Abstract
BACKGROUND
Alterations in hedgehog signaling are implicated in the pathogenesis of basal-cell carcinoma. Although most basal-cell carcinomas are treated surgically, no effective therapy exists for locally advanced or metastatic basal-cell carcinoma. A phase 1 study of vismodegib (GDC-0449), a first-in-class, small-molecule inhibitor of the hedgehog pathway, showed a 58% response rate among patients with advanced basal-cell carcinoma.
METHODS
In this multicenter, international, two-cohort, nonrandomized study, we enrolled patients with metastatic basal-cell carcinoma and those with locally advanced basal-cell carcinoma who had inoperable disease or for whom surgery was inappropriate (because of multiple recurrences and a low likelihood of surgical cure, or substantial anticipated disfigurement). All patients received 150 mg of oral vismodegib daily. The primary end point was the independently assessed objective response rate; the primary hypotheses were that the response rate would be greater than 20% for patients with locally advanced basal-cell carcinoma and greater than 10% for those with metastatic basal-cell carcinoma.
RESULTS
In 33 patients with metastatic basal-cell carcinoma, the independently assessed response rate was 30% (95% confidence interval [CI], 16 to 48; P = 0.001). In 63 patients with locally advanced basal-cell carcinoma, the independently assessed response rate was 43% (95% CI, 31 to 56; P<0.001), with complete responses in 13 patients (21%). The median duration of response was 7.6 months in both cohorts. Adverse events occurring in more than 30% of patients were muscle spasms, alopecia, dysgeusia (taste disturbance), weight loss, and fatigue. Serious adverse events were reported in 25% of patients; seven deaths due to adverse events were noted.
CONCLUSIONS
Vismodegib is associated with tumor responses in patients with locally advanced or metastatic basal-cell carcinoma. (Funded by Genentech; Erivance BCC ClinicalTrials.gov number, NCT00833417.)
Basal-cell carcinoma is the most common cancer. It is estimated that more than 2.1 million new patients were treated for nonmelanoma skin cancer in 2006 in the United States1; approximately 80% of nonmelanoma skin cancers are basal-cell carcinomas. Although most basal-cell carcinomas are readily treated by means of various surgical methods, these lesions occasionally progress to an advanced state that is no longer amenable to surgery or radiation therapy (locally advanced basal-cell carcinoma) or, more rarely, the lesions spread to distant sites (metastatic basal-cell carcinoma). When this study was designed, there was no approved therapy for advanced basal-cell carcinoma.
Molecular and genetic studies have shown that almost all basal-cell carcinomas contain genetic alterations in the hedgehog signaling pathway, resulting in aberrant pathway activation and uncontrolled proliferation of basal cells. Most commonly, these alterations cause loss of function of patched homologue 1 (PTCH1), which normally acts to inhibit the signaling activity of smoothened homologue (SMO), a seven-transmembrane protein.2,3 Vismodegib (GDC-0449, Genentech) is a first-in-class, small-molecule inhibitor of SMO. A phase 1 study of vismodegib involving 33 patients with advanced basal-cell carcinoma showed a 58% confirmed response rate and a median duration of response of 12.8 months.4,5 This phase 2 study (Erivance BCC) was conducted to more fully evaluate the efficacy and safety of vismodegib in patients with locally advanced or metastatic basalcell carcinoma.
METHODS
STUDY DESIGN
A control group was not used in this study, given the small patient population, the historical absence of spontaneous responses, and the lack of available effective therapies. The continuous dosing schedule of 150 mg of vismodegib once daily was chosen on the basis of the pharmacokinetic properties characterized in the phase 1 study.6 Patients received vismodegib until disease progression, unacceptable toxic effects, or discontinuation of the study. Dose interruption for up to 4 weeks was allowed in order for patients to recover from toxic effects.
The primary end point was the objective response rate as assessed by independent review. For metastatic basal-cell carcinoma, we used the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines, version 1.07 (Table 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Because a standard end point for locally advanced basal-cell carcinoma did not exist when this study was designed, response was defined as a decrease of 30% or more in the externally visible or radiographic dimension (if applicable) or complete resolution of ulceration (if present at baseline). The investigators and independent reviewers were instructed to include residual scarring when measuring the externally visible dimension. Responses had to be confirmed at least 4 weeks after initial documentation. Progressive disease was defined as an increase of 20% or more in the externally visible or radiographic dimension (if applicable), new ulceration, or a new lesion. For patients with multiple target lesions, the sum of the longest diameters was used to determine response and progression.
An independent review panel assessed photographs for all patients with locally advanced basal-cell carcinoma, and another panel assessed radiographic scans for all patients with metastatic basal-cell carcinoma and for patients with locally advanced basal-cell carcinoma who had radiographically measurable disease. For patients with locally advanced basal-cell carcinoma who had a response and underwent tumor biopsy during the study, independent pathological evaluation was used to determine whether the response was partial or complete (on the basis of the presence or absence of residual basal-cell carcinoma in the biopsy specimen) (see the Methods section in the Supplementary Appendix).
STUDY OVERSIGHT
The trial was designed jointly by the first author and the sponsor (Genentech). Data were collected by the site investigators under a confidentiality agreement and were retained and analyzed by the sponsor. All authors had full access to the data and vouch for the accuracy and completeness of the data and analysis and the fidelity of the study to the protocol. The first draft of the manuscript was written by the first author and one author who is an employee of the sponsor. All authors contributed to subsequent drafts and decided to submit the manuscript for publication. Writing assistance was provided by F. Hoffmann–La Roche and the sponsor.
The study was reviewed and approved by the institutional review board or ethics committee at each site. All patients provided written informed consent. The protocol, including the statistical analysis plan, is available at NEJM.org.
ELIGIBILITY
Eligible patients were at least 18 years of age, had adequate organ function, and had an Eastern Cooperative Oncology Group performance status of 2 or less, with 0 indicating fully active, 1 restricted in strenuous activity but ambulatory and able to carry out light work, and 2 ambulatory and capable of self-care but unable to work.8 Patients with metastatic basal-cell carcinoma had measurable disease (including nodal metastases), according to the RECIST guidelines, as assessed with computed tomography or magnetic resonance imaging. Patients with locally advanced basal-cell carcinoma had at least one lesion that was 10 mm or more in the longest diameter and was considered inoperable or for which surgery was considered inappropriate, in the opinion of a specialist in Mohs dermatologic, head and neck, or plastic surgery. Acceptable reasons for surgery to be considered inappropriate were one or both of the following: the recurrence of basal-cell carcinoma after two or more surgical procedures and an expectation that curative resection would be unlikely, or substantial morbidity or deformity anticipated from surgery. In the group of patients with locally advanced basal-cell carcinoma, prior radiation therapy to one or more target lesions was required, unless it was contraindicated or inappropriate. A pathology report documenting the diagnosis of basal-cell carcinoma was required for all patients; for patients with metastatic basal-cell carcinoma, the diagnosis needed to be made on the basis of tissue from a metastatic lesion. Patients with the basal-cell nevus syndrome (Gorlin’s syndrome) could enroll in the study if all the other criteria were met.
Women of childbearing potential and men with female partners of childbearing potential were required to use two methods of contraception, owing to the teratogenic potential of vismodegib. Exclusion criteria were major organ dysfunction, pregnancy, lactation, participation in an investigational study in the previous 4 weeks, life expectancy of less than 12 weeks, uncontrolled medical illnesses, other conditions that would contraindicate the use of an investigational drug, and an inability to swallow capsules. Concurrent antitumor therapy was not permitted.
DATA COLLECTION
All patients underwent physical examination and laboratory testing (including pregnancy testing for women of childbearing potential) at baseline and every 4 weeks thereafter. For patients with radiographically measurable disease (all patients with metastatic basal-cell carcinoma and some with locally advanced basal-cell carcinoma), radiographic assessment of tumors was performed at baseline and every 8 weeks thereafter. In patients with locally advanced basal-cell carcinoma, tumors were assessed by means of physical examination (documented by standardized digital photography) at baseline and every 8 weeks thereafter. Data on adverse events were collected for up to 45 days after the last administration of vismodegib or after withdrawal from the study, whichever was later.
HEDGEHOG PATHWAY activity IN STORED TUMOR TISSUE
Archival formalin-fixed, paraffin-embedded samples of tumor tissue were macrodissected for enrichment of tumor content and processed for transcriptional profiling. Expression levels of the hedgehog target genes GLI family zinc finger 1 (GLI1) and patched homologue 2 (PTCH2) were assessed by means of a polymerase-chain-reaction assay (TaqMan, Life Technologies) and calculated by the 2–ΔCt method, in which the cycling threshold (Ct) of GLI1 or PTCH2 was normalized to the Ct of SMO and expressed as a power of 2 (2EXP−[Ct(GLI1)−Ct(SMO)]).4 Control samples of messenger RNA were obtained from formalin-fixed, paraffin-embedded samples of normal skin obtained from commercial sources.
STATISTICAL ANALYSIS
Efficacy and safety analyses were performed according to the protocol-specified time point for the primary analysis, with the use of data collected from the beginning of the study (February 10, 2009) through 9 months after the first treatment of the last enrolled patient (November 26, 2010). The primary objective was to test whether the response rate was greater than 10% among patients with metastatic basal-cell carcinoma and greater than 20% among patients with locally advanced basal-cell carcinoma, as determined with the use of exact binomial one-sided tests. Duration of response was the major secondary end point. The population for the efficacy analysis included all treated patients for whom the independent pathologist confirmed basal-cell carcinoma in tumor tissue (see the Methods section in the Supplementary Appendix). The study had approximately 80% probability of rejecting the null hypothesis, given a true response rate of 37% in the group of patients with metastatic basal-cell carcinoma (with 20 treated patients) and a true response rate of 34% in the group of patients with locally advanced basal-cell carcinoma (with 80 treated patients).
All patients were included in the safety analyses. Adverse events were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf).
RESULTS
PATIENTS
We enrolled 104 patients over a period of 13 months at 31 sites in the United States, Europe, and Australia: 33 patients had metastatic basal-cell carcinoma and 71 had locally advanced basal-cell carcinoma. Eight patients with locally advanced basal-cell carcinoma were excluded from the efficacy analysis because the independent pathologist did not identify basal-cell carcinoma in biopsy specimens obtained at baseline (5 patients did not have basal-cell carcinoma at baseline but did have basal-cell carcinoma in archival tissue, 2 had squamous-cell carcinoma at baseline and basal-cell carcinoma in archival tissue, and 1 did not have basal-cell carcinoma either at baseline or in archival tissue). No patients with metastatic basal-cell carcinoma were excluded. The median age was 62 years in both cohorts; all patients were white (Table 1).
Table 1.
Demographic and Clinical Characteristics of the Patients at Baseline.*
| Characteristic | Metastatic Basal-Cell Carcinoma (N = 33) |
Locally Advanced Basal-Cell Carcinoma (N = 63) |
|---|---|---|
| Age — yr | 61.6±11.4 | 61.4±16.9 |
| Median | 62.0 | 62.0 |
| Range | 38–92 | 21–101 |
| Sex — no.(%) | ||
| Male | 24 (73) | 35 (56) |
| Female | 9 (27) | 28 (44) |
| White race — no. (%)† | 33 (100) | 63 (100) |
| Contraindications to surgery or radiation therapy — no. (%)‡ | ||
| Inoperable tumor | 24 (38) | |
| Surgery inappropriate | 39 (62) | |
| Multiple recurrences | 16 (25) | |
| Substantial morbidity or deformity anticipated | 32 (51) | |
| Radiation therapy previously administered | 13 (21) | |
| Radiation therapy inappropriate or contraindicated | 50 (79) |
Plus–minus values are means ±SD.
Race was determined by the investigators.
Contraindications to surgery or radiation therapy are reported only for patients with locally advanced basal-cell carcinoma. Such contraindications were not part of the eligibility criteria for patients with metastatic basal-cell carcinoma.
The majority of patients with metastatic basal-cell carcinoma (61%) had three or more target lesions. The most frequent sites of target lesions were the lung (67%) and lymph nodes (21%) (Table 2 in the Supplementary Appendix). Prior surgery was reported for 97% of patients with metastatic basal-cell carcinoma, prior radiation therapy for 58%, and prior systemic therapy for 30%.
Among patients with locally advanced basal-cell carcinoma, 38% of those included in the efficacy analysis had cancer that was considered to be inoperable. In the remaining 62% of these patients, surgery was considered to be inappropriate: 25% had multiple recurrences, surgery was anticipated to result in considerable morbidity or deformity in 51%, and both reasons applied in 14%. In this group, 21% of patients had received radiation therapy to a target lesion, whereas radiation therapy was contraindicated or inappropriate in 79%. Prior surgery was reported for 89% of patients in this cohort, prior radiation therapy (for any current or prior basal-cell carcinoma lesions) for 27%, and prior systemic or topical therapy for 11%.
EFFICACY
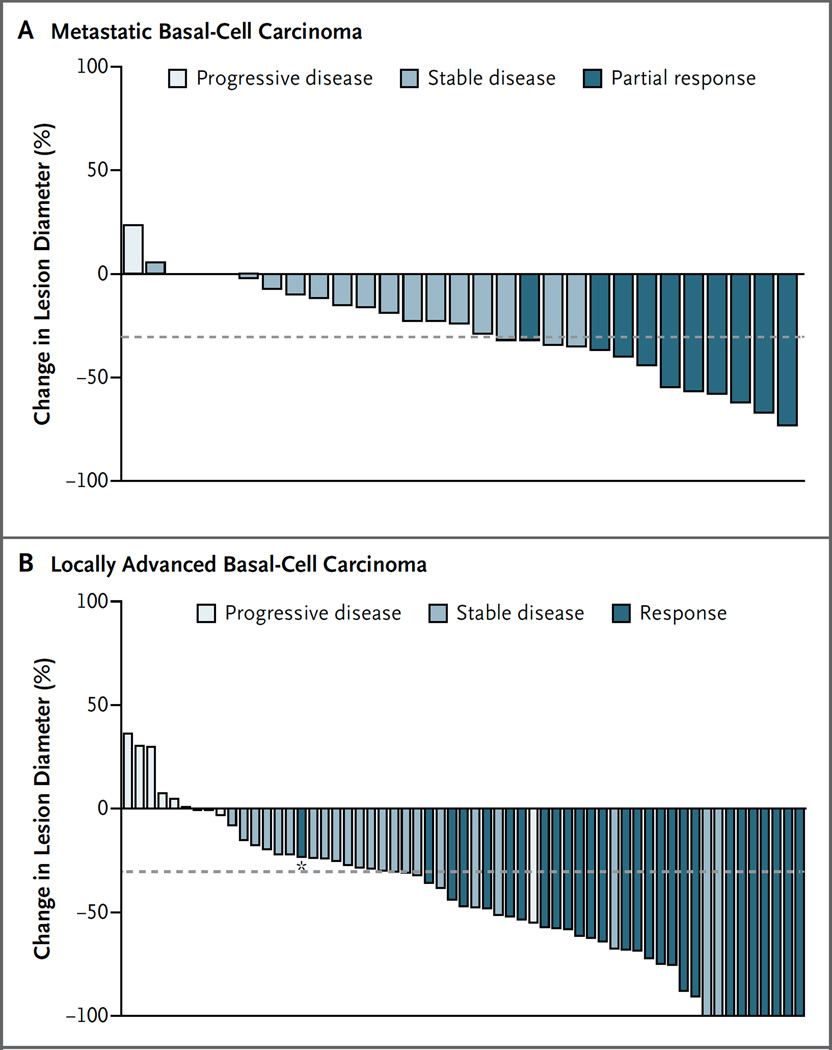
This study met its primary end point of independently assessed objective response in both cohorts (Table 2). In the group of patients with metastatic basal-cell carcinoma, the objective response rate, according to independent review, was 30% (95% confidence interval [CI], 16 to 48), which was significantly greater than the null hypothesis of 10% (P = 0.001). According to the assessments made by the site investigators, responses were observed in 45% of patients (95% CI, 28 to 62). Concordance between the response assessments made by the independent reviewer and those made by the site investigators was 79%. All responses in patients with metastatic basal-cell carcinoma were partial responses. The majority of these patients (24 of 33 [73%]) had tumor shrinkage, according to independent review (Fig. 1A). Three patients with tumor shrinkage of more than 30% had an unconfirmed response and were therefore considered to have stable disease.
Table 2.
Primary and Secondary Efficacy End Points and Treatment Duration.*
| Outcome | Metastatic Basal-Cell Carcinoma (N = 33) |
Locally Advanced Basal-Cell Carcinoma (N = 63) |
||
|---|---|---|---|---|
| Independent Review |
Site Investigators |
Independent Review |
Site Investigators |
|
| Objective response — no. (%) | 10 (30) | 15 (45) | 27 (43) | 38 (60) |
| 95% CI | 16–48 | 28–62 | 30–56 | 47–72 |
| P value | 0.001 | <0.001 | ||
| Stable disease — no. (%) | 21 (64) | 15 (45) | 24 (38) | 15 (24) |
| Progressive disease — no. (%) | 1 (3) | 2 (6) | 8 (13) | 6 (10) |
| Data missing or could not be evaluated — no. (%) | 1 (3) | 1 (3) | 4 (6) | 4 (6) |
| Median duration of response — mo | 7.6 | 12.9 | 7.6 | 7.6 |
| Median progression-free survival, based on independent review — mo | 9.5 | 9.5 | ||
| Duration of treatment — mo† | ||||
| Median | 10.0 | 9.7 | ||
| Range | 0.7–16.4 | 1.1–18.7 | ||
| Patients still receiving treatment — no./total no. (%)‡ | 19/33 (58) | 32/71 (45) | ||
CI denotes confidence interval.
Data were calculated on the basis of all treated patients (33 patients with metastatic basal-cell carcinoma and 71 with locally advanced basal-cell carcinoma).
Data include all treated patients (33 patients with metastatic basal-cell carcinoma and 71 with locally advanced basal-cell carcinoma). Patients were still receiving treatment as of the data-cutoff point (November 26, 2010), which was 9 months after the last patient was enrolled.
Figure 1. Maximum Tumor Shrinkage in the Two Cohorts.
Panel A shows a waterfall plot of maximum tumor shrinkage (the sum of the longest diameters, as compared with baseline), according to the independent review before progression, in the group of 33 patients with metastatic basal-cell carcinoma. Each bar represents a patient; 3 had a best percentage change of 0 in the sum of the longest diameters, accounting for the gap in the bars. Three patients did not have measurements, and 1 could not be evaluated for assessment of best confirmed response; data from these 4 patients were excluded from this figure. Panel B shows a waterfall plot of maximum tumor shrinkage before progression, as assessed by independent review, in the 63 patients with locally advanced basal-cell carcinoma who were included in the efficacy analysis. For patients with target lesions that were assessed only by measuring the externally visible dimension, the maximum tumor shrinkage shown is based on the externally visible dimension. For patients who also underwent radiographic assessment, the waterfall plot shows the assessment approach yielding the greater percent reduction (either radiographic assessment or measurement of the externally visible dimension). Four patients did not have lesion measurements; data from these patients were not included in this figure. The asterisk denotes a patient with tumor shrinkage of less than 30% and a response assessed on the basis of the complete resolution of ulceration. In both panels, the dashed line represents a 30% decrease in the sum of the longest diameters.
As of the data-cutoff point, the median duration of objective response in the group of patients with metastatic basal-cell carcinoma was 7.6 months (range, 2.1 to 11.1), according to independent review. The investigator-determined response duration was 12.9 months (range, 1.9 to 12.9). The median progression-free survival for this cohort was 9.5 months (95% CI, 7.4 to not estimable), according to independent review, and 9.2 months (95% CI, 7.4 to not estimable), according to the assessments made by the site investigators; data on overall survival were not mature.
In the group of patients with locally advanced basal-cell carcinoma, the objective response rate, according to independent review, was 43% (95% CI, 30 to 56), which was significantly greater than the null hypothesis of 20% (P<0.001). Of the 63 patients included in the efficacy analysis, 13 (21%) had a complete response (defined as the absence of residual basal-cell carcinoma on assessment of a biopsy specimen), according to the independent review. As of the data-cutoff point, 10 of the 13 patients who had a complete response had not had disease progression. The response rate, according to the assessments made by the site investigators, was 60% (95% CI, 47 to 72), with 20 patients (32%) having a complete response. Concordance between the independent review and the assessments made by the site investigators was 60%. Of the 8 patients who were excluded from the efficacy analysis because the independent pathologist did not identify basal-cell carcinoma in baseline biopsy specimens, 4 had a response to vismodegib, according to the independent review.
The majority of patients with locally advanced basal-cell carcinoma had tumor shrinkage (Fig. 1B). In some cases, patients with tumor shrinkage of 30% or more were considered not to have had a response (unconfirmed responses), owing to a response followed by one or more assessments of stable disease or progression, missing radiographic or external-dimension assessments, confirmation occurring less than 4 weeks after the initial response, or radiographic evidence of progression at the same time that physical examination showed shrinkage.
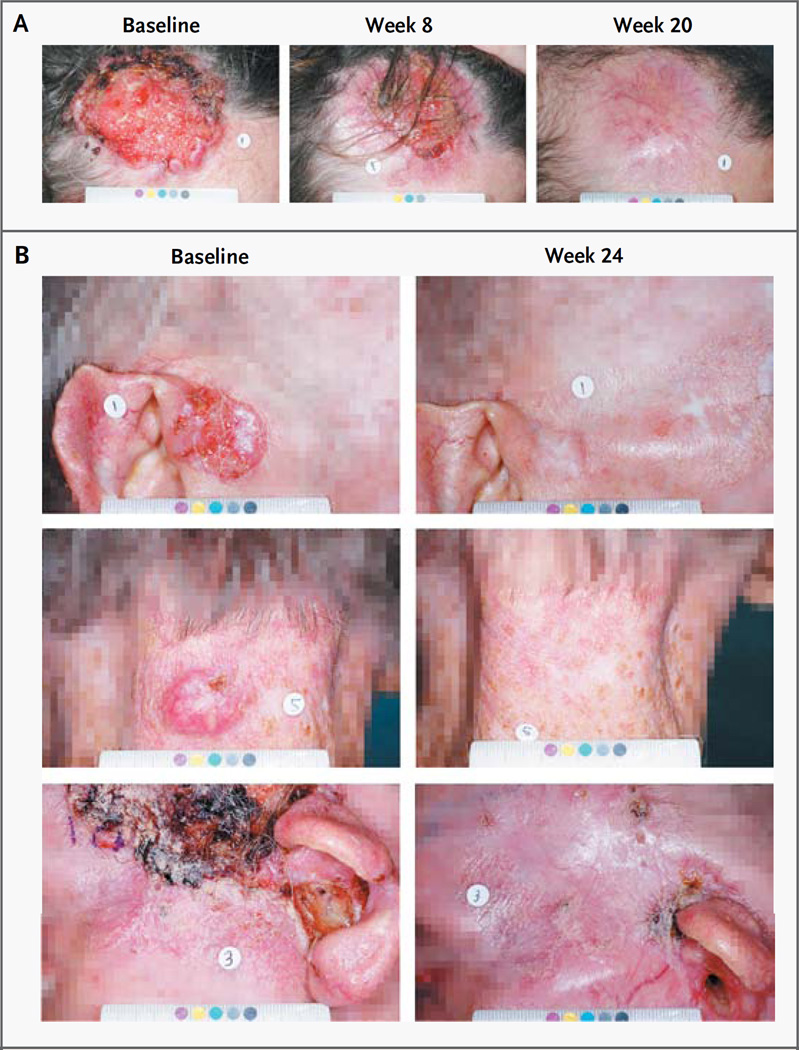
The median duration of response in the group of patients with locally advanced basal-cell carcinoma was 7.6 months (range, 1.0 to 12.9), according to the independent review, and 7.6 months (range, 1.4 to 16.6), according to the site investigators’ assessments. As of the data-cutoff point, the median progression-free survival was 9.5 months (95% CI, 7.4 to 11.9), according to the independent review, and 11.3 months (95% CI, 9.5 to 16.8), according to the investigators’ assessments; data for overall survival were not mature. Overall, biopsy specimens from 34 of the 63 patients (54%) in this group showed no residual basal-cell carcinoma in any target lesions; these 34 patients included patients with a confirmed response, according to the independent review or the investigator’s assessment, as well as patients with stable or progressive disease. Visible reductions in tumor size and improvement in appearance were noted by the site investigators for the majority of patients in this cohort (Fig. 2).
Figure 2. Photographs of Lesions before and during Treatment in Two Patients with Locally Advanced Basal-Cell Carcinoma.
Panel A shows locally advanced basal-cell carcinomas on the scalp and forehead of a 68-year-old woman; substantial deformity was anticipated from surgery. No prior surgeries other than biopsies were reported. Radiation therapy was considered to be contraindicated, owing to multiple coexisting conditions and a risk of damage to the brain. No residual basal-cell carcinoma was detected in biopsy specimens obtained at week 16. This patient was considered to have had a complete response, according to the independent review and the site investigator. Panel B shows three of five target lesions (on the right temple, back of the neck, and left temple and external auditory canal) in an 82-year-old man with locally advanced basal-cell carcinoma. The patient had undergone numerous surgical procedures. The lesions present at enrollment had recurred at least twice and were considered unlikely to be curatively resected or would have substantial anticipated morbidity or deformity from surgery. Radiation therapy was considered to be inappropriate, owing to the extensive disease. Although residual basal-cell carcinoma was detected on biopsy of the remaining ulceration on the left temple at week 24, biopsy specimens of other lesions showed no evidence of basal-cell carcinoma. This patient was considered to have had a partial response, according to the independent review and the site investigator.
After patients had received 150 mg of vismodegib once daily for 8 weeks, the mean (±SD) steady-state plasma concentrations of vismodegib were similar in patients with locally advanced and those with metastatic basal-cell carcinoma (26.3±9.61 µM and 29±9.82 µM, respectively) (Fig. 1 in the Supplementary Appendix). These results are consistent with previous findings.5
Activation of the hedgehog pathway in archival tumor samples from the patients was assessed by relative expression levels of GLI1 and PTCH2, as measured with the TaqMan real-time polymerase-chain-reaction assay; data were available for 76% of patients. The distribution of GLI1 and PTCH2 expression levels was similar in the two cohorts, with higher expression levels than those found in control specimens from normal skin (Fig. 2 in the Supplementary Appendix). These findings are consistent with active hedgehog signaling9 and with the results of the phase 1 study.4
ADVERSE EVENTS
As of the data-cutoff point, approximately half the patients had discontinued the study treatment, and the median duration of drug exposure was approximately 10 months in both cohorts (Table 2). The most common reasons for discontinuation of vismodegib were disease progression in the group of patients with metastatic basal-cell carcinoma (18%) and the patient’s decision in the group of patients with locally advanced basal-cell carcinoma (25%) (Table 3 in the Supplementary Appendix); the reasons for this decision were not documented.
All patients had at least one adverse event during the study; more than half the treated patients (57%) had only grade 1 or 2 adverse events. Adverse events of any grade occurring in 20% or more of patients are summarized in Table 3; these findings are consistent with the pattern of adverse events in the phase 1 study. Adverse events of grade 3 or 4 included muscle spasms, weight loss, fatigue, and loss of appetite. Of the 104 patients in the study, 13 (12%) had an adverse event leading to the discontinuation of the study drug; the most common was muscle spasms, reported in 2 patients.
Table 3.
Commonly Reported Adverse Events, According to Grade.*
| Event | Any Grade | Grade 1 | Grade 2 | Grade 3 or 4 |
|---|---|---|---|---|
| percentage of patients | ||||
| Muscle spasms | 68 | 48 | 16 | 4 |
| Alopecia | 63 | 49 | 14 | 0 |
| Dysgeusia | 51 | 28 | 23 | 0 |
| Decrease in weight | 46 | 27 | 14 | 5 |
| Fatigue | 36 | 27 | 5 | 4 |
| Nausea | 29 | 21 | 7 | 1 |
| Decrease in appetite | 23 | 14 | 6 | 3 |
| Diarrhea | 22 | 16 | 5 | 1 |
These adverse events occurred in at least 20% of all patients and were coded with the use of the Medical Dictionary for Regulatory Activities (MedDRA), version 13.1. The highest grade of event is reported here for each patient.
Serious adverse events were reported in 26 patients (25%) (Table 4 in the Supplementary Appendix). Grade 5 (fatal) adverse events were reported in 7 patients (1 with metastatic basal-cell carcinoma and 6 with locally advanced basal-cell carcinoma): death from an unknown cause (in 3 patients) and hypovolemic shock, myocardial infarction, meningeal disease, and ischemic stroke (in 1 patient each) (for details, see the Supplementary Appendix). A review of these events suggested no definite pattern, and these 7 patients had clinically significant risk factors or coexisting conditions at baseline. The relationship between the study drug and the deaths is unknown.
DISCUSSION
Advanced basal-cell carcinoma is an uncommon, frequently disfiguring, and sometimes life-threatening cancer that has been without effective therapies. New treatment options are urgently needed for patients who have tumors that are not amenable to surgery, for whom surgery would be grossly disfiguring, or who have metastatic disease. To our knowledge, however, no previous clinical trials have focused on this population.
Dysregulation of the hedgehog signaling pathway has been identified in the vast majority of basal-cell carcinomas. Vismodegib is a synthetic, first-in-class, small-molecule inhibitor of SMO, a key component of the hedgehog pathway.
On the basis of the efficacy observed in the phase 1 study, this trial was designed to evaluate the efficacy and safety of vismodegib in patients with advanced basal-cell carcinoma. The study met the protocol-defined primary end point, as measured by the independently assessed tumor response. The majority of patients in both cohorts had tumor shrinkage in response to vismodegib. In addition, 54% of patients with locally advanced basal-cell carcinoma had no residual disease in biopsy specimens obtained during treatment with vismodegib. Furthermore, photographs of patients and comments from treating physicians suggest that the response may have been underestimated for some patients with locally advanced basal-cell carcinoma, such as those with tumor regression and residual scarring, since scarring was included in the measurement of the externally visible dimension.
Concordance between the independent review and the site investigators’ assessment of response was higher in the group of patients with metastatic basal-cell carcinoma than in the group with locally advanced basal-cell carcinoma. In both cohorts, most discordances were attributed to cases in which the site investigator noted a response but the independent reviewer noted a nonresponse. The lower concordance rate for the patients with locally advanced basal-cell carcinoma can be attributed to the multiple components of the end-point definition for response (i.e., visible dimension, ulceration, and RECIST, as applicable) and the amalgamated independent review of each of these components.
Common adverse events observed in this study were generally similar to those seen in prior studies of vismodegib and are hypothesized to be mechanism-related10–13; these included muscle spasms, dysgeusia, alopecia, fatigue, and weight loss. Serious adverse events were reported, including fatal adverse events in seven patients. The relationship of the deaths to the study drug was unknown, but the deaths were considered by the site investigator to be unrelated to vismodegib.
Despite the efficacy observed in this study, some patients chose to discontinue treatment with vismodegib for reasons other than disease progression or adverse events, particularly in the cohort of patients with locally advanced basal-cell carcinoma. Although the reasons for the decision to discontinue therapy were not documented, long-term, low-grade adverse events (e.g., dysgeusia or muscle cramps) or the perception that the maximal benefit had been achieved may have played a role.
These study data suggest that vismodegib is a new treatment option for patients with advanced basal-cell carcinoma, and led to the approval of vismodegib by the Food and Drug Administration. Recently, encouraging data were also reported for vismodegib in the prevention and treatment of basal-cell carcinomas in patients with the basal-cell nevus syndrome, a condition caused by a germline deletion of one copy of PTCH1, which can lead to the development of hundreds to thousands of basal-cell carcinomas in affected persons.14 Ongoing and future studies will help to clarify whether vismodegib may have a role in the treatment of less-advanced basal-cell carcinoma.
Supplementary Material
Acknowledgments
Supported by Genentech.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the patients and their families; the nurses, research coordinators, and data managers; and the research and development staff at Genentech and F. Hoffmann–La Roche.
REFERENCES
- 1.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 2.Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78–81. doi: 10.1038/ng0996-78. [DOI] [PubMed] [Google Scholar]
- 3.Aszterbaum M, Rothman A, Johnson RL, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885–888. doi: 10.1046/j.1523-1747.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- 4.Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 5.LoRusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham RA, Lum BL, Cheeti S, et al. Pharmacokinetics of hedgehog pathway inhibitor GDC-0449 in patients with refractory or untreatable locally advanced or metastatic solid tumors: the role of alpha 1-acid glycoprotein binding. Clin Cancer Res. 2011;17:2512–2520. doi: 10.1158/1078-0432.CCR-10-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Therasse P, Arbuck SG, Eisenhauser EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 8.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 9.Bonifas JM, Pennypacker S, Chuang PT, et al. Activation of expression of hedgehog target genes in basal cell carcinomas. J Invest Dermatol. 2001;116:739–742. doi: 10.1046/j.1523-1747.2001.01315.x. [DOI] [PubMed] [Google Scholar]
- 10.St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- 11.Chiang C, Swan RZ, Grachtchouk M, et al. Essential role for sonic hedgehog during hair follicle morphogenesis. Dev Biol. 1999;205:1–9. doi: 10.1006/dbio.1998.9103. [DOI] [PubMed] [Google Scholar]
- 12.Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263–277. doi: 10.1016/s0012-1606(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 13.Liu H-X, MacCallum DK, Edwards C, Gaffield W, Mistretta CM. Sonic hedgehog exerts distinct, stage-specific effects on tongue and taste papilla development. Dev Biol. 2004;276:280–300. doi: 10.1016/j.ydbio.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 14.Tang JY, Mackay-Wiggan JM, Aszterbaum M, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366:2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.