ABSTRACT
Staphylococcal prosthetic joint infections (PJIs) are associated with biofilm formation, making them difficult to treat; if managed with debridement and implant retention, rifampin-based therapy is usually employed. Rifampin resistance potentially challenges PJI treatment. In investigating the effects of rifampin monotherapy on methicillin-resistant Staphylococcus aureus (MRSA) foreign-body osteomyelitis in rats, we previously demonstrated that rifampin resistance was selected but that it disappeared 14 days following rifampin monotherapy (1) and that rifampin resistance occurred less frequently following two rounds than following one round of rifampin monotherapy (2). Here, we compared rifampin monotherapy followed by rifampin-vancomycin combination therapy to rifampin-vancomycin combination therapy alone in experimental MRSA foreign-body osteomyelitis. Animals treated with rifampin monotherapy followed by rifampin-vancomycin combination therapy had decreased quantities of bacteria 14 days following treatment completion (P = 0.034) compared to those in animals treated with combination therapy alone. Additionally, some isolates recovered from animals treated with combination therapy alone, although still susceptible to rifampin, had higher MIC, minimum biofilm-inhibitory concentration (MBIC), and minimum biofilm-bactericidal concentration (MBBC) values than those of the inoculating strain. This suggests that rifampin may remain a feasible treatment option in foreign-body-associated orthopedic infections following the selection of rifampin resistance.
KEYWORDS: MRSA, rifampin resistance, osteomyelitis, prosthetic joint infection
INTRODUCTION
As the general population ages and life expectancy increases, surgical procedures such as placement of artificial hips, knees, and other joints are commonly performed (3). In 2010, there were approximately 719,000 knee and 332,000 hip arthroplasty procedures done in the United States alone (4). By 2030, the numbers of procedures are expected to increase to over 570,000 and 3.5 million for hips and knees, respectively (5). Unfortunately, prosthetic joint infection (PJI) affects 1 to 2% of prosthetic joints (6, 7). Along with the associated morbidity, the economic burden of PJI is large. Hip- and knee-related PJI had an annual financial load of $320 million in 2001, which increased to $566 million in 2009 and is expected to surpass $1.62 billion by 2020 (6). Staphylococcus aureus and coagulase-negative staphylococci comprise more than half of the organisms causing PJI (7, 8). The surfaces of these implants are conducive to bacterial biofilm formation, complicating treatment. Within biofilms, bacteria grow slowly and evade both host defenses and antimicrobial treatment, being severalfold more resistant to most antimicrobial therapies than their planktonic counterparts (9). Bacterial biofilms are enshrouded by a polymeric matrix composed of nucleic acids, polysaccharides, and/or proteins, which also contributes to resistance to some antimicrobial agents (8). Finally, the area around foreign bodies has decreased microcirculation, which leads to decreased antimicrobial delivery and impairs host defenses (8).
Treatment of PJI is notoriously difficult, time-consuming, and expensive. Management may involve debridement, antimicrobials, and implant retention (DAIR) (9). In spite of medical advances, the rate of failure of PJI treatment remains 10 to 20% (10). Therapy against staphylococcal PJIs is well defined and, when managed by DAIR, almost always involves the use of rifampin due to its bactericidal activity against slow-growing staphylococci (8, 11, 12). Unfortunately, rifampin resistance, caused by one of several single-base mutations in the RNA polymerase rpoB, is easily selected; therefore, rifampin is always administered in conjunction with another antimicrobial agent, and rifampin resistance potentially challenges the use of this otherwise recommended regimen.
We previously observed the emergence and subsequent “disappearance” of rifampin resistance in a rat foreign-body osteomyelitis model (1). Additionally, we recently reported that following two rounds of rifampin monotherapy, rifampin resistance is detected at a lower frequency than after a single round (2). This suggests that resistance may not be durable and that treatment with a rifampin-based regimen may be possible, even if rifampin-resistant bacteria were previously selected and detected at an infected site. To investigate the feasibility of treating infections where rifampin resistance was previously selected with a rifampin-based regimen, we compared the outcomes of treatment of foreign-body osteomyelitis with rifampin monotherapy followed by rifampin-based combination therapy to those of treatment with rifampin-based combination therapy alone.
RESULTS
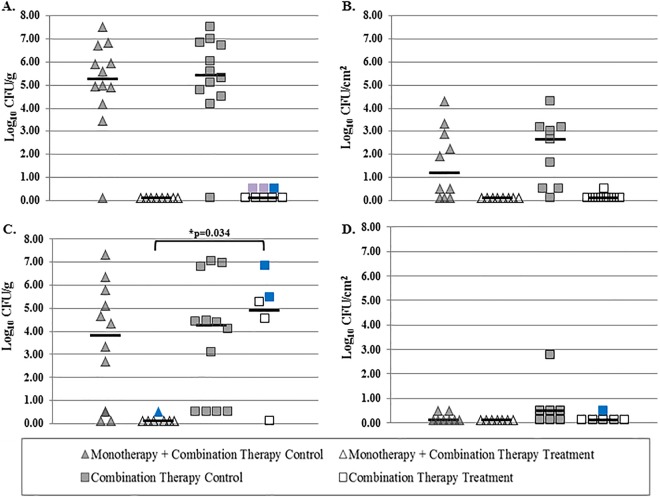
Quantities of bacteria from bones and wires in each treatment group 2 and 14 days following treatment completion are shown in Fig. 1. Rifampin monotherapy followed by combination therapy yielded the lowest level of recovery of bacteria for the two treatment groups.
FIG 1.
(A and B) Quantities of bacteria recovered from bone (A) and wire (B) 2 days following treatment completion. (C and D) Quantities of bacteria recovered from bone (C) and wire (D) 14 days following treatment completion. Rifampin-resistant bacteria are marked in blue, and bacteria with elevated but still susceptible rifampin MICs are marked in purple.
Rifampin monotherapy followed by rifampin-vancomycin combination therapy.
Animals sacrificed 2 days following treatment completion did not have growth on bones or wires. Animals sacrificed 14 days following treatment completion did not have growth on quantitative cultures but had qualitative growth on one animal's bone, which was resistant to rifampin.
Rifampin-vancomycin combination therapy.
Animals sacrificed 2 days following treatment completion had no growth on quantitative cultures but had qualitative growth from 3/8 bones and on 1/8 wires. Bacteria from two of the bones and one wire were susceptible to rifampin; bacteria recovered from the remaining bone were resistant to rifampin. Animals sacrificed 14 days following treatment completion had median bacterial quantities of 4.9 log10 CFU/g of bone and 0.1 log10 CFU/cm2 of wire. MRSA was recovered from 4/6 animal bones and from 1/6 implanted wires. Two animals were not studied further because of contamination, although no S. aureus bacteria were detected. Rifampin resistance was identified in bacteria recovered from two bones and one wire; bacteria from the remaining bones were rifampin susceptible. There was a decrease in the amount of bacteria recovered from the bones of animals 14 days following treatment completion when animals were treated with monotherapy followed by combination therapy compared with those receiving combination therapy alone (0.10 versus 4.26 log10 CFU/g bone; P = 0.034).
There were no differences in bacterial quantities recovered from animals in both control groups 2 days and 14 days following treatment (P = 0.73 and P = 0.77 for bone 2 and 14 days following treatment, respectively, and P = 0.39 and P = 0.18 for wires 2 and 14 days following treatment, respectively).
Calculation of MIC, MBIC, and MBBC values.
MIC, minimum biofilm-inhibitory concentration (MBIC), and minimum biofilm-bactericidal concentration (MBBC) values for rifampin were determined for the parental strain as well as for isolates recovered from the animals 2 and 14 days following treatment completion (Table 1). The parental strain, IDRL-6169, had a rifampin MIC of ≤0.125 μg/ml, an MBIC of ≤0.125 μg/ml, and an MBBC of 4 μg/ml. Isolates that were resistant to rifampin (based on plating on Trypticase soy agar [TSA] with 4 μg/ml rifampin) demonstrated MIC values that were above the 1-μg/ml breakpoint for rifampin (13), MBIC values ranging from 16 to >128 μg/ml, and MBBC values of >128 μg/ml. Isolates that were susceptible to rifampin had MIC values ranging from ≤0.125 to 1 μg/ml, MBIC values ranging from ≤0.125 to 2 μg/ml, and MBBC values ranging from 1 to >128 μg/ml.
TABLE 1.
Rifampin MIC, MBIC, and MBBC values of the parental strain (IDRL-6169) and isolates recovered from animalsa
| Isolate | Posttreatment isolation day | Rifampin resistance detected on plate | MIC (μg/ml) | MBIC (μg/ml) | MBBC (μg/ml) |
|---|---|---|---|---|---|
| IDRL-6169 | Parent strain | N | ≤0.125 | ≤0.125 | 4 |
| 20-2 B | 2 | N | 1 | 2 | >128 |
| 21-2 B | 2 | N | 1 | 2 | >128 |
| 21-2 W | 2 | N | ≤0.125 | ≤0.125 | 1 |
| 22-2 B | 2 | Y | 8 | 32 | >128 |
| 7-1 B | 14 | Y | 16 | 16 | >128 |
| 17-1 B | 14 | Y | 16 | 16 | >128 |
| 17-1 W | 14 | N | ≤0.125 | ≤0.125 | 2 |
| 17-2 B | 14 | N | ≤0.125 | ≤0.125 | 1 |
| 18-2 B | 14 | N | ≤0.125 | ≤0.125 | 1 |
| 24-2 B | 14 | Y | 32 | >128 | >128 |
All isolates were recovered from animals receiving combination therapy only, with the exception of 7-1 B, which was recovered from an animal receiving rifampin monotherapy followed by rifampin-vancomycin combination therapy. B, isolate recovered from bone; W, isolate recovered from implanted wire; N, no; Y, yes.
DISCUSSION
Previous experiments demonstrated that rifampin resistance was undetectable 14 days following the completion of a course of rifampin monotherapy (1). This interesting observation led us to show that two rounds of rifampin monotherapy do not lead to an increase in resistant bacteria and, thus, treatment failure (2). Rifampin resistance was actually observed to emerge at a lower frequency following the second round of treatment. With this in mind, we sought to compare treatment outcomes in animals treated with rifampin monotherapy followed by rifampin-vancomycin combination therapy to those in animals treated with rifampin-vancomycin combination therapy alone.
Bacteria were recovered from the bone of just one animal 14 days following treatment completion in the group treated with rifampin monotherapy followed by rifampin-vancomycin combination therapy. All other bones and wires 2 and 14 days following treatment completion were culture negative. Conversely, animals treated with rifampin-vancomycin combination therapy alone had qualitative bacterial growth in only three bones and one wire 2 days after treatment completion; rifampin resistance was detected in bacteria from one bone, whereas bacteria recovered from the other two bones and one wire were susceptible to rifampin. Quantitative and qualitative growth of bacteria was observed for four bones and qualitative growth alone was observed for one wire 14 days following treatment completion; rifampin resistance was detected in bacteria recovered from 2/4 bones and 1/1 wire.
Combination therapy with rifampin and vancomycin was associated with recovery of bacteria 14 days following treatment completion, suggesting that, in accordance with data from previous studies (1, 14), this often-used treatment regimen may not be ideal. However, our treatment duration was shorter than that which is typically used clinically.
Rifampin MICs, MBICs, and MBBCs corresponded to the absence or presence of colonies on plates containing 4 μg/ml rifampin. Interestingly, there were two isolates (20-2 B and 21-2 B), recovered 2 days following the completion of treatment with combination therapy alone, with 3-fold-higher MICs, 4-fold-higher MBICs, and >10-fold higher MBBCs than those of the parental strain, although they were still susceptible to rifampin. The mechanism underlying this finding is unclear.
With rifampin resistance being so easily acquired, rifampin monotherapy is never used. We initially believed that treatment with rifampin should be avoided if rifampin-resistant bacteria had been detected previously. However, results of this study as well as our previous data (2) suggest that rifampin resistance may not be durable and that rifampin-based combination therapy can be effective even if rifampin-resistant bacteria were previously selected by rifampin exposure. This may expand treatment options utilizing rifampin, which is a cornerstone drug in the treatment of staphylococcal biofilm-associated infections. Further work is needed to identify possible bacterial mutations occurring over the course of treatment that may be exploited to inform treatment options.
Interestingly, we also observed rifampin resistance in bacteria recovered from 2/4 bones and 1/1 wire 14 days following the completion of combination therapy alone. In previous experiments utilizing rifampin monotherapy, resistance was always observed 2 days following treatment completion but was no longer detectable 14 days following treatment completion. When we used rifampin-vancomycin combination therapy, we detected rifampin resistance 14 days following treatment completion, suggesting that combination therapy may prevent susceptible bacteria from outcompeting resistant bacteria, a result which we observed previously (2).
One limitation of this study is that the infection times for the two treatment groups were different. There is, unfortunately, no way to compare quantities of bacteria recovered at identical time points when the treatment approaches studied involve different treatment durations. Another limitation is the assumed rifampin resistance following rifampin monotherapy in the monotherapy/combination therapy treatment group. In this study, bacteria were not harvested following rifampin monotherapy before proceeding to rifampin-vancomycin combination therapy to confirm that rifampin resistance occurred. However, rifampin resistance during rifampin monotherapy has been widely reported (15), and we have shown in two independent studies that rifampin resistance emerges after monotherapy (1, 2). Another limitation is that previous work suggested that the combination of vancomycin and rifampin may be antagonistic during treatment of MRSA biofilms; however, that work either was done only in vitro (16) or investigated the effects of combination treatment on infections other than PJIs. The Infectious Diseases Society of America (IDSA) recommends the use of vancomycin-rifampin combination therapy for the treatment of PJIs caused by MRSA (17).
The results of this study suggest that rifampin-based combination therapy may be effective, even after rifampin resistance was selected for previously. Rifampin monotherapy followed by rifampin-vancomycin combination therapy was more effective than rifampin-vancomycin combination therapy alone in decreasing quantities of bacteria in a rat model of foreign-body osteomyelitis. This work, combined with our previous work (2), informs treatment options that utilize rifampin for the treatment of PJIs.
MATERIALS AND METHODS
Microorganisms.
IDRL-6169, an MRSA isolate previously recovered from a patient with a prosthetic hip infection, was studied.
Antimicrobial agents.
Lyophilized rifampin for intravenous administration (Fresenius Kabi, Lake Zurich, IL) was obtained from the Mayo Clinic Pharmacy and resuspended in 10 ml sterile water according to the manufacturer's instructions to make a stock concentration of 60 mg/ml. Lyophilized vancomycin hydrochloride for injection (Mylan Institutional, Rockford, IL) was obtained from the Mayo Clinic Pharmacy and resuspended in 20 ml sterile water according to the manufacturer's instructions to make a stock concentration of 50 mg/ml.
Experimental rat model.
Chronic foreign-body osteomyelitis was established in male Wistar rats (Charles River Laboratories, Wilmington, MA) weighing 250 to 300 g, as previously described (2, 18). Briefly, general anesthesia was induced by the intramuscular administration of ketamine (60 mg/kg of body weight) and xylazine (6 mg/kg). The left leg of each animal was shaved and prepared with 4% chlorhexidine gluconate (Hibiclens; Mölnlycke Health Care, Norcross, GA). Under sterile conditions, the proximal one-third of the left tibia was surgically exposed, and a 1.5-mm hole was drilled into the medullary cavity. Fifty microliters of a suspension containing 106 CFU of MRSA was injected into the bone. Subsequently, a 5-mm by 1-mm stainless steel wire (Zimmer, Warsaw, IN) was implanted into the bone. The hole was covered with dental gypsum, and the muscle was closed with sized 3-0 vicryl (Ethicon Inc., Somerville, NJ). The skin was closed with Tissuemend II (VPL, Phoenix, AZ) and surgical clips. The wound was sprayed with AluSpray (Neogen Corporation, Lansing, MI) and Chew Guard (Summit Hill Laboratories, Tinton Falls, NJ). Buprenorphine (slow release; 60 mg/kg) and meloxicam (slow release; 2 mg/kg) were administered subcutaneously for analgesia. The study was approved by the Mayo Clinic Institutional Animal Care and Use Committee.
Antimicrobial treatment with rifampin monotherapy followed by rifampin-vancomycin combination therapy.
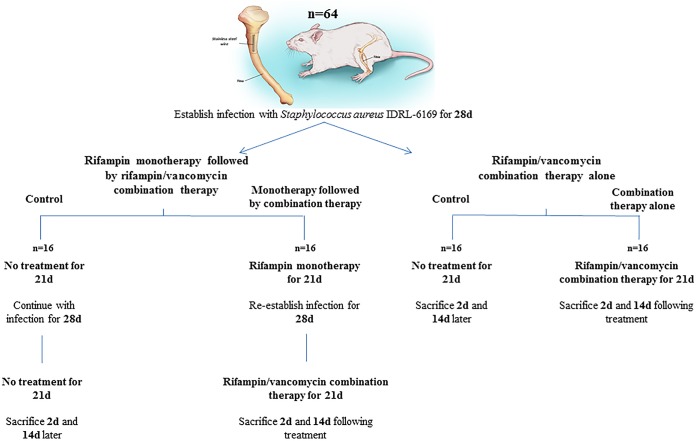
Following a 4-week infection period, animals were treated with rifampin (25 mg/kg intraperitoneally every 12 h) for 21 days. Animals were then left untreated for 28 days, after which they were administered a combination of rifampin (25 mg/kg intraperitoneally every 12 h) and vancomycin (50 mg/kg intraperitoneally every 12 h) for an additional 21 days. The animals were sacrificed 2 or 14 days following treatment completion. Control animals receiving no treatment were infected alongside treated animals and sacrificed at the same time points (Fig. 2).
FIG 2.
Treatment schematic.
Antimicrobial treatment with rifampin-vancomycin combination therapy only.
Following a 4-week infection period, animals were treated with rifampin (25 mg/kg intraperitoneally every 12 h) and vancomycin (50 mg/kg intraperitoneally every 12 h) for 21 days and were sacrificed 2 or 14 days following treatment completion. Control animals receiving no treatment were infected alongside treated animals and sacrificed at the same time points.
Quantification of bacteria.
Animals were sacrificed as previously described (2), using CO2, and the left tibias were aseptically removed and frozen at −70°C. Bone was cut within 5 mm of the implanted stainless steel wire, weighed, and pulverized to separate the bone and wire. Pulverized bone and wire were separately suspended in 2 and 1 ml, respectively, of Trypticase soy broth (TSB), vortexed for 30s, sonicated at 40 kHz for 5 min, vortexed for 30 s, serially diluted, and plated onto both Trypticase soy agar plates containing 5% sheep blood (TSA II; Becton Dickinson, Franklin Lakes, NJ) and TSA plates containing 4 μg/ml rifampin for quantitative culture. Quantitative culture results for bone and wire on blood agar plates were recorded after incubation for 2 days at 37°C and expressed as log10 CFU per gram of bone or log10 CFU per square centimeter of wire surface. Qualitative cultures were performed by incubating TSB containing pulverized bone or wire for 24 h at 37°C followed by subculturing to assess for MRSA.
Calculation of MIC, MBIC, and MBBC values.
The MIC, MBIC, and MBBC values were determined by using the parental strain as well as the isolates recovered from the bones and wires of the animals 14 days following treatment completion. One isolate was recovered from an animal that received rifampin monotherapy followed by rifampin-vancomycin combination therapy (isolate 7-1 B). All other isolates were recovered from animals that had received combination therapy only. The MIC was determined by broth microdilution according to Clinical and Laboratory Standards Institute (CLSI) guidelines (13). A modification (19) of a previously described biofilm assay (20) was used to determine the rifampin MBIC and the MBBC. Tested concentrations of rifampin ranged from 128 to ≤0.125 μg/ml. The MIC, MBIC, and MBBC were defined as the lowest concentrations without turbidity.
Statistical methods.
Statistical analysis was performed as previously described (2). For statistical purposes, the absence of any growth was assigned a value of 0.1 log10 CFU per g of bone or per cm2 of wire. Growth in qualitative broth culture (but not in quantitative cultures) was assigned a value of 0.5 log10 CFU per g of bone or per cm2 of wire. Comparisons of growth on bone and wire between treatment groups were performed by using the Wilcoxon rank sum test. With a sample size of 8 animals per treatment group, a difference of at least 1.5 standard deviations between results of quantitative cultures in two groups was anticipated to yield 80% power (based on a two-sample t test). Analysis was performed by using SAS version 9.3 (SAS Inc., Cary, NC). All tests were two sided; P values of <0.05 were considered significant.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases under award number R21 AI125870 and the National Institute of Arthritis and Musculoskeletal and Skin Diseases for the Musculoskeletal Research Training Program under award number T32 AR56950. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Vergidis P, Schmidt-Malan SM, Mandrekar JN, Steckelberg JM, Patel R. 7 January 2015. Comparative activities of vancomycin, tigecycline and rifampin in a rat model of methicillin-resistant Staphylococcus aureus osteomyelitis. J Infect doi: 10.1016/j.jinf.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Brinkman CL, Tyner HL, Schmidt-Malan SM, Mandrekar JN, Patel R. 2015. Causes and implications of the disappearance of rifampin resistance in a rat model of methicillin-resistant Staphylococcus aureus foreign body osteomyelitis. Antimicrob Agents Chemother 59:4481–4488. doi: 10.1128/AAC.05078-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shuman EK, Urquhart A, Malani PN. 2012. Management and prevention of prosthetic joint infection. Infect Dis Clin North Am 26:29–39. doi: 10.1016/j.idc.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2010. National Hospital Discharge Survey: table, procedures by selected patient characteristics. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf Accessed 20 May 2015. [Google Scholar]
- 5.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. 2007. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 6.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. 2012. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 27:61.e1–65.e1. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Tande AJ, Patel R. 2014. Prosthetic joint infection. Clin Microbiol Rev 27:302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N Engl J Med 351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 9.Esposito S, Leone S. 2008. Prosthetic joint infections: microbiology, diagnosis, management and prevention. Int J Antimicrob Agents 32:287–293. doi: 10.1016/j.ijantimicag.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Berbari EF, Baddour LM. 2016. Epidemiology and prevention of prosthetic joint infections. In Sexton DJ. (ed), UpToDate. UpToDate, Waltham, MA: www.uptodate.com/contents/epidemiology-and-prevention-of-prosthetic-joint-infections. [Google Scholar]
- 11.Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. 1998. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA 279:1537–1541. doi: 10.1001/jama.279.19.1537. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerli W. 2006. Infection and musculoskeletal conditions: prosthetic-joint-associated infections. Best Pract Res Clin Rheumatol 20:1045–1063. doi: 10.1016/j.berh.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing: twenty-sixth informational supplement. CLSI document M100-S26, p 82 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.Saleh-Mghir A, Dumitrescu O, Dinh A, Boutrad Y, Massias L, Martin E, Vandenesch F, Etienne J, Lina G, Cremieux AC. 2012. Ceftobiprole efficacy in vitro against Panton-Valentine leukocidin production and in vivo against community-associated methicillin-resistant Staphylococcus aureus osteomyelitis in rabbits. Antimicrob Agents Chemother 56:6291–6297. doi: 10.1128/AAC.00926-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loeffler AM. 1999. Uses of rifampin for infections other than tuberculosis. Pediatr Infect Dis J 18:631–632. doi: 10.1097/00006454-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Salem AH, Elkhatib WF, Noreddin AM. 2011. Pharmacodynamic assessment of vancomycin-rifampicin combination against methicillin resistant Staphylococcus aureus biofilm: a parametric response surface analysis. J Pharm Pharmacol 63:73–79. doi: 10.1111/j.2042-7158.2010.01183.x. [DOI] [PubMed] [Google Scholar]
- 17.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 18.Vergidis P, Rouse MS, Euba G, Karau MJ, Schmidt SM, Mandrekar JN, Steckelberg JM, Patel R. 2011. Treatment with linezolid or vancomycin in combination with rifampin is effective in an animal model of methicillin-resistant Staphylococcus aureus foreign body osteomyelitis. Antimicrob Agents Chemother 55:1182–1186. doi: 10.1128/AAC.00740-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velez Perez AL, Schmidt-Malan SM, Kohner PC, Karau MJ, Greenwood-Quaintance KE, Patel R. 2016. In vitro activity of ceftolozane/tazobactam against clinical isolates of Pseudomonas aeruginosa in the planktonic and biofilm states. Diagn Microbiol Infect Dis 85:356–359. doi: 10.1016/j.diagmicrobio.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Frank KL, Reichert EJ, Piper KE, Patel R. 2007. In vitro effects of antimicrobial agents on planktonic and biofilm forms of Staphylococcus lugdunensis clinical isolates. Antimicrob Agents Chemother 51:888–895. doi: 10.1128/AAC.01052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]