Abstract
There is an ongoing and significant need for radiation countermeasures to reduce morbidities and mortalities associated with exposure of the heart and lungs from a radiological or nuclear incidents. Radiation-induced late effects occur months to years after exposure, stemming from significant tissue damage and remodeling, resulting in fibrosis and loss of function. TGF-β is reported to play a role in both pulmonary and cardiac fibrosis. We investigated the ability of a small molecule TGF-β receptor 1 inhibitor, IPW-5371, to mitigate the effects of thoracic irradiation in C57L/J mice, a murine model that most closely resembles that observed in humans in the induction of fibrosis and dose response. To simulate a radiological event, radiation was administered in two doses: 5 Gy total-body irradiation (eliciting a whole-body response) and immediately after that, a thoracic “top-up” of 6.5 Gy irradiation, for a total dose of 11.5 Gy to the thorax. IPW-5371 was administered once daily, orally, starting 24 h postirradiation for 6 or 20 weeks at a dose of 10 mg/kg or 30 mg/kg. Animals were monitored for a period of 180 days for survival, and cardiopulmonary injury was assessed by echocardiography, breathing rate and arterial oxygen saturation. Exposure of the thorax (11.5 Gy) induced both pulmonary and cardiac injury, resulting in a reduced life span with median survival of 135 days. IPW-5371 treatment for 6 weeks, at both 10 mg/kg and 30 mg/kg, delayed disease onset and mortality, with median survival of 165 days. Twenty weeks of IPW-5371 treatment at 30 mg/kg preserved arterial O2 saturation and cardiac contractile reserve and resulted in significant decreases in breathing frequency and cardiac and pulmonary fibrosis. This led to dramatic improvement in survival compared to the irradiated, vehicle-treated group (P < 0.001), and was statistically insignificant from the nonirradiated group. We observed that IPW-5371 treatment resulted in decreased pSmad3 tissue levels, confirming the effect of IPW-5371 on TGF-β signaling. These results demonstrate that IPW-5371 represents a potentially promising radiation countermeasure for the treatment of radiation-induced late effects.
INTRODUCTION
Exposure of the civilian population to ionizing radiation, either from a nuclear power plant accident or as a result of nuclear terrorism event, represents a potentially catastrophic public health emergency. Therefore, the National Institute of Allergy and Infectious Disease (NIAID) has made it a priority to fund research to develop radiation medical countermeasures to respond to such incidents.
The health consequences from exposure to radiation depend on both the dose and route of exposure. The initial effects of total-body irradiation (TBI) include the development of acute radiation syndrome involving the most radiosensitive organs, the gastrointestinal and hematopoietic systems (1, 2). For those exposed to potentially lethal doses of radiation, advances in supportive care, such as hematopoietic growth factors, fluids, electrolytes and antibiotics will enable many people to survive acute hematopoietic and gastrointestinal syndromes that in the past have proven fatal. However, these victims and others exposed to potentially toxic doses, will be at high risk for developing late toxicity in radiosensitive organs, such as the heart and lungs (3). Numerous drugs are currently being investigated as radiation countermeasures, but even with these advances in research, only two drugs have been approved by the FDA: filgrastim in March 2015 and pegfilgrastim in November 2015. Both of these drugs are approved to treat adult and pediatric patients exposed to myelosuppressive radiation doses, leaving a significant unmet need with respect to radiation-induced late effects.
Radiation-induced late effects have been extensively characterized. The initial insult is followed by an inflammatory response resulting in increased multifunctional cytokine expression and significant cellular and molecular changes often characterized by the development of fibrosis resulting in loss of organ function (4–7). Transforming growth factor beta (TGF-β) has been implicated as a key mediator of this process, regulating cell proliferation, differentiation and migration. The mRNA and/or protein levels of TGF-β are elevated in almost all fibrotic diseases and experimental models of fibrosis, including radiation-induced lung fibrosis (8–11). TGF-β is a secreted protein that exists in three isoforms, TGF-β1, TGF-β2 and TGF-β3, although studies have linked the formation of lung fibrosis primarily to TGF-β1 and signaling through the canonical pathway involving Smad2/Smad3 activation (12). Use of TGF-β receptor 1 (TGF-βRI) antagonists in the attenuation of pulmonary fibrosis has been well established in both radiation- and bleomycin-induced fibrosis (13–15). While these early studies show great promise for anti-TGF-β therapies as radiation countermeasures, the radiation protocol employed and the animal model chosen may limit their effectiveness as a radiation countermeasure.
In this study, we tested the efficacy of the orally available TGF-βR1 antagonist, IPW-5371, as a suitable countermeasure to extend survival and mitigation of radiation-induced late effects using a murine model with radiosensitivity similar to humans and a radiation protocol mimicking a nuclear accident (16). The mice were evaluated over a period of 180 days for survival as well as physiological markers for pulmonary and cardiac function.
METHODS
Animals
Both male and female C57L/J wild-type 6–8-week-old mice were purchased from Jackson Laboratory (Bar Harbor, ME). The mice were housed with a 12:12 h light-dark schedule and access to water and food ad libitum. The experiments were conducted under the guidelines of laboratory animals for biomedical research published by the National Institutes of Health (rev. 2011). The study protocol was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Experimental Plan
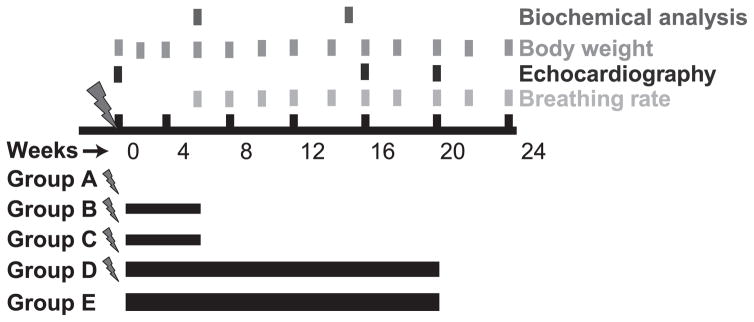
The overall experimental plan is shown in Fig. 1. Mice were randomly distributed among five treatment groups, each with equal numbers of males and females: group A (radiation only); group B (radiation with 10 mg/kg IPW-5371 for 6 weeks); group C (radiation with 30 mg/kg IPW-5371 for 6 weeks); group D (radiation with 30 mg/kg IPW-5371 for 20 weeks); and group E (30 mg/kg IPW-5371 only for 20 weeks). Drug administration began 24 h postirradiation (n = 15–20 mice per group).
FIG. 1.
Treatment and evaluation schedule. Mice were administered 5 Gy TBI plus 6.5 Gy thoracic irradiation on day 0, and 24 h later animals designated to receive IPW-5371 began treatment.
While under ketamine/xylazine anesthesia (100 mg/kg and 10 mg/kg, respectively), mice received 5 Gy TBI, immediately followed by a top-up dose of 6.5 Gy to the thorax, using a Varian 21EX LINAC (Palo Alto, CA) for a total thoracic dose of 11.5 Gy.
Pharmacokinetic Study
All animal experiments were approved by the Jubilant Biosys Institutional Animal Ethics Committee [Bangalore, India (IAEC/JDC/2015/72)] and were in compliance with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Environment, Government of India. Twelve Balb/C mice (22–30 g; age 6–7 weeks) were procured from Bioneeds Preclinical Services (Bangalore, India). Animals were housed in the Jubilant Biosys animal care facility in a temperature- and humidity-controlled room with a 12:12 h light-dark schedule, had free access to food (Provimi, Bangalore, India) and water for one week prior to experiments. After ~4 h fasting (during fasting period animals had free access to water). mice received IPW-5371 orally at a dose of 20 mg/kg (suspension formulation, prepared using 0.5% methylcellulose and Tween® 80). Blood samples (100 μl; sparse sampling was done and at each time point three mice were used for blood sampling) were collected into polypropylene tubes containing Na2-EDTA solution (pre-dose) as an anticoagulant at 0.25, 0.5, 1, 2, 4, 8, 10, 12 and 24 h. Plasma was harvested by centrifuging the blood using Biofuge™ (Heraeus™, Hanau, Germany) at 1,760g for 5 min and stored frozen at −20 ± 10°C until analysis. Animals were allowed to feed 2 h after IPW-5371 administration.
Plasma Sample Processing and Bioanalysis
To an aliquot of 50 μl plasma sample, 200 μl of 10% tetrahydrofuran in acetonitrile containing internal standard (100 ng/ml of tolbutamide) was added and vortex mixed on a cyclo mixer for 5 min. After centrifugation at 14,000 rpm for 5 min (Centrifuge 5430 R; Eppendorf, Hamburg, Germany) at 4°C, clear supernatant (100 μl) was transferred to a pre-labeled HPLC vial and 10 μl was injected onto the LC-MS/MS system for analysis. The linearity range was 1.79–2,063 ng/ml. In-study quality control (QC) samples, supplemented with concentrations of 5.38, 897 and 1,614 ng/ml of IPW-5371, were analyzed with the unknowns. The criteria for acceptance of the analytical runs encompassed the following: 67% of QC sample accuracy must be within 85–115% of the nominal concentration; and 50% of each QC concentration level must meet the acceptance criteria. After analysis was completed for both the linearity and QC samples, values were found to be within the accepted variable limits. Quantitation of IPW-5371 and internal standard was achieved on LC-MS/MS MDS Sciex, API-4000 LC-MS/MS using transition pairs of m/z 458.2 → 369.0 and m/z 271.2 → 155.1, respectively. IPW-5371 and internal standard were chromatographically separated on an Atlantis dC18 (50 × 4.6 mm; 3 μm) using an isocratic mobile phase (acetonitrile: 0.2% formic acid in water; 80:20, v/v) at a flow rate of 0.7 ml/min. The retention times of IPW-5371 and internal standard were 1.06 and 1.15 min, respectively, with a total run time of 2.5 min.
Kinase Activity Assay
In vitro kinase profiling was performed at Reaction Biology Corp. (Malvern, PA) as described previously (17). IPW-5371 was tested in 10-dose IC50 mode with threefold serial dilution starting at 10 μM. Control compounds staurosporine and LDN193189 were tested in 10-dose IC50 mode with threefold serial dilution starting at 20 μM. Reactions were performed at 10 μM ATP.
IPW-5371 Treatment
IPW-5371, developed and provided by Innovation Pathways (Palo Alto, CA), was administered by oral gavage once daily. IPW-5371 was prepared by wetting with Tween 80, triturating with a mortar and pestle and slowly adding 0.5% methylcellulose to the final volume. Mice were sedated with isoflurane and administered 100 μl of IPW-5371 as a suspension at 30 mg/kg or 10 mg/kg. The suspension was stored at room temperature for up to 5 days.
Measurement of Arterial Oxygen Saturation and Breathing Rate
Percutaneous arterial oxygen saturation (SpO2) and breathing rate were measured every 2 weeks starting at week 6 with the MouseOx® system (Starr® Life Sciences Corp., Allison Park, PA), as described previously (18). The MouseOx sensor was attached to the mouse’s thigh under anesthesia with ketamine/xylazine (100 mg/kg and 10 mg/kg, respectively). Measurements were taken for 2 min. All data were analyzed using MouseOx software.
Echocardiography
Transthoracic echocardiography was performed under light anesthesia (sodium pentobarbital 30–50 mg/kg) at baseline, 16 and 20 weeks using the Vevo® 770 imaging system (VisualSonics Inc., Toronto, Canada) using a 30 MHz probe as previously described (19). We measured the left ventricular (LV) end-diastolic and end-systolic areas (LVEDA and LVESA, respectively) at bidimensional mode (B-mode) and the LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), LV anterior wall diastolic and systolic thickness (AWDT and AWST), and LV posterior wall diastolic and systolic thickness (PWDT and PWST) at monodimensional mode (M-mode) as described previously (19, 20) and according to the American Society of Echocardiography recommendations (21). LV fractional shortening, LV ejection fraction (LVEF) and LV mass were calculated as previously described (19, 20). The LV fractional area change (LVFAC) was calculated as [(LVEDA-LVESA)/LVEDA] * 100.
The contractile reserve was assessed with a β-adrenergic receptor agonist (isoproterenol, 10 ng/mouse, Sigma-Aldrich® LLC; St. Louis, MO), as previously described (7). The contractile reserve was calculated as percentage change in LVEF measured at rest (LVEFr) and 3 min after isoproterenol injection (LVEFi) and calculated as [(LVEFi-LVEFr)/LVEFr] * 100.
Right ventricular (RV) dimensions were assessed by measuring the RV end-diastolic area (RVEDA) and RV end-systolic area (RVESA) in the parasternal short-axis view midventricular section, and RV systolic function was estimated by using M-mode and measuring the tricuspid annular plane systolic excursion (TAPSE) (20). The RV fractional area change (RVFAC) was calculated as [(RVEDA-RVESA)/RVEDA] * 100. The investigator performing and reading the echocardiogram was blinded to the treatment allocation.
Hydroxyproline Assay
Hydroxyproline content was determined using the hydroxyproline assay kit (Sigma-Aldrich). In brief, the right lung and heart were removed, dried and weighed followed by homogenization in 0.5 ml of H2O. After centrifugation, pellets were hydrolyzed in 0.5 ml of 12 N HCl for 3 h at 120°C. Each sample (50 μl) was added to a 96-well plate and allowed to dry in 60°C oven. After the addition of 0.1 ml of chloramine-T solution each sample was incubated for 5 min at room temperature, after which 0.1 ml of Ehrlich’s reagent was added and the samples incubated at 60°C for 90 min. Absorbance was measured at 560 nm.
Western Blot Analysis
The left upper lobe of the lung was removed and snap frozen in liquid nitrogen. Samples were stored at −80°C until protein extraction. Samples were homogenized on ice with 5 volumes RIPA buffer with a mortar and pestle. Lysate was centrifuged at 12,000 rpm for 20 min and the resulting supernatant assayed for protein content using the BCA protein assay kit (Thermo Scientific™, Waltham, MA). Proteins were separated by SDS-polyacrylamide gel electrophoresis and probed for pSmad2/3 and GAPDH (Cell Signaling Technology®, Danvers, MA).
Statistical Analysis
Data were expressed as the mean or median and standard error. Student’s t test was used to compare two groups, and values <0.05 were considered significant. Log-rank analysis was used to compare survival among the groups.
RESULTS
IPW-5371 Blocks ALK5/TGF-βR1 Kinase Activity and has a Long Oral Half-Life
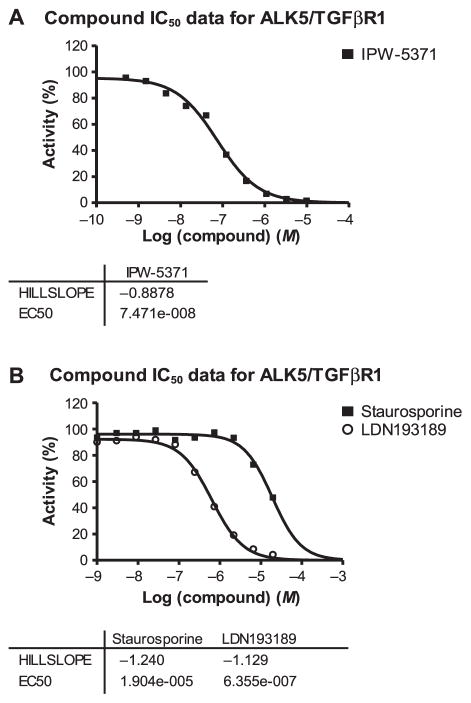
In vitro kinase activity assays were used to evaluate the affinity of IPW-5371 compared to control compounds known to have binding affinity for ALK5/TGF-βR1. Figure 2 shows that IPW-5371 inhibits ALK5/TGF-βR1 with an IC50 of 75 nM. This compares to an IC50 of 636 nM for the ALK2/BMP kinase inhibitor, LDN193189, and an IC50 of 19 μM for the nonspecific kinase inhibitor, staurosporine. The efficacy of IPW-5371 compares favorably with the IC50 = 56 nM and 38 nM of the orally bioavailable TGF-βR1 inhibitor in phase II human clinical trials, LY2157299 [galunisertib (PMID24780821) (22) (patent publication no. US7872020 B2)] Eli Lilly and Co., Indianapolis, IN; and the research tool compound, LY2109761, respectively.
FIG. 2.
In vitro kinase assay. Panel A: The kinase activity of IPW-5371 showed specificity for ALK5/TGF-βRI and potency greater than (panel B) LDN193189 and the nonselective kinase inhibitor staurosporine.
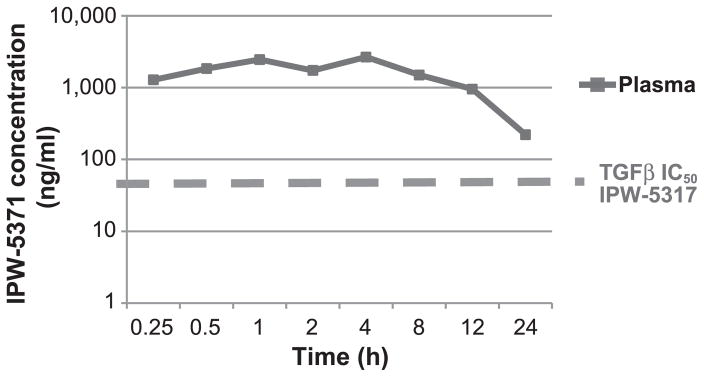
We also evaluated plasma levels of IPW-5371 in mice after a single oral dose of 20 mg/kg. As shown in Fig. 3, plasma levels were maintained well above the IC50 for TGF-βR1 for 24 h. This compares favorably with the short plasma half-life of LY2109761 requiring twice-daily delivery (BID) to achieve efficacy in pharmacology models (PMID22547771) (14). The excellent bioavailability of IPW-5371 led us to choose a once daily oral dose in our studies.
FIG. 3.
Mouse pharmacokinetics. Pharmacokinetic analysis of IPW-5371. One 20 mg/kg IPW-5371 dose was given by oral gavage.
IPW-5371 Enhances Survival after Irradiation
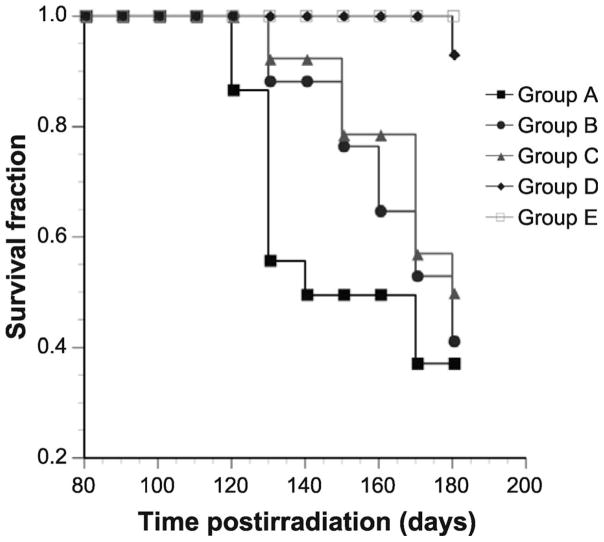
We evaluated the ability of IPW-5371 to mitigate radiation-induced mortality in C57L/J mice. A total 11.5 Gy dose to the thorax (5 Gy TBI followed with 6.5 Gy dose to the thoracic) resulted in a significant reduction in survival with a median survival of 130 days [group A (P < 0.001) compared to group E, no radiation] (Fig. 4). Mice treated for six weeks with IPW-5371, regardless of the dose, (groups B and C) had a median survival of 165 days, a significant delay in mortality (P < 0.05); however, the overall survival after 180 days was similar to that of group A (radiation only). In contrast, group D [radiation with IPW-5371 (30 mg/kg for 20 weeks)] showed marked improvement in survival compared to group A (radiation only) (P < 0.001), and was statistically insignificant from group E [IPW-5371 only (no radiation)].
FIG. 4.
Kaplan-Meier curve. Survival for C57L/J mice that received 5 Gy TBI and immediately thereafter 6.5 Gy irradiated to the thorax (total dose to the thorax was 11.5 Gy). Group A (radiation only), n = 15; group B [radiation with IPW-5371 (10 mg/kg for 6 weeks)], n = 16; group C [radiation with IPW-5371 (30 mg/kg for 6 weeks)], n =14; group D [radiation with IPW-5371 (30 mg/kg for 20 weeks)], n =14; group E [IPW-5371 only (30 mg/kg for 20 weeks), n =12. P < 0.01 between radiation alone and radiation with IPW-5371 for 20 weeks.
IPW-5371 Improves Respiratory Function in Irradiated Mice
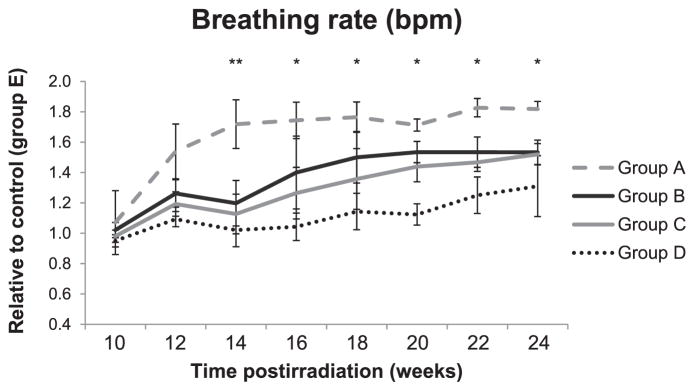
Respiratory function was evaluated using the MouseOx system on anesthetized mice. The pulse oximetry system is able to monitor heart rate (bpm), breath distention, breathing rate and arterial oxygen saturation (SpO2). Here we used the breathing rate as well as the SpO2 to determine the respiratory health of both the irradiated and the irradiated with IPW-5371 treated mice. Figure 5 shows that mice receiving 11.5 Gy to the thorax developed radiation-induced lung injury (RILI) significantly sooner than mice that received radiation with IPW-5371 as measured by an increase in breathing rate.
FIG. 5.
Mouse breathing rate. Change in breathing rate over 24 weeks after 5 Gy TBI with 6.5 Gy thoracic irradiation with and without IPW-5371 treatment. Measurements were made under ketamine/xylazine anesthesia. At 10–14 weeks: group A, n =17; group B, n =18; group C, n =16; group D, n =16; and group E, n =14. At 16 weeks: group A, n =13, group B, n =16, group C, n =14; and group D (n =14) and group E (n =12) remained the same for the duration of the study. At 18 weeks: group A, n =9; group B, n =15; and group C, n =13. At 20 weeks: group A, n =8; group B, n =14; and group C, n =12. At 22 weeks: group A, n =8; group B, n =12; and group C, n =10. At 24 weeks: group A, n =6; group B, n =9; and group C, n =9. Groups are defined as in Fig. 1. Data are reported as the standard deviation of the median. **P < 0.05 for groups receiving IPW-5371 vs. radiation alone. *P < 0.05 for group D vs. group A.
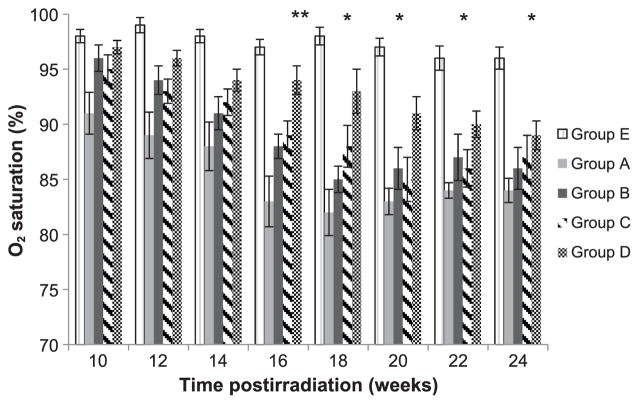
It has been reported that hypoxia is observed in animals and patients suffering from pulmonary fibrosis. To assess hypoxia and determine overall lung function we measured the SpO2. In Fig. 6 we show a significant reduction in SpO2 in mice after 11.5 Gy thorax irradiation that is shifted towards normal with IPW-5371 treatment. All groups receiving IPW-5371 showed a statistically significant improvement in SpO2, P < 0.05 out to 20 weeks. However, mice administered 20 weeks of IPW-5371, group D, showed a statistically significant improvement in SpO2 for the duration of the study, up to 24 weeks, P < 0.01.
FIG. 6.
Evaluation of blood oxygen levels in mice. Pulse oximetry was used to determine arterial O2 saturation in mice every two weeks postirradiation. “n” for each group is the same as for the breathing rate (see Fig. 5). Groups are defined as in Fig 1. Data are presented as means SEM. **P < 0.05 for all groups receiving radiation and drug (groups B–D) compared with radiation only (group A). *P < 0.01 group D compared with group A.
In summary, measurements of animal survival, breathing rate and SpO2 demonstrate that treatment with IPW-5371 resulted in a significant delay in disease onset as well as an overall physiologic improvement.
Effects of IPW-5371 on Radiation-Induced Cardiomyopathy
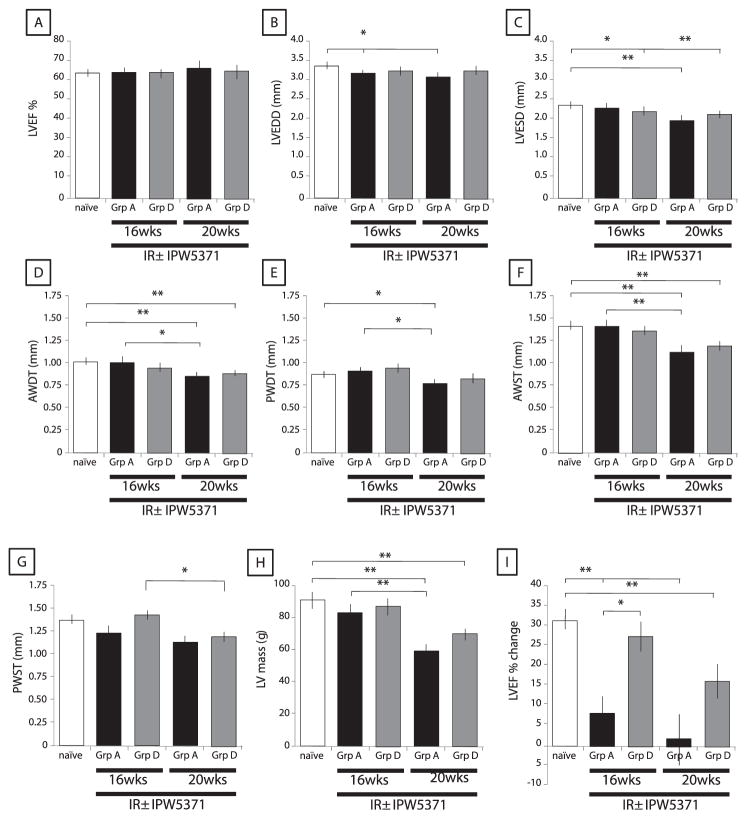
Thoracic irradiation not only affects the lungs, but has also been shown to induce cardiomyopathy. We evaluated the impact of our TBI model with a thoracic top-up dose on the dimensions and function of the left and right ventricle as well as the contractile reserve in response to isoproterenol. Values were compared in animals with and without IPW-5371. The LV function was preserved in both groups of irradiated mice with a reduction in LV dimension in irradiated mice compared to naïve mice (LV diastolic and systolic diameters, wall thickness and LV mass) (Fig. 7 and Table 1).
FIG. 7.
IPW-5371 ameliorates radiation-induced cardiomyopathy. Echocardiography was used to assess cardiac function (panel A), diameters in diastole and systole (panels B and C), wall thickness in diastole and systole (panels D–G) and LV mass (panel H). The contractile reserve, determined by the change in left ventricular ejection fraction (LVEF), was measured in mice at 16 and 20 weeks postirradiation before and 3 min after β-adrenergic stimulation. Groups are defined as in Fig. 1. Data are presented as means SEM. n = 6 at 16 weeks; n =5–7 at 20 weeks. LVEDD =LV end diastolic diameter; LVESD =LV end systolic diameter; AWDT = anterior wall diastolic thickness; OWDT = posterior wall diastolic thickness; AWST = anterior wall systolic thickness; and PWST = posterior wall systolic thickness. *P < 0.05, **P < 0.005.
TABLE 1.
Cardiac Parameters at 20 Weeks
| naïve | Group A | Group D | |
|---|---|---|---|
| LVFS (%) | 33.1 ± 0.61 | 35.4 ± 2.4 | 34.4 ± 2.2 |
| LVEF (%) | 63.0 ± 0.86 | 66.0 ± 3.1 | 64.7 ± 2.8 |
| LVEDD (mm) | 3.43 ± 0.09 | 3.05 ± 0.12# | 3.19 ± 0.04 |
| LVESD (mm) | 2.34 ± 0.04 | 1.94 ± 0.11# | 2.02 ± 0.10# |
| AWDT (mm) | 1.00 ± 0.03 | 0.80 ± 0.04# | 0.83 ± 0.04# |
| PWDT (mm) | 0.87 ± 0.02 | 0.75 ± 0.45# | 0.81 ± 0.05 |
| AWST (mm) | 1.45 ± 0.04 | 1.12 ± 0.08# | 1.23 ± 0.04# |
| PWST (mm) | 1.33 ± 0.06 | 1.14 ± 0.05 | 1.21 ± 0.06 |
| LV mass | 91.9 ± 5.04 | 58.9 ± 6.6# | 67.9 ± 3.7# |
| LVEDA (mm2) | - | 7.19 ± 0.76 | 8.24 ± 0.44 |
| LVESA (mm2) | - | 3.67 ± 0.51 | 4.15 ± 0.24 |
| LVFAC (%) | - | 49.7 ± 2.7 | 48.7 ± 4.2 |
| RVEDA (mm2) | - | 4.39 ± 0.55 | 5.39 ± 0.59 |
| RVESA (mm2) | - | 2.90 ± 0.44 | 3.93 ± 0.37 |
| RVFAC (%) | - | 34.2 ± 3.6 | 26.2 ± 4.0 |
| TAPSE (mm) | 1.09 ± 0.05 | 1.23 ± 0.08 | 0.96 ± 0.06* |
| HR | 373 ± 15 | 381 ± 41 | 416 ± 13 |
Notes.
vs naïve;
Group D vs. group A (P < 0.05.
A slight reduction in the right ventricular function was observed in mice treated with IPW-5371 compared to untreated irradiated mice (RVFAC and TAPSE), that was not significant to compared to naïve mice (Table 1).
There was, however, loss of contractile reserve at both 16 and 20 weeks postirradiation, when measured as the percent change in LVEF after isoproterenol injection (P < 0.05) (Fig. 7). The loss in contractile reserve observed in the irradiated mice was mostly reversed by daily treatment with IPW-5371 at 16 and 20 weeks.
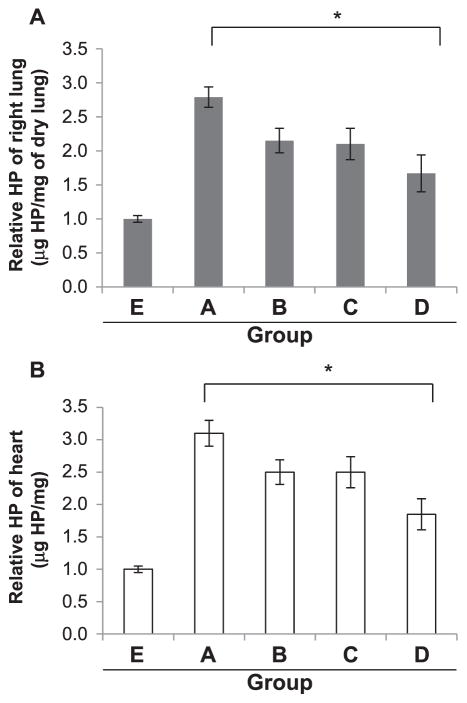
IPW-5371 Reduced Collagen Deposition in the Lung and the Heart
To assess the extent of fibrosis in the lung and heart we measured hydroxyproline (HP) levels as a determinant of collagen formation. Group A showed a >2.5-fold increase in HP content in the lung and a 3-fold increase in the heart compared to group E at 15 weeks postirradiation, with 31.4 μg/mg and 12 μg/mg of dry lung, respectively (Fig. 8A and B). Groups B and C (radiation with TPW-5371 for 6 weeks) both showed less, but no significant decrease in HP content. In contrast, group D (radiation with TPW-5371 for 20 weeks) showed a significant decrease of >40% (*P ≤ 0.05) in HP content in both the lung and heart compared to group A (radiation only).
FIG. 8.
Hydroxyproline (HP) content. The right lung (panel A) and heart (panel B) from each mouse was removed at 15 weeks postirradiation and assessed for collagen content. Groups are defined as in Fig. 1. Values expressed as means SEM. *P < 0.05 n =2 mice/group.
IPW-5371 Reduces Canonical TGF-β Signaling
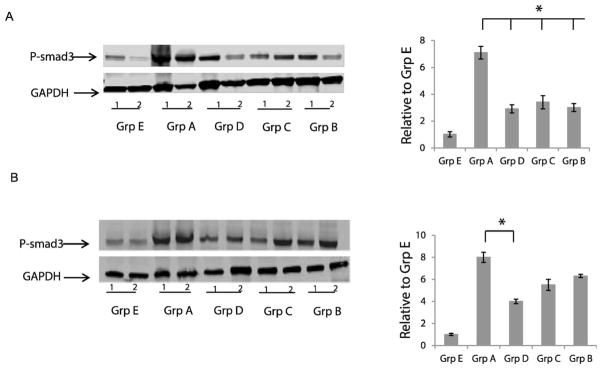
Based on the results of the kinase activity assay we evaluated the effect of IPW-5371 on TGF-βR1 activity, measured by Smad3 phosphorylation. Western blot analysis of the left upper lobe of the lung at 6 and 15 weeks postirradiation demonstrated a greater than sevenfold increase in pSmad3 levels of irradiated animals compared to nonirradiated animals (Fig. 9A and B). All drug-treated groups showed significantly decreased Smad3 phosphorylation at 6 weeks postirradiation, confirming IPW-5371’s actions on TGF-β signaling. At 15 weeks postirradiation only those mice continuing to receive IPW-5371 had significantly decreased levels of pSmad3 (P < 0.05).
FIG. 9.
IPW-5371 reduces pSmad3. Panel A: Western blot analysis of pSmad3 in lung tissue homogenates at 6 weeks postirradiation, n = 2. Panel B: Western blot analysis of pSmad3 at 15 weeks postirradiation, n = 2. Quantification of 3 Western blots normalized to GAPDH. *P < 0.05.
DISCUSSION
In determining the efficacy of IPW-5371 as a radiation countermeasure, our goal was not only to demonstrate improved survival but also improved cardiopulmonary function. Our study has shown that at 24 h postirradiation the administration of orally potent and efficacious TGF-βRI inhibitor, IPW-5371, to C57L/J mice that received 11.5 Gy thoracic irradiation, dramatically improved survival as well as reduced development of fibrosis in both the lung and heart. We have also shown that IPW-5371 treatment improves pulmonary function (decreased breathing rate and improved blood oxygenation levels) and cardiac contractile reserve.
While our current study was designed with the primary end point of survival, which was highly significant at 180 days postirradiation with 20 weeks of IPW-5371 treatment, we were also able to obtain some promising functional data. Our animal model, radiation protocol, drug and our physiological measurements are relevant and translatable to humans. A requirement for a compound to be considered for use as a radiation countermeasure is that it demonstrates efficacy in one or more animal models expected to react with a response similar to humans. Many studies utilize the C57Bl/6 mouse model to study radiation countermeasures; however, these mice are known to succumb to pleural effusions, a sequela typically not developed and not lethal to humans after irradiation. In the current study we used the C57L/J mouse model, which has a clinical presentation and pathogenesis of lung injury after irradiation that closely resembles the known outcome in humans. The C57L/J mouse also has a similar lethal dose for 50% within the first 180 days (LD50/180) to humans, 11.35 Gy compared to 10.6 Gy. The LD50/180 for C57Bl/6J mice is 14.10 Gy (22).
A network of Centers for Medical Countermeasures against Radiation has been established and provided guidelines for radiation protocols when studying countermeasures against RILI. It was proposed that researchers should use a sublethal dose that induces the hematopoietic syndrome immediately followed by lung-only top-up treatment (16). We used this radiation protocol in our study; the two-hit model is a preferred approach due to its inclusion of total-body-irradiation response, instead of limiting to the thorax. This type of exposure more closely resembles a radiological event compared to hemi- or whole-thoracic lung irradiation. An important finding from our studies was the validation of this two-hit model. When we compare our survival results to those reported in the literature for 11.5 Gy whole-thoracic lung irradiation in C57L/J mice, we obtain similar survival results after 180 days, 37% in our studies compared to slightly over 40% (22).
Since the duration of treatment for a radiation countermeasure may be weeks to months, when considering patient compliance, it is better to have as few required doses as possible. IPW-5371 has an IC50 of 75 nM against TGF-βR1, which is comparable to the inhibitor LY2157299 (IC50 of 56 nM) that is currently in phase II trials. However, IPW-5371 has a significantly longer plasma half-life compared to LY2157299 in mice (23). The longer half-life of IPW-5371 makes it a more effective compound in terms of compliance, since only BID dosing of LY2157299 was needed. Additionally, to have a compound approved by the FDA as a radiation countermeasure, it must be evaluated under the “Animal Rule”. This will likely require demonstrating efficacy in a nonhuman primate (NHP) model of radiation damage. Oral dosing of NHPs more than once a day can be a logistical challenge that might prevent a compound requiring BID dosing from completion of long-term survival and toxicology studies.
The development of RILI results from an immediate postirradiation inflammatory response of increased infiltration of inflammatory cells, elevated pro-inflammatory cytokine expression and increased generation of reactive oxygen species/reactive nitrogen species (ROS/RNS), which then lead to physiological changes including hypoxia and decreases in lung perfusion (4, 5). These early responses are often transient with cytokine levels returning to baseline or near baseline (11). During the development of RILI, this early acute phase is often followed by a latency period before the onset of an acute phase resulting from a second round of inflammation characterized by the increased expression of TGF-β and pro-inflammatory cytokines, as well as increased ROS/RNS resulting in the development of pneumonitis. The molecular changes that occur during this phase are often irreversible, such as the expression of TGF-β, resulting in progression to the late phase characterized by the development of fibrosis (24).
Cardiac fibrosis is a clinical manifestation of radiation-induced cardiomyopathy and has been demonstrated to occur through similar mechanisms: propagation of an inflammatory and cytokine response leading to activation of myofibroblasts resulting in fibrosis (6). As in RILI, cardiac fibrosis has been associated with cardiac damage leading to a decrease in cardiac function (7). TGF-β is known to play a critical role in cardiac injury, repair and remodeling (25). Using a TGF-β agonist, Boerma et al. recently reported on a direct effect of TGF-β on radiation-induced cardiac injury (26).
Published reports using rats have demonstrated that while irradiation of the heart and lung induce cardiac and pulmonary dysfunction, respectively, whole-thoracic lung irradiation results in significantly enhanced morbidities (27, 28). Clinical studies have also demonstrated that incidental irradiation of the heart increases the risk of radiation-induced pneumonitis in patients receiving radiotherapy for non-small cell lung cancer (NSCLC) (29). Fatigue and impaired exercise capacity with reduced peak oxygen consumption has been observed in long-term cancer survivors that received radiotherapy to the chest (30, 32). Decreased peak oxygen consumption is a measure of contractile reserve in patients and is a hallmark of heart failure. As already observed for low-dose exposure to the heart (7), there were no differences in LV function measured at rest in all groups of mice (see Table 1); however, as previously reported (7, 33), we observed a decrease in cardiac performance after β-adrenergic stimulation after irradiation. Long-term treatment with IPW-5371 preserved the contractile reserve in irradiated mice, however the improvement was not associated with a preservation of LV dimensions, the loss of which characterizes the development of restrictive cardiomyopathy (32, 34, 35).
TGF-β is involved in the pathogenesis of several cardiac diseases, affecting both the inflammatory and reparative responses to injury (25). The timing and levels of TGF-β inhibition appear to be crucial for preventing inflammation and/or excessive collagen deposition leading to cardiac dysfunction (26). IPW-5371 preserved the response to β-adrenergic stimulation and reduced collagen deposition after irradiation; however, we observed a slight reduction in right ventricular function (RVFAC and TAPSE) associated with IPW-5371 treatment. Basal levels of TGF-β signaling may be necessary to preserve right ventricular function after radiation injury. The association with reduced RV function will require further investigation.
Studies using TGF-β antagonists as antifibrotic agents have employed several different approaches, including treatment before, during and after irradiation. However, in mice treated twice daily with LY2109761, Flechsig et al. reported that postirradiation treatment, compared to treatment for four weeks before and during irradiation, was the most effective strategy for attenuating radiation-induced fibrosis and improving survival (14). Taking this data into consideration along with simulating a potential treatment response to a radiological event, we chose to begin treatment 24 h postirradiation. It is possible that beginning treatment at a later time point may prove to be more effective. Fibrosis has been associated with unchecked wound repair involving aberrant sustained TGF-β signaling. Dosing at a later time may allow some necessary wound repair to occur while blocking the deleterious effects of sustained TGF-β activity that lead to fibrosis. This delay would fit with the biphasic TGF-β response to radiation with an early wave within hours of irradiation and a second wave peaking from 2–8 weeks (36, 37).
Postirradiation treatments with TGF-β inhibitors have been administered starting from 1–3 days after exposure, and lasting from days to weeks (13, 38). We have shown that long-term administration of IPW-5371 is more effective at prolonging mouse survival than short-term administration. While there was no difference among median survival groups E (no radiation) and D (radiation), both of which received 30 mg/kg IPW-5371 for 20 weeks, both groups survived significantly longer than groups B and C, the latter received radiation and 6 weeks of IPW-5371 treatment. The relative effectiveness of long-term vs. short-term treatment observed here is consistent with other studies examining the mitigation of radiation-induced late effects. Treatment of irradiated rats with the TGF-β inhibitor SM16 for six months compared to three weeks resulted in significantly decreased breathing rate, lung fibrosis and macrophage activation (13). Delanian et al. have reported that patients treated with the combination of pentoxifylline and alpha-tocopherol (vitamin E) showed a decrease in radiation-induced fibrosis. The effect was greater the longer the patient was treated. In fact, they found that there was a risk of rebound effect if the treatment was too short (39, 40). The optimal time to begin treatment, the duration of treatment and the minimum efficacious dose of the drug we administer are all areas that require further investigation.
TGF-β-induced phosphoSmad3 has been demonstrated to be important for both radiation- and bleomycin-induced fibrosis. Use of mice with a targeted Smad3 deletion, Smad3−/−, showed significantly reduced pulmonary fibrosis (41, 42). Our current study demonstrates a similar finding in that we have significantly decreased TGF-β-induced phosphoSmad3, specifically phosphoSmad3, resulting in significant decreases in pulmonary and cardiac fibrosis. Experiments to determine non-TGF-β-mediated Smad2/3 activity as well as nonSmad2/3 TGF-β activity, such as Rho proteins, MARPK, PI3K and JNK are needed to completely elucidate the mechanism by which IPW-5371 reduces fibrosis.
While the involvement of TGF-β in fibrotic disease has been well documented there are alternative pathways leading to fibrosis. PDGF stimulates proliferation and is upregulated in some models of fibrosis (43). NFκB is an active transcription factor in the adaptive response to radiation and TNF-α mRNA levels have been reported to be elevated after irradiation (44). The role of TNF-α in fibrotic disease has been well documented (45). These alternative pathways may be important in the development in radiation-induced fibrosis in our model and future studies are needed to examine their role.
Several approaches to prevent radiation-induced late effects have been examined, including interruption of the inflammatory response by administering free radical scavengers, steroids, cytokines and their receptor blockers (46–49). These compounds, however, have usually been examined in the context of therapeutic radiation, have shown limited long-term success or have been administered prophylactically, limiting their use as a countermeasure. TGF-β signaling is a viable target in the mitigation of radiation-induced late effects. Early alterations in TGF-β gene expression have been reported as an indicator for RILI in mouse lung irradiation (50). In humans, TGF-β changes in plasma have been reported to be predictive of the development of radiation-induced pneumonitis (51) and analysis of the TGF-β1 promoter has demonstrated an association between the development of RILI and the presence of SNP rs1800469 in the promoter (52). TGF-β is also an active area of pharmaceutical investigation. Small molecule inhibitors and antibodies against TGF-β are active in phase I and II clinical trials in many forms of cancer (53, 54). It has been reported that TGF-β inhibitors offer an additional advantage as radiation countermeasures, since they were shown to be more effective RILI inhibitors when administered after irradiation (14). The finding that IPW-5371 may improve both pulmonary and cardiac health is of great importance; if either is compromised, this can lead to mortality associated with radiation-induced late effects. The established safety of these types of drugs in humans combined with our results suggests that further development of IPW-5371 as a radiation countermeasure is warranted.
Acknowledgments
Pilot project funding was provided by the National Institutes of Health (grant no. NIH/A1 U19 AI0991036; JP Williams). The authors would like to thank PS Suresh and Ramesh Mullangi from Jubilant Biosys. Ltd. (Bangalore, India) for their contributions to the manuscript and their DMPK services. We would also like to thank Reaction Biology Corp. (Malvern, PA) for performing the TGF-βR1 kinase assays.
References
- 1.Fasano A. Pathophysiology and management of radiation injury of the gastrointestinal tract. In: Ricks RC, Berger ME, O’Hara FM, editors. The medical basis for radiation-accident preparedness. Boca Raton: CRC Press; 2002. pp. 149–60. [Google Scholar]
- 2.Maekawa K. Overview of medical care for highly exposed victims in the Tokaimura accident. In: Ricks RC, Berger ME, O’Hara FM, editors. The medical basis for radiation-accident preparedness. Boca Raton: CRC Press; 2002. pp. 313–8. [Google Scholar]
- 3.UNSCEAR Report to the General Assembly, Annex J: Exposures and effects of Chernobyl accident. New York: United Nations; 2000. Sources, effects and risks of ionizing radiation. [Google Scholar]
- 4.Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN, et al. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33:99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- 5.Vujaskovic Z, Anscher MS, Feng QF, Rabbani ZN, Amin K, Samulski TS, et al. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int J Radiat Oncol Biol Phys. 2001;50:851–5. doi: 10.1016/s0360-3016(01)01593-0. [DOI] [PubMed] [Google Scholar]
- 6.Taunk NK, Haffty BG, Kostis JB, Goyal S. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol. 2015;5:39. doi: 10.3389/fonc.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mezzaroma E, Mikkelsen RB, Toldo S, Mauro AG, Sharma K, Marchetti C, et al. Role of interleukin-1 in radiation-induced cardiomyopathy. Mol Med. 2015;21:210–8. doi: 10.2119/molmed.2014.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellmers LJ, Scott NJ, Medicherla S, Pilbrow AP, Bridgman PG, Yandle TG, et al. Transforming growth factor-beta blockade down-regulates the renin-angiotensin system and modifies cardiac remodeling after myocardial infarction. Endocrinology. 2008;149:5828–34. doi: 10.1210/en.2008-0165. [DOI] [PubMed] [Google Scholar]
- 9.Ng YY, Chen YM, Tsai TJ, Lan XR, Yang WC, Lan HY. Pentoxifylline inhibits transforming growth factor-beta signaling and renal fibrosis in experimental crescentic glomerulonephritis in rats. Am J Nephrol. 2009;29:43–53. doi: 10.1159/000150600. [DOI] [PubMed] [Google Scholar]
- 10.Rube CE, Uthe D, Schmid KW, Richter KD, Wessel J, Schuck A, et al. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys. 2000;47:1033–42. doi: 10.1016/s0360-3016(00)00482-x. [DOI] [PubMed] [Google Scholar]
- 11.Chung EJ, Hudak K, Horton JA, White A, Scroggins BT, Vaswani S, et al. Transforming growth factor alpha is a critical mediator of radiation lung injury. Radiat Res. 2014;182:350–62. doi: 10.1667/RR13625.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ask K, Bonniaud P, Maass K, Eickelberg O, Margetts PJ, Warburton D, et al. Progressive pulmonary fibrosis is mediated by TGF-beta isoform 1 but not TGF-beta3. Int J Biochem Cell Biol. 2008;40:484–95. doi: 10.1016/j.biocel.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anscher MS, Thrasher B, Zgonjanin L, Rabbani ZN, Corbley MJ, Fu K, et al. Small molecular inhibitor of transforming growth factor-beta protects against development of radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2008;71:829–37. doi: 10.1016/j.ijrobp.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 14.Flechsig P, Dadrich M, Bickelhaupt S, Jenne J, Hauser K, Timke C, et al. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-beta and BMP-associated proinflammatory and proangiogenic signals. Clin Cancer Res. 2012;18:3616–27. doi: 10.1158/1078-0432.CCR-11-2855. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Lu W, Ma Z, Li Z, et al. Oxymatrine attenuates bleomycin-induced pulmonary fibrosis in mice via the inhibition of inducible nitric oxide synthase expression and the TGF-beta/Smad signaling pathway. Int J Mol Med. 2012;29:815–22. doi: 10.3892/ijmm.2012.923. [DOI] [PubMed] [Google Scholar]
- 16.Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173:557–78. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR, et al. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nature Biotechnol. 2011;29:1039–45. doi: 10.1038/nbt.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka K, Azuma A, Miyazaki Y, Sato K, Mizushima T, et al. Effects of lecithinized superoxide dismutase and/or pirfenidone against bleomycin-induced pulmonary fibrosis. Chest. 2012;142:1011–9. doi: 10.1378/chest.11-2879. [DOI] [PubMed] [Google Scholar]
- 19.Abbate A, Salloum FN, Vecile E, Das A, Hoke NN, Straino S, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–83. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 20.Toldo S, Bogaard HJ, Van Tassell BW, Mezzaroma E, Seropian IM, Robati R, et al. Right ventricular dysfunction following acute myocardial infarction in the absence of pulmonary hypertension in the mouse. PloS One. 2011;6:e18102. doi: 10.1371/journal.pone.0018102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porter TR, Shillcutt SK, Adams MS, Desjardins G, Glas KE, Olson JJ, et al. Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015;28:40–56. doi: 10.1016/j.echo.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Jackson IL, Xu PT, Nguyen G, Down JD, Johnson CS, Katz BP, et al. Characterization of the dose response relationship for lung injury following acute radiation exposure in three well-established murine strains: developing an interspecies bridge to link animal models with human lung. Health Phys. 2014;106:48–55. doi: 10.1097/HP.0b013e3182a32ccf. [DOI] [PubMed] [Google Scholar]
- 23.Bueno L, de Alwis DP, Pitou C, Yingling J, Lahn M, Glatt S, et al. Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur J Cancer. 2008;44:142–50. doi: 10.1016/j.ejca.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Mattos MD, Kimura ET, Silva MR, Egami MI, Segreto RA, Segreto HR. Latent TGFbeta1 activation in the lung irradiated in vivo. Revista da Associacao Medica Brasileira. 2002;48:329–34. doi: 10.1590/s0104-42302002000400039. in Portuguese. [DOI] [PubMed] [Google Scholar]
- 25.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–6. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boerma M, Wang J, Sridharan V, Herbert JM, Hauer-Jensen M, et al. Pharmacological induction of transforming growth factor-beta1 in rat models enhances radiation injury in the intestine and the heart. PloS One. 2013;8:e70479. doi: 10.1371/journal.pone.0070479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghobadi G, van der Veen S, Bartelds B, de Boer RA, Dickinson MG, de Jong JR, et al. Physiological interaction of heart and lung in thoracic irradiation. Int J Radiat Oncol Biol Phys. 2012;84:e639–46. doi: 10.1016/j.ijrobp.2012.07.2362. [DOI] [PubMed] [Google Scholar]
- 28.van Luijk P, Novakova-Jiresova A, Faber H, Schippers JM, Kampinga HH, Meertens H, et al. Radiation damage to the heart enhances early radiation-induced lung function loss. Cancer Res. 2005;65:6509–11. doi: 10.1158/0008-5472.CAN-05-0786. [DOI] [PubMed] [Google Scholar]
- 29.Huang EX, Hope AJ, Lindsay PE, Trovo M, El Naqa I, Deasy JO, et al. Heart irradiation as a risk factor for radiation pneumonitis. Acta Oncol. 2011;50:51–60. doi: 10.3109/0284186X.2010.521192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM, et al. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010;102:14–25. doi: 10.1093/jnci/djp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bower JE. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, Diller L, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–48. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 33.Mezzaroma E, Di X, Graves P, Toldo S, Van Tassell BW, Kannan H, et al. A mouse model of radiation-induced cardiomyopathy. Int J Cardiol. 2012;156:231–3. doi: 10.1016/j.ijcard.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipshultz SE, Adams MJ. Cardiotoxicity after childhood cancer: beginning with the end in mind. J Clin Oncol. 2010;28:1276–81. doi: 10.1200/JCO.2009.26.5751. [DOI] [PubMed] [Google Scholar]
- 35.Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, Kodama K, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76:656–65. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rube CE, Wilfert F, Palm J, König J, Burdak-Rothkamm S, Liu L, et al. Irradiation induces a biphasic expression of pro-inflammatory cytokines in the lung. Strahlenther Onkol. 2004;180:442–8. doi: 10.1007/s00066-004-1265-7. [DOI] [PubMed] [Google Scholar]
- 37.Haiping Z, et al. Prevention of radiation-induced pneumonitis by recombinant adenovirus-mediated transferring of soluble TGF-beta type II receptor gene. Cancer Gene Ther. 2006;13:864–72. doi: 10.1038/sj.cgt.7700959. [DOI] [PubMed] [Google Scholar]
- 38.Nishioka A, Ogawa Y, Kariya S, Hamada N, Nogami M, Inomata T, et al. Reduction of fibroproliferative changes in irradiated rat lung with soluble transforming growth factor-beta receptor. Mol Med Rep. 2015;11:2659–63. doi: 10.3892/mmr.2014.3064. [DOI] [PubMed] [Google Scholar]
- 39.Delanian S, Balla-Mekias S, Lefaix JL. Striking regression of chronic radiotherapy damage in a clinical trial of combined pentoxifylline and tocopherol. J Clin Oncol. 1999;17:3283–90. doi: 10.1200/JCO.1999.17.10.3283. [DOI] [PubMed] [Google Scholar]
- 40.Delanian S, Porcher R, Rudant J, Lefaix JL. Kinetics of response to long-term treatment combining pentoxifylline and tocopherol in patients with superficial radiation-induced fibrosis. J Clin Oncol. 2005;23:8570–9. doi: 10.1200/JCO.2005.02.4729. [DOI] [PubMed] [Google Scholar]
- 41.Epperly MW, Franicola D, Zhang X, Nie S, Wang H, Bahnson AB, et al. Reduced irradiation pulmonary fibrosis and stromal cell migration in Smad3−/− marrow chimeric mice. In Vivo. 2006;20:573–82. [PubMed] [Google Scholar]
- 42.Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85:47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine & growth factor reviews. 2004;15:255–73. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Hallahan DE, Spriggs DR, Beckett MA, Kufe DW, Weichselbaum RR. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci U S A. 1989;86:10104–7. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oikonomou N, Harokopos V, Zalevsky J, Valavanis C, Kotanidou A, Szymkowski DE, et al. Soluble TNF mediates the transition from pulmonary inflammation to fibrosis. PloS one. 2006;1:e108. doi: 10.1371/journal.pone.0000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vujaskovic Z, Feng QF, Rabbani ZN, Samulski TV, Anscher MS, Brizel DM, et al. Assessment of the protective effect of amifostine on radiation-induced pulmonary toxicity. Exp Lung Res. 2002;28:577–90. doi: 10.1080/01902140290096791. [DOI] [PubMed] [Google Scholar]
- 47.Magana E, Crowell RE. Radiation pneumonitis successfully treated with inhaled corticosteroids. South Med J. 2003;96:521–4. doi: 10.1097/01.SMJ.0000054502.81803.A3. [DOI] [PubMed] [Google Scholar]
- 48.Koukourakis MI, Panteliadou M, Abatzoglou IM, Sismanidou K, Sivridis E, Giatromanolaki A, et al. Postmastectomy hypofractionated and accelerated radiation therapy with (and without) subcutaneous amifostine cytoprotection. Int J Radiat Oncol Biol Phys. 2013;85:e7–13. doi: 10.1016/j.ijrobp.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 49.Ding NH, Li JJ, Sun LQ. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr Drug Targets. 2013;14:1347–56. doi: 10.2174/13894501113149990198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finkelstein JN, Johnston CJ, Baggs R, Rubin P, et al. Early alterations in extracellular matrix and transforming growth factor beta gene expression in mouse lung indicative of late radiation fibrosis. Int J Radiat Oncol Biol Phys. 1994;28:621–31. doi: 10.1016/0360-3016(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 51.Anscher MS, Murase T, Prescott DM, Marks LB, Reisenbichler H, Bentel GC, et al. Changes in plasma TGF beta levels during pulmonary radiotherapy as a predictor of the risk of developing radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1994;30:671–6. doi: 10.1016/0360-3016(92)90954-g. [DOI] [PubMed] [Google Scholar]
- 52.Alam A, Mukhopadhyay ND, Ning Y, Reshko LB1, Cardnell RJ, Alam O, et al. A preliminary study on racial differences in HMOX1, NFE2L2, and TGFbeta1 gene polymorphisms and radiation-induced late normal tissue toxicity. Int J Radiat Oncol Biol Phys. 2015;93:436–43. doi: 10.1016/j.ijrobp.2015.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buijs JT, Stayrook KR, Guise TA. The role of TGF-beta in bone metastasis: novel therapeutic perspectives. Bonekey Rep. 2012;1:96. doi: 10.1038/bonekey.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]