifampin (n = 15), or vancomycin (n = 15). Quantitative bone and wire cultures were performed after treatment completion and also 1 week after infection in a separate group of five rats. The median quantity of staphylococci in bone after the 1-week infection period was 4.89 log10 CFU/g bone (interquartile range, 3.83 to 5.33 log10 CFU/g bone); staphylococci were recovered from all associated wires. A median quantity of staphylococci of 3.70 log10 CFU/g bone was detected in bones of untreated control rats after 3 weeks. Quantities of staphylococci in bones of all treatment groups except the group receiving vancomycin alone (2.78 log10 CFU/g) were significantly lower than those for untreated controls, with no staphylococci being detected in the groups receiving rifampin monotherapy, tedizolid-plus-rifampin combination therapy, and vancomycin-plus-rifampin combination therapy. Quantities of staphylococci on wires from all treatment groups that included rifampin were significantly lower than those for untreated controls. No resistance to rifampin, tedizolid, or vancomycin was detected. Tedizolid combined with rifampin was active in a rat model of MRSE foreign body-associated osteomyelitis.
KEYWORDS: foreign body, osteomyelitis, Staphylococcus epidermidis
INTRODUCTION
Over one-half of chronic device-associated infections are caused by staphylococci, with Staphylococcus aureus and Staphylococcus epidermidis being the most common (1). Delayed-onset infections, usually acquired during implantation, have indolent presentations and are typically caused by low-virulence organisms. S. epidermidis, an important commensal bacterium, has emerged as the most significant pathogen in delayed-onset infections related to implanted foreign materials (2), and such strains are often drug resistant. In the United States and Europe, over 80% of coagulase-negative staphylococcal strains causing prosthetic joint infection (PJI) are methicillin resistant (3, 4), and S. epidermidis strains associated with implant infections are often multidrug resistant (5). These frequently long-lasting infections involve biofilm formation (6). Within biofilms, an altered microenvironment compromises antimicrobial action as a result of the presence of persister cells; decreased activity of growth-dependent (e.g., cell wall-active) antibiotics; modification of some antimicrobial agents by inactivating enzymes; and, to some extent and with certain antimicrobial agents, decreased penetration (7). For these reasons, implant-associated infections due to methicillin-resistant S. epidermidis (MRSE) can be difficult to treat; their management typically requires the removal of all prosthetic components or, in selected cases, debridement and implant retention. In both situations, long-term antimicrobial therapy is necessary. Safe and effective antimicrobial regimens, especially those that can be used in the outpatient setting, are therefore needed.
To date, vancomycin has been the treatment of choice for MRSE infections (8), but several studies have pointed out its potential toxicity, limited bactericidal action, and low level of activity against staphylococcal biofilms as well as its inability to reach optimal pharmacodynamic parameters due to the relatively high vancomycin MIC values of MRSE (9, 10). Rifampin has shown activity in biofilm-related infections (11, 12). However, selection of resistance is a risk, and for this reason, rifampin is not used as monotherapy (13). Linezolid is a consideration but may have variable interindividual pharmacokinetics; furthermore, interactions with monoamine oxidase inhibitors may occur, as can myelosuppression, lactic acidosis, and/or hepatic dysfunction (14, 15).
Tedizolid is an oxazolidinone with high oral bioavailability approved by the U.S. Food and Drug Administration for acute bacterial skin and skin structure infections; it has greater potency against methicillin-resistant staphylococci than does linezolid, and data from comparative clinical trials suggest that it may have less toxicity than linezolid (16). Moreover, tedizolid is administered once daily.
To the best of our knowledge, there has not been an animal model of tedizolid activity against MRSE described in the literature. Here, we established a rat model of foreign body-associated osteomyelitis with a PJI-associated MRSE strain and used it to compare tedizolid alone and in combination with rifampin to rifampin monotherapy, vancomycin monotherapy, and vancomycin combined with rifampin.
RESULTS
MICs, MBICs, and MBBCs.
The MICs of the MRSE study isolate for each antimicrobial were as follows: >128 μg/ml for oxacillin, 2 μg/ml for vancomycin, ≤0.015 μg/ml for rifampin, and 0.5 μg/ml for tedizolid. The minimum biofilm-inhibitory concentrations (MBICs) of the isolate were 2 μg/ml for vancomycin, 0.001 μg/ml for rifampin, and 2 μg/ml for tedizolid. The minimum biofilm-bactericidal concentrations (MBBCs) of the isolate were 128 μg/ml for vancomycin, 2 μg/ml for rifampin, and >32 μg/ml for tedizolid.
Experimental rat model.
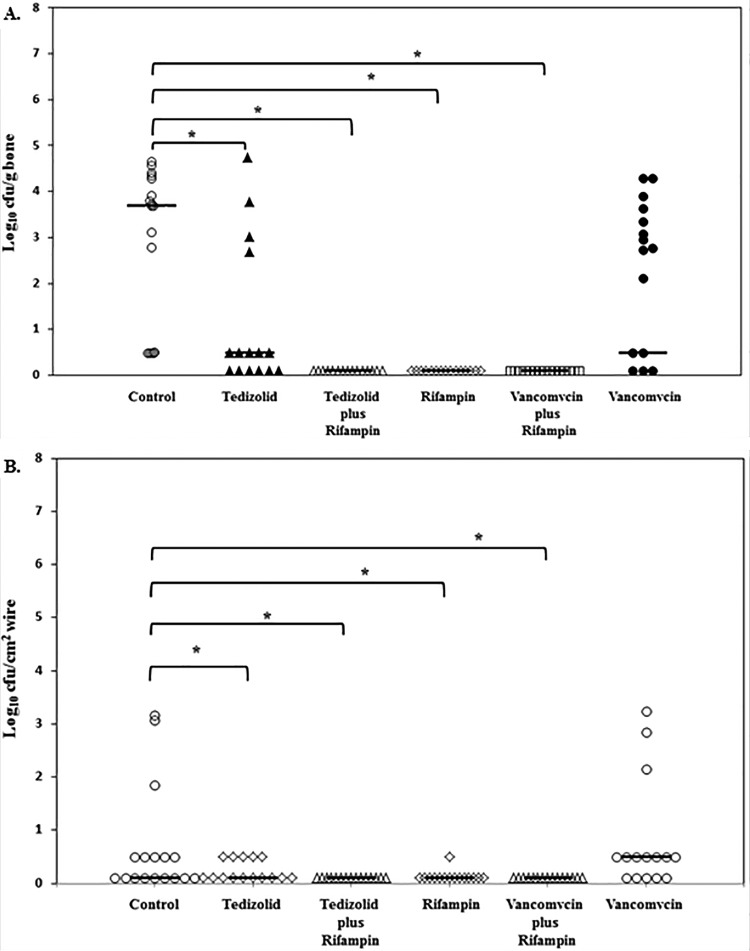
The median quantity of methicillin-resistant coagulase-negative staphylococci recovered from bone after the 1-week infection period was 4.89 log10 CFU/g bone (interquartile range [IQR], 3.83 to 5.33 log10 CFU/g bone), with methicillin-resistant coagulase-negative staphylococci being detected on all wires. Results of quantitative cultures of bone after treatment are summarized in Fig. 1A. Methicillin-resistant coagulase-negative staphylococci were cultured from all harvested bones in the control group at a median of 3.70 log10 CFU/g of bone (IQR, 0.5 to 4.29 log10 CFU/g of bone) after 3 weeks of infection. After 2 weeks of treatment, the median counts of methicillin-resistant coagulase-negative staphylococci were 0.50 log10 CFU/g of bone (IQR, 0.1 to 2.68 log10 CFU/g of bone) in the tedizolid group and 2.78 log10 CFU/g of bone (IQR, 0.50 to 3.64 log10 CFU/g of bone) in the vancomycin group. No methicillin-resistant coagulase-negative staphylococci were detected in the bones of animals in the groups receiving rifampin, tedizolid plus rifampin, or vancomycin plus rifampin. Differences in colony counts compared to those for the control group were significant for all treatment groups (P ≤ 0.005), except for vancomycin monotherapy. Also, there was no significant difference between the tedizolid and vancomycin treatment groups (P = 0.11). However, there was a significant difference between the group receiving tedizolid alone and treatment groups that included rifampin (P = 0.0008) and between the group receiving vancomycin and the treatment groups that included rifampin (P = 0.0003).
FIG 1.
In vivo results. (A) Results of quantitative bone culture expressed as log10 CFU per gram of bone. *, P ≤ 0.005. (B) Results of quantitative wire culture expressed as log10 CFU per square centimeter of wire surface. *, P ≤ 0.005. Median values and individual results are shown for each study group. Horizontal lines represent median values.
Quantitative cultures of stainless steel wires were also obtained (Fig. 1B). Differences in colony counts compared to those of the control group were significant for the treatment groups that included rifampin (P ≤ 0.05). Results of qualitative cultures were as follows (P values shown are with reference to control rats). Methicillin-resistant coagulase-negative staphylococci were cultured from stainless steel wires in 8/17 control rats. There was growth in 1/15 rats in the rifampin treatment group (P = 0.02), 5/15 rats in the tedizolid treatment group (P = 0.43), 0/15 rats in the tedizolid-plus-rifampin treatment group (P = 0.003), 0/15 rats in the vancomycin-plus-rifampin treatment group (P = 0.003), and 10/15 rats in the vancomycin treatment group (P = 0.27).
Histopathology.
Histopathologically, there was considerable bony remodeling with osteoblastic activity in all groups (Fig. 2A), but there was no evidence of acute inflammation in any specimen examined. A wire track was apparent in the medullary cavity on a cross section of bone from an animal from the vancomycin treatment group (Fig. 2B).
FIG 2.
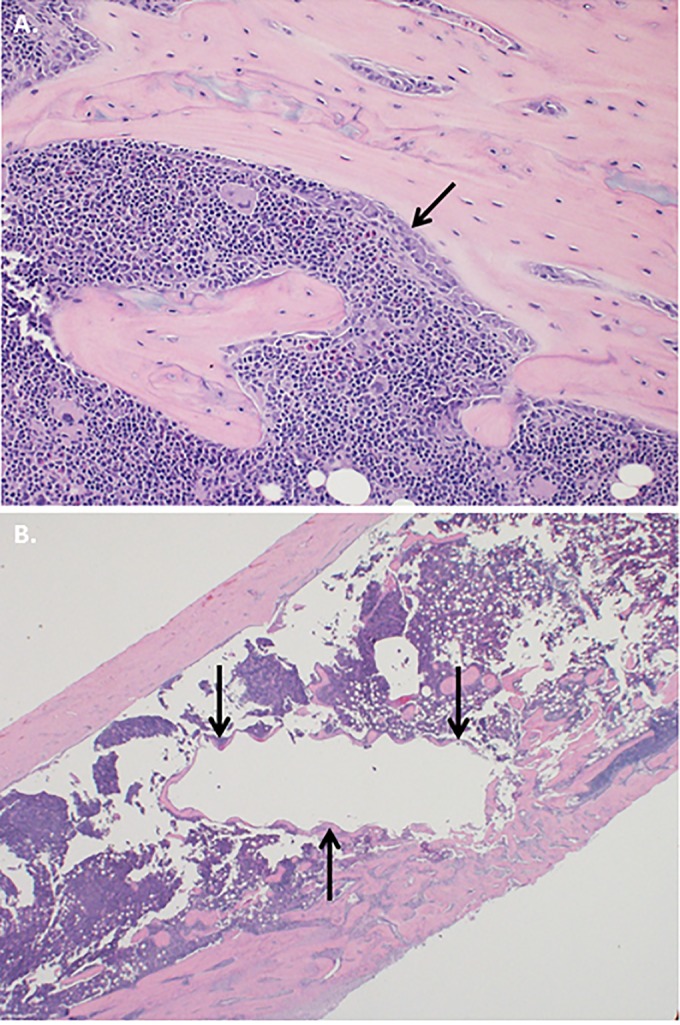
Histopathology. (A) Bony remodeling with osteoblasts lining the marrow cavity was apparent in all groups (arrow) (hematoxylin and eosin stain) (original magnification, ×200). (B) A wire tract was seen in the medullary cavity on a cross section of bone from an animal from the vancomycin group (arrows) (hematoxylin and eosin stain) (original magnification, ×20).
Emergence of resistance.
There was no emergence of resistance to tedizolid or vancomycin in the respective treatment groups. The single isolate recovered from a wire in the group receiving rifampin monotherapy was rifampin susceptible.
DISCUSSION
In the present study, we established a rat model of foreign body-associated osteomyelitis with a PJI-associated MRSE strain. We demonstrated that the combination of tedizolid plus rifampin or vancomycin plus rifampin was more active than no treatment in this model, in which the infected foreign body was retained. Although the activity of tedizolid against methicillin-resistant S. aureus (MRSA) has been analyzed in several experimental studies, such as in a model of experimental endocarditis in rabbits (17) and a model of catheter-related biofilm infection in mice (18), the role of tedizolid against MRSE in experimental osteomyelitis has not been well studied. Successful animal models of osteomyelitis and foreign body infection have been established in rabbits, dogs, chicks, guinea pigs, and rats (19, 20). However, animal models can differ by parameters such as animal species, bacterial load, bacterial strain, implant configuration, implant location, and whether or not surgical management (i.e., explantation or debridement) is carried out. While there have been a few studies of experimental rabbit models of MRSE foreign body osteomyelitis (21, 22), rabbits are more difficult to handle and are more expensive than rats. For this reason, we developed an experimental foreign body-associated osteomyelitis model in rats and used it to assess tedizolid activity against MRSE. Previously, we used a similar model with MRSA, where infection was established over 4 weeks following injection of bacteria into the medullary cavity, with treatment subsequently being administered for 3 weeks (23). However, MRSE did not establish an adequate infection by using this approach; it appeared that S. epidermidis was spontaneously cleared over 3 weeks in this model. Therefore, we made several modifications to our MSRA model to adapt it for use with MRSE, including increasing the inoculum and precolonizing the wire with MRSE biofilm. We also added arachidonic acid as a sclerosing agent to facilitate bone infection by occluding the microvasculature and decreased the infection and treatment intervals to 1 and 2 weeks, respectively. In the present study, we observed that the quantity of methicillin-resistant coagulase-negative staphylococci decreased over time (medians of 4.89 log10 CFU/g bone at 1 week and 3.7 log10 CFU/g bone at 3 weeks). Although there was no evidence of acute inflammation after 3 weeks of infection, there was bony remodeling and osteoblastic activity, likely due to the introduction of foreign material into the bone. Recently, our group observed no acute inflammation after 3 and 6 weeks in a rat model of Propionibacterium acnes foreign body-associated osteomyelitis (24). Using a rat femur fracture model that included metal implants, Lovati et al. reported that intraoperative low-grade S. epidermidis contamination might prevent bone healing, even in the absence of overt findings of infection (25). Like P. acnes, S. epidermidis is a low-virulence organism. The absence of acute inflammation in these animal studies suggests that in humans, infection with such organisms may not always be associated with acute inflammation.
In this study, we observed that vancomycin monotherapy did not reduce bacterial counts, whereas tedizolid monotherapy reduced bacterial counts compared to those with no treatment. The median quantities of methicillin-resistant coagulase-negative staphylococci in bones and on wires in the tedizolid monotherapy group were lower than those in the vancomycin monotherapy group, although there was no statistically significant difference. The quantity of methicillin-resistant coagulase-negative staphylococci was significantly reduced in the tedizolid-plus-rifampin combination therapy group compared to that with no treatment, and the combination of tedizolid plus rifampin was as effective as vancomycin-plus-rifampin combination therapy. While vancomycin is considered the therapy of choice, its activity against staphylococci is debated due to tolerance and heteroresistance, compounded by the occasional presence of vancomycin-intermediate staphylococci (26). Outpatient oral treatment of indolent infections, such as osteomyelitis, caused by low-virulence organisms, such as MRSE, would provide convenience in clinical practice. Recently, Bayer et al. reported that tedizolid had better efficacy in reducing MRSA densities than did linezolid and vancomycin in a murine model of subcutaneous catheter-related biofilm infection (18). We recently performed a study of tedizolid activity in MRSA experimental foreign body-associated osteomyelitis in which tedizolid monotherapy as well as tedizolid-plus-rifampin combination therapy were active (27). The in vitro potency of tedizolid is 2- to 8-fold higher than that of vancomycin against staphylococci, and its prolonged subinhibitory MIC and postantibiotic effect against staphylococci are greater than those of linezolid (28). The study by Bayer et al. reported that in in vitro time-kill studies, tedizolid prevented regrowth at between 6 and 24 h of incubation and that, as determined by bioluminescent in vivo imaging, tedizolid was not associated with relapse after discontinuation of therapy (18). Our present study suggests that combination treatment with tedizolid plus rifampin may be a treatment option for MRSE foreign body-associated osteomyelitis.
There are several limitations of our study. First, we used a single bacterial strain. Second, because the duration of infection was short, the model may not reflect chronic osteomyelitis. Third, we were unable to thoroughly address the emergence of rifampin resistance. Fourth, confirmation of the species and strain of recovered bacteria was not performed.
In conclusion, the present study describes a new rat model of foreign body-associated MRSE osteomyelitis that can be used for comparison of antimicrobial treatment regimens. Tedizolid-plus-rifampin combination therapy was active against MRSE in the model described here.
MATERIALS AND METHODS
Microorganism and antimicrobial agents.
MRSE IDRL-8883, originally recovered from a patient with PJI, was studied. Vancomycin was obtained from Hospira Inc. (Lake Forest, IL). Tedizolid was obtained from Merck & Co. Inc. (Whitehouse Station, NJ). Rifampin was obtained from Akorn Inc. (Lake Forest, IL). The MIC of each antimicrobial agent was determined by broth microdilution using an inoculum of 5 × 105 CFU/ml according to Clinical and Laboratory Standards Institute (CLSI) guidelines (29). For biofilm assays, the minimum biofilm inhibitory concentration (MBIC) and the minimum biofilm bactericidal concentration (MBBC) were determined as previously described (30). S. aureus ATCC 29213 was used for quality control.
Animal model.
This study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Mayo Clinic. Experimental foreign body-associated osteomyelitis was established in male Wistar rats weighing 250 to 350 g, as previously described, with some modifications (23). Briefly, general anesthesia was induced by intraperitoneal administration of ketamine (60 mg/kg of body weight) and xylazine (6 mg/kg), after which the left legs of the rats were shaved, the proximal one-third of the left tibia was surgically exposed, and a 1.5-mm hole was drilled into the medullary cavity. Ten microliters of the sclerosing agent, arachidonic acid sodium salt (99%; Sigma-Aldrich Company, St. Louis, MO), in normal saline at a final concentration of 50 μg/ml was injected into the medullary cavity (31). One hundred microliters of a 108-CFU/ml suspension of the MRSE isolate was injected into the bone. Before wire implantation, each 5-mm by 1-mm stainless steel wire (Zimmer, Warsaw, IN) was incubated in a 1-ml suspension of MRSE at a 4 McFarland standard in Trypticase soy broth at 37°C with shaking for 2 h. The wire with preformed biofilm was implanted into the bone. The hole was covered with dental gypsum. The fascia and skin were closed with sutures, and the wound was sprayed with antiseptic. Extended-release buprenorphine (0.05 mg/kg) was administered subcutaneously for analgesia.
To confirm the establishment of infection, quantitative bone and wire cultures were performed after 1 week of infection in five rats. One week after infection was established, treatment was initiated in 98 animals. Animals were randomly assigned to one of six study arms: no treatment (n = 18), tedizolid alone (n = 16), tedizolid plus rifampin (n = 16), rifampin alone (n = 16), vancomycin alone (n = 16), or vancomycin plus rifampin (n = 16). Rifampin dosing was selected to simulate the concentration above the MIC observed in humans. Vancomycin dosing was selected to simulate the time above the MIC observed in humans. Tedizolid dosing was selected to simulate the free area under the concentration-time curve (AUC) observed in humans after the administration of 200 mg. Tedizolid was administered at 30 mg/kg intraperitoneally once daily, rifampin was administered at 25 mg/kg intraperitoneally twice daily, and vancomycin was administered at 50 mg/kg intraperitoneally twice daily. Treatment was administered for 14 days.
Twenty-four hours after the completion of tedizolid therapy and 12 h after the completion of nontedizolid antimicrobial therapy, rats were sacrificed by using CO2. Times of sacrifice were selected based on the half-lives of the individual antimicrobial agents. The left tibia was aseptically removed and, if being submitted for culture, frozen at −80°C. One rat tibia from each group was used for histopathologic evaluation and therefore was not cultured. For those bones being cultured, after being frozen for at least 4 h at −80°C, bone within 5 mm of the implanted stainless steel was cut and pulverized by using a custom instrument developed by the Mayo Clinic Department of Physiology and Biomedical Engineering. During the pulverization process, the stainless steel wire was aseptically removed. The pulverized bone was weighed. The pulverized bone and wire were each suspended in 2 ml of Trypticase soy broth, vortexed for 30 s, and sonicated at 40 kHz for 5 min. Quantitative bone or wire cultures were performed as previously described, with results being expressed as log10 CFU per gram of bone or log10 CFU per square centimeter of wire surface, respectively (23). If there was no growth in quantitative cultures, qualitative cultures were performed by adding 8 ml of sterile Trypticase soy broth to the remaining 2 ml of broth and incubating the culture for 48 h at 37°C. Recovered colonies were identified as methicillin-resistant coagulase-negative Staphylococcus species by using a Staphaurex test (Thermo Scientific, Waltham, MA) combined with a cefoxitin or oxacillin disc test (Thermo Scientific). For broth with evidence of growth, subculturing was performed to confirm the presence of methicillin-resistant coagulase-negative Staphylococcus species.
Assessment of antimicrobial resistance.
For methicillin-resistant coagulase-negative staphylococci recovered from bone or wire after treatment, the MIC of tedizolid, rifampin, or vancomycin, as appropriate, was determined by using three colonies from the quantitative culture plates (unless fewer than three colonies were detected).
Histopathology.
One randomly chosen removed tibia from each group was fixed for 2 days in 10% formaldehyde and decalcified in a 5% nitric acid solution. The tibia was cut in half longitudinally, embedded in paraffin with the medullary cavity surface on top, sectioned longitudinally, and stained with hematoxylin and eosin.
Statistical methods.
Descriptive statistics for staphylococcal log10 CFU per gram of bone or log10 CFU per square centimeter of wire surface were summarized as medians and IQRs. For statistical purposes, the absence of growth was assigned a value of 0.1 log10 CFU per g of bone or cm2 of wire, and growth in qualitative broth culture only (but not on quantitative culture plates) was assigned a value of 0.5 log10 CFU per g of bone or cm2 of wire. Differences in median log10 CFU per gram of bone or log10 CFU per square centimeter of wire among the groups were compared by using the Kruskal-Wallis test. Further pairwise comparisons of median log10 numbers of CFU of methicillin-resistant coagulase-negative staphylococci per gram of bone or log10 numbers of CFU of methicillin-resistant coagulase-negative staphylococci per square centimeter of wire among the six groups (i.e., the control group and the five treatment groups) were made by using the Wilcoxon rank sum test.
The false discovery rate (FDR) approach was used for adjustment of multiple comparisons (32). Data for qualitative broth cultures of wires in untreated and treated groups were compared by using Fisher's exact test. All tests were two sided; P values of <0.05 were considered statistically significant. Analysis was performed by using SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
ACKNOWLEDGMENT
Funding for this study was provided by Merck.
REFERENCES
- 1.Del Pozo JL, Patel R. 2009. Clinical practice. Infection associated with prosthetic joints. N Engl J Med 361:787–794. doi: 10.1056/NEJMcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N Engl J Med 351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 3.Garvin KL, Hinrichs SH, Urban JA. 1999. Emerging antibiotic-resistant bacteria. Their treatment in total joint arthroplasty. Clin Orthop Relat Res 369:110–123. [PubMed] [Google Scholar]
- 4.Lutro O, Langvatn H, Dale H, Schrama JC, Hallan G, Espehaug B, Sjursen H, Engesaeter LB. 2014. Increasing resistance of coagulase-negative staphylococci in total hip arthroplasty infections: 278 THA-revisions due to infection reported to the Norwegian arthroplasty register from 1993 to 2007. Adv Orthop 2014:580359. doi: 10.1155/2014/580359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park KH, Greenwood-Quaintance KE, Patel R. 2016. In vitro activity of ceftaroline against staphylococci from prosthetic joint infection. Diagn Microbiol Infect Dis 84:141–143. doi: 10.1016/j.diagmicrobio.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Vuong C, Otto M. 2002. Staphylococcus epidermidis infections. Microbes Infect 4:481–489. doi: 10.1016/S1286-4579(02)01563-0. [DOI] [PubMed] [Google Scholar]
- 7.Del Pozo JL, Patel R. 2007. The challenge of treating biofilm-associated bacterial infections. Clin Pharmacol Ther 82:204–209. doi: 10.1038/sj.clpt.6100247. [DOI] [PubMed] [Google Scholar]
- 8.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 9.Ahlstrand E, Svensson K, Persson L, Tidefelt U, Soderquist B. 2011. Glycopeptide resistance in coagulase-negative staphylococci isolated in blood cultures from patients with hematological malignancies during three decades. Eur J Clin Microbiol Infect Dis 30:1349–1354. doi: 10.1007/s10096-011-1228-8. [DOI] [PubMed] [Google Scholar]
- 10.Rogers KL, Fey PD, Rupp ME. 2009. Coagulase-negative staphylococcal infections. Infect Dis Clin North Am 23:73–98. doi: 10.1016/j.idc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Widmer AF, Frei R, Rajacic Z, Zimmerli W. 1990. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J Infect Dis 162:96–102. doi: 10.1093/infdis/162.1.96. [DOI] [PubMed] [Google Scholar]
- 12.Drancourt M, Stein A, Argenson JN, Roiron R, Groulier P, Raoult D. 1997. Oral treatment of Staphylococcus spp. infected orthopaedic implants with fusidic acid or ofloxacin in combination with rifampicin. J Antimicrob Chemother 39:235–240. doi: 10.1093/jac/39.2.235. [DOI] [PubMed] [Google Scholar]
- 13.Zavasky DM, Sande MA. 1998. Reconsideration of rifampin: a unique drug for a unique infection. JAMA 279:1575–1577. doi: 10.1001/jama.279.19.1575. [DOI] [PubMed] [Google Scholar]
- 14.Gould FK. 2011. Linezolid: safety and efficacy in special populations. J Antimicrob Chemother 66(Suppl 4):iv3–iv6. doi: 10.1093/jac/dkr071. [DOI] [PubMed] [Google Scholar]
- 15.Dehghanyar P, Burger C, Zeitlinger M, Islinger F, Kovar F, Muller M, Kloft C, Joukhadar C. 2005. Penetration of linezolid into soft tissues of healthy volunteers after single and multiple doses. Antimicrob Agents Chemother 49:2367–2371. doi: 10.1128/AAC.49.6.2367-2371.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomson KS, Goering RV. 9 April 2013. Activity of tedizolid (TR-700) against well-characterized MRSA strains of diverse epidemiological origin. Antimicrob Agents Chemother doi: 10.1128/AAC.00274-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan LC, Basuino L, Dip EC, Chambers HF. 2015. Comparative efficacies of tedizolid phosphate, vancomycin, and daptomycin in a rabbit model of methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother 59:3252–3256. doi: 10.1128/AAC.04376-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayer AS, Abdelhady W, Li L, Gonzales R, Xiong YQ. 2016. Comparative efficacies of tedizolid phosphate, linezolid, and vancomycin in a murine model of subcutaneous catheter-related biofilm infection due to methicillin-susceptible and -resistant Staphylococcus aureus. Antimicrob Agents Chemother 60:5092–5096. doi: 10.1128/AAC.00880-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Reilly T, Mader JT. 1999. Handbook of animal models of infection. Academic Press, London, United Kingdom. [Google Scholar]
- 20.An YH, Friedman RJ. 1998. Animal models of orthopedic implant infection. J Invest Surg 11:139–146. doi: 10.3109/08941939809032193. [DOI] [PubMed] [Google Scholar]
- 21.An YH, Bradley J, Powers DL, Friedman RJ. 1997. The prevention of prosthetic infection using a cross-linked albumin coating in a rabbit model. J Bone Joint Surg Br 79:816–819. doi: 10.1302/0301-620X.79B5.7228. [DOI] [PubMed] [Google Scholar]
- 22.Tan HL, Ao HY, Ma R, Lin WT, Tang TT. 2014. In vivo effect of quaternized chitosan-loaded polymethylmethacrylate bone cement on methicillin-resistant Staphylococcus epidermidis infection of the tibial metaphysis in a rabbit model. Antimicrob Agents Chemother 58:6016–6023. doi: 10.1128/AAC.03489-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vergidis P, Rouse MS, Euba G, Karau MJ, Schmidt SM, Mandrekar JN, Steckelberg JM, Patel R. 2011. Treatment with linezolid or vancomycin in combination with rifampin is effective in an animal model of methicillin-resistant Staphylococcus aureus foreign body osteomyelitis. Antimicrob Agents Chemother 55:1182–1186. doi: 10.1128/AAC.00740-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tyner H, Greenwood-Quaintance KE, Patel R. 2016. Animal model for Propionibacterium acnes prosthetic joint infection: bacterial presence and histopathology in an experimental animal model of chronic Propionibacterium acnes foreign body infection, abstr 539. Abstr ASM Microbe 2016. [Google Scholar]
- 25.Lovati AB, Romano CL, Bottagisio M, Monti L, De Vecchi E, Previdi S, Accetta R, Drago L. 2016. Modeling Staphylococcus epidermidis-induced non-unions: subclinical and clinical evidence in rats. PLoS One 11:e0147447. doi: 10.1371/journal.pone.0147447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmes NE, Johnson PD, Howden BP. 2012. Relationship between vancomycin-resistant Staphylococcus aureus, vancomycin-intermediate S. aureus, high vancomycin MIC, and outcome in serious S. aureus infections. J Clin Microbiol 50:2548–2552. doi: 10.1128/JCM.00775-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park KH, Greenwood-Quaintance KE, Mandrekar J, Patel R. 2016. Activity of tedizolid in methicillin-resistant Staphylococcus aureus experimental foreign body-associated osteomyelitis. Antimicrob Agents Chemother 60:6568–6572. doi: 10.1128/AAC.01248-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Locke JB, Zurenko GE, Shaw KJ, Bartizal K. 2014. Tedizolid for the management of human infections: in vitro characteristics. Clin Infect Dis 58(Suppl 1):S35–S42. doi: 10.1093/cid/cit616. [DOI] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 30.Schmidt-Malan SM, Greenwood Quaintance KE, Karau MJ, Patel R. 2016. In vitro activity of tedizolid against staphylococci isolated from prosthetic joint infections. Diagn Microbiol Infect Dis 85:77–79. doi: 10.1016/j.diagmicrobio.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Rissing JP, Buxton TB, Fisher J, Harris R, Shockley RK. 1985. Arachidonic acid facilitates experimental chronic osteomyelitis in rats. Infect Immun 49:141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glickman ME, Rao SR, Schultz MR. 2014. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol 67:850–857. doi: 10.1016/j.jclinepi.2014.03.012. [DOI] [PubMed] [Google Scholar]