ABSTRACT
Doravirine is a novel, highly potent, nonnucleoside reverse transcriptase inhibitor that is administered once daily and that is in development for the treatment of HIV-1 infection. In vitro and clinical data suggest that doravirine is unlikely to cause significant drug-drug interactions via major drug-metabolizing enzymes or transporters. As a common HIV-1 infection comorbidity, hypercholesterolemia is often treated with statins, including the commonly prescribed atorvastatin. Atorvastatin is subject to drug-drug interactions with cytochrome P450 3A4 (CYP3A4) inhibitors. Increased exposure due to CYP3A4 inhibition may lead to serious adverse events (AEs), including rhabdomyolysis. Furthermore, atorvastatin is a substrate for breast cancer resistance protein (BCRP), of which doravirine may be a weak inhibitor; this may increase atorvastatin exposure. The potential of doravirine to affect atorvastatin pharmacokinetics was investigated in a two-period, fixed-sequence study in healthy individuals. In period 1, a single dose of atorvastatin at 20 mg was administered followed by a 72-h washout. In period 2, doravirine at 100 mg was administered once daily for 8 days, with a single dose of atorvastatin at 20 mg concomitantly being administered on day 5. Sixteen subjects were enrolled, and 14 completed the trial; 2 discontinued due to AEs unrelated to the treatment. The atorvastatin area under the curve from time zero to infinity was similar with and without doravirine (geometric mean ratio [GMR] for doravirine-atorvastatin/atorvastatin, 0.98; 90% confidence interval [CI], 0.90 to 1.06), while the maximum concentration decreased by 33% (GMR for doravirine-atorvastatin/atorvastatin, 0.67; 90% CI, 0.52 to 0.85). These changes were deemed not to be clinically meaningful. Both of the study drugs were generally well tolerated. Doravirine had no clinically relevant effect on atorvastatin pharmacokinetics in healthy subjects, providing support for the coadministration of doravirine and atorvastatin.
KEYWORDS: CYP3A4, atorvastatin, doravirine, human immunodeficiency virus, hypercholesterolemia, pharmacokinetics
INTRODUCTION
Doravirine (MK-1439) is a novel, highly potent nonnucleoside reverse transcriptase inhibitor (NNRTI) that is administered once daily (QD) and that is in development for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in combination with existing antiretroviral therapies (ARTs). To date, doravirine has been demonstrated to have both good tolerability and efficacy in a number of clinical trials (1–3). When doravirine was administered at 25 to 200 mg QD in combination with tenofovir disoproxil fumarate-emtricitabine, robust and sustained viral load suppression was demonstrated through 48 weeks in a phase 2 trial in HIV-1-infected ART-naive patients (2). Furthermore, in the above-described study (2), doravirine at 100 mg induced fewer treatment-emergent central nervous system adverse events (AEs) than the commonly prescribed NNRTI efavirenz, an antiviral therapy for HIV-1 infection included in the World Health Organization's Model List of Essential Medicines (4). On the basis of these promising findings, clinical evaluation of 100 mg doravirine is continuing in ongoing phase 3 trials.
Doravirine is primarily metabolized by hepatic cytochrome P450 3A4 (CYP3A4) (5). In addition, doravirine does not inhibit or induce CYP enzymes, including CYP3A4, or inhibit major drug transporters in vitro, suggesting that it has a low potential to cause clinically meaningful drug-drug interactions (5). Consistent with in vitro data, clinical studies have demonstrated that doravirine does not perpetrate clinically meaningful drug interactions with substrates for CYP3A4, breast cancer resistance protein (BCRP), organic cation transporter member 2 (OCT2), organic anion transporter 1 (OAT1), and OAT3 (1, 6, 7; data on file).
Patients infected with HIV-1 frequently concomitantly receive a variety of medications, so understanding the drug-drug interaction profiles for any emerging ART is of great importance. HIV infection is now considered a chronic disease, and as the upper age range of the patient base increases, the prevalence of comorbid conditions will increase, suggesting an expanded need for the concomitant use of medications (8, 9). The complex mixture of comorbidities and associated medications drives the need to understand potential drug-drug interactions and how treatment will be affected.
Hypercholesterolemia is a significant comorbidity of HIV and is exacerbated when it occurs in combination with ARTs (10–12). 3-Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors are a mainstay in treatment, with atorvastatin being a commonly prescribed agent. Atorvastatin undergoes hepatic metabolism and is a substrate of the hepatic uptake transporter organic anion-transporting polypeptide 1B1 (OATP1B1); P-glycoprotein (P-gp), also known as multidrug resistance protein 1 (MDR1); and BCRP (13, 14). Similarly to doravirine, atorvastatin is metabolized by CYP3A4 (13), and CYP3A4 modulators have demonstrated a marked effect on its pharmacokinetics (PK) (15). Despite this shared pathway, atorvastatin is not anticipated to alter the PK of CYP3A4 substrates significantly. The lack of modulation induced by atorvastatin was demonstrated using the 4β-hydroxycholesterol/cholesterol ratio, a marker of CYP3A4 activity (16). The ratio was shown to decrease by only 13% following chronic treatment with atorvastatin at 20 mg, and these results, in addition to others obtained with drugs that are CYP3A4 substrates, indicate weak/moderate inhibition of CYP3A4 by atorvastatin (16–18). On the basis of their individual profiles, coadministration of doravirine and atorvastatin is not projected to result in clinically meaningful alterations to the PK of either drug. As doravirine neither significantly induces nor inhibits CYP3A4, a clinically meaningful modulation of CYP3A4 activity is unlikely (1). In a previous drug-drug interaction study, doravirine slightly increased the area under the concentration-time curve (AUC) and the maximum concentration (Cmax) of dolutegravir when doravirine was administered at a high dose (200 mg) (7). This slight alteration in dolutegravir PK was likely due to inhibition of intestinal BCRP in a concentration-dependent interaction. Therefore, there is a potential for doravirine to inhibit BCRP and increase the level of exposure to atorvastatin, though this is considered unlikely at the clinical dose of doravirine used, 100 mg. Given that doravirine is a weak BCRP inhibitor (half-maximal inhibitory concentration, 51 μM), any drug interaction with BCRP substrates is anticipated to be limited to the gut. While BCRP is also a key transporter for the liver canalicular membrane, systemic doravirine concentrations are expected to be about 15-fold below the half-maximal inhibitory concentration, too low to have an inhibitory effect on liver BCRP and therefore unlikely to have an effect on the PK of BCRP substrates, such as atorvastatin.
Atorvastatin has a relatively complex disposition profile, and significant AEs, including rhabdomyolysis, may occur in the presence of high atorvastatin concentrations caused by drug-drug interactions. Due to the concern of myopathy with increased concentrations, a drug-drug interaction study was conducted to confirm the lack of a clinically meaningful effect of multiple-dose doravirine on the PK of atorvastatin. As atorvastatin is not anticipated to alter the PK of doravirine, the effect of atorvastatin on doravirine was not investigated in this trial.
RESULTS
Study population.
A total of 16 subjects were enrolled in the study. The demographic characteristics of the subjects are listed in Table 1. Of the 16 subjects, 14 completed the trial and 2 discontinued due to AEs. One subject discontinued while receiving atorvastatin due to back pain, headache, and feeling feverish in period 1. Another subject discontinued due to fever prior to doravirine dosing in period 2. All of the AEs affecting the two subjects who discontinued were deemed to be not related to the treatment.
TABLE 1.
Study population disposition and demographic characteristicsa
| Characteristic | Value |
|---|---|
| No. (%) of subjects enrolled | 16 (100) |
| Mean (range) age (yr) | 33 (20–45) |
| No. (%) of subjects by sex | |
| Male | 8 (50) |
| Female | 8 (50) |
| No. (%) of subjects by race | |
| Black or African-American | 12 (75) |
| White | 4 (25) |
| Mean ± SD wt (kg) | 74.1 ± 11.6 |
| Mean ± SD ht (cm) | 172.7 ± 10.4 |
| Mean ± SD BMI (kg/m2) | 24.8 ± 3.0 |
| No. (%) of subjects who: | |
| Completed the trial | 14 (87.5) |
| Discontinued the trial | 2 (12.5) |
AE, adverse event; BMI, body mass index.
Plasma PK of atorvastatin.
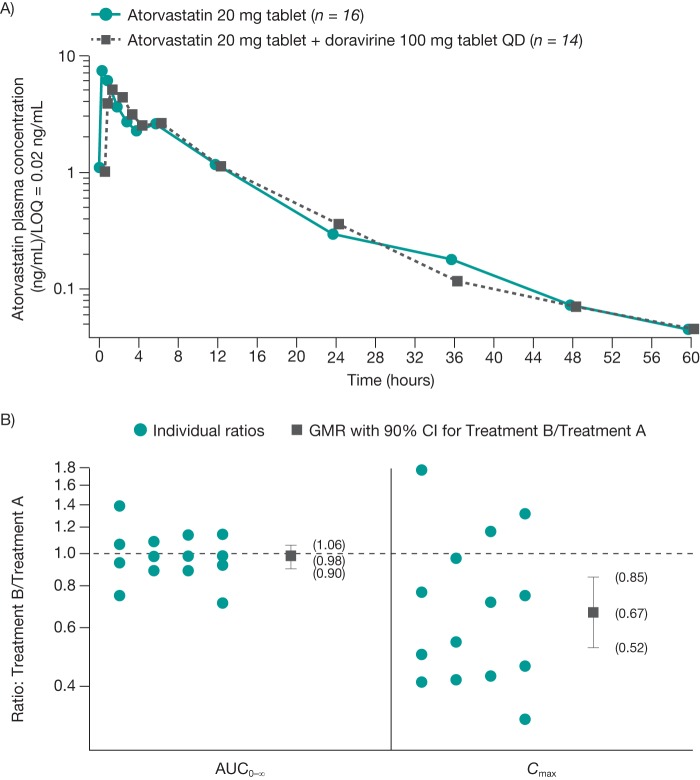
The mean atorvastatin concentration-time profiles after atorvastatin administration in the absence or presence of doravirine are shown in Fig. 1A. At 60 h postdosing, the atorvastatin concentration was below the limit of detection for 1 subject in the doravirine arm and 3 subjects in the concomitant doravirine and atorvastatin arm. The individual ratios, geometric mean ratio (GMR), and 90% confidence intervals (CIs) for the atorvastatin AUC from time zero to infinity (AUC0–∞) and Cmax are shown in Fig. 1B. Summary statistics for atorvastatin PK are shown in Table 2. The atorvastatin AUC0–∞, AUC from time zero to the last measurement (AUC0–last), apparent terminal elimination half-life (t1/2), apparent total clearance (CL/F), and apparent volume of distribution (V/F) were similar when atorvastatin was administered alone or with doravirine, though the atorvastatin Cmax decreased by 33% after coadministration with doravirine. While the median time to Cmax (Tmax) for atorvastatin was essentially unchanged by coadministration with doravirine, increasing by 30 min, individual Tmax values suggested that a delay occurred for some subjects. Achievement of steady-state concentrations for doravirine was not assessed in this study. However, doravirine demonstrates linear PK and has a terminal half-life of approximately 15 h (1); therefore, steady-state exposure is anticipated to be reached following 3 days of dosing. Thus, assessment of atorvastatin PK on day 5 of once-daily doravirine dosing is appropriate, as steady-state conditions for doravirine are achieved at this time.
FIG 1.
Mean ± standard deviation concentration-time profiles of atorvastatin (A) and individual atorvastatin ratios and geometric mean ratios (B) after administration of a single dose of atorvastatin at 20 mg alone and following administration of doravirine at 100 mg once daily for 5 days in healthy subjects. Treatment A, atorvastatin alone; treatment B, atorvastatin plus doravirine. AUC0–∞, area under the curve from time zero to infinity; CI, confidence interval; Cmax, maximum concentration; GMR, geometric mean ratio; LOQ, limit of quantification; PK, pharmacokinetics; QD, once daily.
TABLE 2.
Atorvastatin PK following a single dose of atorvastatin at 20 mg administered alone and administered following treatment with doravirine at 100 mg QD for 5 days in healthy subjectsa
| Treatment | GM or GMRb (95% CI) |
Median (range) Tmax (h) | GM (% CVc) |
||||
|---|---|---|---|---|---|---|---|
| AUC0–∞ (ng · h/ml) | AUC0–last (ng · h/ml) | Cmax (ng/ml) | t1/2 (h) | CL/F (liters/h) | V/F (liters) | ||
| Atorvastatin alone (n = 16)d | 39.9 (32.0–49.8) | 39.2 (31.4–49.0) | 7.87 (5.93–10.4) | 0.5 (0.5–1.0) | 11.1 (28.2) | 501 (43.5) | 7,990 (58.2) |
| Atorvastatin + doravirine (n = 14)e | 39 (31.2–48.8) | 38.2 (30.4–48.0) | 5.25 (3.54–7.78) | 1 (0.5–6.0) | 10.8 (38.3) | 508 (44.9) | 7,910 (69.6) |
| Atorvastatin + doravirine/atorvastatin alone | 0.98 (0.90–1.06) | 0.97 (0.90–1.06) | 0.67 (0.52–0.85) | ||||
AUC0–∞, area under the curve from time zero to infinity; AUC0-last, area under the curve from time zero to the last measurement; CI, confidence interval; CL/F, apparent total clearance; Cmax, maximum concentration; CV, coefficient of variation; GM, geometric mean; GMR, geometric mean ratio; Tmax, time to Cmax; t1/2, elimination half-life; V/F, apparent volume of distribution.
Data for atorvastatin alone and atorvastatin plus doravirine are geometric means, and data for atorvastatin plus doravirine/atorvastatin alone are geometric mean ratios. The data represent the back-transformed least-squares means (95% CIs) from the linear mixed-effects model performed on natural log-transformed values. For AUC0–∞, AUC0–last, and Cmax, the intrasubject coefficients of variation were 12.1, 12.6, and 36, respectively.
Percent CV was calculated as 100 · [exp(s2) − 1], where s2 is the observed between-subject variance on the natural log scale.
In both periods, single oral doses of atorvastatin at 20 mg were administered following an overnight fast.
Multiple oral doses of doravirine at 100 mg were administered QD for 8 consecutive days with coadministration of a single oral dose of atorvastatin at 20 mg on day 5. With the exception of day 5 in period 2, a light breakfast was given approximately 30 min prior to doravirine administration.
Safety.
The study drugs were generally well tolerated, and no serious AEs occurred. Eight (50%) subjects reported a total of 19 AEs, 4 of which were considered by the investigators to be related to the study medication. The most commonly reported AEs were abdominal pain, back pain, and headache, each of which was reported by two subjects. Two subjects discontinued treatment due to AEs, but the AEs were not considered to be drug related in either subject. There were no clinically meaningful trends in laboratory values, electrocardiographic findings, or vital signs.
DISCUSSION
Doravirine is an NNRTI in development for the treatment of HIV-1 infection in combination with other antiretrovirals. Hypercholesterolemia is a relatively common comorbidity in patients being treated for HIV infection. A primary mode of therapy for hypercholesterolemia is statin treatment, with atorvastatin frequently being prescribed. Though doravirine was not expected to affect the exposure of atorvastatin via OATP1B1, BCRP, or CYP3A4 inhibition, this study was conducted to provide guidance for the future concomitant use of doravirine and atorvastatin.
Consistent with in vitro and clinical drug-drug interaction findings to date (1, 5–7), data from the current investigation support the hypothesis that doravirine does not have a clinically meaningful effect on CYP3A4 metabolism or OATP1B1, MDR1, and BCRP transporters, which are implicated in the disposition of atorvastatin (13, 14). This observation was justified by the lack of changes in exposure, clearance, and half-life associated with atorvastatin when it was administered both alone and concomitantly with doravirine.
While the GMR was close to unity for the atorvastatin AUC, Cmax was decreased by approximately 33% when atorvastatin and doravirine were coadministered compared with the Cmax obtained when atorvastatin was administered alone. However, this decrease is not considered clinically relevant, as similar decreases associated with food intake and nighttime dosing have been observed, while efficacy was maintained when these other factors were present (19–21). Extensive meta-analysis indicated that the efficacy of statins correlates with total daily exposure rather than with peak exposure. On the basis of this analysis, the effect of doravirine alone on the Cmax is not expected to be clinically meaningful. The additivity of the effect of doravirine to other factors that lower Cmax, such as food and diurnal administration, is unknown. However, a comparison of twice-daily versus once-daily dosing data for fluvastatin, lovastatin, and pravastatin indicates equal or better efficacy with twice-daily administration, despite significant reductions in Cmax (22). This analysis is also applicable to atorvastatin. Thus, an unexpected additive effect between doravirine and other factors on the Cmax of atorvastatin is not anticipated to have significant consequences for its efficacy. Interestingly, the observed decrease in the Cmax of atorvastatin administered in the presence of doravirine compared with that of atorvastatin administered alone is in contrast to the findings of a previously conducted drug-drug interaction study of the coadministration of doravirine at 200 mg QD with dolutegravir, another BCRP substrate (7). In the doravirine-dolutegravir study, the dolutegravir Cmax was increased by 43% (7). In addition to BCRP, dolutegravir is also a substrate for uridine 5′-diphospho-glucuronosyltransferase 1A3 (UGT1A3), UGT1A9, and P-gp and is primarily metabolized by UGT1A1, with some contribution coming from CYP3A4 (23). In this earlier study, the observed modest, though not clinically significant, 43% increase in Cmax was attributed to concentration-dependent BCRP inhibition at the gut level. This effect was not observed in the current study with doravirine and atorvastatin, possibly due to the lower dose of doravirine of 100 mg administered. A delay in the time to the peak concentration of atorvastatin was also observed in some individuals upon coadministration with multiple doses of doravirine compared with the time to the peak concentration with the administration of atorvastatin alone. The mechanisms for the delayed Tmax and the reduction in Cmax are currently unknown.
The terminal half-life of atorvastatin was approximately 11 h for both treatment conditions, indicating that the disposition of atorvastatin was unaffected upon administration alone or coadministration with multiple doses of doravirine. The clearance and volume of distribution of atorvastatin were relatively similar in both treatments, further supporting a lack of an effect on the elimination or distribution of atorvastatin.
This study was conducted with a 20-mg dose of atorvastatin, as this is one of the most commonly prescribed doses (24) and may be anticipated to be as sensitive as higher doses to drug interactions. Therefore, the lack of observation of an effect of doravirine on atorvastatin exposure could perhaps be extrapolated to higher doses of atorvastatin. The single-dose design of the study was chosen, as atorvastatin has been demonstrated to have dose proportionality and a lack of accumulation over multiple doses (25). Furthermore, the decreased atorvastatin Cmax in combination with a delayed Tmax in the absence of changes in AUC or elimination half-life strongly suggests that the interaction occurs during absorption and, consequently, is independent of treatment duration. In this study, only the PK of atorvastatin were measured. Because the exposure of atorvastatin was not affected, changes in metabolic profiles are anticipated to be minimal.
The effect of atorvastatin on doravirine was not measured in this study. Though this is a potential limitation, atorvastatin is not expected to meaningfully alter CYP3A4-mediated doravirine PK. This is due to atorvastatin demonstrating only a weak to moderate inhibitory effect on CYP3A4 activity (16–18). Furthermore, exposure to doravirine at 200 mg was previously well tolerated in a phase 2 trial (2). Therefore, the anticipated small, if any, increase in exposure achieved with doravirine at 100 mg when it is coadministered with atorvastatin would not be expected to be clinically meaningful.
Of the currently administered ARTs, atorvastatin and other HMG-CoA reductase inhibitors, such as simvastatin and lovastatin, are either contraindicated or used with caution when coadministered with delavirdine, ritonavir, nelfinavir, or other protease inhibitors. This is due to the increases in atorvastatin exposure caused by drug-drug interactions mediated by these ARTs (26). Data from the current study suggest that doravirine may be a more suitable ART for coadministration with atorvastatin than other ARTs, many of which are known CYP3A4 inhibitors/inducers, such as ritonavir, delavirdine, nevirapine, and efavirenz (26). Furthermore, data obtained in vitro in transporter inhibition assays, combined with data from clinical interaction studies, suggest that doravirine has a low potential for interaction with statins as a class, which are, to various degrees, substrates for OATP1B1/OATP1B3, BCRP, and CYP3A4. With regard to the potential for atorvastatin to impact doravirine PK, atorvastatin does not induce significant drug-drug interactions and would not be expected to have a significant effect on doravirine PK (19).
Conclusions.
Doravirine has no clinically meaningful effect on atorvastatin PK. Due to the overall lack of meaningful drug-drug interactions caused by doravirine, this novel ART has the potential to be an alternative more favorable than existing therapies that are known to inhibit or induce CYP3A4.
MATERIALS AND METHODS
Study design.
This open-label, two-period, two-treatment, fixed-sequence study was conducted in healthy adults in December 2014 (Merck protocol 1439-036). On day 1 of period 1, subjects received a single oral dose of atorvastatin at 20 mg. This was followed by a washout period of 72 h, to allow time for the sufficient elimination of atorvastatin (the atorvastatin half-life is ∼14 h [27]). Starting on day 1 of period 2, subjects received an oral dose of doravirine at 100 mg QD for 8 days and a single dose of atorvastatin at 20 mg was coadministered on day 5, by which time the doravirine concentrations were anticipated to have reached steady state. Subjects fasted for at least 8 h prior to and 4 h after atorvastatin administration in both periods. On days 1 to 4 and days 6 to 8 of period 2, the subjects received a light breakfast ∼30 min prior to doravirine administration. Plasma samples for determination of atorvastatin concentrations were collected prior to atorvastatin dosing and up to 60 h postdosing in periods 1 and 2. Safety was evaluated throughout the conduct of the study.
This single-center study was performed at Pharma Medica Research Inc. (St. Charles, MO, USA). The study was approved by the Salus Institutional Review Board (Austin, TX, USA) and performed according to good clinical practice guidelines.
Study population.
Subjects were considered eligible for the study if they were healthy adults aged 18 to 65 years inclusive with a body mass index of between 19.0 and 33.0 kg/m2. The results of a PK evaluation of this healthy volunteer population are projected to mirror those of a PK evaluation of individuals with HIV-1 infection, as no major differences in the PK of the agents under investigation in the two populations are anticipated. This lack of anticipated difference is based upon results for doravirine from a previous trial (28) and by the lack of an indication in the prescribing information of the need for atorvastatin dose adjustments for HIV-infected patients (19). All participants gave written informed consent to participate in the trial.
PK evaluations.
Blood samples were collected prior to dosing and at 0.25, 0.5, 1, 2, 3, 4, 6, 12, 24, 36, 48, and 60 h after atorvastatin administration. The blood samples were then centrifuged at approximately 4°C for 10 min at 1,851 × g to isolate plasma for analysis. Atorvastatin concentration-time profiles were derived from an analysis of the plasma samples by validated liquid chromatography with tandem mass spectrometry detection with a lower limit of detection of 0.02 ng/ml (Pharma Medica Research Inc., Mississauga, Ontario, Canada).
The following PK parameters for atorvastatin were assessed using a noncompartmental approach in Phoenix WinNonlin software (version 6.3; Pharsight, Mountain View, CA, USA): Cmax, AUC from time zero to infinity (AUC0–∞), AUC from time zero to the last measurement (AUC0–last), time to Cmax (Tmax), apparent terminal elimination half-life (t1/2), apparent total clearance (CL/F), and apparent volume of distribution (V/F).
Safety assessments.
Throughout the study, all AEs were reported and assessed to be mild, moderate, or severe in intensity. Safety evaluations, including vital signs, 12-lead electrocardiogram (ECG), physical examination, and clinical laboratory assessments, were conducted prior to drug administration and 24 h after the last drug administration in each period.
Data analysis and statistics.
All subjects with available data were included in the PK analysis, and all subjects enrolled were evaluated for safety.
Statistical analyses were performed using Statistical Analysis System (SAS) software (version 9.3; SAS Institute Inc., Cary, NC, US). The Cmax and AUC0–∞ values for atorvastatin were natural log transformed prior to analysis and evaluated separately using a linear mixed-effects model with a fixed-effects term for treatment in periods 1 and 2. An unstructured covariance matrix was used to allow unequal treatment variances and to model the correlation between the treatment measurements within each subject via the Repeated statement in the SAS Proc Mixed program. The method of Kenward and Roger was used to calculate the number of degrees of freedom in the denominator for the fixed effects (29). In the PK analysis, the value for any samples that had concentrations that were below the lower limit of quantification of 0.02 ng/ml was treated as 0 for the calculation of arithmetic mean concentrations and as missing for calculation of geometric mean concentrations and half-life values.
The atorvastatin AUC0–∞, AUC0–last, and Cmax were compared for atorvastatin administered with doravirine and atorvastatin administered alone. A two-sided 90% confidence interval (CI) for the true mean difference (between atorvastatin plus doravirine and atorvastatin alone) for the atorvastatin AUC0–∞ and Cmax on the log scale was computed from the above-described linear mixed-effect model. These confidence limits were then exponentiated to obtain the 90% CIs for the true geometric mean ratios (GMRs) (atorvastatin plus doravirine/atorvastatin) for the atorvastatin AUC0–∞ and Cmax.
ACKNOWLEDGMENTS
We thank the trial participants and clinical study site staff. Medical writing assistance was provided by Edward Rochford of Complete Medical Communications, Hackensack, NJ, USA.
Funding for this research was provided by Merck & Co., Inc., Kenilworth, NJ, USA. The medical writing assistance was funded by Merck & Co., Inc., Kenilworth, NJ, USA. Noha Maklad works under a fellowship in partnership with the Ernest Mario School of Pharmacy, Rutgers, NJ, USA.
S.K., K.L.Y., R.I.S., I.T., L.F., and M.I. are current or former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock and/or stock options. N.M. has no conflict of interest. H.J. and M.M. are employees of Pharma Medica Research Inc.
REFERENCES
- 1.Anderson MS, Gilmartin J, Cilissen C, De Lepeleire I, van Bortel L, Dockendorf MF, Tetteh E, Ancona JK, Liu R, Guo Y, Wagner JA, Butterton JR. 2015. Safety, tolerability and pharmacokinetics of doravirine, a novel HIV non-nucleoside reverse transcriptase inhibitor, after single and multiple doses in healthy subjects. Antivir Ther 20:397–405. [DOI] [PubMed] [Google Scholar]
- 2.Gatell JM, Morales-Ramirez JO, Hagins DP, Thompson M, Keikawus A, Hoffmann C, Rugina S, Osiyemi O, Escoriu S, Dretler R, Harvey C, Xu X, Teppler H. 2014. Forty-eight-week efficacy and safety and early CNS tolerability of doravirine (MK-1439), a novel NNRTI, with TDF/FTC in ART-naive HIV-positive patients. J Int AIDS Soc 17(4 Suppl 3):19532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gatell JM, Raffi F, Plettenberg A, Smith D, Portilla J, Hoffmann C, Arasteh K, Thompson M, Hagins DP, Morales-Ramirez JO, Xu X, Teppler H. 2015. Efficacy and safety of doravirine 100mg QD versus efavirenz 600mg QD with TDF/FTC in ART-naive HIV-infected patients: week 24 results, abstr TUAB0104. Abstr 8th Int AIDS Soc Conf HIV Pathog Treatment Prev. [Google Scholar]
- 4.World Health Organization. 2015. WHO model list of essential medicines, 19th list World Health Organization, Geneva, Switzerland: http://www.who.int/medicines/publications/essentialmedicines/en/. [Google Scholar]
- 5.Sanchez RI, Fillgrove K, Hafey M, Palamanda J, Newton D, Lu B, Bleasby K. 2015. In vitro evaluation of doravirine potential for pharmacokinetic drug interactions, abstr P95. Abstr 20th North Am Meet Int Soc Study Xenobiotics (ISSX), Orlando, FL. [Google Scholar]
- 6.Anderson MS, Kaufman D, Castranuovo P, Tetteh E, Lai KA, Armas DR, Wagner JA, Butterton JR. 2015. Effect of doravirine (MK-1439) on the pharmacokinetics of an oral contraceptive (ethinyl estradiol [EE] and levonorgestrel [LNG]), HIV treatment bulletin. 16th Int Workshop Clin Pharmacol HIV Hepatitis Ther. http://regist2.virology-education.com/abstractbook/2015_4.pdf. [Google Scholar]
- 7.Anderson MS, Khalilieh SG, Yee KL, Liu R, Fan L, Rizk M, Shah V, Hussaini A, Song I, Ross L, Butterton JR. 2015. A 2-way steady state pharmacokinetic interaction study of doravirine (MK-1439) and dolutegravir, NATAP Pharm_40. 16th Int Workshop Clin Pharmacol HIV Hepatitis Ther http://www.natap.org/2015/Pharm/Pharm_40.htm. [DOI] [PubMed] [Google Scholar]
- 8.Deeks SG. 2011. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nachega JB, Hsu AJ, Uthman OA, Spinewine A, Pham PA. 2012. Antiretroviral therapy adherence and drug-drug interactions in the aging HIV population. AIDS 26(Suppl 1):S39–S53. doi: 10.1097/QAD.0b013e32835584ea. [DOI] [PubMed] [Google Scholar]
- 10.Husain NE, Ahmed MH. 2014. Managing dyslipidemia in HIV/AIDS patients: challenges and solutions. HIV AIDS (Auckl) 7:1–10. doi: 10.2147/HIV.S46028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mothe B, Perez I, Domingo P, Podzamczer D, Ribera E, Curran A, Vilades C, Vidal F, Dalmau D, Pedrol E, Negredo E, Molto J, Paredes R, Perez-Alvarez N, Gatell JM, Clotet B. 2009. HIV-1 infection in subjects older than 70: a multicenter cross-sectional assessment in Catalonia, Spain. Curr HIV Res 7:597–600. doi: 10.2174/157016209789973691. [DOI] [PubMed] [Google Scholar]
- 12.Hejazi N, Rajikan R, Choong CL, Sahar S. 2013. Metabolic abnormalities in adult HIV infected population on antiretroviral medication in Malaysia: a cross-sectional survey. BMC Public Health 13:758. doi: 10.1186/1471-2458-13-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellick KA, Bottorff M, Toth PP, The National Lipid Association's Safety Task Force. 2014. A clinician's guide to statin drug-drug interactions. J Clin Lipidol 8:S30–S46. doi: 10.1016/j.jacl.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Ulvestad M, Skottheim IB, Jakobsen GS, Bremer S, Molden E, Asberg A, Hjelmesaeth J, Andersson TB, Sandbu R, Christensen H. 2013. Impact of OATP1B1, MDR1, and CYP3A4 expression in liver and intestine on interpatient pharmacokinetic variability of atorvastatin in obese subjects. Clin Pharmacol Ther 93:275–282. doi: 10.1038/clpt.2012.261. [DOI] [PubMed] [Google Scholar]
- 15.Neuvonen PJ. 2010. Drug interactions with HMG-CoA reductase inhibitors (statins): the importance of CYP enzymes, transporters and pharmacogenetics. Curr Opin Investig Drugs 11:323–332. [PubMed] [Google Scholar]
- 16.Hukkanen J, Puurunen J, Hyotylainen T, Savolainen MJ, Ruokonen A, Morin-Papunen L, Oresic M, Piltonen T, Tapanainen JS. 2015. The effect of atorvastatin treatment on serum oxysterol concentrations and cytochrome P450 3A4 activity. Br J Clin Pharmacol 80:473–479. doi: 10.1111/bcp.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mc Donnell CG, Shorten G, Van Pelt FN. 2005. Effect of atorvastatin and fluvastatin on the metabolism of midazolam by cytochrome P450 in vitro. Anaesthesia 60:747–753. doi: 10.1111/j.1365-2044.2005.04110.x. [DOI] [PubMed] [Google Scholar]
- 18.Choi DH, Shin WG, Choi JS. 2008. Drug interaction between oral atorvastatin and verapamil in healthy subjects: effects of atorvastatin on the pharmacokinetics of verapamil and norverapamil. Eur J Clin Pharmacol 64:445–449. doi: 10.1007/s00228-007-0447-5. [DOI] [PubMed] [Google Scholar]
- 19.FDA. 2009. Full prescribing information: Lipitor (atorvastatin). FDA, Rockville, MD: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020702s056lbl.pdf. [Google Scholar]
- 20.Whitfield LR, Stern RH, Sedman AJ, Abel R, Gibson DM. 2000. Effect of food on the pharmacodynamics and pharmacokinetics of atorvastatin, an inhibitor of HMG-CoA reductase. Eur J Drug Metab Pharmacokinet 25:97–101. doi: 10.1007/BF03190074. [DOI] [PubMed] [Google Scholar]
- 21.Cilla DD Jr, Gibson DM, Whitfield LR, Sedman AJ. 1996. Pharmacodynamic effects and pharmacokinetics of atorvastatin after administration to normocholesterolemic subjects in the morning and evening. J Clin Pharmacol 36:604–609. doi: 10.1002/j.1552-4604.1996.tb04224.x. [DOI] [PubMed] [Google Scholar]
- 22.Vargo R, Adewale A, Behm MO, Mandema J, Kerbusch T. 2014. Prediction of clinical irrelevance of PK differences in atorvastatin using PK/PD models derived from literature-based meta-analyses. Clin Pharmacol Ther 96:101–109. doi: 10.1038/clpt.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reese MJ, Savina PM, Generaux GT, Tracey H, Humphreys JE, Kanaoka E, Webster LO, Harmon KA, Clarke JD, Polli JW. 2013. In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab Dispos 41:353–361. doi: 10.1124/dmd.112.048918. [DOI] [PubMed] [Google Scholar]
- 24.QuintilesIMS. 2016. USA national disease and therapeutic index June 2016. IMS Dataview, version 7.0.1. QuintilesIMS, Durham, NC. [Google Scholar]
- 25.Lins RL, Matthys KE, Verpooten GA, Peeters PC, Dratwa M, Stolear JC, Lameire NH. 2003. Pharmacokinetics of atorvastatin and its metabolites after single and multiple dosing in hypercholesterolaemic haemodialysis patients. Nephrol Dial Transplant 18:967–976. doi: 10.1093/ndt/gfg048. [DOI] [PubMed] [Google Scholar]
- 26.Fichtenbaum CJ, Gerber JG. 2002. Interactions between antiretroviral drugs and drugs used for the therapy of the metabolic complications encountered during HIV infection. Clin Pharmacokinet 41:1195–1211. doi: 10.2165/00003088-200241140-00004. [DOI] [PubMed] [Google Scholar]
- 27.Shin J, Pauly DF, Pacanowski MA, Langaee T, Frye RF, Johnson JA. 2011. Effect of cytochrome P450 3A5 genotype on atorvastatin pharmacokinetics and its interaction with clarithromycin. Pharmacotherapy 31:942–950. doi: 10.1592/phco.31.10.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schurmann D, Sobotha C, Gilmartin J, Robberechts M, De Lepeleire I, Yee KL, Guo Y, Liu R, Wagner F, Wagner JA, Butterton JR, Anderson MS. 2016. A randomized, double-blind, placebo-controlled, short-term monotherapy study of doravirine in treatment-naive HIV-infected individuals. AIDS 30:57–63. doi: 10.1097/QAD.0000000000000876. [DOI] [PubMed] [Google Scholar]
- 29.Kenward MG, Roger JH. 1997. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997. doi: 10.2307/2533558. [DOI] [PubMed] [Google Scholar]