ABSTRACT
Avermectins are powerful endectocides with an established potential to reduce the incidence of vector-borne diseases. Here, we show that several avermectins inhibit the hepatic stage of Plasmodium infection in vitro. Notably, ivermectin potently inhibits liver infection in vivo by impairing parasite development inside hepatocytes. This impairment has a clear impact on the ensuing blood stage parasitemia, reducing disease severity and enhancing host survival. Ivermectin has been proposed as a tool to control malaria transmission because of its effects on the mosquito vector. Our study extends the effect of ivermectin to the early stages of mammalian host infection and supports the inclusion of this multipurpose drug in malaria control strategies.
KEYWORDS: avermectins, ivermectin, Plasmodium, liver stage, malaria
INTRODUCTION
Despite recent achievements, malaria remains a formidable public health problem, primarily affecting children in the poorest regions of the world. Plasmodium parasites, the causative agents of malaria, are transmitted to humans as sporozoites (spz) through the bite of infected Anopheles mosquitoes. Injected spz travel to the liver and initiate an asymptomatic, yet obligatory, replication and differentiation phase (1). This intrahepatic developmental process culminates in the release of thousands of merozoites into the bloodstream, where they cyclically infect red blood cells, causing disease symptoms and originating gametocytes that warrant the progress of infection in the mosquito vector. Historically, successful malaria control interventions have combined effective vector control strategies, capable of interrupting transmission, and strong antiparasitic drugs that prevent disease and death. However, current tools are precarious and calls have recently been made for the development of new drug formulations or the repurposing of old drug formulations as valuable interventions to help control malaria infection (2).
Avermectins are a family of macrocyclic lactones that includes compounds presenting not only a best-in-class antiparasitic activity but also a strong insecticidal effect (3). Their impact on vector populations led to the suggestion of a potential role for avermectins in reducing the incidence of vector-borne disease (4). In particular, ivermectin, a semisynthetic member of the avermectin family, revolutionized the treatment of nematode and arthropod parasites in animals and is commonly used to treat neglected tropical diseases such as onchocerciasis, lymphatic filariasis, and strongyloidiasis (5–7). More recently, ivermectin has emerged as a potential tool for malaria control (4, 8–10), given its insecticidal effect (11–16), its ability to inhibit Plasmodium falciparum sporogony (17), its inhibitory effect on the blood stages of Plasmodium berghei (in vivo) and P. falciparum (in vitro) (18), and its ability to disrupt parasite transmission (19–21). However, no reports on the effect of avermectins on Plasmodium liver stages exist so far.
Here, we investigated the effects of avermectins on the liver stages of P. berghei parasites and showed that ivermectin is remarkably active against this stage of the malaria parasite's life cycle. These results provide further support for the potential of ivermectin, a drug that is already employed in mass drug administration (MDA) in regions where malaria is endemic and has an established impact on malaria transmission, as a multipurpose, multistage tool for malaria control.
RESULTS
Avermectins inhibit Plasmodium infection of human hepatoma cells in vitro.
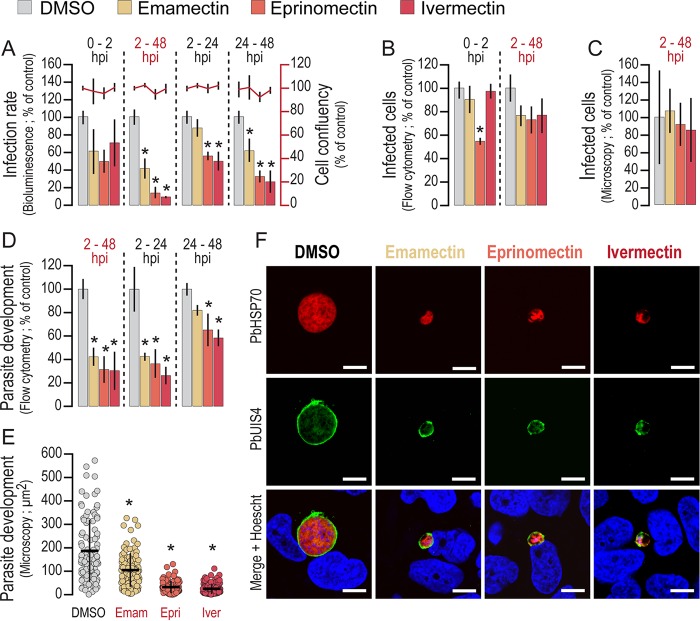
The effect of emamectin, eprinomectin, and ivermectin on the in vitro infection of Huh7 cells by luciferase-expressing P. berghei parasites was measured by using a bioluminescence assay (22). Our data show that all three avermectins tested are active against Plasmodium liver stages, with 50% inhibitory concentrations (IC50s) of ∼2 μM, equivalent to 2.6, 2.2, and 2.1 μg/ml for emamectin, eprinomectin, and ivermectin, respectively (see Fig. S1 in the supplemental material). Treatment at different periods of infection further indicated that these avermectins act mainly during the parasite's intrahepatic development phase, as the strongest effect occurs when the drugs are added to the cells after completion of the invasion process (Fig. 1A). The invasion and development phenotypes in the presence of the compounds were further analyzed by a flow cytometry-based approach (23). These data suggested an effect of eprinomectin on the ability of the parasites to invade Huh7 cells, which was not observed with either emamectin or ivermectin (Fig. 1B). Furthermore, quantification of the infected cells at 48 h postinfection (hpi) by either flow cytometry (Fig. 1B) or immunofluorescence microscopy (Fig. 1C) showed no significant changes in the overall number of intracellular Plasmodium parasites. On the contrary, avermectins strongly impair the parasite's ability to develop inside cells. Measurement of green fluorescent protein (GFP) intensity in cells at 48 hpi, a correlate of parasite development, showed that avermectins inhibit parasite replication, particularly when present between 2 and 24 h of infection (Fig. 1D). This effect was confirmed by immunofluorescence microscopy analysis of infected cells incubated with drugs at their IC90s throughout the 48-h infection period (Fig. 1E and F). Nonetheless, treatment with avermectins does not disrupt parasitophorous vacuole membrane (PVM) formation and does not affect the localization of the PVM-resident protein, upregulated in spz 4, PbUIS4 (Fig. 1F) or of the circumsporozoite protein PbCSP (see Fig. S2). Avermectin treatment does significantly impact somatic integrity and schizogony, as the parasite's DNA appears to be strongly condensed (Fig. 1F; see Fig. S2). Overall, our results show that avermectins inhibit Plasmodium hepatic infection by impairing the parasite's ability to develop inside cells.
FIG 1.
Avermectins inhibit the development of Plasmodium hepatic stages in vitro. (A) Huh7 cells were infected with luciferase-expressing P. berghei spz and treated with the IC90s of avermectins or with equivalent amounts of dimethyl sulfoxide (DMSO; control) for the times indicated. Total parasite loads (bioluminescence) and cell viability were assessed at 48 hpi. (B) Parasite invasion was quantified by flow cytometry at 2 hpi with GFP-expressing P. berghei spz by determining the percentage of GFP+ cells. (C) Hepatic parasite numbers at 48 hpi were quantified by immunofluorescence microcopy. (D, E) Parasite development at 48 hpi was assessed by flow cytometry (D) and immunofluorescence microscopy (E) by determining the fluorescence intensity of GFP+ cells and the area of developing parasites, respectively. (F) The effect of avermectin treatment on PVM structure, somatic integrity, and schizogony was analyzed by immunofluorescence microcopy employing antibodies against PbUIS4 (a PVM protein, in green), PbHSP70 (a heat shock protein that localizes to the parasite soma, in red), and the nuclear stain Hoechst (in blue). Representative confocal images show P. berghei hepatic forms treated with the IC90s of different avermectins or with dimethyl sulfoxide (control) from 2 to 48 hpi. Scale bars, 10 μm. Plots represent the mean values of at least three independent experiments with error bars indicating the standard deviations. The Mann-Whitney test was employed to assess the statistical significance of differences (*, P < 0.05).
Ivermectin decreases the liver Plasmodium load in vivo.
We further evaluated the effect of avermectins in the context of a physiologically relevant Plasmodium liver infection. Emamectin, eprinomectin, and ivermectin were administered to mice by oral gavage on a three-dose administration schedule of 10-mg/kg solutions in soybean oil (Fig. 2A). Mice were infected by mosquito bite injection of P. berghei spz 24 h after the first drug administration, and the parasite load was assessed 44 to 46 h after parasite administration. Our data show that ∼80% inhibition of liver infection in vivo is observed upon treatment with 10 mg/kg ivermectin but not upon treatment with emamectin or eprinomectin, an effect comparable to that obtained with an equivalent administration of primaquine, the only licensed liver stage antiplasmodial drug (24) (Fig. 2B and C). It should be noted that upon administration of 10 mg/kg ivermectin, some mice displayed behavioral signs of neurotoxicity. However, equally of note, strong impairment of infection was also observed when lower doses of ivermectin (1, 5, and 8 mg/kg) were administered following a similar regimen (see Fig. S3). Since avermectins have a strong insecticidal effect on Anopheles mosquitoes (8), we examined whether the observed effect on liver infection resulted from deficient mosquito-to-mammal parasite transmission because of an alteration of the mosquito feeding behavior on drug-treated mice. To investigate this, we initially inspected the mosquitoes' feeding pattern on drug- and vehicle-treated mice. Our data show that mosquito feeding behavior was not altered by the presence of avermectins in circulation (see Fig. S4A), whereas, not surprisingly, they strongly affected mosquito survival after feeding (see Fig. S4B). We then questioned whether the amount of Plasmodium spz deposited into the skin would be influenced by the circulating avermectins. The bioluminescence signal corresponding to the parasites deposited into mouse skin was measured immediately after a mosquito blood meal, revealing no significant differences between groups of mice (see Fig. S4C and D). Overall, our data show that although the presence of avermectins in circulation has no effect on the feeding behavior of Plasmodium-infected mosquitoes or on spz deposition into mammalian host skin, ivermectin displays a marked effect on P. berghei liver infection in vivo.
FIG 2.
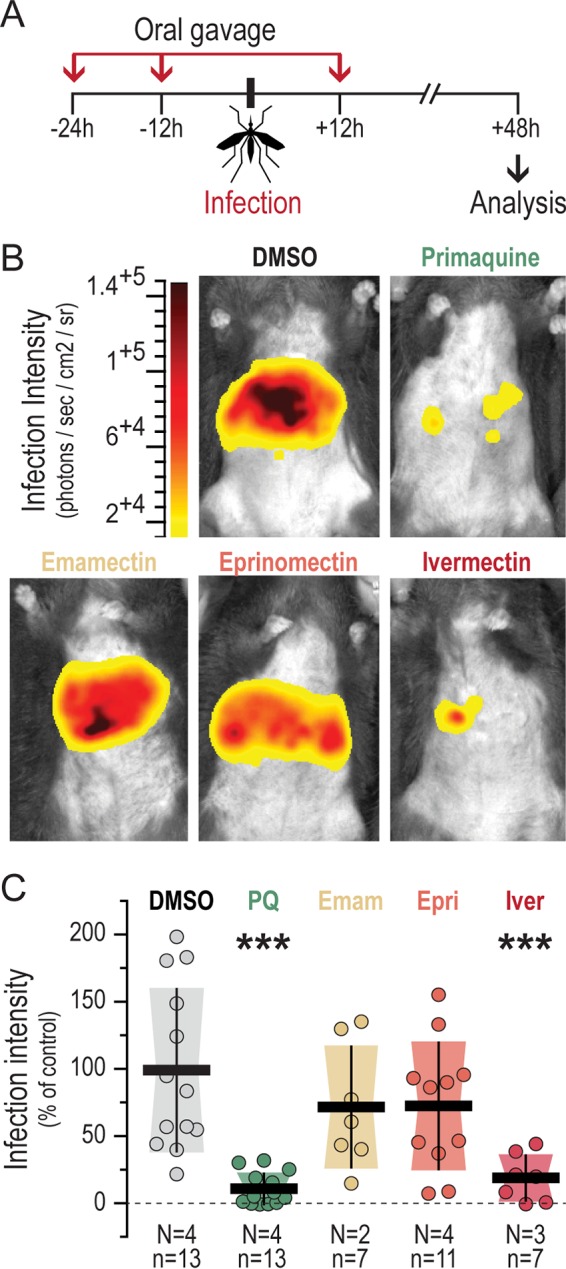
Ivermectin decreases liver infection in vivo. (A) Schematic illustration of the in vivo experimental setup. The arrows indicate when each avermectin was orally administered at 10 mg/kg. Vehicle- or primaquine (10 mg/kg)-treated mice were used as controls. P. berghei spz were delivered by the bites of 5 to 7 mosquitoes. (B) Representative bioluminescence images of mouse livers at 48 hpi. (C) Liver parasite infection loads in mice treated with the various compounds, relative to those in DMSO-treated controls. Dots represent the radiance intensity of individual liver areas relative to the average radiance of all DMSO-treated mice. The total number of mice (n) in each data set and the number of independent biological replicate experiments (N) performed are indicated. The statistical significance of differences from the DMSO-treated mice was calculated by employing the nonparametric Mann-Whitney test. P values are indicated above each data set (***, P < 0.001). Horizontal dark lines indicate the relative mean liver parasite infection load, whereas the vertical bars and shaded area represent the standard deviation of the mean. PQ, primaquine; Emam, emamectin; Epri, eprinomectin; Iver, ivermectin.
Ivermectin impairs parasite development in the liver and impacts host survival.
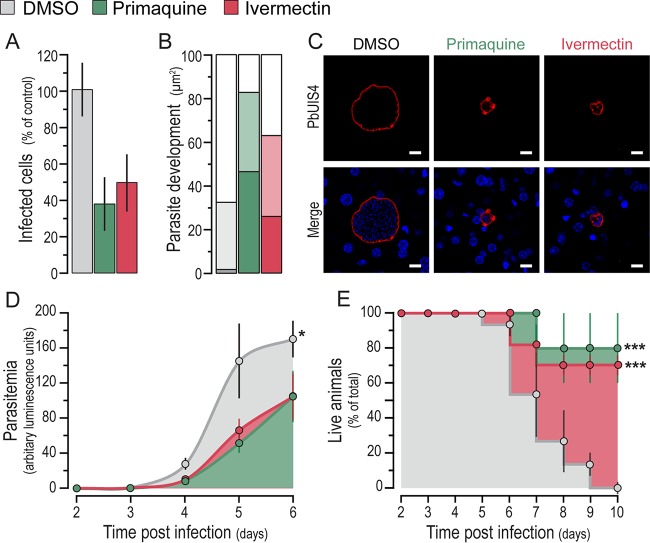
To investigate the basis of the observed impairment of in vivo liver infection by ivermectin, infected mouse livers were analyzed by immunofluorescence microscopy at 46 hpi. Our analysis showed that ivermectin-treated mice had fewer (Fig. 3A) and significantly less developed (Fig. 3B and C) liver parasites than those of vehicle-treated control mice. We then investigated whether the ivermectin-dependent reduction of liver infection would impact the appearance of parasites in the blood and the ensuing pathology. To this end, infection was allowed to proceed past the liver stage and blood stage parasitemia, disease symptoms, and mouse survival were monitored for 10 days following parasite administration. Our results show that the impairment of liver parasite development caused by ivermectin treatment affects the onset of blood parasitemia (Fig. 3D) and, most importantly, has a clear impact on host survival, with 80% of the treated mice surviving for 10 days, compared with all of the control mice dying with symptoms of experimental cerebral malaria (ECM) within the same period (Fig. 3E). Overall, these results show that ivermectin significantly abrogates liver infection by impairing parasite development and survival in hepatic cells, which impacts the ensuing blood parasite burden and contributes to enhanced host survival.
FIG 3.
Ivermectin treatment inhibits parasite development in hepatocytes and improves the outcome of disease. (A) Parasite density per square millimeter of liver section following treatment with ivermectin, primaquine, or the vehicle at 48 hpi. (B) Effect of treatment on the parasite area as a correlate of parasite development at 48 hpi. Darker colors indicate parasite areas of <250 μm2, intermediate shading indicates areas between 250 and 750 μm2, and lighter bars indicate parasite areas of >750 μm2. (C) Representative confocal microscopy images of liver parasites in treated and control mice. Green, PbUIS4 labeling showing the PVM; blue, Hoechst nuclear staining. Scale bars,10 μm. (D) Assessment of the prepatency period and blood parasitemia by bioluminescence assays at various time points after spz administration and drug treatment as in Fig. 2A. The mean bioluminescence ± the standard errors of the pooled data of 15 mice from three biological replicate experiments is presented for each of the time points analyzed. A statistical analysis of the mean parasitemia observed across time was performed by using the nonparametric Friedman test, which indicated a significant difference, with a P value of <0.05 (*). (E) Mouse survival following spz administration and drug treatment as in Fig. 2A. The mean percentage of live animals ± the standard errors of the pooled data of 15 mice from three biological replicate experiments is shown for daily records, starting at the second day after spz administration and drug treatment and up to 10 days pi. The Mantel-Cox (log rank) test was employed to compare survival curves, indicating statistically significant differences, with a P value of <0.001 (***) for both the primaquine- and ivermectin-treated groups compared to the DMSO-treated controls.
DISCUSSION
Avermectins are widely used for protection against a wide spectrum of parasitic diseases. Various avermectins have been synthesized, with different antiparasitic efficacies and pharmacokinetic profiles, including one, ivermectin, that is safe and well tolerated in humans. Several studies have shown that ivermectin has a strong insecticidal effect that could be explored as a potential tool against malaria transmission (9). Besides, inhibitory effects of ivermectin against the sporogonic and blood stages of the Plasmodium life cycle have been described, highlighting its potential as a multistage malaria intervention strategy (17, 18).
The effect of avermectins on the liver stage of Plasmodium infection had hitherto not been assessed. Nevertheless, the relatively low numbers of parasites that infect hepatocytes, coupled with the obligatory and asymptomatic natures of this phase of infection, make the liver a privileged target for prophylactic intervention (1, 25). Moreover, the existence of Plasmodium species capable of forming hypnozoites, parasite forms that remain dormant in the liver for long periods of time, demands effective ways of clearing hepatic parasites before they can cause disease relapses. We originally described a potential inhibition of Plasmodium hepatic infection by ivermectin as part of the results obtained in a drug screen targeting Plasmodium liver stages (26). We now demonstrate that ivermectin is the only compound of the avermectin family assessed in the present study that is active against Plasmodium liver stages in vivo. We confirm that ivermectin treatment strongly decreases parasite load in vivo, to a degree that is comparable to that of primaquine. Ivermectin strongly inhibits parasite development in the liver, which has a strong impact on the ensuing pathology and host survival, decreasing the magnitude of the blood stage infection and protecting from cerebral disease.
The exact mechanism of action of primaquine remains elusive but may involve impairment of the parasites' mitochondrial metabolism or the production of highly reactive metabolites that generate intracellular oxidative potentials (reviewed in references 24 and 27). Azithromycin, another drug with demonstrated activity against P. berghei liver stages, is proposed to act by blocking apicoplast development, leading to impaired parasite maturation (28). However, the molecular mechanism of ivermectin inhibition of Plasmodium liver stages is currently unknown. In the mosquito, the primary target of ivermectin is the invertebrate glutamate-gated chloride channel (GluCl) (29). Ivermectin has also been shown to kill the parasite's blood stages by blocking the nuclear import of the P. falciparum signal recognition particle (SRP), a family of six polypeptides involved in protein targeting to the endoplasmic reticulum (18). Recently, ivermectin was shown to be a ligand for farnesoid X receptor (NR1H4) (30), whose activation has a critical role in regulating the homeostasis of glucose, a molecule that plays a pivotal role during hepatic Plasmodium infection (31).
A recent modeling study has shown that adding ivermectin to mass treatment strategies with artemether-lumefantrine can help reduce/interrupt malaria transmission (32), and efforts are being made to develop a suitable slow-release formulation (33). Our results now show that besides its recognized effect on mosquitoes, ivermectin inhibits Plasmodium development inside hepatic cells, the obligatory initial stage of the malaria parasite's infection of its mammalian host. Thus, our study lends further support to the use of ivermectin as a tool for malaria control and warrants further investigation of the impact of ivermectin MDA on this devastating disease.
MATERIALS AND METHODS
Mice, parasites, and reagents.
Male C57BL/6 mice 6 to 8 weeks old were purchased from Charles River and housed in facilities of the Instituto de Medicina Molecular (iMM), Lisbon. Experimental procedures were performed in accordance with the Federation of European Laboratory Animal Science Associations guidelines and iMM regulations. In experiments involving blood stage infections, mice were euthanized at the first behavioral signs of onset of ECM, and this was considered the experimental endpoint. When no ECM was observed, mice were euthanized at day 10 postinfection (pi), the end of the time frame for the development of this phenotype. A GFP/luciferase-expressing P. berghei ANKA transgenic parasite line (676m1cl1) was used in all experiments. Mosquitoes were bred at the insectary facility of the iMM. All of the chemicals used were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Quantification of in vitro parasite infection.
For IC50 and IC90 determinations, compounds were added to the cells prior to spz addition and incubated for 48 h. In all other experiments, compounds were used at their IC90s and incubated for the times indicated. The infection load of human hepatoma Huh7 cells was determined by bioluminescence measurements as previously described (22). Invasion and intracellular parasite development were assessed by determining the percentage of GFP+ cells at 2 hpi and GFP intensity at 48 hpi, respectively, as previously described (23). The Mann-Whitney test was employed to assess the statistical significance of differences (P < 0.05; GraphPad Prism v5).
Immunofluorescence microscopy analysis of in vitro and in vivo hepatic infections.
For in vitro studies, cells seeded onto coverslips were fixed at 48 hpi with 4% (vol/vol) paraformaldehyde for 10 min at room temperature (RT), permeabilized/blocked with 0.1% (vol/vol) Triton X-100–1% (wt/vol) bovine serum albumin in 1× phosphate-buffered saline (PBS) for 30 min at RT, and incubated with anti-Hsp70 and anti-UIS4 antibodies for 1 h at RT, followed by adequate secondary antibodies and Hoechst 33342 for 1 h at RT. For in vivo studies, fixed sections of livers collected at 48 hpi (intravenous [i.v.] administration of 30,000 spz) were similarly stained. Images were acquired with a Zeiss 510 Meta confocal microscope and a Zeiss Axiovert 200 M microscope and then processed with the ImageJ software.
Quantification of in vivo hepatic infection and blood stage parasitemia.
In vivo parasite loads in mice were determined by real-time in vivo imaging 30 min and 44 to 46 h after 15 min of exposure to five to seven infected Anopheles stephensi mosquitoes with the in vivo IVIS Lumina Imaging System as previously described (22). Additionally, real-time PCR analysis of specific P. berghei 18S rRNA and mouse hypoxanthine guanine phosphoribosyl transferase (HPRT)-encoding housekeeping genes in infected livers collected at 44 to 46 hpi was performed as previously described (34). The nonparametric Mann-Whitney test was applied to assess the statistical significance of differences between liver parasite loads (P < 0.001; GraphPad Prism v5). Blood stage parasitemia following the i.v. injection of 5,000 spz was assessed by collecting 5 μl of tail blood between days 2 and 6 pi in 50 μl of lysis buffer. Bioluminescence was then measured following the addition of 50 μl of d-luciferin dissolved in firefly luciferase assay buffer to 30 μl of lysate with an Infinite M200 multiplate reader (Tecan). The nonparametric Friedman test was applied to assess the statistical significance of differences between the mean parasitemias observed across time in mice subjected to the different drug treatments (P < 0.05; GraphPad Prism v5).
Supplementary Material
ACKNOWLEDGMENTS
We thank Ana Filipa Teixeira and Ana Parreira for mosquito production, as well as the iMM facilities for technical support. We are grateful to Maria M. Mota (iMM) for advice and reagents.
A.M.M., P.M., and M.P. acknowledge the Fundação para a Ciência e Tecnologia, Portugal, for grants SFRH/BPD/80693/2011, SFRH/BD/71098/2010, and Investigador FCT (2013), respectively. This work was supported by Fundação para a Ciência e Tecnologia (FCT, Portugal) grant PTDC/SAU-MIC/117060/2010.
The authors report no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02005-16.
REFERENCES
- 1.Prudêncio M, Rodriguez A, Mota MM. 2006. The silent path to thousands of merozoites: the Plasmodium liver stage. Nat Rev Microbiol 4:849–856. doi: 10.1038/nrmicro1529. [DOI] [PubMed] [Google Scholar]
- 2.Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, Collins F, Doumbo OK, Greenwood B, Hall BF, Levine MM, Mendis K, Newman RD, Plowe CV, Rodriguez MH, Sinden R, Slutsker L, Tanner M. 2011. A research agenda to underpin malaria eradication. PLoS Med 8:e1000406. doi: 10.1371/journal.pmed.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson ML. 1993. Avermectins in arthropod vector management—prospects and pitfalls. Parasitol Today 9:83–87. doi: 10.1016/0169-4758(93)90210-7. [DOI] [PubMed] [Google Scholar]
- 4.Foy BD, Kobylinski KC, da Silva IM, Rasgon JL, Sylla M. 2011. Endectocides for malaria control. Trends Parasitol 27:423–428. doi: 10.1016/j.pt.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotez PJ. 2007. Control of onchocerciasis—the next generation. Lancet 369:1979–1980. doi: 10.1016/S0140-6736(07)60923-4. [DOI] [PubMed] [Google Scholar]
- 6.Moncayo AL, Vaca M, Amorim L, Rodriguez A, Erazo S, Oviedo G, Quinzo I, Padilla M, Chico M, Lovato R, Gomez E, Barreto ML, Cooper PJ. 2008. Impact of long-term treatment with ivermectin on the prevalence and intensity of soil-transmitted helminth infections. PLoS Negl Trop Dis 2:e293. doi: 10.1371/journal.pntd.0000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tambo E, Khater EI, Chen JH, Bergquist R, Zhou XN. 2015. Nobel prize for the artemisinin and ivermectin discoveries: a great boost towards elimination of the global infectious diseases of poverty. Infect Dis Poverty 4:58–65. doi: 10.1186/s40249-015-0091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaccour CJ, Kobylinski KC, Bassat Q, Bousema T, Drakeley C, Alonso P, Foy BD. 2013. Ivermectin to reduce malaria transmission: a research agenda for a promising new tool for elimination. Malar J 12:153–160. doi: 10.1186/1475-2875-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaccour CJ, Rabinovich NR, Slater H, Canavati SE, Bousema T, Lacerda M, Ter Kuile F, Drakeley C, Bassat Q, Foy BD, Kobylinski K. 2015. Establishment of the Ivermectin Research for Malaria Elimination Network: updating the research agenda. Malar J 14:243–250. doi: 10.1186/s12936-015-0691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steketee RW, Ter Kuile FO. 2015. Ivermectin as a complementary strategy to kill mosquitoes and stop malaria transmission? Clin Infect Dis 60:366–368. doi: 10.1093/cid/ciu802. [DOI] [PubMed] [Google Scholar]
- 11.Chaccour C, Lines J, Whitty CJ. 2010. Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans: the potential of oral insecticides in malaria control. J Infect Dis 202:113–116. doi: 10.1086/653208. [DOI] [PubMed] [Google Scholar]
- 12.Foley DH, Bryan JH, Lawrence GW. 2000. The potential of ivermectin to control the malaria vector Anopheles farauti. Trans R Soc Trop Med Hyg 94:625–628. doi: 10.1016/S0035-9203(00)90211-6. [DOI] [PubMed] [Google Scholar]
- 13.Fritz ML, Walker ED, Miller JR. 2012. Lethal and sublethal effects of avermectin/milbemycin parasiticides on the African malaria vector, Anopheles arabiensis. J Med Entomol 49:326–331. doi: 10.1603/ME11098. [DOI] [PubMed] [Google Scholar]
- 14.Kobylinski KC, Deus KM, Butters MP, Hongyu T, Gray M, da Silva IM, Sylla M, Foy BD. 2010. The effect of oral anthelmintics on the survivorship and re-feeding frequency of anthropophilic mosquito disease vectors. Acta Trop 116:119–126. doi: 10.1016/j.actatropica.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouédraogo AL, Bastiaens GJ, Tiono AB, Guelbeogo WM, Kobylinski KC, Ouédraogo A, Barry A, Bougouma EC, Nebie I, Ouattara MS, Lanke KH, Fleckenstein L, Sauerwein RW, Slater HC, Churcher TS, Sirima SB, Drakeley C, Bousema T. 2015. Efficacy and safety of the mosquitocidal drug ivermectin to prevent malaria transmission after treatment: a double-blind, randomized, clinical trial. Clin Infect Dis 60:357–365. doi: 10.1093/cid/ciu797. [DOI] [PubMed] [Google Scholar]
- 16.Sylla M, Kobylinski KC, Gray M, Chapman PL, Sarr MD, Rasgon JL, Foy BD. 2010. Mass drug administration of ivermectin in south-eastern Senegal reduces the survivorship of wild-caught, blood fed malaria vectors. Malar J 9:365–374. doi: 10.1186/1475-2875-9-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobylinski KC, Foy BD, Richardson JH. 2012. Ivermectin inhibits the sporogony of Plasmodium falciparum in Anopheles gambiae. Malar J 11:381–389. doi: 10.1186/1475-2875-11-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panchal M, Rawat K, Kumar G, Kibria KM, Singh S, Kalamuddin M, Mohmmed A, Malhotra P, Tuteja R. 2014. Plasmodium falciparum signal recognition particle components and anti-parasitic effect of ivermectin in blocking nucleo-cytoplasmic shuttling of SRP. Cell Death Dis 5:e994. doi: 10.1038/cddis.2013.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alout H, Krajacich BJ, Meyers JI, Grubaugh ND, Brackney DE, Kobylinski KC, Diclaro JW II, Bolay FK, Fakoli LS, Diabate A, Dabire RK, Bougma RW, Foy BD. 2014. Evaluation of ivermectin mass drug administration for malaria transmission control across different West African environments. Malar J 13:417–426. doi: 10.1186/1475-2875-13-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobylinski KC, Alout H, Foy BD, Clements A, Adisakwattana P, Swierczewski BE, Richardson JH. 2014. Rationale for the coadministration of albendazole and ivermectin to humans for malaria parasite transmission control. Am J Trop Med Hyg 91:655–662. doi: 10.4269/ajtmh.14-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobylinski KC, Sylla M, Chapman PL, Sarr MD, Foy BD. 2011. Ivermectin mass drug administration to humans disrupts malaria parasite transmission in Senegalese villages. Am J Trop Med Hyg 85:3–5. doi: 10.4269/ajtmh.2011.11-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ploemen IH, Prudêncio M, Douradinha BG, Ramesar J, Fonager J, GJ van Gemert Luty AJ, Hermsen CC, Sauerwein RW, Baptista FG, Mota MM, Waters AP, Que I, Lowik CW, Khan SM, Janse CJ, Franke-Fayard BM. 2009. Visualisation and quantitative analysis of the rodent malaria liver stage by real time imaging. PLoS One 4:e7881. doi: 10.1371/journal.pone.0007881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prudêncio M, Rodrigues CD, Ataide R, Mota MM. 2008. Dissecting in vitro host cell infection by Plasmodium sporozoites using flow cytometry. Cell Microbiol 10:218–224. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues T, Prudêncio M, Moreira R, Mota MM, Lopes F. 2012. Targeting the liver stage of malaria parasites: a yet unmet goal. J Med Chem 55:995–1012. doi: 10.1021/jm201095h. [DOI] [PubMed] [Google Scholar]
- 25.Prudêncio M, Mota MM, Mendes AM. 2011. A toolbox to study liver stage malaria. Trends Parasitol 27:565–574. doi: 10.1016/j.pt.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 26.da Cruz FP, Martin C, Buchholz K, Lafuente-Monasterio MJ, Rodrigues T, Sonnichsen B, Moreira R, Gamo FJ, Marti M, Mota MM, Hannus M, Prudêncio M. 2012. Drug screen targeted at Plasmodium liver stages identifies a potent multistage antimalarial drug. J Infect Dis 205:1278–1286. doi: 10.1093/infdis/jis184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vale N, Moreira R, Gomes P. 2009. Primaquine revisited six decades after its discovery. Eur J Med Chem 44:937–953. doi: 10.1016/j.ejmech.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Stanway RR, Witt T, Zobiak B, Aepfelbacher M, Heussler VT. 2009. GFP-targeting allows visualization of the apicoplast throughout the life cycle of live malaria parasites. Biol Cell 101:415–430. doi: 10.1042/BC20080202. [DOI] [PubMed] [Google Scholar]
- 29.Meyers JI, Gray M, Kuklinski W, Johnson LB, Snow CD, Black WC IV, Partin KM, Foy BD. 2015. Characterization of the target of ivermectin, the glutamate-gated chloride channel, from Anopheles gambiae. J Exp Biol 218:1478–1486. doi: 10.1242/jeb.118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin L, Feng X, Rong H, Pan Z, Inaba Y, Qiu L, Zheng W, Lin S, Wang R, Wang Z, Wang S, Liu H, Li S, Xie W, Li Y. 2013. The antiparasitic drug ivermectin is a novel FXR ligand that regulates metabolism. Nat Commun 4:1937–1944. doi: 10.1038/ncomms2924. [DOI] [PubMed] [Google Scholar]
- 31.Meireles P, Sales-Dias J, Andrade CM, Mello-Vieira J, Mancio-Silva L, Simas JP, Staines HM, Prudêncio M. 12 July 2016. GLUT1-mediated glucose uptake plays a crucial role during Plasmodium hepatic infection. Cell Microbiol doi: 10.1111/cmi.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slater HC, Walker PG, Bousema T, Okell LC, Ghani AC. 2014. The potential impact of adding ivermectin to a mass treatment intervention to reduce malaria transmission: a modelling study. J Infect Dis 210:1972–1980. doi: 10.1093/infdis/jiu351. [DOI] [PubMed] [Google Scholar]
- 33.Chaccour C, Barrio A, Gil Royo AG, Martinez Urbistondo D, Slater H, Hammann F, Del Pozo JL. 2015. Screening for an ivermectin slow-release formulation suitable for malaria vector control. Malar J 14:102–110. doi: 10.1186/s12936-015-0618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruña-Romero O, Hafalla JC, Gonzalez-Aseguinolaza G, Sano G, Tsuji M, Zavala F. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int J Parasitol 31:1499–1502. doi: 10.1016/S0020-7519(01)00265-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.