Abstract
Purpose
To conduct a phase I clinical trial exploring the safety and efficacy of ruxolitinib, a JAK1/2 inhibitor, for chronic myelomonocytic leukemia (CMML).
Experimental Design
Patients with CMML-1 were included without regard to previous therapy. Key exclusion criteria included an absolute neutrophil count (ANC) <0.25 × 103 cells/dL and a platelet count <35 × 103 cells/dL. Four cohorts were enrolled using a "rolling six" study design, with doses ranging from 5 to 20 mg twice daily of ruxolitinib in 5-mg dose escalations.
Results
Between March 2013 and January 2015, 20 patients were enrolled and treated with ruxolitinib. Seventy percent of patients had the proliferative subtype and 47% had higher risk disease by the Global MD Anderson Scoring System. Eight patients (42%) received a prior hypomethylating agent. No dose-limiting toxicities for ruxolitinib were identified. One subject had grade (G)3 thrombocytopenia with no other drug-associated G3 or G4 adverse events. The mean duration of therapy was 122 days (range, 28–409 days). Four had hematologic improvement and one patient had a partial response per 2006 International Working Group (IWG) criteria. Five of 9 patients with splenomegaly had a reduction in spleen size. Ten of 11 patients with reported disease-related symptoms had clinically meaningful or complete resolution. When combining IWG and spleen responses, a total response rate of 35% (n = 7) was identified. Correlative analysis demonstrated a reduction in inflammatory cytokines and GM-CSF–dependent STAT5 phosphorylation.
Conclusions
The recommended phase II dose of ruxolitinib is 20 mg twice daily. We demonstrate that ruxolitinib has promising activity in CMML with particular benefit in those with disease-related B symptoms that warrants further study.
Introduction
Chronic myelomonocytic leukemia (CMML) is an aggressive adult myeloid neoplasm classified as a myelodysplastic syndrome/myeloproliferative neoplasm (MDS/MPN) and characterized by peripheral blood monocytosis, bone marrow dysplasia, frequent occurrence of extramedullary disease, and a propensity for progression to acute myeloid leukemia (AML; ref. 1). The morbidity that accompanies this diagnosis can include transfusion-dependent cytopenias, symptomatic splenomegaly, and disease-related fatigue or other constitutional symptoms.
Although the DNA hypomethylating agents (HMA) azacitidine and decitabine are approved for use in CMML by the FDA, a paucity of CMML patients were included in registration trials, and no treatment has been shown to impact the natural history of this disease (2). CMML is a disease entity distinct from pure MDS or MPN, with a unique biology, mutational landscape, disease manifestations, and natural history (3, 4). It can be further classified as MDS-CMML or MPN-CMML based on the absence or presence of leukocytosis, respectively (5). HMA therapy can temporarily improve cytopenias in MDS-CMML, but is associated with less favorable outcomes in MPN-CMML, which constitutes 50% of CMML cases (6–8). Cytoreductive agents such as hydroxyurea or cytarabine can reduce leukocytosis and splenomegaly in MPN-CMML, but usually worsen cytopenias and have modest impact on disease-associated symptoms. Given the limited treatment options for CMML, there is a clear unmet need for the development of novel therapeutics.
Although the genetic landscape of CMML is heterogeneous, we have previously shown that the majority of CMML cases display GM-CSF hypersensitivity, as has also been reported in the pediatric myeloid neoplasm juvenile myelomonocytic leukemia (9, 10). We further demonstrated that this pathway is a potential therapeutic target by reducing CMML cell proliferation via GM-CSF neutralization with anti-GM-CSF antibodies or JAK2 inhibitors in vitro (9). On the basis of this preclinical evidence, we conducted a phase I study of the JAK1/2 inhibitor ruxolitinib, which is approved by the FDA for patients with intermediate or higher risk myelofibrosis or polycythemia vera, to explore its safety and, as a secondary objective, its efficacy in patients with CMML (11, 12).
Patients and Methods
Study population
Patients had a diagnosis of CMML per World Health Organization (WHO) criteria that was confirmed at each treatment center. These criteria require that patients have a sustained peripheral monocyte count greater than 1 × 103 cells/dL, evidence of dysplasia or clonality, and the exclusion of other hematologic malignancies such as chronic myeloid leukemia or other MPNs. Both treatment-naïve and pretreated patients were allowed on study. Eligibility criteria included a platelet count of greater than 35 × 103 cells/dL, an absolute neutrophil count (ANC) greater than 250 cells/dL, a serum creatinine ≤2.0 mg/dL, and a serum total bilirubin <1.5 times the laboratory upper limit of normal. Other eligibility criteria included age greater than 18 years and an Eastern Cooperative Oncology Group performance status score of 0, 1, or 2. Concurrent GM-CSF treatment was not allowed and patients with a history of metastatic malignancy within the last 2 years were excluded. Erythropoietin and G-CSF recombinant products were allowed provided the patient was on therapy for at least 8 weeks. Previous clinical therapies required a wash-out period of at least 28 days. The study was approved by each of the MDS Clinical Research Consortium centers' respective institutional review board and investigators abided by good clinical practice guidelines. Each participant provided informed consent before initiating study procedures. The study was registered at clinicaltrial.gov (NCT01776723).
Study design
A dose escalation, "rolling six" phase I study design was employed (13). Subjects were allocated to starting daily doses of ruxolitinib of 10 to 40 mg/day, divided in two equal doses, and escalated by increments of 10 mg/day for each cohort according to the predetermined algorithm outlined in Supplementary Table S1. Each cohort could include up to 6 subjects. One cycle was defined as 4 weeks of therapy without interruption. Patients were treated for a total of 16 weeks but were allowed to continue therapy until disease progression or prohibitive toxicity. Dose-limiting toxicities (DLT) were defined as any grade 4 hematologic toxicity and any grade 3 or greater nonhematologic toxicity except nausea that is controlled by antiemetic therapy based on the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. To discern disease-specific versus drug-specific myelosuppression, grade 4 thrombocytopenia was defined as a decrease of ≥ 50% from baseline pretreatment values and a level of < 25,000/dL. Grade 4 neutropenia was defined as a decrease of ≥ 50% from baseline and a level of <500/dL. For dose-escalation purposes, subjects were evaluated for DLTs during the first and second cycles of therapy. The MTD was defined as the highest dose where less than 33% of subjects experience a drug-related predefined DLT.
Response criteria
Blood and bone marrow response and progression were assessed according to the International Working Group (IWG) 2006 criteria for MDS (14). Best responses were annotated and reported. Symptoms were assessed with the EORTC-QLQ-C30 and the FACT-Leu scales (15, 16). Chart review and investigator interview were also performed to determine whether ruxolitinib resulted in a clinically meaningful improvement in disease-related symptoms. Spleen length was assessed by physical exam. A spleen response was recorded if the spleen length below the left costal margin was reduced by >50% as recorded by a tape measure from the costal margin to the spleen tip on physical exam. Bone marrow biopsies were performed during screening and at the end of cycles 2 and 4. Complete blood counts were assessed at screening and weekly during treatment cycles at local laboratories. Patients were considered evaluable for response if they received at least one dose of ruxolitinib.
Mutational analysis
Using a portion of cryopreserved bone marrow mononuclear cells (BMNC) from aspirates, 50 to 100 µg of DNA was isolated as described previously (17). Amplicon-based targeted next-generation sequencing with a custom Qiagen GeneRead myeloid panel was performed to determine the presence of recurrent JAK2, CBL, NRAS, KRAS, RUNX1, TET2, SRSF2, EZH2, IDH1/2, KIT, ASXL1, and SF3B1 gene mutations, including variant allele frequency from pretreatment and end of study, or best response samples when available. Sequential samples from the same patient were only used if the DNA was derived from the same tissue source. Sequencing was performed on a Miseq personal sequencer (illumina) and analysis performed with NextGene (Softgenetics LLC) and GeneRead DNA (Qiagen) genomic software as described previously (17). Variants present in the Exome Variant Server (NHLBI; Bethesda, MD) or the 1000-Genome Project databases at a frequency of >1% were considered likely to be germ line. Only mutations occurring in the coding region were considered. Frame-shift, nonsense, and missense variants occurring at a variant frequency of >5%that had a PolyPhen-2 and SIFT prediction score of probably damaging or higher were included in the final analysis (18, 19).
pSTAT5 and cytokine analysis
Cryopreserved BMNCs were thawed and aliquots were either treated with 10 ng/mL of GM-CSF for 15 minutes or left untreated. Cells were fixed, permeabilized, and stained with an anti-pSTAT5 Alexa647 antibody as described previously (9). The 95th percentile of cells in the stimulated gate was measured under basal and GM-CSF–stimulated conditions for pretreatment and end of study or best response samples when available using Cytobank flow cytometry (Cytobank Inc; ref. 20). Bone marrow plasma was isolated by collecting the supernatant of Ficoll-separated, red blood cell–lysed whole bone marrow. Proinflammatory cytokine levels were measured in pretreatment and end of study or week 17 samples when available, using an Inflammatory Cytokine Human 30-Plex Panel (Luminex).
Plasma inhibitory assay
Frozen peripheral blood plasma samples isolated prior to the first dose, 3 hours after the first dose of ruxolitinib, and while on therapy (mean 10 weeks, range 5–16 weeks). The plasma inhibitory assay (PIA) was performed as previously described for FLT3 inhibitors (21). For each condition above 0.75 × 106 THP1 cells were incubated with 0.75 mL of plasma at 37°C for 2 hours in duplicate, adding 10 ng/µL of GM-CSF in the last 30 minutes of incubation for one of the arms. After incubation, the cells were washed twice with PBS and prepared for pSTAT5 analysis as stated above.
Results
Patient characteristics
Between March 2013 and January 2015, 20 patients were enrolled at 3 MDS clinical research consortium sites and treated with ruxolitinib. Table 1 describes the baseline characteristics of these patients. The median age was 71 (48–84) years and 75% of patients were male. All patients had CMML-1 by the WHO criteria and 70% (n = 14) had MPN-CMML by French–American–British (FAB) criteria. Forty-five percent of patients (n = 9) had palpable splenomegaly and 30% of patients had intermediate-2 or higher risk disease as measured by the Global MD Anderson Prognostic Scoring System (22). Eleven patients had disease-related symptoms (55%) and 11 (55%) received prior therapy, 8 of whom had been treated with a HMA.
Table 1.
Baseline characteristics of CMML patients treated with ruxolitinib
| Baseline characteristic | (n = 20) |
|---|---|
| Median age (range) | 69 (48–84) |
| Male (%) | 15 (75%) |
| WHO classification | |
| CMML-1 (%) | 20 (100%) |
| CMML-2 (%) | 0 (0%) |
| FAB classification | |
| MDS-CMML (%) | 6 (30%) |
| MPN-CMML (%) | 14 (70%) |
| Risk stratification (MDASC) | |
| Lower risk (%) | 70% (14) |
| Higher risk (%) | 30% (6) |
| Hypomethylating agent status | |
| Treatment (%) | 40% (8) |
| No treatment (%) | 60% (12) |
| Splenomegaly (%) | 9 (45%) |
| Median WBC × 103/dL (range) | 22 (2.3–92.9) |
| Median AMC × 103/dL (range) | 2.93 (0.61–5.83) |
| Median HGB g/dL | 10.4 (6.3–14.2) |
| Median PLT × 103/dL | 152 (41–488) |
| Disease-related/constitutional symptomsa | 11 (55%) |
Abbreviations: AMC, absolute monocyte count; HGB, hemoglobin; MDASC, Global MD Anderson Scoring System; PLT, platelets; WBC, white blood cell count.
As reported by medical record abstraction.
Dose escalation and toxicity
Four, twice-daily dose levels were evaluated without respect to food (23; 5, 10, 15, and 20 mg). Six patients were enrolled in cohort 1 (5 mg twice daily), 4 patients in cohort 2 (10 mg twice daily), 5 patients in cohort 3 (15 mg twice daily), and 5 patients were enrolled in cohort 4 (20 mg twice daily). All 20 patients enrolled were evaluable for toxicity. No DLTs were observed in any of the cohorts during the 2-cycle evaluation period. Summaries of possible, probable, or definite treatment-emergent toxicities occurring at a frequency of greater than 10% are in Table 2 and all toxicities recorded are available in Supplementary Table S2. There were no grade 3 or 4 treatment-emergent hematologic toxicities at any dose level, which is distinct from phase I toxicity data reported in primary myelofibrosis (24). The median hemoglobin for cohorts 1 to 4 was 9.9, 10.2, 11.8, and 10.2 g/dL, respectively (median 10.5 g/dL, range 6.3–14.2 g/dL). A total of 8 patients (40%) were packed red blood cell transfusion dependent at study entry. The median platelet count of cohorts 1 to 4 was 143, 200, 156, and 120 cells × 103/dL, respectively (median of study 152 × 103 cells/dL, range 41–488 × 103 cells/dL). No patients were platelet transfusion dependent at study entry. Weekly hemoglobin and platelet counts did not differ significantly while on ruxolitinib therapy and are plotted in Supplementary Fig. S1.
Table 2.
Treatment-emergent toxicities occurring at a frequency of greater than or equal to 10%
| Toxicity | Grade 1/2 | Grade 3/4 | Total (%) |
|---|---|---|---|
| Fatigue/weakness | 6 | 2 | 8 (40) |
| Nausea/vomiting | 8 | 0 | 8 (40) |
| Shortness of Breath/dyspnea | 5 | 2 | 7 (30) |
| Diarrhea | 5 | 1 | 6 (26) |
| Anemia | 3 | 0 | 3 (15) |
| Headache | 3 | 0 | 3 (15) |
| Rash/pruritus | 2 | 0 | 2 (10) |
| Constipation | 2 | 0 | 2 (10) |
| Mucositis | 2 | 0 | 2 (10) |
| Gastroesophageal reflex disease | 2 | 0 | 2 (10) |
| Cramping | 2 | 0 | 2 (10) |
Clinical response and duration
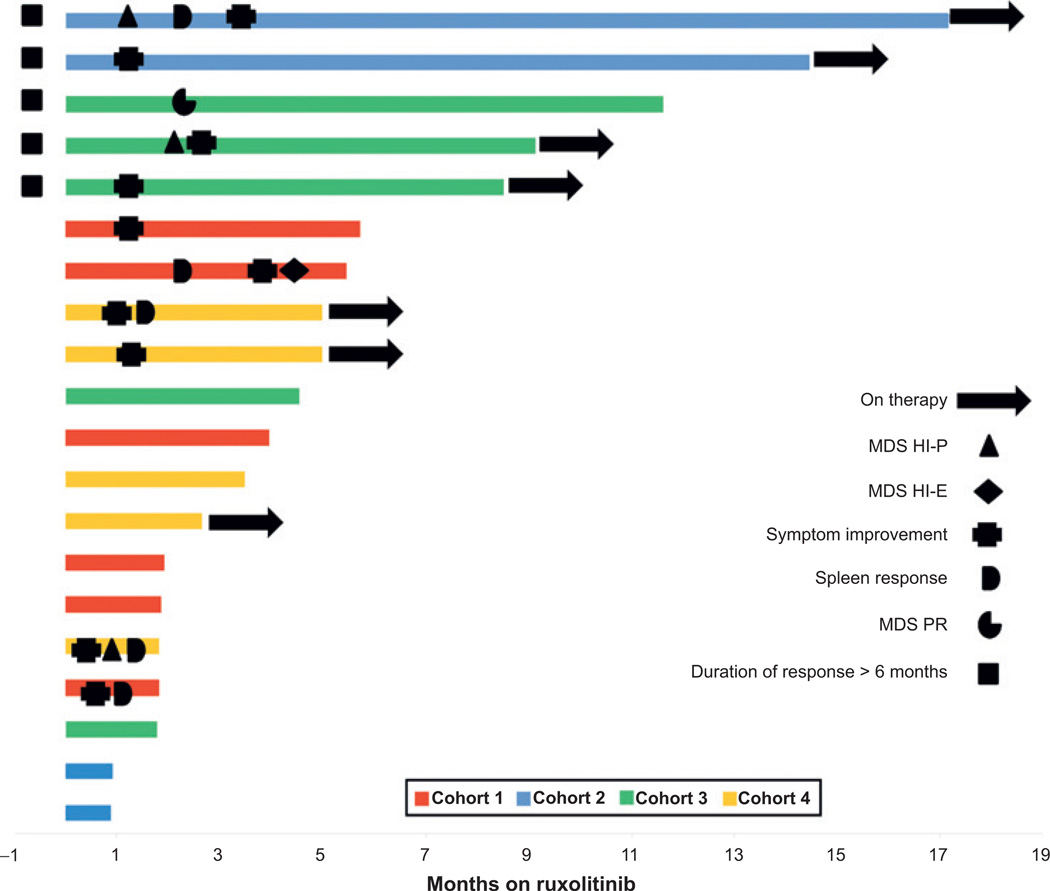
Hematologic and bone marrow responses were assessed by the IWG 2006 MDS guidelines a priori. Four patients met criteria for hematologic improvement by IWG MDS criteria and one patient achieved a partial bone marrow response. The details of each patient who achieved a response are summarized in Supplementary Table S3. In total, 5 of 9 patients with palpable splenomegaly had >50% improvement in spleen size. Eleven of 20 patients had constitutional symptoms or severe fatigue preventing patients from completing activities of daily living, at baseline. Of these 11 patients, 10 had resolution or clinically meaningful improvement in these symptoms. However, the EORTC-QLQ30 and FACT-Leu were not able to identify interval improvement in symptoms despite clear benefit noted by patients to investigators (Supplementary Fig. S2). The duration of therapy and responses are demonstrated by the Swimmer plot in Fig. 1 (mean duration of therapy was 122 days; range, 28–409 days). The most frequent reason for therapy discontinuation was disease progression. However, no patients transformed to AML while on therapy. A detailed description of reasons for discontinuation is located in Supplementary Table S3. When combining spleen and MDS IWG responses, the overall response rate was 35% (n = 7) for the entire cohort. There was no difference in response based on FAB subclassification of MDS-CMML versus MPN-CMML (P = 0.6).
Figure 1.
Swimmer plot of clinical response and DoT. Plot demonstrating duration of therapy in months. Legend in plot describes symbols and colors denote cohort. Information regarding treatment discontinuation is located in Supplementary Table S3.
Correlative analysis
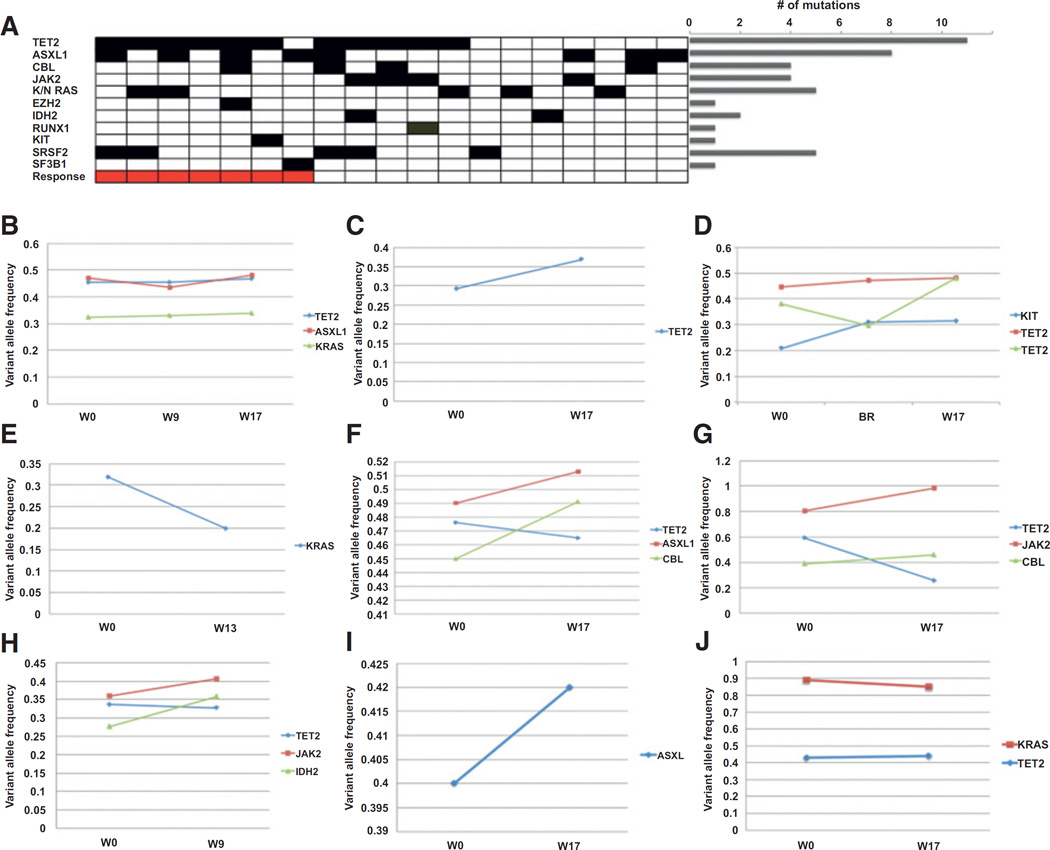
Mutational profiling
Nineteen of 20 patients had pretreatment DNA from blood or bone marrow available for mutational profiling and 9 of 20 had sequential samples available for analysis. The spectrum of mutations in this cohort is shown in Fig. 2A. The most frequent gene mutated was TET2 (58%, n = 11), followed by ASXL1 (42%, n = 8) and SRSF2 (26%, n = 5) as previously described for CMML (25). The frequency of mutations in JAK2 (21%, n = 4) was higher and the frequency of SRSF2 and RUNX1 (5%, n = 1) were lower than previously published (25). This may be explained by the increased number of patients enrolled with MPN-CMML and the ineligibility of patients with severe thrombocytopenia compared with historical cohorts for JAK2 and RUNX1, respectively (26). No statistically significant differences in response were seen when the comparison was grouped at the gene level (i.e., TET2, SRSF2, JAK2) or the pathway level (i.e., signaling, splicing, and epigenetic pathway mutations). In those cases in which sequential sequencing was performed at screening and week 17 (± 2 weeks) or end of study, no difference in the pattern of mutant allelic burden was identified between responders (n = 3) and nonresponders (n = 7), as shown in Fig. 2B–J. Minimal change in mutant allele frequency was identified during therapy, consistent with previous reports of short-term ruxolitinib therapy in myelofibrosis (11).
Figure 2.
Mutational profiling of CMML patients treated with ruxolitinib. A, the mutational landscape of CMML patients on trial. Patients are represented in columns and gene mutations in rows. Cases sorted by combined MDS IWG 2006 and spleen response. The number of mutations seen in the entire cohort is denoted in the bar plot (L). B–D, sequential variant allele frequency of responding patients from 0 to 1.00. E–J, sequential variant allele frequency of nonresponding patients. x-axis denotes week (W) and/or best response (BR).
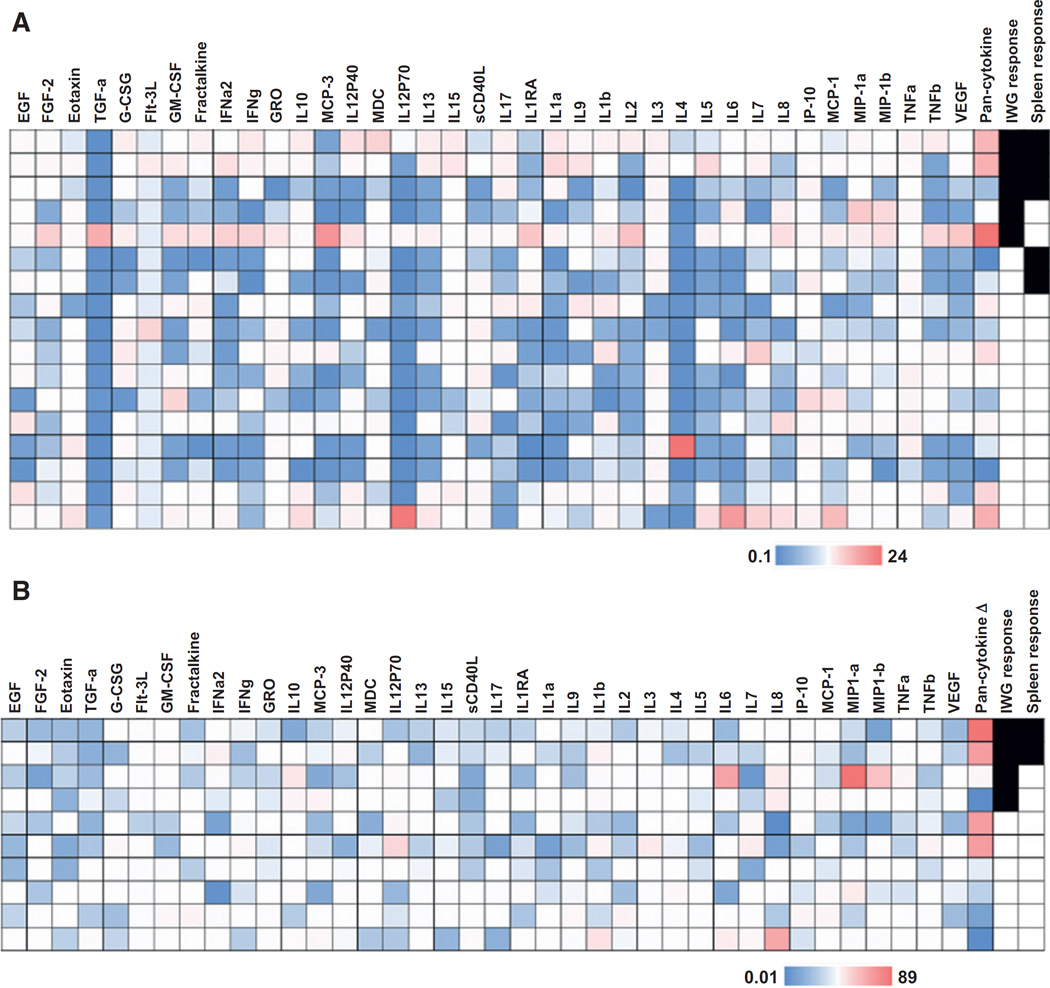
Inflammatory cytokine analysis
Given previous data demonstrating the capacity of ruxolitinib to downregulate a broad array of inflammatory cytokines in myelofibrosis, we explored the effect of ruxolitinib on cytokine levels in CMML and correlation with response (24). Seventeen of 20 cases had bone marrow plasma available for cytokine profiling and 10 of 20 cases had sequential samples available for analysis. Pretreatment levels of VEGF, MCP-1, IP-10, IL8, IL1RA, sCD40L, MDC, MCP-3, GRO, TGFα, eotaxin, and FGF-2 were increased in our cohort relative to other cytokines tested (data not shown). There was no association between any single cytokine and clinical response. Furthermore, there were no statistical associations but a trend identified with response for those patients that multiple increased cytokines (pan-cytokine column), as denoted in Fig. 3A. In those cases in which sequential bone marrow plasma samples were present at screening and week 17 (± 2 weeks) or end of study, EGF, FGF-2, eotaxin, TGFα, IFNγ, GRO, MCP-3, IL12P40, IL15, sCD40L, IL1RA, IL9, IL2, MIP-1a, TNFβ, and VEGF were downregulated in 50% or more of cases analyzed. When comparing downregulation of cytokines to response, 2 of 4 cases with >20-fold downregulated cytokines had both a MDS IWG and spleen response (Fig. 3B). The other two cases had a clinically meaningful improvement in symptoms and duration of treatment (DoT) of 509 (remains on treatment) and 172 days (mean DoT 122), suggesting clinical benefit not captured by IWG response criteria.
Figure 3.
Inflammatory cytokine profiling of CMML patients treated with ruxolitinib. Heatmap demonstrating cytokine levels in the context of ruxolitinib. Legend is denoted on the figure. Red=higher levels, Blue=lower levels. Cases that achieved an MDS IWG 2006 response and/or spleen response are denoted. A, heatmap demonstrating cytokine levels relative to the mean level of each respective cytokine. "Pan-cytokine" is defined as the number of cytokines elevated above the mean in any given subject (n = 17). B, heatmap demonstrating downregulation of cytokines after week 17 in 10 subjects. "Pan-cytokine Δ" is the number of cytokines downregulated after therapy in any given subject. Those in red had at least 20 cytokines decreased compared with pretreatment levels. Responses denoted as in (A).
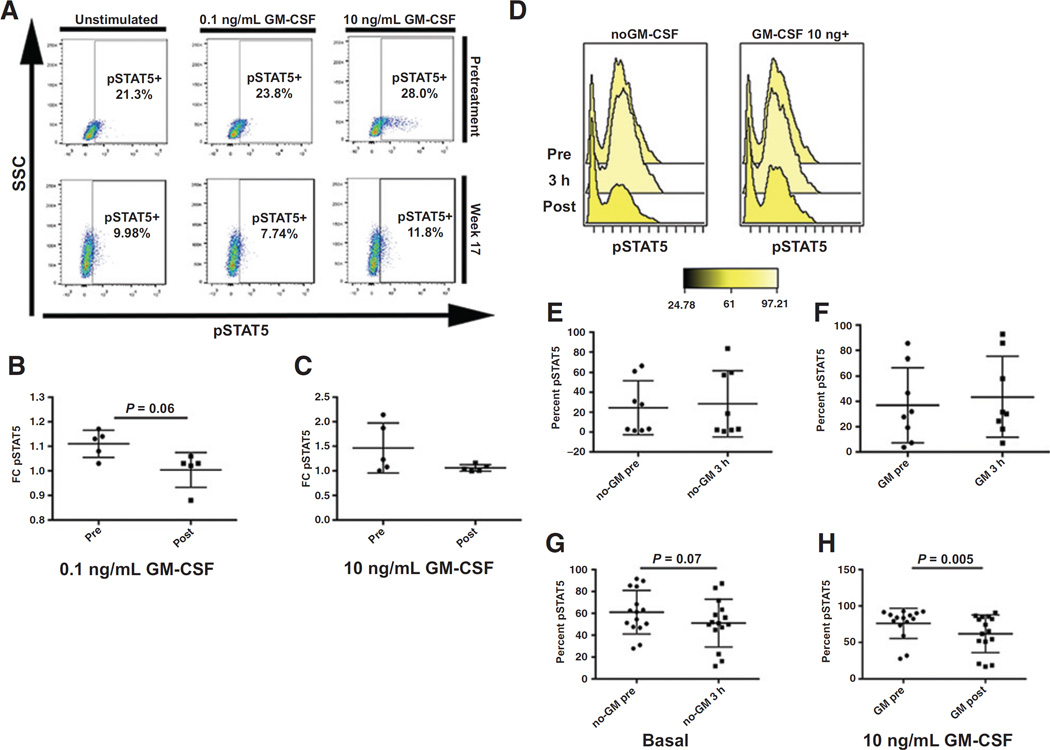
GM-CSF–dependent activation of pSTAT5
We tested the basal and GM-CSF induction of pSTAT5 in 16 of 20 cases on study. As shown in Fig. 4A and B, no differences were seen in prestudy GM-CSF pSTAT5 induction between responding and nonresponding groups when comparing the fold-change increase in pSTAT5 from basal levels. There was also no difference in basal pSTAT5 levels when comparing responders and nonresponders (data not shown). In 5 cases with informative sequential samples, GM-CSF pSTAT5 induction was reduced (P = 0.06) in week 17 ± 2 weeks when compared with its prestudy counterpart (Fig. 4A–C).
Figure 4.
Ruxolitinib suppresses GM-CSF–dependent pSTAT5 activation. A, representative sample demonstrating change in pSTAT5 activation while on ruxolitinib therapy. Percent of cells in pSTAT5 gate are noted. B, fold change of GM-CSF 0.1 ng/mL stimulated pSTAT5 levels relative to basal pSTAT5 in pretreatment and week 17 (± 2 weeks) bone marrow samples (n = 5). C, fold change of GM-CSF 10 ng/mL stimulated pSTAT5 levels relative to basal pSTAT5 in pretreatment and week 17 (±2 weeks) bone marrow samples (n = 5). D–H, PIA results demonstrating the percent of THP-1 cells in the pSTAT5 gate comparing representative sample of THP-1 cells treated with patient plasma (D), basal pretreatment or screening samples (pre) with samples taken 3 hours after the first dose (3 hours; n = 11; E, GM-CSF 10 ng/mL stimulated pretreatment or screening samples (pre) with samples taken 3 hours after the first dose (3 hours; n = 11; F, basal pretreatment or screening samples (pre) with samples taken while patient was on study for an average of 10 weeks (post). (n = 15; G). H, GM-stimulated 10 ng/mL pretreatment or screening samples (pre) with samples taken while patient was on study for an average of 10 weeks (post). (n = 15).
To determine whether sufficient free drug was available in the peripheral blood to downregulate pSTAT5, we performed the PIA assay as previously described. Using THP-1 cells, we tested the capacity of peripheral plasma from patients 3 hours posttreatment or while on study (median 10 weeks) to downregulate pSTAT5 in the cell line relative to the corresponding screening or pretreatment plasma. As shown in Fig. 4D–H, on-treatment plasma (n = 15) was able to downregulate both basal and GM-CSF–dependent pSTAT5 suggesting that ruxolitinib was able to effectively target the JAK/STAT pathway in CMML. No difference in downregulation of pSTAT5 was seen in between cohorts or doses.
Discussion
There are only a few active therapies for CMML. Therapies that can be useful in MDS, such as HMAs, are of limited value in treatment of splenomegaly and other MPN-like symptoms in CMML. The majority of agents tested in the CMML context have either had modest activity or unacceptable toxicity (27–30). In part, CMML therapy has lagged behind that for MDS because CMML has been grouped with MDS historically despite biologic differences from MDS and clinically distinct behavior, and also because it's reported incidence rate is low: on the order of 0.4 per 100,000 persons per year in the United States (31). However, CMML incidence may be underestimated, in part, because no CMML-specific therapeutic strategy exists that would provide compelling reason to stratify CMML from MDS in clinical practice.
The spleen and symptom-related activity of ruxolitinib in myelofibrosis and our preclinical in vitro data identified JAK2 as a potential therapeutic target in CMML and provided the rationale for the current study. Given the reported high rate of myelosuppression in myelofibrosis and polycythemia vera seen with ruxolitinib, we performed a phase I clinical trial to determine the MTD in CMML and to preliminarly explore efficacy. In our study, ruxolitinib was well tolerated at all dose levels with minimal toxicity. We did not observe the severe hematologic toxicity reported in MPNs, despite similar dosing, suggesting a disease-specific favorable toxicity profile. Although the underlying biology for this is not clear, these findings provide a platform to begin to understand the microenvironmental or cell intrinsic mechanisms by which hematologic toxicity is spared in CMML.
Although data generated from murine JAK2 knockout models indicate that the hematopoietic suppression of ruxolitinib is secondary to "on target" cytokine suppression, emerging clinical data suggests a more complex underlying etiology (32). For example, pacritinib and momelotinib, two JAK inhibitors in clinical development, have been tested in myelofibrosis with less hematologic toxicity compared with ruxolitinib (33, 34).
In addition to a favorable hematologic toxicity profile, 3 patients in this study achieved durable hematologic responses by MDS IWG criteria. Furthermore, 5 of 9 patients achieved a >50% reduction in splenomegaly, (26) and the majority of patients derived improvement in physician-reported disease-related symptomatology. The latter is particularly notable because, to our knowledge, there are no large studies reporting the incidence or quality of these symptoms in CMML. In our experience, the magnitude of these symptoms was only fully appreciated after evaluating responding patients on treatment.
Although abstraction of medical records and investigator interview confirm a dramatic improvement in fatigue and B-symptoms, the patient reported FACT-Leu and EORTC-QLQ-C30 scales did not support this. This discrepancy is explained by either an overestimation of effect detailed in the medical record or a lack of sensitivity of the tested scales to measure the improvement in symptoms. We favor the latter explanation because the tools used in this study were exploratory and have not been validated in CMML. Furthermore, anecdotes of patients who did not wish to be removed from study for objective disease progression because of dramatic improvement in fatigue were common on study.
The correlative laboratory studies demonstrate a downregulation of GM-CSF–dependent pSTAT5 posttreatment, suggesting adequate on target effect. However, we did not find a correlation between basal GM-CSF–dependent pSTAT5 activation and response to ruxolitinib. In addition, when profiling mutational composition and inflammatory cytokines we additional found no statistically associated basal marker that predicted clinical response. However, sequential profiling did reveal that ruxolitinib appears to have a similar effect on inflammatory cytokines in CMML compared with myelofibrosis, and a lack of alteration in mutation burden, also similar to MPN (24).
To our knowledge, this study represents among the first CMML-specific, biologically driven clinical trials. We demonstrated that ruxolitinib is safe and has a favorable hematologic toxicity profile in the context of CMML at a recommended phase II dose of 20 mg twice daily. We observed a broad range of activity that includes hematologic, spleen, and symptom response and correlative analysis highlighted the frequency of ruxolitinib-associated cytokine depletion in this disease. A phase II study is planned that will incorporate the MPN-Symptom Assessment Form, which has been preliminarily demonstrated to have sensitivity in CMML and is also incorporated in recent MDS/MPN–specific response criteria that were not available when this trial was conducted (35).
Supplementary Material
Translation Relevance.
Chronic myelomonocytic leukemia (CMML) is an aggressive leukemia with no available disease-modifying therapies. There is a specific void in the therapeutic armamentarium of CMML for those patients who have been treated with hypomethylating agents and those that have myeloproliferative symptoms. This phase I clinical trial identifies ruxolitinib as a clinical therapeutic in CMML with notable efficacy in those patients with splenomegaly and constitutional symptoms even after treatment with hypomethylating agents. We additionally find that ruxolitinib is well tolerated and, in contrast to myelofibrosis, is not associated with significant hematologic toxicity even at 20 mg twice daily. Taken together, this study provides the first clinical evidence that ruxolitinib, and by extension the JAK/STAT pathway, is a targetable and clinically relevant vulnerability in CMML.
Acknowledgments
Grant Support
This study was supported (in part) by research funding from the Evans Foundation (E. Padron and R.S. Komrokji), the American Society of Hematology Junior Faculty Scholar Award (E. Padron), and Incyte Corporation (E. Padron).
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Prior presentation: Preliminary results were presented, in part, at the 2015 ASCO Annual Meeting.
Disclosure of Potential Conflicts of Interest
E. Padron reports receiving commercial research grants from Cell Therapeutics and Incyte Corporation, speakers bureau honoraria from Novartis, and is a consultant/advisory board member for Cell Therapeutics. K. Vaddi has ownership interest (including patents) in Incyte Corporation. S. Tinsley reports receiving speakers bureau honoraria from Incyte Corporation. D.P. Steensma is a consultant/advisory board member for Amgen, Celgene, Incyte Corporation, and Novartis. R.S. Komrokji reports receiving speakers bureau honoraria from and is a consultant/advisory board member for Incyte Corporation. No potential conflicts of interest were disclosed by the other authors.
Authors' Contributions
Conception and design: E. Padron, K. Vaddi, D.P. Steensma, A.F. List, M.A. Sekeres, R.S. Komrokji
Development of methodology: E. Padron, M. Andrade-Campos, Q. Zhang, R.S. Komrokji
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): E. Padron, P. Scherle, Q. Zhang, Y. Ma, M.E. Balasis, S. Tinsley, C. Zimmerman, G.J. Roboz, J.E. Lancet, M.A. Sekeres, R.S. Komrokji
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): E. Padron, A. Dezern, Q. Zhang, M.E. Balasis, D.P. Steensma, M.A. Sekeres, R.S. Komrokji
Writing, review, and/or revision of the manuscript: E. Padron, A. Dezern, M. Andrade-Campos, P. Scherle, M.E. Balasis, D.P. Steensma, G.J. Roboz, J.E. Lancet, A.F. List, M.A. Sekeres, R.S. Komrokji
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): Q. Zhang, H. Ramadan, C. Zimmerman Study supervision: E. Padron, C. Zimmerman, R.S. Komrokji
Other (performed physical examination of study participants and collected patients history concerning side effects of treatment): S. Tinsley
References
- 1.Padron E, Komrokji R, List AF. The clinical management of chronic myelomonocytic leukemia. Clin Adv Hematol Oncol. 2014;12:172–178. [PubMed] [Google Scholar]
- 2.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padron E, Steensma DP. Cutting the cord from myelodysplastic syndromes: chronic myelomonocytic leukemia-specific biology and management strategies. Curr Opin Hematol. 2015;22:163–170. doi: 10.1097/MOH.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 4.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 5.Bennett JM, Catovsky D, Daniel M-T, Flandrin G, Galton DAG, Gralnick HR, et al. Proposals for the Classification of the Acute Leukaemias French-American-British (FAB) Co-operative Group. Br J Haematol. 1976;33:451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 6.Ades L, Sekeres MA, Wolfromm A, Teichman ML, Tiu RV, Itzykson R, et al. Predictive factors of response and survival among chronic myelomonocytic leukemia patients treated with azacitidine. Leuk Res. 2013;37:609–613. doi: 10.1016/j.leukres.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Costa R, Abdulhaq H, Haq B, Shadduck RK, Latsko J, Zenati M, et al. Activity of azacitidine in chronic myelomonocytic leukemia. Cancer. 2011;117:2690–2696. doi: 10.1002/cncr.25759. [DOI] [PubMed] [Google Scholar]
- 8.Braun T, Itzykson R, Renneville A, de Renzis B, Dreyfus F, Laribi K, et al. Molecular predictors of response to decitabine in advanced chronic myelomonocytic leukemia: a phase 2 trial. Blood. 2011;118:3824–3831. doi: 10.1182/blood-2011-05-352039. [DOI] [PubMed] [Google Scholar]
- 9.Padron E, Painter JS, Kunigal S, Mailloux AW, McGraw K, McDaniel JM, et al. GM-CSF-dependent pSTAT5 sensitivity is a feature with therapeutic potential in chronic myelomonocytic leukemia. Blood. 2013;121:5068–5077. doi: 10.1182/blood-2012-10-460170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emanuel PD, Bates LJ, Castleberry RP, Gualtieri RJ, Zuckerman KS. Selective hypersensitivity to granulocyte-macrophage colony-stimulating factor by juvenile chronic myeloid leukemia hematopoietic progenitors. Blood. 1991;77:925–929. [PubMed] [Google Scholar]
- 11.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 13.Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. 2008;26:190–195. doi: 10.1200/JCO.2007.12.7712. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 15.Cella D, Jensen SE, Webster K, Hongyan D, Lai JS, Rosen S, et al. Measuring health-related quality of life in leukemia: the functional assessment of cancer therapy-leukemia (FACT-Leu) questionnaire. Value Health. 2012;15:1051–1058. doi: 10.1016/j.jval.2012.08.2210. [DOI] [PubMed] [Google Scholar]
- 16.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQC30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 17.Padron E, Yoder S, Kunigal S, Mesa T, Teer JK, Al Ali N, et al. ETV6 and signaling gene mutations are associated with secondary transformation of myelodysplastic syndromes to chronic myelomonocytic leukemia. Blood. 2014;123:3675–3677. doi: 10.1182/blood-2014-03-562637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11:863–874. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotecha N, Krutzik PO, Irish JM. Web-based analysis and publication of flow cytometry experiments. Curr Protoc Cytom. 2010 doi: 10.1002/0471142956.cy1017s53. Chapter 10: Unit10 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levis M, Brown P, Smith BD, Stine A, Pham R, Stone R, et al. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood. 2006;108:3477–3483. doi: 10.1182/blood-2006-04-015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kantarjian H, O'Brien S, Ravandi F, Cortes J, Shan J, Bennett JM, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113:1351–1361. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shilling AD, Nedza FM, Emm T, Diamond S, McKeever E, Punwani N, et al. Metabolism, excretion, and pharmacokinetics of [14C]INCB018424, a selective Janus tyrosine kinase 1/2 inhibitor, in humans. Drug Metab Dispos. 2010;38:2023–2031. doi: 10.1124/dmd.110.033787. [DOI] [PubMed] [Google Scholar]
- 24.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itzykson R, Kosmider O, Renneville A, Gelsi-Boyer V, Meggendorfer M, Morabito M, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J Clin Oncol. 2013;31:2428–2436. doi: 10.1200/JCO.2012.47.3314. [DOI] [PubMed] [Google Scholar]
- 26.Padron E, Garcia-Manero G, Patnaik MM, Itzykson R, Lasho T, Nazha A, et al. An international data set for CMML validates prognostic scoring systems and demonstrates a need for novel prognostication strategies. Blood Cancer J. 2015;5:e333. doi: 10.1038/bcj.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Manero G, Gore SD, Cogle C, Ward R, Shi T, Macbeth KJ, et al. Phase I study of oral azacitidine in myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J Clin Oncol. 2011;29:2521–2527. doi: 10.1200/JCO.2010.34.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldman EJ, Cortes J, DeAngelo DJ, Holyoake T, Simonsson B, O'Brien SG, et al. On the use of lonafarnib in myelodysplastic syndrome and chronic myelomonocytic leukemia. Leukemia. 2008;22:1707–1711. doi: 10.1038/leu.2008.156. [DOI] [PubMed] [Google Scholar]
- 29.Wattel E, Guerci A, Hecquet B, Economopoulos T, Copplestone A, Mahe B, et al. A randomized trial of hydroxyurea versus VP16 in adult chronic myelomonocytic leukemia. Groupe Francais des Myelodysplasies and European CMML Group. Blood. 1996;88:2480–2487. [PubMed] [Google Scholar]
- 30.Bejanyan N, Tiu RV, Raza A, Jankowska A, Kalaycio M, Advani A, et al. A phase 2 trial of combination therapy with thalidomide, arsenic trioxide, dexamethasone, and ascorbic acid (TADA) in patients with overlap myelodysplastic/myeloproliferative neoplasms (MDS/MPN) or primary myelofibrosis (PMF) Cancer. 2012;118:3968–3976. doi: 10.1002/cncr.26741. [DOI] [PubMed] [Google Scholar]
- 31.Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 32.Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 33.Komrokji RS, Seymour JF, Roberts AW, Wadleigh M, To LB, Scherber R, et al. Results of a phase 2 study of pacritinib (SB1518), a JAK2/JAK2 (V617F) inhibitor, in patients with myelofibrosis. Blood. 2015;125:2649–2655. doi: 10.1182/blood-2013-02-484832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pardanani A, Lasho T, Smith G, Burns CJ, Fantino E, Tefferi A. CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia. 2009;23:1441–1445. doi: 10.1038/leu.2009.50. [DOI] [PubMed] [Google Scholar]
- 35.Savona MR, Malcovati L, Komrokji R, Tiu RV, Mughal TI, Orazi A, et al. An international consortium proposal of uniform response criteria for myelodysplastic/myeloproliferative neoplasms (MDS/MPN) in adults. Blood. 2015;125:1857–1865. doi: 10.1182/blood-2014-10-607341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.