Abstract
Background
Adrenocortical carcinoma (ACC) is a rare and aggressive malignancy with limited therapeutic options beyond surgical resection. The characteristics of actual long-term survivors following surgical resection for ACC have not been previously reported.
Method
Patients who underwent resection for ACC at one of 13 academic institutions participating in the US Adrenocortical Carcinoma Group from 1993 to 2014 were analyzed. Patients were stratified into four groups: early mortality (died within 2 years), late mortality (died within 2–5 years), actual 5-year survivor (survived at least 5 years), and actual 10-year survivor (survived at least 10 years). Patients with less than 5 years of follow-up were excluded.
Results
Among the 180 patients available for analysis, there were 49 actual 5-year survivors (27%) and 12 actual 10-year survivors (7%). Patients who experienced early mortality had higher rates of cortisol-secreting tumors, nodal metastasis, synchronous distant metastasis, and R1 or R2 resections (all P < 0.05). The need for multi-visceral resection, perioperative blood transfusion, and adjuvant therapy correlated with early mortality. However, nodal involvement, distant metastasis, and R1 resection did not preclude patients from becoming actual 10-year survivors. Ten of twelve actual 10-year survivors were women, and of the seven 10-year survivors who experienced disease recurrence, five had undergone repeat surgery to resect the recurrence.
Conclusion
Surgery for ACC can offer a 1 in 4 chance of actual 5-year survival and a 1 in 15 chance of actual 10-year survival. Long-term survival was often achieved with repeat resection for local or distant recurrence, further underscoring the important role of surgery in managing patients with ACC.
Keywords: adrenocortical carcinoma, survival, surgery, adrenal cancer
INTRODUCTION
Adrenocortical carcinoma (ACC) is a rare malignancy with an incidence of approximately one per million in the United States [1,2]. Approximately 40% of ACC tumors do not secrete hormones and, therefore, may present as large tumors causing local mass effect [3]. As a result, most patients present at an advanced stage of disease and have a median survival time of less than 12 months [2,4]. Even with surgical resection, ACC can still be associated with a poor long-term prognosis, with estimated 5-year survival ranging from 16% to 45% [5–13]. Surgery, however, offers the best chance to achieve long-term survival even if recurrence is common and occurs in as many as 70% of patients following complete resection of ACC [1]. Although several studies have reported survival outcomes following curative-intent surgery for ACC, characteristics of the actual 5- and 10-year survivors have not been previously reported.
In fact, to date, only eight case reports and one small case series of six patients in the literature have reported on patients with ACC who have achieved long-term survival of more than 10 years [10,14–21]. Of note, various clinical and pathologic factors such as tumor size [22,23], lymphadenectomy [24,25], major venous involvement [26], margin status [27], and higher Weiss score [28] have not been consistently associated with long-term survival following resection of ACC. Therefore, the objective of the current study was to use a multi-institutional collaborative database of ACC patients in the United States to identify characteristics of actual 5- and 10-year survivors following resection of ACC.
METHODS
Patient Population and Study Design
Patients who underwent surgical resection for ACC were identified using a multi-institutional database of 13 academic institutions participating in the US Adrenocortical Carcinoma Study Group: Stanford University, Stanford, CA; John Hopkins Hospital, Baltimore, MD; Emory University, Atlanta, GA; Washington University, St. Louis, MO; Wake Forest University, Winston-Salem, NC; University of Wisconsin, Madison, WI; The Ohio State University, Columbus, OH; Medical College of Wisconsin, Milwaukee, WI; New York University, New York, NY; University of California at San Diego, San Diego, CA; University of California at San Francisco, San Francisco, CA; University of Texas Southwestern Medical Center, Dallas, TX; and Vanderbilt University Medical Center, Nashville, TN. Institutional Review Board approval was obtained by each of the participating institutions. Patients were stratified into four groups based on survival: early mortality (died within 2 years), late mortality (died within 2–5 years), actual 5-year survivor (survived at least 5 years) and actual 10-year survivor (survived at least 10 years). Patients alive at the last encounter with less than 5 years of follow-up were excluded.
Data on patient demographics, clinicopathologic characteristics, perioperative outcomes, and overall survival were collected. Preoperative variables included age, race, comorbidities, presence of hormonal excess, and symptoms. Pathologic variables included tumor size, tumor weight, TNM stage, and histologic characteristics. Postoperative morbidity was graded using the modified Clavien-Dindo classification of surgical complications [29]. The seventh edition of the American Joint Commission on Cancer (AJCC) Staging Manual was used to determine TNM classification [30]. Tumors were classified as “atypical ACC” when fewer than 3 Weiss criteria were present on histologic analysis [31].
Statistical Analysis
Continuous variables were reported as median values with interquartile range (IQR) and compared using ANOVA or Kruskal–Wallis test, where appropriate. Categorical variables were presented as frequency and percentages and compared using chi-square or Fisher’s exact test, where applicable. Overall survival was calculated using the Kaplan–Meier method and compared using log-rank test. Clinical and pathologic data were analyzed using univariate and multivariate Cox regression methods. Variables with P < 0.05 in the univariate analysis were entered into the Cox’s proportional hazard model to determine predictors of long-term survival. Covariates that did not satisfy the Cox proportional hazard assumption were excluded from the final multivariate model. All statistical analyses were performed using the STATA 13.0 statistical software package (STATA Corp., College Station, TX) and SPSS version 23.0 (IBM, Chicago, IL). Significance was set at a P value of <0.05 (two-tailed).
RESULTS
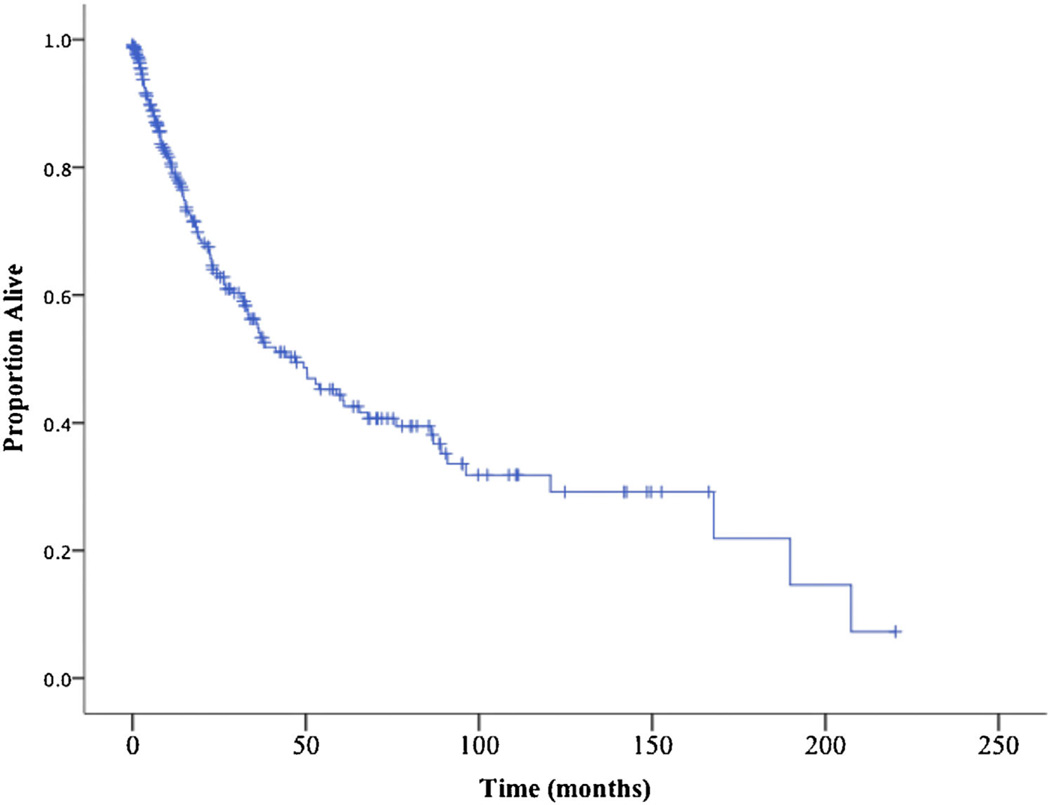
A total of 265 patients underwent surgery for ACC at one of 13 academic institutions participating in the U.S. Adrenocortical Carcinoma Study Group during the study period (1993–2014). The 5- and 10-year actuarial survival estimates were 44.3% and 31.8%, respectively (Fig. 1). Among the 265 patients, 180 patients met the inclusion criterion of at least 5 years follow-up. Actual 5- and 10-year survival among this subset of patients was 27% and 7%, respectively.
Fig. 1.
Kaplan–Meier curve demonstrating overall survival of 256 patients who underwent resection of ACC (median survival 47 months).
Stratification of these 180 patients into four groups as described in the Methods demonstrated comparable clinical characteristics among the groups with regard to age, sex, race, and comorbidities (Table I). While the proportion of incidental ACC tumors was similar across the four groups, non-secreting tumors were more common among actual 10-year survivors. On pathological analysis, tumor size and weight were comparable in the four groups. Factors associated with a longer survival included early T-stage, R0 resection, and lack of metastatic disease (Table I). Patients with ACC tumors that did not have lymphovascular invasion (LVI) or capsular invasion were also more likely to have a longer survival. Long-term survivors had a lower incidence of multiorgan resection, intraoperative blood transfusion and lower blood loss (all P < 0.05). In contrast, there were no differences in survival among patients with atypical ACC or tumors that had histologic evidence of high mitotic rate, microvascular invasion, or necrosis. The majority of patients did not undergo lymphadenectomy and nodal status was not different among the four groups.
TABLE I.
Clinicopathologic Characteristics of 180 Patients with Adrenocortical Carcinoma Stratified by Their Survival Outcome
| Alive <2 yrs (n = 73) |
Alive 2–5 yrs (n = 57) |
Alive 5–10 yrs (n = 37) |
Alive >10 yrs (n = 12) |
P-value | |
|---|---|---|---|---|---|
| Preoperative | |||||
| Age (median, IQR) | 54 (44–65) | 56 (47–62) | 52 (44–58) | 49 (37–52) | 0.180 |
| Female | 42 (57.5) | 34 (58.6) | 23 (62.2) | 10 (83.3) | 0.395 |
| Non-White | 16 (22.5) | 9 (15.8) | 7 (20.6) | 1 (9.1) | 0.639 |
| CAD | 7 (9.7) | 7 (12.3) | 3 (8.1) | 1 (9.1) | 0.939 |
| CHF | 4 (5.6) | 1 (1.8) | 0 (0) | 1 (9.1) | 0.197 |
| COPD | 6 (8.2) | 4 (7) | 0 (0) | 2 (16.7) | 0.120 |
| CKD | 5 (6.8) | 3 (5.3) | 0 (0) | 0 (0) | 0.444 |
| DM | 20 (27.4) | 9 (15.8) | 4 (10.8) | 1 (8.3) | 0.126 |
| BMI (median, IQR) | 29 (24–35) | 27 (24–31) | 26 (23–29) | 25.5 (25–36) | 0.326 |
| Incidentaloma | 27 (37.5) | 19 (35.8) | 17 (48.6) | 4 (36.4) | 0.651 |
| Weight loss (n = 166) | 12 (17.1) | 8 (15.4) | 6 (18.2) | 0 (0) | 0.581 |
| Abdominal pain (n = 165) | 32 (44.4) | 30 (57.7) | 11 (35.5) | 3 (30) | 0.155 |
| Palpable Mass (n = 162) | 7 (9.9) | 12 (24.5) | 4 (12.5) | 2 (20) | 0.147 |
| Leg Edema (n = 159) | 20 (29.4) | 8 (16.3) | 2 (6.3) | 1 (10) | 0.033 |
| Hormonal hypersecretion | |||||
| Non-secreting | 33 (46.5) | 35 (66) | 16 (48.5) | 8 (72.7) | 0.013 |
| Cortisol-secreting | 23 (32.4) | 4 (7.5) | 8 (24.2) | 0 (0) | |
| Non-cortisol | 11 (15.5) | 11 (20.8) | 6 (18.2) | 3 (27.3) | |
| Pathology | |||||
| Tumor size (median, IQR) | 13 (10–15.8) | 12 (7–16.5) | 11 (8.5–13.7) | 11.7 (9–15) | 0.506 |
| Tumor weight (grams) (median, IQR) | 487.5 (239.9–1365) | 355.5 (133.1–1226) | 370 (210–1030) | 610 (360–1050) | 0.647 |
| T-stage (n = 159) | |||||
| T1 | 0 (0) | 2 (4) | 2 (6) | 1 (11.1) | 0.007 |
| T2 | 18 (26.9) | 27 (54) | 16 (48.5) | 4 (44.4) | |
| T3 | 31 (46.3) | 17 (34) | 12 (36.4) | 3 (33.3) | |
| T4 | 18 (26.9) | 4 (8) | 3 (9.1) | 1 (11.1) | |
| N stage | |||||
| N0 | 15 (20.5) | 14 (24.1) | 9 (24.3) | 2 (16.7) | 0.060 |
| N1 | 15 (20.5) | 6 (10.3) | 0 (0) | 2 (16.7) | |
| Nx | 43 (58.9) | 38 (65.5) | 28 (75.7) | 8 (66.7) | |
| M1 | 31 (44.3) | 6 (10.7) | 1 (3) | 1 (9.1) | <0.001 |
| Margins | |||||
| R0 | 30 (45.5) | 42 (84) | 25 (78.1) | 6 (85.7) | <0.001 |
| R1 | 26 (39.4) | 5 (10) | 7 (21.9) | 1 (14.3) | |
| R2 | 10 (15.2) | 3 (6) | 0 (0) | 0 (0) | |
| Lymphatic invasion (n = 105) | 30 (65.2) | 18 (50) | 3 (18.8) | 3 (42.9) | 0.012 |
| MVI (n = 93) | 32 (74.4) | 18 (60) | 7 (41.2) | 1 (33.3) | 0.056 |
| High mitotic rate (n = 76) | 21 (65.6) | 16(61.5) | 8 (57.1) | 2 (50) | 0.883 |
| Necrosis (n = 139) | 50 (86.2) | 42 (91.3) | 25 (96.2) | 9 (100) | 0.489 |
| Capsular invasion (n = 119) | 40 (78.4) | 18 (50) | 12 (48) | 4 (57.1) | 0.012 |
| Atypical (vs. ACC) (n = 127) | 4 (7.5) | 2 (4.4) | 3 (14.3) | 1 (12.5) | 0.391 |
| Operative | |||||
| Other organs resected | 46 (63.9) | 20 (36.4) | 13 (36.1) | 4 (33.3) | 0.004 |
| OR time minutes (median, IQR) | 251 (171–366) | 181 (123–294) | 179 (132–230) | 173 (106–173) | 0.152 |
| EBL (per mL) (median, IQR) | 1150 (300–2350) | 450 (150–1175) | 500 (200–900) | 400 (200–400) | 0.010 |
| Open (vs. laparoscopic) | 63 (88.7) | 43 (78.1) | 30 (81.1) | 8 (80) | 0.377 |
| Intraoperative blood transfusion (n = 122) | 31 (56.4) | 12 (33.3) | 5 (20) | 1 (16.6) | 0.006 |
Parenthesis represent percentages unless otherwise indicated. CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; DM, diabetes mellitus; BMI, body mass index; IQR, interquartile range; LVI, lymphovascular invasion; yrs, years; MVI, microvascular invasion
Table II details postoperative variables following initial resection of ACC. Overall morbidity was slightly higher among patients who had a shorter survival, but the incidence of major complications (grade 3 or more) was similar in the four groups. There were no significant differences in rates of specific complications, reoperation, readmission, and length of stay. While the use of adjuvant chemotherapy and radiation were similar in the four groups, mitotane was more commonly used in patients with shorter survival.
TABLE II.
Postoperative Outcomes Following Adrenocortical Carcinoma Resection
| Alive <2 yrs (n = 73) |
Alive 2–5 yrs (n = 57) |
Alive 5–10 yrs (n = 37) |
Alive >10 yrs (n = 12) |
P-value | |
|---|---|---|---|---|---|
| Any complication | 27 (40.9) | 17 (35.4) | 3 (10.3) | 3 (37.5) | 0.019 |
| Grade >3 complication (n = 127) | 10 (18.2) | 4 (9.8) | 1 (4.2) | 1 (14.3) | 0.310 |
| Reoperation (n = 149) | 5 (7.7) | 0 (0) | 1 (3.2) | 0 (0) | 0.218 |
| Intra-abdominal abscess (n = 149) | 3 (4.6) | 0 (0) | 0 (0) | 0 (0) | 0.417 |
| Respiratory failure (n = 148) | 6 (9.4) | 1 (2.2) | 0 (0) | 1 (12.5) | 0.132 |
| Renal failure requiring dialysis (n = 148) | 4 (6.3) | 0 (0) | 0 (0) | 0 (0) | 0.239 |
| Pulmonary embolism (n = 147) | 3 (4.6) | 2 (4.4) | 0 (0) | 0 (0) | 0.781 |
| Length of stay in days (median, IQR) | 7 (5–10) | 6 (4–8) | 6 (4–7) | 6 (4–10) | 0.4352 |
| Readmission within 90 days (n = 136) | 17 (27.4) | 4 (10.2) | 3 (10.7) | 2 (28.5) | 0.082 |
| Adjuvant Chemotherapy (n = 168) | 16 (25.4) | 11 (19) | 4 (11.4) | 1 (8.3) | 0.334 |
| Adjuvant Mitotane (n = 151) | 30 (51.7) | 25 (46.3) | 7 (23.3) | 0 (0) | 0.002 |
| Adjuvant Radiation (n = 147) | 5 (8.8) | 5 (9.6) | 3 (10.7) | 0 (0) | 0.917 |
IQR, interquartile range; yrs, years.
Table III shows details on actual 10-year survivors (n¼12, 7%). Most patients were women (83.3%) and had non-secreting tumors (66.7%). The mean tumor size was 12.2-cm (range 4.5–20 cm). Two of the three patients who underwent lymphadenectomy had evidence of lymph node involvement and one patient had synchronous metastatic disease. The absence of IVC tumor thrombus was a universal factor shared by these actual 10-year survivors. None of the patients received adjuvant mitotane after initial resection for adrenocortical carcinoma. Recurrence was eventually observed in 7 of the 12 actual 10-year survivors, and 5 (71%) of these patients underwent repeat resection for recurrent ACC. The longest survivor remains alive 18 years after initial resection and without evidence of recurrence.
TABLE III.
Clinicopathologic Characteristics of 12 10-Year Survivors After Resection of ACC
| Age (years) |
Sex | Hormonal secretion | Size (cm) |
TNM | Margins | Adjuvant therapy |
Recurrence | DFI (months) |
Treatment for Recurrence |
Status | Survival (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 46 | F | Nonsecreting | 15.0 | T2NxM0 | R0 | None | No | NA | NA | NED | 143 |
| 49 | M | Nonsecreting | NR | NR | R0 | None | No | NA | NA | NED | 220 |
| 73 | F | Nonsecreting | 4.5 | T1NxM0 | R0 | None | Lung (distant) | 108.8 | None | DOD | 168 |
| 65 | F | Virilizing/feminizing | 13.0 | T2NxM0 | R0 | None | No | NA | NA | DOD | 207 |
| 49 | F | Nonsecreting | 9.8 | T2NxM0 | NR | None | No | NA | NA | NED | 125 |
| 50 | F | Nonsecreting | 8 | T2NxM0 | R0 | None | No | NA | NA | DOC | 190 |
| 33 | F | NR | 20.0 | T3NxM0 | NR | None | Spine (distant) | 36.1 | Mitotane and radiation | DOD | 121 |
| 2 | F | Virilizing/feminizing | 19.5 | T3N1M0 | R1 | Cisplatin, VP-16 |
LN (local) | 2.3 | Surgery and chemotherapy |
NED | 166 |
| 32 | F | Virilizing/feminizing | NR | NR | NR | None | Lung (distant) | 4.3 | Surgery only | AWD | 142 |
| 49 | F | Nonsecreting | 13.0 | T3N0M0 | R0 | None | Distal Pancreas (local) |
39.1 | Surgery only | AWD | 149 |
| 42 | F | Nonsecreting | 9.0 | T2NxM0 | NR | None | Liver (local) | 98.4 | Surgery and mitotane | AWD | 150 |
| 55 | M | Nonsecreting | 10.5 | T4N1M1 | NR | None | Liver (local) | 48.5 | Surgery, SBRT, chemotherapy |
AWD | 153 |
NR, not recorded; F, female; M, male; N/A, not applicable; NED, no evidence of disease; DOD, died of disease; DOC, died of other causes; AWD, alive with disease; DFI, disease free interval.
DISCUSSION
In one of the largest multi-institutional studies on patients with ACC, we demonstrated that 7% of those who underwent curative intent surgery were actual 10-survivors. To our knowledge, only one small series in the existing literature described actual 10-year survivors in patients with ACC, noting a prevalence of 3% (six patients) over a 28-year period [15]. Thus, our study is the largest series from the United States characterizing factors associated with 10-year actual survival following resection of ACC. Factors associated with early mortality include positive margins, capsular invasion, distant metastasis, and vena cava involvement. The findings of this study underscore the importance of complete resection as well as the prognostic significance of tumor biology and pathology in determining prognosis.
We found several distinct characteristics shared by long-term survivors who underwent curative intent resection for ACC. For example, none of the actual 10-year survivors had Cushing’s syndrome. This finding can be attributed to the negative effects of cortisol production on tumor cell growth and cell immunity [32]. We also observed that long-term survivors universally lacked IVC involvement. Although the benefit of surgery in patients with ACC with vena cava involvement has mostly been limited to case reports, one of the largest series consisting of 15 patients with ACC extending into the IVC demonstrated a median survival of only 8 months following curative intention resection and no 5-year survivors [26]. These data suggest that although tumors with caval involvement may be technically resectable using surgical techniques such as total hepatic vascular exclusion with or without venovenous or cardiopulmonary bypass, the anticipated limited survival benefit should weigh carefully into the decision to offer surgery in light of the risk of serious postoperative complications. Therefore, while ACC with vena cava involvement should not be considered an absolute contraindication for curative intent resection, it should be considered an indicator of a highly aggressive tumor biology and a limited chance of cure should be anticipated.
In the current study, we observed a high recurrence rate (58%) after resection of primary ACC even within the 10-year survivors group. Several studies have suggested that surgery for recurrent disease can provide a survival benefit [33–36], a second recurrence should be universally anticipated as 94% of patients who had undergone any intervention for recurrent disease eventually experienced repeat recurrence [34]. In our study, 7 of the 12 10-year survivors experienced disease recurrence after the 10-year mark, a finding that underscores the fact that 10-year survival does not equal cure for this disease. Surveillance for recurrence should continue even among those who have reached the 10-year milestone, as these patients should not be considered cured of their disease, especially if they have recurred previously, but underwent successful treatment for this.
A common dilemma faced by clinicians is whether adjuvant therapy is merited after complete macroscopic resection of ACC. The benefit of mitotane after resection of ACC remains controversial given the limited data on long-term outcomes. One promising retrospective study consisting of 177 patients demonstrated prolonged recurrence-free survival, but equivocal overall survival benefit when compared to one of the control groups in the study [37]. Other studies on the efficacy of adjuvant mitotane have revealed no benefit in terms of disease-free or overall survival. One study from M.D. Anderson Cancer Center consisting of 19 patients who underwent resection of ACC demonstrated no survival advantage in patients who received mitotane indefinitely compared to those who received mitotane for a short period (2–12 months) and those who did not receive mitotane after resection [38]. Similarly, a recent study published from our collaborative group did not identify a significant impact in survival even after adjusting for tumor and treatment related factors [39]. In the current study, we found a significant proportion of patients (51.7%) who died within 2 years were treated with adjuvant mitotane (likely prompted by unfavorable pathologic characteristics of the tumor) whereas none of the actual 10-year survivors received adjuvant mitotane. This difference may partly be a function of selection bias, as patients who received mitotane were likely those with the greatest risk of recurrence.
There are several major limitations in this study. First, despite the multi-institutional nature of the study consisting of 13 major academic hospitals in the United States, the sample size is still small, with each institution performing on average about one resection for primary ACC per year. Second, specific data on the Weiss criteria was not universally available due to variation in pathology reporting by each of the participating institutions. Nevertheless, the heterogeneous population in this multi-institutional study allows for generalizable results for a very rare malignancy.
In conclusion, actual 10-year survival is possible in 7% of patients undergoing surgery for adrenocortical carcinoma. Vena cava involvement, R2 resection, and glucocorticoid secretion are factors precluding 10-year survival. On the other hand, long-term survival beyond the 10-year milestone can still be achieved in the presence of lymph node or even distant metastasis and R1 resection. The vast majority (83%) of 10-year survivors were women and a substantial portion of them (42%) had undergone repeat resection for recurrence. Until more effective systemic therapies become available, these data can help clinicians counsel patients on the long-term outcomes of surgery for this challenging malignancy.
Footnotes
Conflicts of interest: None.
REFERENCES
- 1.Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States: Treatment utilization and prognostic factors. Cancer. 2008;113:3130–3136. doi: 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- 2.Kebebew E, Reiff E, Duh QY, et al. Extent of disease at presentation and outcome for adrenocortical carcinoma: Have we made progress? World J Surg. 2006;30:872–878. doi: 10.1007/s00268-005-0329-x. [DOI] [PubMed] [Google Scholar]
- 3.Przytulska J, Rogala N, Bednarek-Tupikowska G. Current and emerging therapies for adrenocortical carcinoma—Review. Adv Clin Exp Med. 2015;24:185–193. doi: 10.17219/acem/30645. [DOI] [PubMed] [Google Scholar]
- 4.Sidhu S, Sywak M, Robinson B, et al. Adrenocortical cancer: Recent clinical and molecular advances. Curr Opin Oncol. 2004;16:13–18. doi: 10.1097/00001622-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Crucitti F, Bellantone R, Ferrante A, et al. The Italian registry for adrenal cortical carcinoma: Analysis of a multiinstitutional series of 129 patients. the ACC Italian registry study group. Surgery. 1996;119:161–170. doi: 10.1016/s0039-6060(96)80164-4. [DOI] [PubMed] [Google Scholar]
- 6.Haak HR, Hermans J, van de Velde CJ, et al. Optimal treatment of adrenocortical carcinoma with mitotane: Results in a consecutive series of 96 patients. Br J Cancer. 1994;69:947–951. doi: 10.1038/bjc.1994.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: Surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg. 2001;25:891–897. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 8.Luton JP, Cerdas S, Billaud L, et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med. 1990;322:1195–1201. doi: 10.1056/NEJM199004263221705. [DOI] [PubMed] [Google Scholar]
- 9.Pommier RF, Brennan MF. An eleven-year experience with adrenocortical carcinoma. Surgery. 1992;112:963–970. discussion 970–961. [PubMed] [Google Scholar]
- 10.Sakamoto K, Ariyoshi A, Okazaki M. Metastatic adrenocortical carcinoma treated by repeated resection: A case report of long-term survival over 18 years. Int J Urol. 1995;2:50–52. [PubMed] [Google Scholar]
- 11.Schulick RD, Brennan MF. Adrenocortical carcinoma. World J Urol. 1999;17:26–34. doi: 10.1007/s003450050101. [DOI] [PubMed] [Google Scholar]
- 12.Vassilopoulou-Sellin R, Schultz PN. Adrenocortical carcinoma. Clinical outcome at the end of the 20th century. Cancer. 2001;92:1113–1121. doi: 10.1002/1097-0142(20010901)92:5<1113::aid-cncr1428>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Venkatesh S, Hickey RC, Sellin RV, et al. Adrenal cortical carcinoma. Cancer. 1989;64:765–769. doi: 10.1002/1097-0142(19890801)64:3<765::aid-cncr2820640333>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.De Leon DD, Lange BJ, Walterhouse D, et al. Long-term (15 years) outcome in an infant with metastatic adrenocortical carcinoma. J Clin Endocrinol Metab. 2002;87:4452–4456. doi: 10.1210/jc.2001-011978. [DOI] [PubMed] [Google Scholar]
- 15.Hermsen IG, Gelderblom H, Kievit J, et al. Extremely long survival in six patients despite recurrent and metastatic adrenal carcinoma. Eur J Endocrinol. 2008;158:911–919. doi: 10.1530/EJE-07-0723. [DOI] [PubMed] [Google Scholar]
- 16.Ilias I, Alevizaki M, Philippou G, et al. Sustained remission of metastatic adrenal carcinoma during long-term administration of low-dose mitotane. J Endocrinol Invest. 2001;24:532–535. doi: 10.1007/BF03343888. [DOI] [PubMed] [Google Scholar]
- 17.Meyer A, Behrend M. 32-year survival with metastatic adrenal cortical carcinoma—Update of a case report. Anticancer Res. 2007;27:1045–1046. [PubMed] [Google Scholar]
- 18.Orlando R, Pelizzo MR, Lirussi F. Adrenocortical carcinoma: A 15-year survival after complete resection and repeated resection. A retrospective study in a patient with an expected poor prognosis. Anticancer Res. 2003;23:2929–2931. [PubMed] [Google Scholar]
- 19.van Aalderen W, van Seters AP, Backer ET, et al. A case of recurrent adrenocortical carcinoma, with observations on long-term o,p’-DDD therapy and complications. Neth J Med. 1992;41:161–170. [PubMed] [Google Scholar]
- 20.Bergeat D, Rayar M, Beuzit L, et al. An unusual case of adrenocortical carcinoma with liver metastasis that occurred at 23 years after surgery. Hepatobiliary Surg Nutr. 2016;5:265–268. doi: 10.21037/hbsn.2016.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mawardi M, Al-Judaibi B, Marotta P. Hepatic metastasis from adrenocortical carcinoma fifteen years after primary resection. Saudi J Gastroenterol. 2012;18:140–142. doi: 10.4103/1319-3767.93821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berruti A, Baudin E, Gelderblom H, et al. Adrenal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:vii131–vii138. doi: 10.1093/annonc/mds231. [DOI] [PubMed] [Google Scholar]
- 23.Canter DJ, Mallin K, Uzzo RG, et al. Association of tumor size with metastatic potential and survival in patients with adrenocortical carcinoma: An analysis of the National Cancer Database. Can J Urol. 2013;20:6915–6921. [PubMed] [Google Scholar]
- 24.Nilubol N, Patel D, Kebebew E. Does lymphadenectomy improve survival in patients with adrenocortical carcinoma? A population-Based study. World J Surg. 2016;40:697–705. doi: 10.1007/s00268-015-3283-2. [DOI] [PubMed] [Google Scholar]
- 25.Reibetanz J, Jurowich C, Erdogan I, et al. Impact of lymphadenectomy on the oncologic outcome of patients with adrenocortical carcinoma. Ann Surg. 2012;255:363–369. doi: 10.1097/SLA.0b013e3182367ac3. [DOI] [PubMed] [Google Scholar]
- 26.Chiche L, Dousset B, Kieffer E, et al. Adrenocortical carcinoma extending into the inferior vena cava: Presentation of a 15-patient series and review of the literature. Surgery. 2006;139:15–27. doi: 10.1016/j.surg.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Margonis GA, Kim Y, Prescott JD, et al. Adrenocortical carcinoma: Impact of surgical margin status on long-Term outcomes. Ann Surg Oncol. 2016;23:134–141. doi: 10.1245/s10434-015-4803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bulzico D, de Faria PA, de Paula MP, et al. Recurrence and mortality prognostic factors in childhood adrenocortical tumors: Analysis from the Brazilian National Institute of Cancer experience. Pediatr Hematol Oncol. 2016;33:248–258. doi: 10.3109/08880018.2016.1173148. [DOI] [PubMed] [Google Scholar]
- 29.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 30.Edge SB. American Joint Committee on Cancer American Cancer Society: AJCC cancer staging manual. New York, London: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 31.Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol. 1984;8:163–169. doi: 10.1097/00000478-198403000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Abiven G, Coste J, Groussin L, et al. Clinical and biological features in the prognosis of adrenocortical cancer: Poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab. 2006;91:2650–2655. doi: 10.1210/jc.2005-2730. [DOI] [PubMed] [Google Scholar]
- 33.Jensen JC, Pass HI, Sindelar WF, et al. Recurrent or metastatic disease in select patients with adrenocortical carcinoma. Aggressive resection vs chemotherapy. Arch Surg. 1991;126:457–461. doi: 10.1001/archsurg.1991.01410280059008. [DOI] [PubMed] [Google Scholar]
- 34.Erdogan I, Deutschbein T, Jurowich C, et al. The role of surgery in the management of recurrent adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:181–191. doi: 10.1210/jc.2012-2559. [DOI] [PubMed] [Google Scholar]
- 35.Dy BM, Wise KB, Richards ML, et al. Operative intervention for recurrent adrenocortical cancer. Surgery. 2013;154:1292–1299. doi: 10.1016/j.surg.2013.06.033. discussion 1299. [DOI] [PubMed] [Google Scholar]
- 36.Gaujoux S, Al-Ahmadie H, Allen PJ, et al. Resection of adrenocortical carcinoma liver metastasis: Is it justified? Ann Surg Oncol. 2012;19:2643–2651. doi: 10.1245/s10434-012-2358-7. [DOI] [PubMed] [Google Scholar]
- 37.Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356:2372–2380. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]
- 38.Vassilopoulou-Sellin R, Guinee VF, Klein MJ, et al. Impact of adjuvant mitotane on the clinical course of patients with adrenocortical cancer. Cancer. 1993;71:3119–3123. doi: 10.1002/1097-0142(19930515)71:10<3119::aid-cncr2820711037>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 39.Postlewait LM, Ethun CG, Tran TB, et al. Outcomes of adjuvant mitotane after resection of adrenocortical carcinoma: A 13-Institution study by the US Adrenocortical Carcinoma Group. J Am Coll Surg. 2016;222:480–490. doi: 10.1016/j.jamcollsurg.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]