Abstract
The Sensory Organization Test (SOT) of Computerized Dynamic Posturography (EquiTest™ equipment) is a valuable tool for investigating how an individual uses balance system sensory input (vestibular, vision, proprioception/somatosensory) to maintain quiet stance; however, it is limited as a screening tool for identifying peripheral vestibular system dysfunction. Previous research has shown that adding horizontal head-shake to portions of the standard SOT battery improved the identification of peripheral vestibular system asymmetry; however, flaws in the methods were noted. The objective of this work was to evaluate the sensitivity and specificity of the modified head-shake SOT (HS-SOT) protocol for identification of peripheral vestibular system lesion. Fifteen patients with chief complaint of instability, vertigo, and/or lightheadedness, with and without a caloric unilateral weakness (UW) and fifteen age-matched healthy controls were included in the final analysis. Ten of the 15 patients demonstrated a caloric UW ≥ 25%. Participants completed standard conditions 2 and 5 of SOT with head still and during four horizontal head-shaking tasks (i.e., HS-SOT2–60°/s, HS-SOT2–120°/s, HS-SOT5–15°/s, and HS-SOT5–60°/s). Average equilibrium scores decreased as condition difficulty increased (SOT2, HS-SOT2–60°/s, HS-SOT2–120°/s, SOT 5, HS-SOT5–15°/s, and HS-SOT5–60°/s) for each group; as expected, a lower decline was noted for controls (slope = −6.59) compared to patients (slope = −11.69). The HS-SOT5–15°/s condition was superior for identifying peripheral vestibular asymmetry (AUC = 0.90 sensitivity = 70%, specificity = 100%), with the strongest correlation to caloric UW% (rs = −0.743, p = 0.000006). HS-SOT5–15°/s appears to be a promising screening measure for peripheral vestibular asymmetry.
Keywords: Head-shake, Posturography, Unilateral weakness, Caloric weakness, Peripheral vestibular, Sensory organization test, Head-shake posturography, Calorics, Peripheral vestibular asymmetry
1. Introduction
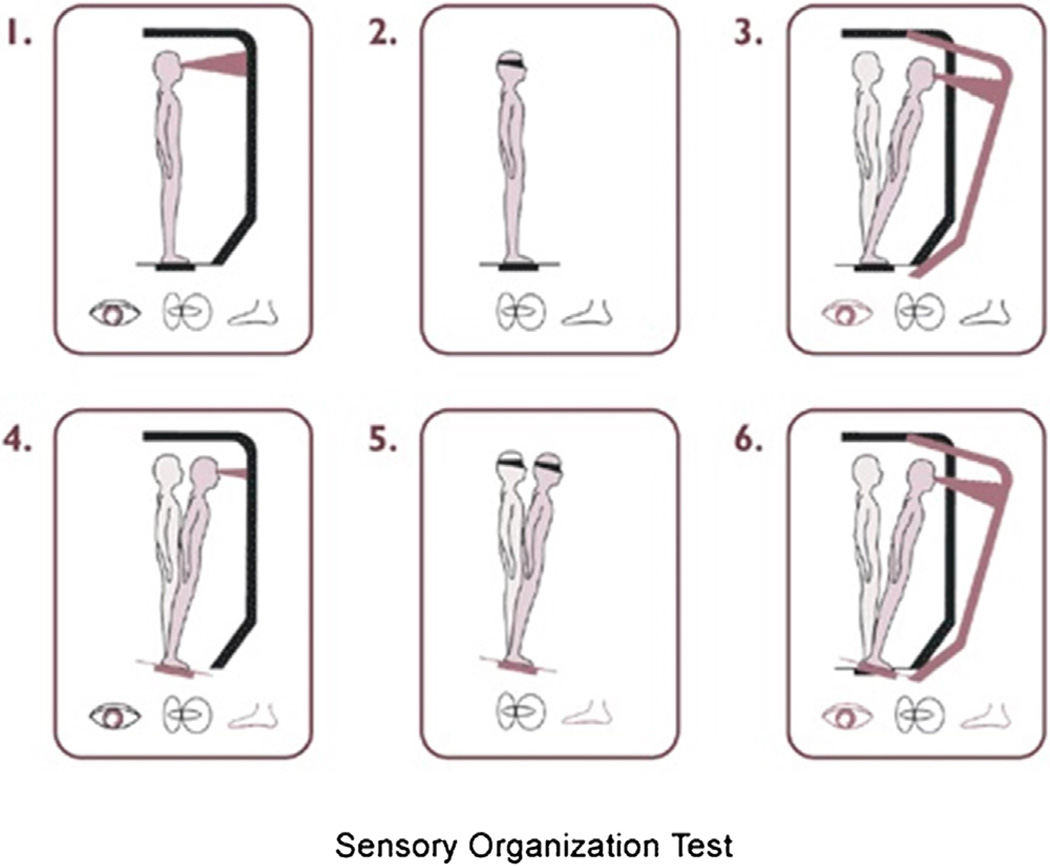
Our ability to maintain balance is influenced by coordination of sensory input (vestibular, vision, and proprioception) and motor output, which sends commands to lower extremities and muscles. The Sensory Organization Test (SOT) of Computerized Dynamic Posturography evaluates the ability to utilize vision, vestibular and biomechanical sensors at the joints and on the plantar surface of the foot to maintain balance [1,2]. Changes in center of pressure are quantified during six increasingly challenging conditions that disrupt portions of balance sensory input (Fig. 1). An equilibrium score for each condition trial is calculated by comparing the angular difference between the patient’s calculated maximum and minimum sagittal plane body sway to a theoretical maximum displacement (12.5°), and referenced as a score between 100 (no body sway) to 0 (fall) [3].
Fig. 1.
Graphic of the Sensory Organization Test (SOT) depicting the six increasingly challenging conditions. Reprinted with permission from Neurocom International, online resources: http://resoursesonbalance.com/program/role/cdp/protocols.aspx.
The SOT measures the functional ability to coordinate balance after an injury or disease affects the balance system [4,5]; however, it is a limited tool to screen for peripheral vestibular asymmetry, with respect to site-of-lesion [4–8]. In many cases, measures of postural control will be normal within a short period of time after unilateral vestibular loss [9–11].
The addition of horizontal head-shake during standard SOT testing decreases postural control ability [12,13] and improves SOT performance for identifying unilateral vestibular loss [14]. During head-shake, the vestibular system is stimulated; therefore, the individual’s postural control system is challenged [15]. Furthermore, when the individual is receiving inaccurate visual and/or proprioceptive sensory information and the vestibular system is activated (i.e., head-shake), discrimination between the head-shake and body sway must be made by the brain [16]. Individuals with vestibular dysfunction are unable to distinguish between the body sway and the vestibular system input, which ultimately leads to increased body sway (i.e., reduced postural control). Thus, head-shake SOT (HS-SOT) may be appropriate for individuals presenting with persistent symptoms, but appear to be compensated due to normal or near-normal SOT performance.
Mishra et al. [14] examined HS-SOT 60°/s during conditions 2 (eyes closed and stable support surface; HS-SOT2–60°/s) and 5 (eyes closed and sway reference support; HS-SOT5–60°/s); however, there were ceiling and floor effects. A modification to the HS-SOT protocol was then evaluated in 40 healthy controls [15] that included the same head-shake conditions proposed by Mishra et al. [14], but included head-shake with peak head velocity at 120°/s during SOT condition 2 (HS-SOT2–120°/s), and head-shake with peak head velocity at 15°/s during SOT condition 5 (HS-SOT5–15°/s). The inclusion of the HS-SOT2–120°/s and HS-SOT5–15°/s eliminated the observed ceiling and floor effects, respectively.
Therefore, the purpose of this study was to evaluate the performance of the modified HS-SOT test proposed by Honaker et al. [15] in patients with and without peripheral vestibular asymmetry. We hypothesize the modified HS-SOT will increase sensitivity and specificity for identifying unilateral vestibular dysfunction.
2. Methods
2.1. Subjects
Patients were randomly selected from individuals referred to the University of Nebraska-Lincoln (UNL) and Boys Town National Research Hospital (BTNRH) vestibular clinics. Consistent with Mishra et al. [14] all patients presented with the chief complaint of instability, vertigo, and/or lightheadedness, with and without peripheral vestibular system asymmetries as determined by caloric testing. All patients received open loop bithermal (44 °C and 30 °C) caloric irrigations, which were analyzed using Jongkee’s formula [17] to determine presence or absence of clinically significant caloric unilateral weakness (UW; ≥ 25% slow-phase eye velocity asymmetry between right and left ears). Standard SOT was performed to verify inclusion for the study. Patients were excluded from participation based on the following criteria: (1) central nervous system involvement; (2) bilateral hearing loss that would interfere with communication; (3) orthopedic condition that would interfere with standing balance; (4) cervical range-of-motion limitations that would interfere with horizontal head movements; (5) inability to complete SOT, and (6) fall reactions on SOT conditions 5 and 6.
Also included were age-matched controls recruited from community sources near the participating care facilities. Through a screening interview, all controls verified negative history of: (1) dizziness lasting longer than 1 hour in duration or recurring for greater than 1 day; (2) active middle ear disease; (3) perceived unilateral hearing loss; (4) disorders interfering with mobility and stance; (5) disorders limiting cervical range-of-motion that would interfere with horizontal head movements; (6) central nervous system involvement; and (7) alcohol consumption within 24 hours of participation. Standard SOT was performed to verify inclusion for the study; control subjects were excluded if they were unable to complete SOT or if fall reactions on SOT conditions 5 and 6 were observed. The healthy control subjects did not receive caloric testing.
2.2. Procedure
All participants provided written informed consent approved by the Institutional Review Boards (IRB) at UNL and BTNRH. Participants were instructed to stand on the EquiTest™ System (NeuroCom International, Inc), force plate with shoes removed while wearing a safety harness; proper positioning of the feet was executed [2]. Participants were asked to maintain quiet upright stance, without touching the walls or harness, and to keep their eyes closed during each task. First, three 20 second trials of standard SOT conditions 2 and 5 were performed. Next, a NeuroCom International software, version 8.3.0 (Clackamas, OR, USA), head mounted rate sensor (InertiaCube2+, 3DOF gyro) was placed on each participant’s head to monitor horizontal head movement (15 ° excursions to the right and left) and velocity (°/s). The participants repeated three 20 second trials of SOT conditions 2 and 5 with horizontal head-shake to the right and left during different head velocities in the following order: 1) SOT condition 2 at 60°/s (HS-SOT2–60°/s), 2) SOT condition 2 at 120°/s (HS-SOT2–120°/s), 3) SOT condition 5 at 15°/s (HS-SOT5–15°/s), and 4) SOT condition 5 at 60°/s (HS-SOT5–60°/s). An audible signal cued by a metronome was used during all trials to maintain appropriate head velocity. These conditions were not randomized based on a pragmatic decision to improve the application of the test to use in a routine clinical population. Breaks were offered as needed to reduce the effects of fatigue.
2.3. Statistical Analysis
Descriptive statistics, means (M), standard deviations (SD) and ranges, of the modified HS-SOT equilibrium scores were calculated within each group. Linear regression slopes between condition task difficulty rank (condition 1 = SOT 2, condition 2 = HS-SOT2–60°/s, condition 3 = HS-SOT2–120°/s, condition 4 = SOT 5, condition 5 = HS-SOT5–15°/s, condition 6 = HS-SOT5–60°/s) and equilibrium score were calculated to quantify change in performance with increasing difficulty of the task. While learning effects are noted across condition trials [18], the standard clinical analysis approach for calculating equilibrium score was performed [1]. Specifically, an equilibrium score was calculated for each trial, and the average score across all three trials was used [1]. Subjects who were unable to maintain the 20 second SOT trial due to fall reactions were given a score of zero [1]. To test the hypothesis that performance on the modified HS-SOT was different between groups, mean slopes were compared using Mann-Whitney U-test; justification for this approach was due to unequal variances between groups and small sample size. Two subjects could not complete HS-SOT 15°/s, and three could not complete HS-SOT 120°/s (missing data); therefore, a SAS mixed procedure (equivalent to multivariate repeated-measures analysis of variance, accounting for missing data) was used to estimate a mixed-model with an unstructured variance, comparing performance on all six conditions between the two groups. To calculate the sensitivity and specificity of the modified HS-SOT for idenfying peripheral vestibular assymetry, clinical utility indices (sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio) were calculated based on linear regression slope, and individual equilibrium scores for each modified HS-SOT condition, using the following formulas [19]:
Sensitivity = True positives (proportion of individuals with dis-ease)/(true positive + false negatives (proportion of individuals shown as free of the disease when disease is present) × 100 = %
Specificity = True negatives (proportion of individuals without disease)/(true negatives + false positives (proportion of individuals shown as having the disease when disease is absent) × 100 = %
Positive likelihood ratio = Sensitivity/(100% − specificity)
Negative likelihood ratio = (100% − sensitivity)/specificity
Caloric UW (% ≥25) was used as the gold standard to define unilateral peripheral vestibular paresis to assess the diagnostic accuracy of the modified HS-SOT protocol for the patient sample. HS-SOT slope and individual conditions were further analyzed using receiver operator characteristic (ROC) [20], area under the curve (AUC) [21] and Spearman Correlation Coeffients analysis to determine clinical utility of the modified HS-SOT protocol. Significance level was set to p < 0.05. To reduce type I errors, Bonferroni adjustment procedure was performend for multiple comparsions [22]. Analyses were performed using SPSS (Version 22, Chicago, IL, USA) and SAS (Version 9.2, Cary, NC, USA).
2.4. Results
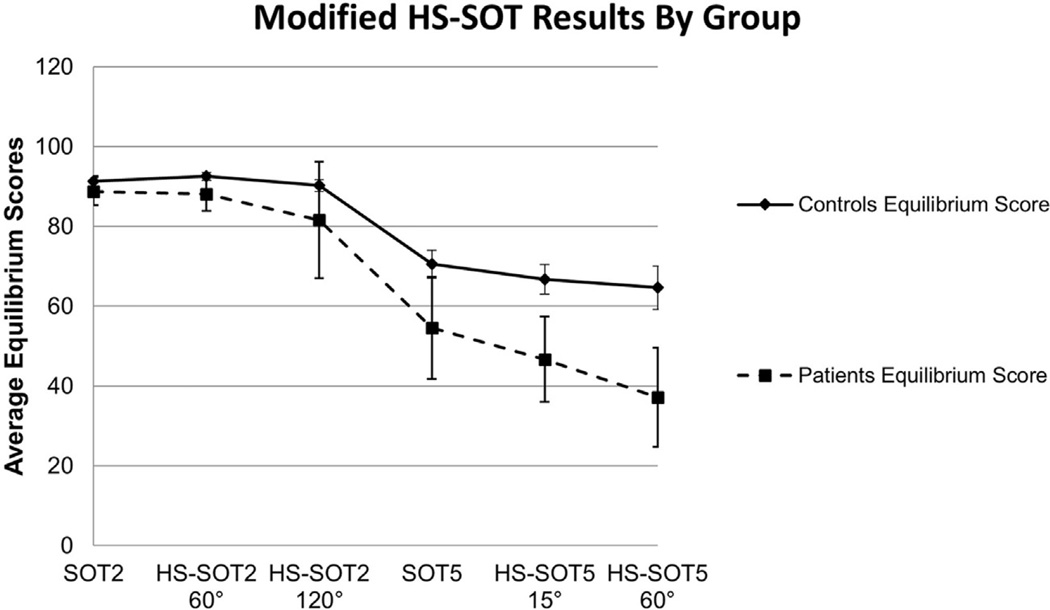
This study consisted of 30 participants, 15 patients with complaints of vertigo, instability, lightheadedness (age (M) = 53 years, (SD) = 11.92, age range: 34–74 years) and 15 age-matched subjects without symptoms and history of vestibular or balance disorders (age (M) = 53.53, (SD) = 12.21, age range: 33–74 years). Average patient group caloric UW was 42.93 (SD = 31.7); ten patients demonstrated caloric UW ≥ 25%. Average equilibrium scores decreased as HS-SOT protocol condition difficulty increased (SOT2, HS-SOT2–60°/s, HS-SOT2–120°/s, SOT5, HS-SOT5–15°/s, and HS-SOT5–60°/s) for each group (controls slope = −6.59, p = 0.008, 95% CI −10.36 to −2.81; patients slope = −11.69, p = 0.002, 95% CI −16.28 to −7.11). As expected, a significantly greater decline was noted for the patients as compared to controls (Mann-Whitney U = 43, p = 0.004; Fig. 2). See Table 1 for descriptive statistics for all conditions.
Fig. 2.
Slopes of linear regression best fit lines with 95% confidence interval (CI) bars between condition difficulty rank (condition 1 = SOT 2, condition 2 = HS SOT2–60°/s, condition 3 = HS SOT2–120°/s, condition 4 = SOT 5, condition 5 = HS SOT5–15°/s, condition 6 = HS SOT5–60°/s) equilibrium score for patients (dashed line) and age-matched healthy controls (solid line). A greater decline was noted for the patients (slope = −11.69) as compared to healthy controls (slope = −6.59).
Table 1.
Equilibrium Score data (Mean (SD) and range) of the six modified head-shake posturography test conditions for patients and healthy controls.
| Patients | Healthy Controls | |
|---|---|---|
| SOT 2 | ||
| Mean (SD) | 88.78 (6.28) | 91.29 (2.53) |
| Range | 68.67–96.33 | 87.00–95.00 |
| n = 15 | n = 15 | |
| HS-SOT2 60°/s | ||
| Mean (SD) | 88.05 (7.67) | 92.50 (1.81) |
| Range | 68.67–96.67 | 89.67–96.00 |
| n = 15 | n = 15 | |
| HS-SOT2 120°/s | ||
| Mean (SD) | 81.54 (26.39) | 90.20 (2.68) |
| Range | 0–95.33 | 85.00–94.00 |
| n = 12 | n = 15 | |
| SOT 5 | ||
| Mean (SD) | 54.51 (23.14)* | 70.53 (6.36) |
| Range | 9.0–90.00 | 59.67–82.00 |
| n = 15 | n = 15 | |
| HS-SOT5 15°/s | ||
| Mean (SD) | 46.65 (19.28)* | 66.68 (6.69) |
| Range | 0–78.00 | 53.33–83.33 |
| n = 13 | n = 15 | |
| HS-SOT5 60°/s | ||
| Mean (SD) | 37.17 (22.37)* | 64.60 (9.72) |
| Range | 0–75.33 | 42.67–86.67 |
| n = 15 | n = 15 |
Note:
denotes significant group differences (p < 0.05).
Based on the mixed procedure approach (6 conditions × 2 groups), there was a significant effect of condition, F (5, 25.2) = 57.46, p < 0.0001, a significant effect of group, F (1, 26.1) = 13.15, p = 0.0012, and a significant interaction, F (5, 16.8) = 3.07, p = 0.038. Therefore, simple effects were explored, and there were significant differences between groups. The normal control group performed significantly better than the patient group for HS-SOT5–60 °/s condition (t (23.7) = 4.16, p = 0.0004), HS-SOT5–15°/s condition (t (28) = 4.36, p = 0.0002) and SOT 5 condition (t (28) = 2.59, p = 0.0152); however, no significant differences were noted between groups for HS-SOT2–120 °/s (t (19.9) = 1.63, p = .1197), HS-SOT2–60 °/s (t (28) = 2.19, p = 0.0373) or SOT 2 (t (28) = 1.43, p = .1627) conditions. In the patient group, there was a significant within-subject difference between SOT 5 and HS-SOT 5–15 deg/s (F (3, 6) = 5.72, p. = 034), with higher equilibrium scores observed on SOT 5 (M = 60.79, 95% CI: 50.32–71.27) than HS-SOT 5–15 deg/s (M = 46.65, 95% CI: 35.00–58.30).
Next, clinical utility indices were calculated and ROC curves were constructed to determine AUC for the accuracy of the six modified HS-SOT equilibrium scores and the slope between condition task difficulty rank for identifying unilateral peripheral vestibular asymmetry (i.e., caloric weakness ≥ 25%) in the patient sample. Table 2 provides the clinical utility indices for each condition, including criterion value for distinguishing between patients with and without peripheral vestibular asymmetry. The HS-SOT5–15°/s condition was identified as the superior condition for identifying peripheral vestibular asymmetry (AUC, 0.90; 95% CI: 0.70–1.0, p =.04), with a criterion equilibrium score value of ≤ 51.67 (sensitivity = 70%, specificity = 100%). Poor clinical performance to separate between groups was identified for the remaining conditions (SOT2, SOT5, HS-SOT2–120°/s, HS-SOT2–60°/s, and HS-SOT5–60°/s). However, two patients were unable to complete the HS-SOT5–15°/s condition (i.e., floor effects). HS-SOT5–15°/s was also found to have the strongest association with caloric UW (r = −0.743, p = 0.000006), followed by HS-SOT5–60°/s (r = −0.−665, p < 0.00006), and the slope of all six conditions (r = −615, p = 0.0003), and SOT5 (r = −0.412, p = 0.02); no additional conditions were correlated with caloric UW (p > 0.05).
Table 2.
Clinical utility indices for modified HS-SOT test conditions for distinguishing between patients with caloric unilateral weakness ≥ 25% (n = 10) and patients without significant caloric unilateral weakness (n = 5).
| Condition | Sensitivity | Specificity | False Negative Rate (100− sensitivity) |
False Positive Rate (100− specificity) |
Positive Likelihood Ratio |
Negative Likelihood Ratio |
AUC | Cut-point* |
|---|---|---|---|---|---|---|---|---|
| SOT2a | 80 | 20 | 20 | 80 | 1.00 | 0.50 | 0.44 | ≤91.15 |
| HS 2 60°/sb | 60 | 40 | 40 | 60 | 1.00 | 1.00 | 0.45 | ≤92.00 |
| HS 2 120°/ sc |
44 | 33 | 56 | 67 | 0.65 | 1.69 | 0.37 | ≤89.34 |
| SOT5d | 50 | 40 | 50 | 60 | 0.83 | 1.25 | 0.34 | ≤62.17 |
| HS 5 15°/se | 70 | 100 | 30 | 0 | 0 | 0.3 | 0.90 | ≤51.67 |
| HS 5 60°/sf | 70 | 60 | 30 | 40 | 1.75 | 0.75 | 0.69 | ≤37.58 |
| Slopeg | 60 | 60 | 40 | 40 | 1.5 | 0.66 | 0.70 | ≤−13.06 |
Cut-point value represents equilibrium score discriminating between patients and healthy controls for identifying peripheral vestibular system involvement.
Sensory Organization Test (SOT) Conditions 2.
Head-shake posturography SOT condition 2 with horizontal head movements at 60°/s.
Head-shake posturography SOT condition 2 with horizontal head movements at 120°/s.
Sensory Organization Test (SOT) Condition 5.
Head-shake posturography SOT condition 5 with horizontal head movements at 15°/s.
Head-shake posturography SOT condition 2 with horizontal head movements at 60°/s.
Slope of linear regression best fit line between condition task difficulty (a–f) equilibrium scores.
A separate ROC analysis was performed to identify the performance of modified HS-SOT for identifying patients with complaints of vertigo, instability, and lightheadedness (n = 15) as compared to age-matched controls (n = 15) regardless of caloric UW. Again, HS-SOT5–15°/s condition was identified as the superior task to distinguish between groups (AUC, 0.89; 95% CI: 0.75–1.0, p = 0.0004). Table 3 provides the clinical performance indices for each condition, including criterion value for distinguishing between patients with complaints of vertigo, lightheadedness, and instability as compared to age-matched controls.
Table 3.
Clinical utility indices for modified HS-SOT test conditions for distinguishing between those with complaints of vertigo, lightheadedness, and instability as compared to age-matched controls.
| Condition | Sensitivity | Specificity | False Negative Rate (100−sensitivity) |
False Positive Rate (100−specificity) |
Positive Likelihood Ratio | Negative Likelihood Ratio | AUC | Cut-point* |
|---|---|---|---|---|---|---|---|---|
| SOT2a | 80 | 40 | 20 | 60 | 1.33 | 0.50 | 0.62 | ≤91.2 |
| HS 2 60°/sb | 60 | 47 | 40 | 53 | 1.13 | 0.85 | 0.65 | ≤92.17 |
| HS 2 120°/sc | 58 | 33 | 42 | 67 | 0.87 | 1.27 | 0.56 | ≤91.42 |
| SOT5d | 67 | 67 | 33 | 33 | 2.03 | 0.49 | 0.75 | ≤66.34 |
| HS 5 15°/se | 85 | 73 | 15 | 27 | 3.15 | 0.21 | 0.89 | ≤62.67 |
| HS 5 60°/sf | 80 | 67 | 20 | 33 | 2.42 | 0.30 | 0.85 | ≤61.12 |
| Slopeg | 73 | 67 | 27 | 33 | 2.21 | 0.40 | 0.80 | ≤−7.31 |
Cut-point value represents equilibrium score discriminating between patients with complaints of vertigo, instability and/or lightheadedness and healthy age-matched controls.
Sensory Organization Test (SOT) Conditions 2.
Head-shake posturography SOT condition 2 with horizontal head movements at 60°/s.
Head-shake posturography SOT condition 2 with horizontal head movements at 120°/s.
Sensory Organization Test (SOT) Condition 5.
Head-shake posturography SOT condition 5 with horizontal head movements at 15°/s.
Head-shake posturography SOT condition 2 with horizontal head movements at 60°/s.
Slope of linear regression best fit line between condition task difficulty (a–f) equilibrium scores.
3. Discussion
Our results demonstrated that stance is compromised in patients with unilateral peripheral vestibular loss during a head-shake postural control task. These findings are consistent with the literature suggesting that patients with unilateral peripheral hypofunction demonstrate reduced performance on SOT with the addition of provocative head movements [14,23] and changes in head orientation [24–26]. Research was conducted in the early 1990’s exploring the effects of head position on postural control results [24–26]; patients with unilateral vestibular loss demonstrated increased postural sway with changes in head orientation [23,26,27]. Postural instability following horizontal head-shake is likely due to asymmetrical neural input within the velocity storage integrator and vestibular nuclei after stimulation of the horizontal semicircular canals [14,15]. The head-shaking task produces enhanced vestibular asymmetry within the velocity storage integrator [28]. Clinically this may manifest in post-headshake nystagmus and postural instability via asymmetrical neural output via the vestibular-spinal reflex. Thus, we hypothesized that incorporation of HS-SOT may uncover unmasked postural control deficits resulting from unilateral vestibular lesions.
Our modification of a HS-SOT protocol demonstrated improved performance to identify unilateral peripheral vestibular hypo-function in a small sample of patients relative to healthy controls compared to Mishra et al. [15]. The modified HS-SOT method (slope of all six conditions) was found to identify unilateral peripheral vestibular impairment with a sensitivity of 60% and specificity of 60%; however, HS-SOT5–15°/s condition demonstrated superior performance for distinguishing between groups (sensitivity = 70%, specificity = 100%). The improved performance of HS-SOT5–15°/s may be due to its strong correlation with the gold standard comparison of caloric UW, which we attribute to their comparative frequency ranges. Specifically, caloric irrigations artificially stimulate the peripheral vestibular system at low frequencies (0.002–0.004 Hz), which is equivalent to a slow head movement [29]. Head movements at 15°/s, when using yaw plane head excursions of 30°, are equal to 0.16 Hz, a lower head rotation frequency than 60°/s (0.64 Hz) or 120°/s (1.28 Hz) [15].
HS-SOT5–15°/s was found to have superior performance, suggesting that it could be used as the single measure to identify peripheral vestibular loss; however, two patients were unable to complete the task (floor effects); interestingly of these two patients, only one had a caloric UW > 25%. All 15 patients were able to complete HS-SOT5–60°/s; however, sensitivity (70%) and specificity (60%) were low and the AUC was not significant. The decline in specificity was similar to Mishra et al. [14] who also show low specificity (22%), however, higher sensitivity based on their sample (95%). The high incidence of false positives and misses with the HS-SOT5–60°/s suggest this task alone is clinically unacceptable for identifying individuals with peripheral asymmetry, further supporting the use of HS-SOT5–15°/s. The floor effects observed during HS-SOT5–15°/s may provide further evidence of peripheral vestibular loss as those without peripheral vestibular loss are able to complete this task [15].
Decline in vestibulo-ocular reflex function during rapid head motion is also apparent in patients with otherwise normal caloric irrigations [30]. Two of five (40%) patients with symptoms of dizziness, imbalance and/or lightheadedness without caloric UW ≥ 25% had declined performance on both HS-SOT5–60°/s and HS-SOT5–15°/s; and one patient only had declined performance on HS-SOT5–15°/s. Mishra et al. [14] also observed postural instability in patients with reported symptoms of dizziness and/or imbalance. Thus HS-SOT may identify decrements in peripheral vestibular function not identified with caloric irrigations. Studies reporting increased sway with head tilt tasks attributed the results to alterations in utricular receptors [7]. Thus, declined performance may be due to alterations in otolith function, or the combination of a motor task (head movements) with a postural control task (i.e., dual task) [7].
While our results are promising for using HS-SOT5–15°/s as a screening tool for peripheral vestibular hypofunction, there are limitations. First, the small sample size may not support the use of our data for immediate clinical use, and the comparison of HS-SOT performance in patients and healthy controls introduces a spectrum bias. The screening accuracy for the proposed protocol requires further testing in patient groups (similar to Mishra et al. [14]), given that the protocol would not be applied to healthy controls. Also, there is the possibility of sub-clinical pathological vestibular system changes which cannot be ruled out given the healthy controls did not receive caloric testing. Additionally, we only included horizontal head-shake movements. The use of horizontal head-shake is consistent with stimulation of the horizontal canals – the same structures evaluated during caloric irrigations; however, activities of daily living involve stimulation of the vertical canals and thus pitch and roll plane SOT testing is warranted. Also, the use of video head impulse testing is quickly emerging as a high frequency test of peripheral vestibular (semicircular canal) function. Use of this equipment was not available at the beginning of this study. Therefore, further examination of the clinical performance of HS-SOT should be compared to this test. Finally, the reliability of measuring postural control with EquiTest™ is poor due to learning effects observed across condition trials [18], and inclusion of different postural control measures [31] are warranted to minimize these effects. In light of this, the test-retest reliability of HS-SOT is excellent – good in a cohort of younger and older participants [32], and thus screening for vestibular loss over time may still be of value with the EquiTest™ system.
4. Conclusion
The modified HS-SOT, utilizing head-shake at 15°/s, significant-ly improved sensitivity and specificity for identifying peripheral vestibular asymmetry. While these results are based on a small sample, test performance on HS-SOT-15°/s, suggests it could be added to traditional SOT testing and used as a screening tool for peripheral vestibular hypofunction.
Abbreviations
- CDP
computerized dynamic posturography
- HS-SOT
head-shake posturography
- SOT
sensory organization test
- UW
unilateral weakness.
Biographies
Julie Honaker is an employee of the University of Nebraska-Lincoln. Julie is a Board Member of the American Balance Society and American Speech, Language, Hearing Association National Advisory Committee. Julie received grant funding from the NCAA-DoD and American Academy of Audiology Foundation for previous work, and is currently funded by the American Nursing Foundation and Bryan Heart Foundation.
Kristen Janky is an employee of Boys Town National Research Hospital, and a consultant for Otometrics/Audiology systems. Kristen is a Board Member of the American Balance Society.
Jessie Patterson is student at the University of Nebraska-Lincoln. Jessie is a Board Member of the American Balance Society.
Neil Shepard is an employee of Mayo Clinic-Rochester and Board Member of the American Balance Society. Neil is a course instructor for the Vestibular rehabilitation: a competency-based course, Emory University School of Medicine. In addition, he is co-editor of textbook, Balance Function Assessment and Management.
References
- 1.Nashner LM. Computerized dynamic posturography. In: Jacobson GP, Shepard NT, editors. Balance Function Assessment and Management. San Diego: Plural Publishing; 2016. [Google Scholar]
- 2.NeuroCom Internation, Inc. Balance Manager Systems Clinical Interpretation Guide. Computerized Dynamic Posturography. D102559-00. [Google Scholar]
- 3.Whipple R, Wolfson L, Derby C, Devender S, Tobin J. Altered sensory function and balance in older persons. J. Gerontol. 1993;48:71–76. doi: 10.1093/geronj/48.special_issue.71. [DOI] [PubMed] [Google Scholar]
- 4.El-Kashlan HK, Shepard NT, Asher AM, Smith-Wheelock M, Telian SA. Evaluation of clinical measures of equilibrium. Laryngoscope. 1998;108:311–319. doi: 10.1097/00005537-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Nashner LM. Computerized dynamic posturography. In: Jacobson GP, Newman CW, Kartush JM, editors. Handbook of Balance Function Testing. San Diego: Singular Publishing Group; 1993. [Google Scholar]
- 6.Allum JHJ, Shepard NT. An overview of the clinical use of dynamic posturography in the differential diagnosis of balance disorders. J. Vestib. Res. 1999;9:223–252. [PubMed] [Google Scholar]
- 7.Chandra NS, Shepard NT. Clinical utility of lateral head tilt Posturography. Am. J. Otol. 1996;17(2):271–277. [PubMed] [Google Scholar]
- 8.Shepard NT, Schulz A, Alexander NB, Gu MJ, Boismier T. Postural control in young and elderly adults when stance is challenged: clinical versus laboratory measurements. Ann. Otol. Rhinol. Laryngol. 1993;102(7):508–517. doi: 10.1177/000348949310200704. [DOI] [PubMed] [Google Scholar]
- 9.Black FO, Shupert CL, Peterka RJ, Nashner LM. Effects of unilateral loss of vestibular function on the vestibulo-ocular reflex and postural control. Ann. Otol. Rhinol. Laryngol. 1989;98(11):884–889. doi: 10.1177/000348948909801109. [DOI] [PubMed] [Google Scholar]
- 10.Furman J. Posturography: uses and limitations. Baillieres Clin. Neurol. 1994;3(3):501–513. [PubMed] [Google Scholar]
- 11.Fetter M, Diener HC, Dichgans J. Recovery of postural control after an acute unilateral vestibular lesion in humans. J. Vestib. Res. 1990;1(4):373–383. [PubMed] [Google Scholar]
- 12.Shepard NT, Speers RA. Head movement modification to sensory organization protocol of EquiTest: clinical utility in a random sample of balance disorder patients; Paper presented at the 20th Barany Society Meeting; 1998; Wuerzburg, Germany. [Google Scholar]
- 13.Paloski WH, Wood SJ, Feiverson AH, Black FO, Hwang EY, Reschke MF. Destabilization of human balance control by static and dynamic head tilts. Gait Posture. 2006;23:315–323. doi: 10.1016/j.gaitpost.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Mishra A, Davis S, Speers R, Shepard NT. Head-shake computerized dynamic posturography in peripheral vestibular lesions. Am. J. Audiol. 2009;18(1):53–59. doi: 10.1044/1059-0889(2009/06-0024). [DOI] [PubMed] [Google Scholar]
- 15.Honaker JA, Converse CM, Shepard NT. Modified Head Shake Computerized Dynamic Posturography. Am. J. Audiol. 2009;18:108–113. doi: 10.1044/1059-0889(2009/09-0012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters JF. Computerized dynamic posturography (CDP) and the assessment of balance with active head movements. J. Korean Balance Soc. 2007;6(2):243–247. [Google Scholar]
- 17.Jongkee LB, Maas J, Philipszoon A. Clinical nystagmography: a detailed study of electronystagmography in 341 patients with vertigo. Pract. Otorhinolaryngol. (Basel) 1962;24:65–93. [PubMed] [Google Scholar]
- 18.Leitner C, Mair P, Paul M, Wick F, Mittermaier C, Sycha T, Ebenbichler G. Reliability of posturographic measurements in the assessment of impaired sensorimotor function in chronic low back pain. J. Electromyog. Kinesiol. 2009;19:380–390. doi: 10.1016/j.jelekin.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Lalkhen AG, McCluskey A. Clinical tests: sensitivity and specificity. Contin. Educ. Anaesth. Crit. Care. 2008;8(6):221–223. [Google Scholar]
- 20.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:39–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 21.DeLong ER, DeLong DM, Clarke-Pearson DJ. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 22.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th. Boston, MA: Pearson; 2007. [Google Scholar]
- 23.Clark S, Iltis P. Effects of dynamic head tilts on sensory organization test performance: a comparison between college-age athletes and nonathletes. J. Orthop. Sports Phys. Ther. 2008;38(5):262–268. doi: 10.2519/jospt.2008.2406. [DOI] [PubMed] [Google Scholar]
- 24.Jackson RT, Epstein CM. Effect of head extension on equilibrium in normal subjects. Ann. Otol. Rhinol. Laryngol. 1991;100(1):63–67. doi: 10.1177/000348949110000110. [DOI] [PubMed] [Google Scholar]
- 25.Jackson RT, Epstein CM, Boyette JE. Enhancement of posturography testing with head tilt and energy measurements. Am. J. Otol. 1991;12(6):420–425. [PubMed] [Google Scholar]
- 26.Barin K, Seitz CM, Welling B. Effect of head orientation on the diagnostic sensitivity of posturography in patients with compensated unilateral lesions. Otolaryngol. Head Neck Surg. 1992;106(4):355–362. doi: 10.1177/019459989210600407. [DOI] [PubMed] [Google Scholar]
- 27.Brandt T, Krafczyk S, Malsbenden I. Postural imbalance with head extension: improvement by training as a model for ataxia therapy. Ann. N.Y. Acad. Sci. 1981;374:636–649. doi: 10.1111/j.1749-6632.1981.tb30907.x. [DOI] [PubMed] [Google Scholar]
- 28.Panosian MMS, Paige GD. Nystagmus and postural instability after headshake in patients with vestibular dysfunction. Otolaryngol. Head Neck Surg. 1995;112:399–404. doi: 10.1016/S0194-59989570273-3. [DOI] [PubMed] [Google Scholar]
- 29.Shepard NT, Telian SA. Practical management of the balance disorder patient. San Diego: Singular Publishing Group; 1996. [Google Scholar]
- 30.Schubert MC, Tusa RJ, Grine LE, Herdman SJ. Optimizing the sensitivity of the head thrust test for identifying vestibular hypofunction. Phys. Ther. 2004;84:151–158. [PubMed] [Google Scholar]
- 31.Schwesig R, Becker S, Fischer D. Intraobserver reliability of posturography in healthy subjects. Somatosens. Mot. Res. 2014;31(1):16–22. doi: 10.3109/08990220.2013.819797. [DOI] [PubMed] [Google Scholar]
- 32.Pang MYC, Lam FM, Wong GH, Au IH, Chow DL. Balance performance in head-shake computerized dynamic posturography: aging effects and test-retest reliability. Phys. Ther. 2016;91(2):246–253. doi: 10.2522/ptj.20100221. [DOI] [PubMed] [Google Scholar]