Abstract
Objective
The economic implications of strategies to improve prenatal screening for congenital heart disease (CHD) in low-risk mothers have not been explored. The aim was to perform a cost-effectiveness analysis of different screening methods.
Methods
We constructed a decision analytic model of CHD prenatal screening strategies (four-chamber screen (4C), 4C + outflow, nuchal translucency (NT) or fetal echocardiography) populated with probabilities from the literature. The model included whether initial screens were interpreted by a maternal–fetal medicine (MFM) specialist and different referral strategies if they were read by a non-MFM specialist. The primary outcome was the incremental cost per defect detected. Costs were obtained from Medicare National Fee estimates. A probabilistic sensitivity analysis was undertaken on model variables commensurate with their degree of uncertainty.
Results
In base–case analysis, 4C + outflow referred to an MFM specialist was the least costly strategy per defect detected. The 4C screen and the NT screen were dominated by other strategies (i.e. were more costly and less effective). Fetal echocardiography was the most effective, but most costly. On simulation of 10 000 low-risk pregnancies, 4C+ outflow screen referred to an MFM specialist remained the least costly per defect detected. For an additional $580 per defect detected, referral to cardiology after a 4C + outflow was the most cost-effective for the majority of iterations, increasing CHD detection by 13 percentage points.
Conclusions
The addition of examination of the outflow tracts to second-trimester ultrasound increases detection of CHD in the most cost-effective manner. Strategies to improve outflow-tract imaging and to refer with the most efficiency may be the best way to improve detection at a population level.
Keywords: congenital heart disease, cost, cost analysis, nuchal translucency measurement, prenatal diagnosis, prenatal ultrasound
INTRODUCTION
Congenital heart disease (CHD) is the most common birth defect, affecting slightly less than 1% of all live births1. Screening for fetal CHD is challenging, requiring population screening because approximately 80% of CHD occurs in fetuses of mothers without risk factors2,3. For low-risk mothers in the USA, recent revisions in consensus guidelines (April 2013) mandate that fetal cardiac screening includes views of the four chambers and both outflow tracts4. This updated previous recommendations for views of the outflow tracts only if ‘feasible’5–7 and is similar to more recent mandates in the UK and Canada8,9. Detection of a potential abnormality results in referral for higher-level imaging, specifically a fetal echocardiogram, usually resulting in a definitive diagnosis.
Several studies have shown that detection of CHD can be as high as 85% with ultrasound screening10,11. However, despite high rates of ultrasound use, the population detection rate of CHD is 30–50% in the – USA and in most developed countries12–16. Several alternative screening methods have been studied, including the use of first-trimester screening with nuchal translucency (NT)17,18, and, more recently, the use of three-dimensional (3D) or four-dimensional (4D) imaging of the fetal heart19,20. Although the efficacy of some alternative strategies has been studied, few economic analyses have been conducted. Screening strategies for CHD that improve detection may be more costly at the outset, but may prove more cost-effective with regard to the cost per defect detected. Published cost-effectiveness analyses (CEAs) in CHD have focused primarily on screening strategies for one high-risk group, namely diabetic mothers21. A recent CEA of prenatal CHD screening in low-risk women evaluated the cost-effectiveness of using telemedicine for expert review of second-trimester ultrasound scans, but did not consider different screening protocols22. Understanding the trade-off between cost and effectiveness of CHD screening in low-risk populations may provide further evidence for current recommendations, consideration of alternative strategies and/or focusing efforts for effective implementation in practice. Thus, the objective of this study was to perform an economic analysis of prenatal CHD screening in low-risk mothers to determine the cost-effectiveness of available strategies in terms of healthcare costs per heart defect detected.
METHODS
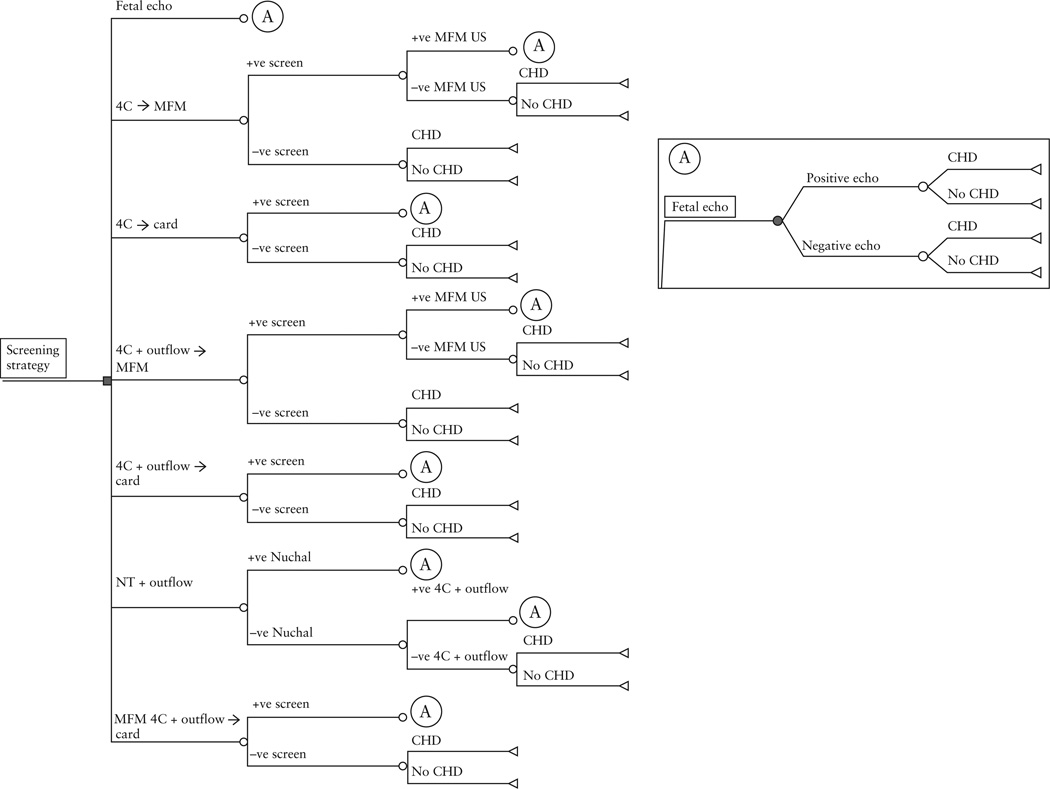
We constructed a decision analytic model of seven prenatal screening strategies for CHD using TreeAge Pro 11 (DATA Pro) (Figure 1). For the purposes of the model we assumed that women presented for prenatal care before 12 weeks’ gestation so that all screening options would be available and that all women were at low risk for delivering a child with CHD. Our model also took into account variation in the probability that a screening ultrasound could be performed at a tertiary/academic center and read by an MFM specialist, or performed at a non-tertiary center and read by a radiologist or a general obstetrician. It also accounted for different choices in referral for an abnormal screen interpreted by a non-tertiary practitioner, either to an MFM specialist first or directly to a pediatric cardiologist. The seven prenatal screening strategies considered were:
A second-trimester ultrasound screen with a four-chamber view alone, with an abnormal screen resulting in referral to an MFM specialist first (4C → MFM). A subsequent abnormal screen on detailed ultrasound performed by an MFM specialist would result in referral to a pediatric cardiologist for a fetal echocardiogram.
A second-trimester ultrasound screen with a four-chamber view alone, with an abnormal screen resulting in direct referral to a pediatric cardiologist (4C → card).
A second-trimester ultrasound screen with four-chamber and outflow-tract views, with an abnormal screen resulting in referral to an MFM specialist first. A subsequent abnormal screen on detailed ultrasound performed by an MFM specialist would result in referral to a pediatric cardiologist for a fetal echocardiogram (4C + outflow → MFM).
A second-trimester ultrasound screen with four-chamber and outflow-tract views, with an abnormal scan resulting in direct referral to a pediatric cardiologist (4C + outflow → card).
A second-trimester ultrasound with four-chamber and outflow-tract views performed at a tertiary obstetric center (and interpreted by an MFM specialist), with referral to a pediatric cardiologist if abnormal (MFM 4C + outflow → card)
A tiered screen that initially evaluated NT with first-trimester ultrasound. Those who screened negative would then undergo a second-trimester ultrasound screen with both a four-chamber and outflow-tract view of the heart (NT + outflow).
Use of universal fetal echocardiography to screen low-risk mothers (fetal echo).
Figure 1.
Decision tree structure for each strategy arm for prenatal screening of congenital heart disease. 4C → MFM, standard screen with second-trimester ultrasound four-chamber view of the heart, with referral to a maternal-fetal medicine (MFM) specialist if abnormal; 4C → card, standard screen with second-trimester ultrasound of the four-chamber view of the heart, with referral to a pediatric cardiologist if abnormal; 4C + outflow → MFM, screen with second-trimester ultrasound four-chamber and outflow views of the heart (read by a non-MFM specialist), with referral to an MFM specialist if abnormal; 4C + outflow → card, screen with second-trimester ultrasound four-chamber and outflow views of the heart (read by a non-MFM specialist), with referral to a pediatric cardiologist if abnormal; MFM 4C + outflow → card, screen with second-trimester ultrasound four-chamber and outflow views of the heart, interpreted by an MFM specialist and referred to a pediatric cardiologist if abnormal; NT + outflow, tiered screen with first-trimester nuchal translucency and referral directly for fetal echocardiography if abnormal; if normal, screen with second-trimester ultrasound four-chamber and outflow views. Fetal echo, universal screen with fetal echocardiography.
We chose to model the use of NT rather than ductus venosus in the first-trimester ultrasound, as the sensitivity of an abnormal NT is reported to be higher for detecting CHD and is more widely used18,23.
The values of the model parameters were determined from a review of the literature. The probability estimates and test characteristics (sensitivity and specificity) of screening tests were determined using the following medical search (MeSH) terms to search English publications in PubMed: ultrasonography, prenatal; heart defects, congenital; sensitivity and specificity, NT. Baseline estimates and ranges were assessed from this literature search (Table 1). Although the literature was searched as far back as 1992, estimates of test characteristics were taken preferentially from the last 15 years, if available. Baseline values were calculated using averages when several studies were available. Cost data were obtained from the Medicare National Fee schedule for 2012 (Table 2). Costs specified are global costs (including both the technical and professional components). A screen using a four-chamber view alone was considered a basic second-trimester ultrasound. Given the change in guidelines, an outflow tract was considered as part of a standard ultrasound in our baseline model. A scan that included outflows and was read by a MFM specialist was considered as a detailed ultrasound. The cost of a fetal echocardiogram included Doppler and color add on (Table 2).Those with a positive fetal echocardiogram (concerning CHD) had an assumed cost for a detailed ultrasound to look for other congenital defects (if one had not already been performed) in the process of screening. Medical costs for screening were considered only from the time of screening initiation to the time of detection of CHD (or a negative screen). Costs were only considered from a health-payer perspective and therefore did not include patient, family or other costs that may be relevant from a societal perspective.
Table 1.
Baseline estimates, obtained from the literature, of test characteristics for different screening approaches and of incidence of congenital heart disease (CHD): input values for the decision-analysis model
| Variable/Reference | Baseline estimate |
|
|---|---|---|
| Sensitivity (range) | Specificity (range) | |
| 4C view43–48 | 0.492 (0.210–0.810) | 0.990 (0.988–0.999) |
| 4C + outflow views28,29,45,48–50 |
0.67 (0.31–0.86) | 0.990 (0.970–0.999) |
| 4C + outflow views by MFM16,30,51–53 |
0.77 (0.55–1.00) | 0.99 (0.97–1.00) |
| First-trimester NT18,54 |
0.246 (0.080–0.560) | 0.982 (0.965–0.992) |
| MFM referral ultrasound16,52,55 |
0.80 (0.60–1.00) | 0.982 (0.924–0.999) |
| Pediatric cardiology fetal echo- cardiogram3,12,55 |
0.940 (0.900–0.976) | 0.995 (0.993–1.000) |
| –Incidence of CHD40–42 | 0.0075 (0.003–0.011) | |
4C, four chamber; MFM, maternal–fetal medicine specialist; NT, nuchal translucency.
Table 2.
Cost of procedures using Medicare 2012 National Fee Estimate56 (including professional and technical components)
| Test | Code | National Medicare Fee Estimate 2012 |
|---|---|---|
| Obstetric ultrasound (>14 weeks) |
76805 | $167 |
| Obstetric ultrasound, detailed | 76811 | $211 |
| Obstetric ultrasound, NT single or first gestation |
76813 | $140 |
| Fetal echocardiography | 76825 | $245 |
| Doppler fetal echocardiography |
76827 | $71 |
| Doppler color, add on | 93325 | $31 |
NT, nuchal translucency.
The decision tree describing the sequence of events in the screening process was analyzed to determine the incremental cost-effectiveness ratio (ICER) of the screening strategies. The ICER compares two competing strategies and is constructed by dividing the difference in the average costs between the two strategies by the difference in the average effectiveness. The effectiveness measure in our model was CHD cases detected. Therefore, the ICER represents the additional costs or savings for screening per additional congenital heart defect detected. In this case, as more than two strategies were being considered, all strategies were compared with one another in an incremental analysis. As costs were only measured to the time of detection of the heart defect, there was no need to discount costs or effects over time.
We performed a limited number of one-way sensitivity analyses to determine the variability in outcome based on the range of the test-input variables. The minimum and maximum values used for the deterministic sensitivity analysis were the lowest and highest results found in the literature (Table 1). One such analysis looked at the impact of varying the sensitivity of a 4C + outflow screen performed at a non-tertiary practice (by a non-MFM specialist) to look at how results would be influenced by the lowest and highest reported sensitivities of this screen. We also performed an additional sensitivity analysis in which we repeated the model comparing an arm of 4C + outflow → MFM (Strategy 3) with an arm that incorporated varying probabilities that a 4C + outflow screening ultrasound would be performed at a tertiary center and interpreted by an MFM specialist (MFM 4C + outflow) compared with a non-MFM specialist and referred to an MFM only if abnormal (4C + outflow → MFM). This resulted in an arm that combined Strategies 5 and 3 with varying probabilities of proceeding down one branch or the other.
Additionally, a probabilistic sensitivity analysis using 10 000 second-order Monte Carlo simulations was performed to assess the impact of the range of uncertainty in all the model variables. The minimum and maximum values used were the lowest and highest results found in our literature review.
RESULTS
The results for the model under baseline assumptions are illustrated in Table 3. In this base–case analysis, the least costly strategy for prenatal screening for CHD was out-flow → MFM, with an average cost per patient of $169.33. The same strategy, with referral to a pediatric cardiologist first, was more effective and specifically was more likely to identify a true positive diagnosis in the population (4.42 per 1000 vs 3.53 per 1000, assuming a population incidence of seven per 1000 for CHD) but was also more costly. The most effective strategy was fetal echocardiography (with the likelihood of identifying a true positive diagnosis of 6.6 per 1000). However, this strategy was extremely costly compared with other strategies, with an ICER of $112 560. Use of 4C alone was dominated (i.e. was more costly and less effective) by the use of four-chamber and outflow views, regardless of referral pattern. Additionally, NT + outflow was less effective and more costly than a linear combination of two alternative strategies, a concept called extended dominance. An MFM 4C + outflow screen was also more effective and identified 5.1 cases of CHD per 1000 births but with an ICER of almost $31 000.
Table 3.
Cost-effectiveness base–case analysis for prenatal screening of congenital heart disease (CHD)
| Strategy | Cost | Incremental cost |
Effectiveness* (cases detected per 1000) |
Incremental effectiveness (per 1000) |
ICER | Cases found† (%) |
|---|---|---|---|---|---|---|
| 4C + outflow → MFM | $169.33 | — | 3.53 | — | — | 50.0 |
| 4C + outflow → card | $169.84 | $0.51 | 4.42 | 0.9 | $579 | 63.1 |
| 4C → MFM | $170.37 | $1.04 | 2.37 | −1.16 | Dominated‡ | 33.9 |
| 4C → card | $171.76 | $2.43 | 2.96 | −0.57 | Dominated‡ | 42.4 |
| MFM 4C + outflow → card | $216.53 | $47.20 | 5.07 | 1.54 | $30 591 | 72.5 |
| NT + outflow | $316.58 | $147.25 | 5.06 | 1.53 | Extended dominance§ | 72.4 |
| Fetal echo | $513.72 | $344.39 | 6.59 | 3.06 | $112 560 | 94.2 |
Effectiveness is the population likelihood of a true-positive diagnosis.
Cases found represents the effectiveness/population likelihood of CHD (assumed incidence is seven per 1000 births).
A strategy is dominated when it is more costly and less effective than the strategy to which it is being compared.
A strategy is dominated by extended dominance when a combination of two other strategies is less expensive and more effective. 4C, four-chamber view; 4C+ outflow, four-chamber and outflow-tract views; card, pediatric cardiologist; Fetal echo, fetal echocardiography; ICER, incremental cost-effectiveness ratio; MFM, maternal–fetal medicine specialist; NT, nuchal translucency.
We also performed a one-way sensitivity analysis in which we only varied the sensitivity and specificity of a screen with four-chamber and outflow-tract views (Table 4). With a sensitivity as low as 31%, the use of the 4C + outflow → MFM remained the least expensive strategy. At its highest reported sensitivity of 86%, the ICER for this strategy with referral to cardiology first (4C + outflow →card), compared with referral to an MFM specialist first, dropped to an additional $520 per CHD detected.
Table 4.
One-way sensitivity analysis, varying the sensitivity of second-trimester ultrasound screening with four-chamber and outflow-tract views (4C + outflow) and the resulting incremental cost-effectiveness ratios (ICER) compared with the strategy involving a 4C+ outflow referred to a maternal–fetal medicine specialist (4C + outflow → MFM)
| Sensitivity of 4C + outflow |
ICER |
|||||
|---|---|---|---|---|---|---|
| 4C → MFM | 4C → card | 4C + outflow → card | MFM 4C + outflow → card | NT + outflow | Fetal echo | |
| 0.31 | $3105 | $2755 | $919 | $14079 | $83346 | $69822 |
| 0.42 | $12604 | $4385 | $748 | $16803 | $86729 | $79004 |
| 0.53 | Dominated* | $17 137 | $660 | $20912 | $90962 | $90994 |
| 0.64 | Dominated* | Dominated* | $595 | $27825 | $95017 | $107 319 |
| 0.75 | Dominated* | Dominated* | $549 | $41890 | $99455 | $130 843 |
| 0.86 | Dominated* | Dominated* | $520 | $86207 | $104 355 | $167 679 |
A strategy is dominated when it is more costly and less effective than the strategy to which it is being compared. 4C, four chamber; card, pediatric cardiologist; Fetal echo, fetal echocardiography; NT, nuchal translucency.
We examined the impact of having a varying proportion of 4C + outflow screening ultrasound scans read by an MFM specialist vs a non-MFM specialist, attributing the cost of an ultrasound read by an MFM specialist as that of a detailed ultrasound only if it was abnormal. If MFM specialists (MFM 4C + outflow) interpreted 20% of ultrasounds and non-MFM specialists interpreted 80%, then 55% of defects would be detected with an ICER of $26 204 compared to if 100% of screening ultrasounds were read by non-MFM specialists (Table 5). The ICER increased to $30 187 and the sensitivity to 68% if 80% of scans were interpreted by an MFM specialist.
Table 5.
Sensitivity analysis comparing the incremental cost-effectiveness for a strategy which compared the probability that a second-trimester obstetric ultrasound (with four-chamber (4C) and outflow views) will be interpreted by a maternal–fetal medicine (MFM) specialist (rather than non-MFM specialist) with a strategy in which all ultrasound images with 4C and outflow views are read by non-MFM specialists (4C + outflow → MFM)
| Probability of a screening ultrasound read by an MFM specialist |
Effectiveness (cases detected per 1000)* |
ICER compared with 4C + outflow → MFM |
|---|---|---|
| 20% | 2.89 | $26 204 |
| 40% | 4.18 | $29 026 |
| 60% | 4.48 | $29 789 |
| 80% | 4.78 | $30 187 |
| 100% | 5.10 | $30 591 |
Assuming a congenital heart disease incidence of 0.007 per live birth. 4C + outflow, four-chamber and outflow-tract views; ICER, incremental cost-effectiveness ratio.
Inputs for testing probabilities were varied based on their range of values in 10 000 second-order Monte Carlo simulations. Cost-effectiveness rankings and results remained similar to the baseline case for these simulations and were extrapolated to calculate the costs and effectiveness of screening 10 000 low-risk mothers, as presented in Table 6. Assuming an incidence of CHD of seven per 1000, there were 70 cases of CHD in this population. The least expensive strategy in multiway sensitivity analysis remained 4C + outflow → MFM, with a total cost of $1 690 000 to screen 10 000 mothers and an average cost of $47 268 per CHD detected. This strategy identified 36 (51%) cases. Use of a 4C alone remained more expensive and less effective. Although the use of NT + outflow was more effective (identifying 73% of cases), it was much more expensive compared with 4C + outflow → MFM (incremental cost of $1 472 634). Universal fetal echocardiography was highly effective, identifying 94% of CHD cases, but cost $5 million to screen 10 000 women. 4C + outflow →card had an incremental cost of $5168 more, but identified 45 (64%) cases and had an incremental cost-effectiveness of an additional $574 for each additional CHD detected. MFM 4C + outflow →card identified 73% of defects at a cost of $2 million to screen 10 000 women.
Table 6.
Summary of screening results in second-order Monte Carlo simulations of 10000 low-risk mothers
| Screening strategy | Total cost (× 106) |
Effectiveness (n (%) of cases detected)* |
Average CE |
Incremental cost |
Incremental effectiveness (%) |
ICER ($/cases detected) |
|---|---|---|---|---|---|---|
| 4C → MFM | $1.70 | 24 (34) | $71 701 | $10 005 | −12 (−30) | Dominated† |
| 4C → card | $1.72 | 30 (43) | $57 774 | $23 994 | −6 (−22) | Dominated† |
| 4C + outflow → MFM (Ref) | $1.69 | 36 (51) | $47 268 | — | — | — |
| 4C + outflow → card | $1.70 | 45 (64) | $37 885 | $5168 | 9 (13) | $574 |
| MFM 4C + outflow → card | $2.17 | 51 (73) | $42 548 | $471672 | 15 (21) | $31 320 |
| NT + outflow | $3.17 | 52 (74) | $61 889 | $1 472 634 | 15 (21) | Extended dominance‡ |
| Fetal echo | $5.14 | 66 (94) | $77 907 | $3 443 591 | 30 (43) | $114367 |
Assuming an incidence of congenital heart disease (CHD) of 0.007 per live birth and 70 cases of CHD in this cohort.
A strategy is dominated when it is more costly and less effective than the strategy to which it is being compared.
A strategy is dominated by extended dominance when a combination of two other strategies is less expensive and more effective. 4C, four-chamber view; 4C + outflow, four-chamber and outflow-tract views; card, pediatric cardiologist; CE, cost effectiveness; Fetal echo, fetal echocardiography; ICER, incremental cost-effectiveness ratio; MFM, maternal–fetal medicine specialist; NT, nuchal translucency; Ref, reference.
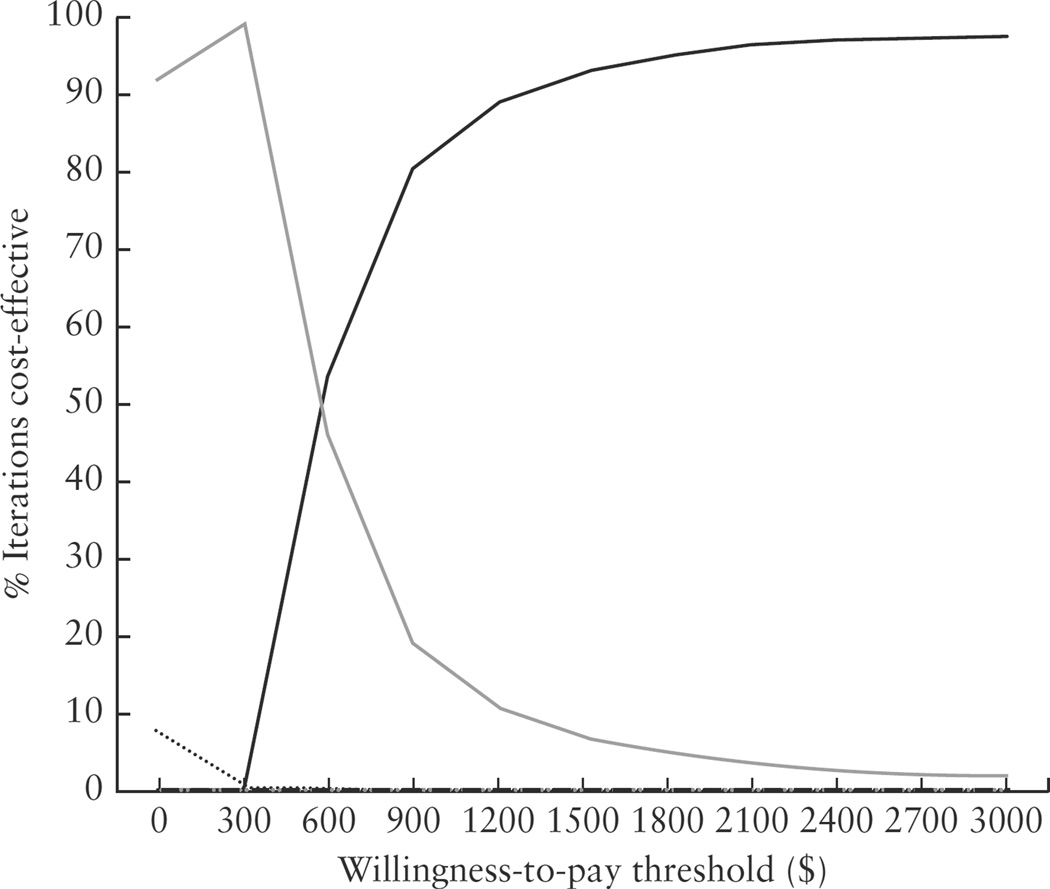
Figure 2 shows the results from the probabilistic sensitivity analysis as cost-effectiveness acceptability curves. These curves depict the proportion of the 10 000 Monte Carlo simulations for which each strategy is cost-effective, given a certain threshold value for willingness to pay for an additional detected case of CHD. Use of 4C + outflow → MFM was cost-effective for the majority of simulations until the threshold of $574, when it shifted in favor of referral to a pediatric cardiologist first. For willingness-to-pay thresholds greater than $2000 per defect, 4C + outflow → card was cost-effective for almost 100% of the simulations.
Figure 2.
Cost-effectiveness acceptability curve depicting the percentage of iteration strategies that are cost-effective based on willingness-to-pay per congenital heart disease detected. ………., 4C→MFM, standard screen with second-trimester ultrasound four-chamber view of the heart, with referral to a maternal–fetal medicine (MFM) specialist if abnormal;  , 4C→card, standard screen with second-trimester ultrasound of the four-chamber view of the heart, with referral to a pediatric cardiologist if abnormal;
, 4C→card, standard screen with second-trimester ultrasound of the four-chamber view of the heart, with referral to a pediatric cardiologist if abnormal;  , 4C + outflow → MFM, screen with second-trimester ultrasound four-chamber and outflow views of the heart (read by a non-MFM specialist), with referral to an MFM specialist if abnormal;
, 4C + outflow → MFM, screen with second-trimester ultrasound four-chamber and outflow views of the heart (read by a non-MFM specialist), with referral to an MFM specialist if abnormal;  , 4C + outflow → card, screen with second-trimester ultrasound four-chamber and outflow views of the heart (read by a non-MFM specialist), with referral to a pediatric cardiologist if abnormal;
, 4C + outflow → card, screen with second-trimester ultrasound four-chamber and outflow views of the heart (read by a non-MFM specialist), with referral to a pediatric cardiologist if abnormal;  , MFM 4C + outflow → card, screen with second-trimester ultrasound four-chamber and outflow views of the heart, interpreted by an MFM specialist and referred to a pediatric cardiologist if abnormal;
, MFM 4C + outflow → card, screen with second-trimester ultrasound four-chamber and outflow views of the heart, interpreted by an MFM specialist and referred to a pediatric cardiologist if abnormal;  , NT + outflow, tiered screen with first-trimester nuchal translucency and referral directly for fetal echocardiography if abnormal; if normal, screen with second-trimester ultrasound four chamber and outflow views.
, NT + outflow, tiered screen with first-trimester nuchal translucency and referral directly for fetal echocardiography if abnormal; if normal, screen with second-trimester ultrasound four chamber and outflow views.  , Fetal echo, universal screen with fetal echocardiography.
, Fetal echo, universal screen with fetal echocardiography.
DISCUSSION
To our knowledge, this is the first study to examine the relative cost-effectiveness of prenatal screening strategies for CHD in low-risk mothers in the USA. While we found universally that fetal echocardiography was the most effective strategy, it was also exceedingly costly. The addition of an outflow-tract view to the standard screen was more effective and less expensive than almost all other strategies for prenatal CHD screening, lending further support for recent changes in consensus recommendations in the USA and by the International Society of Ultrasound in Obstetrics and Gynecology8,24. This finding remained robust across wide simultaneous variations of estimated inputs.
Our study reinforces previous findings of the UK’s National Institute for Clinical Excellence (NICE) in an unpublished CEA of prenatal screening for d-transposition of the great arteries in low-risk mothers. The study concluded that inclusion of outflow-tract views was cost-effective compared with a four-chamber view alone, but did not consider other proposed screening strategies9. Several publications have reported the association of abnormalities in early first-trimester scans (primarily abnormal NT or ductus venosus tracings) with a higher CHD risk17,18,23. In our model, a tiered screening with first-trimester ultrasound to measure NT for all low-risk women was more effective, but was considerably more expensive than the use of second-trimester ultrasound with four-chamber and outflow views. Use of non-invasive screening for aneuploidy through cell-free fetal DNA may result in fewer women undergoing NT for identification of aneuploidy25,26. Thus, determining the value of NT for detecting CHD may become increasingly important. Our findings would argue against focusing on this strategy for detection of CHD in low-risk mothers.
Improving the prenatal CHD screening strategy also requires consideration of optimal referral patterns for an initial screen and abnormal screening results. Previous economic evaluations of prenatal CHD screening have centered on higher-risk pregnancies (primarily diabetic mothers)21,27. Some conclude that a well-executed obstetric scan is cost effective compared with referral of these mothers directly for fetal echocardiography. However, this conclusion depends heavily on the quality of the ultrasound and its interpretation. The sensitivity of second-trimester ultrasound screening is currently quite variable and operator dependent, probably because of challenges in obtaining the outflow-tract view28,29. As our model shows, having all such ultrasounds interpreted by MFM specialists who have a higher accuracy is very effective, but is also more costly. However, previous studies have shown that, at present, ultrasounds are interpreted primarily outside tertiary centers by non-MFM specialists16,30. Telemedicine may prove a cost-effective way to address access barriers to specialist interpretation22. Alternatively, resources could be invested in improving skills at non-tertiary centers. One study, looking at cost savings in postnatal care for prenatally diagnosed infants with CHD, extrapolated that increased training of sonographers would be cost-effective. However, the study primarily focused on costs related to the transport of newborns with CHD31.
In our model, if an initial screening ultrasound was read by a non-MFM specialist, referral to an MFM specialist after an abnormal screen was less expensive, but missed almost 50% of heart defects. The optimal strategy for referral depends on society’s willingness to pay for increased effectiveness. Historical consensus-based thresholds for willingness to pay are based on quality-adjusted life years (QALYs), a commonly used effectiveness measure for CEAs32. However, thresholds for willingness to pay for prenatal detection of diseases are unclear. Currently, the average cost of prenatal screening programs for trisomy 21 ranges from $27 000 to $78 000 per defect detected, depending on the strategy33–35. CHD is much more common and more lethal than trisomy 211,36 and compares favorably in terms of costs per defect detected, being an average of $38 000 to $47 000 in our study using second-trimester ultrasound. In this setting, an additional $580–2100 per defect may be acceptable to society.
This study considered costs and effectiveness for a limited time horizon, specifically only to the time of CHD detection. This was a purposeful decision. Few studies have compared the costs of prenatally vs postnatally diagnosed infants37, and in those that have, the findings are unclear. Further investigation of the impact of prenatal detection on outcomes is required to provide reliable inputs for a broader economic analysis. Calculating QALYs for children with CHD requires determining their additional life years saved and multiplying this by the utility (the value of each year depending on the quality of their life). However, there are few utilities available for survivors with different types of CHD. Most previous studies, acknowledging these limitations, have used utilities for adult heart-failure patients22,38. Looking beyond detection of CHD also requires modeling how prenatal diagnosis impacts pregnancy terminations. Careful consideration of costs and benefits are needed for future models navigating the complexities of addressing this politically sensitive issue39.
There were additional limitations to our study. Our model assumed that all women presented early for prenatal care. We did not consider other strategies proposed for prenatal screening for CHD, such as the use of 3D or 4D imaging19,20. As the equipment and operator familiarity with 3D imaging is currently limited primarily to tertiary or academic centers, this technology has yet to be widely used in practice, and reliable data on effectiveness outside such centers are lacking. The strategies considered in this study are available at most centers providing obstetric ultrasound services. As more data become available, future economic analyses should consider additional strategies.
In summary, this study shows that, of the methods currently widely available in practice, examination of both the four-chamber and outflow-tract views during second-trimester ultrasound is the least expensive strategy to screen for CHD prenatally. This adds to a strong body of evidence to prioritize effective implementation of new consensus guidelines in the USA and similar guidelines previously mandated in Canada, Britain and other European countries over other current alternatives. It also encourages the adoption of similar guidelines in other nations. Further inquiry by health professionals and policy makers is required to decide on optimal referral strategies for initial screens and abnormal results based on economic and clinical consequences.
Acknowledgments
This study was supported by the National Cancer Institute’s Translational and Comparative Effectiveness Scholars Program NIH Career Enhancement Award (Award Number 1KM1CA156723-01) and the University of Utah Center For Clinical and Translational Science’s Pilot Project Award (Award Number UL1RR025764) for Dr Pinto.
REFERENCES
- 1.Hoffman JI. Incidence of congenital heart disease: II. Prenatal incidence. Pediatr Cardiol. 1995;16:155–165. doi: 10.1007/BF00794186. [DOI] [PubMed] [Google Scholar]
- 2.Kleinert S. Routine prenatal screening for congenital heart disease. Lancet. 1996;348:836. doi: 10.1016/S0140-6736(05)64727-7. [DOI] [PubMed] [Google Scholar]
- 3.Stumpfen I, Stumpfen A, Wimmer M, Bernaschek G. Effect of detailed fetal echocardiography as part of routine prenatal ultrasonographic screening on detection of congenital heart disease. Lancet. 1996;348:854–857. doi: 10.1016/S0140-6736(96)04069-X. [DOI] [PubMed] [Google Scholar]
- 4.AIUM Practice Guideline for the Performance of Obstetric Ultrasound Examinations. J Ultrasound Med. 2013;32:1083–1101. doi: 10.7863/ultra.32.6.1083. [DOI] [PubMed] [Google Scholar]
- 5.AIUM Practice Guideline for the Performance of Obstetric Ultrasound Examinations. J Ultrasound Med. 2010;29:157–166. doi: 10.7863/jum.2010.29.1.157. [DOI] [PubMed] [Google Scholar]
- 6.American College of Radiology Practice Guideline for the Performance of Obstetrical Ultrasound. Reston, VA: American College of Radiology; 2007. [Google Scholar]
- 7.ACOG Practice Bulletin No 77: screening for fetal chromosomal abnormalities. Obstet Gynecol. 2007;109:217–227. doi: 10.1097/00006250-200701000-00054. [DOI] [PubMed] [Google Scholar]
- 8.Cargill Y, Morin L. Content of a complete routine second trimester obstetric ultrasound examination and report. J Obstet Gynaecol Can. 2009;31:272–275. doi: 10.1016/S1701-2163(16)34127-5. [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Health and Clinical Excellence. Antenatal Care: Routine Care for the Healthy Pregnant Woman. London: NICE; 2008. [Google Scholar]
- 10.Bromley B, Estroff JA, Sanders SP, Parad R, Roberts D, Frigoletto FD, Jr, Benacerraf BR. Fetal echocardiography: accuracy and limitations in a population at high and low risk for heart defects. Am J Obstet Gynecol. 1992;166:1473–1481. doi: 10.1016/0002-9378(92)91622-h. [DOI] [PubMed] [Google Scholar]
- 11.Chew C, Halliday JL, Riley MM, Penny DJ. Population-based study of antenatal detection of congenital heart disease by ultrasound examination. Ultrasound Obstet Gynecol. 2007;29:619–624. doi: 10.1002/uog.4023. [DOI] [PubMed] [Google Scholar]
- 12.Khoo NS, Van Essen P, Richardson M, Robertson T. Effectiveness of prenatal diagnosis of congenital heart defects in South Australia: a population analysis 1999–2003. Aust N Z J Obstet Gynaecol. 2008;48:559–563. doi: 10.1111/j.1479-828X.2008.00915.x. [DOI] [PubMed] [Google Scholar]
- 13.Stoll C, Garne E, Clementi M. Evaluation of prenatal diagnosis of associated congenital heart diseases by fetal ultrasonographic examination in Europe. Prenat Diagn. 2001;21:243–252. doi: 10.1002/pd.34. [DOI] [PubMed] [Google Scholar]
- 14.Marek J, Tomek V, Skovranek J, Povysilova V, Samanek M. Prenatal ultrasound screening of congenital heart disease in an unselected national population: a 21-year experience. Heart. 2011;97:124–130. doi: 10.1136/hrt.2010.206623. [DOI] [PubMed] [Google Scholar]
- 15.McBrien A, Sands A, Craig B, Dornan J, Casey F. Major congenital heart disease: antenatal detection, patient characteristics and outcomes. J Matern Fetal Neonatal Med. 2009;22:101–105. doi: 10.1080/14767050802483106. [DOI] [PubMed] [Google Scholar]
- 16.Pinto NM, Keenan HT, Minich LL, Puchalski MD, Heywood M, Botto LD. Barriers to prenatal detection of congenital heart disease: a population-based study. Ultrasound Obstet Gynecol. 2012;40:418–425. doi: 10.1002/uog.10116. [DOI] [PubMed] [Google Scholar]
- 17.Makrydimas G, Sotiriadis A, Ioannidis JP. Screening performance of first-trimester nuchal translucency for major cardiac defects: a meta-analysis. Am J Obstet Gynecol. 2003;189:1330–1335. doi: 10.1067/s0002-9378(03)00645-8. [DOI] [PubMed] [Google Scholar]
- 18.Clur SA, Ottenkamp J, Bilardo CM. The nuchal translucency and the fetal heart: a literature review. Prenat Diagn. 2009;29:739–748. doi: 10.1002/pd.2281. [DOI] [PubMed] [Google Scholar]
- 19.Cohen L, Mangers K, Grobman WA, Gotteiner N, Julien S, Dungan J, Fonseca L, Platt LD. Three-dimensional fast acquisition with sonographically based volume computer-aided analysis for imaging of the fetal heart at 18 to 22 weeks’ gestation. J Ultrasound Med. 2010;29:751–757. doi: 10.7863/jum.2010.29.5.751. [DOI] [PubMed] [Google Scholar]
- 20.Espinoza J, Lee W, Comstock C, Romero R, Yeo L, Rizzo G, Paladini D, Viñals F, Achiron R, Gindes L, Abuhamad A, Sinkovskaya E, Russell E, Yagel S. Collaborative study on 4-dimensional echocardiography for the diagnosis of fetal heart defects: the COFEHD study. J Ultrasound Med. 2010;29:1573–1580. doi: 10.7863/jum.2010.29.11.1573. [DOI] [PubMed] [Google Scholar]
- 21.Odibo AO, Coassolo KM, Stamilio DM, Ural SH, Macones GA. Should all pregnant diabetic women undergo a fetal echocardiography? A cost-effectiveness analysis comparing four screening strategies. Prenat Diagn. 2006;26:39–44. doi: 10.1002/pd.1322. [DOI] [PubMed] [Google Scholar]
- 22.Mistry H, Gardiner HM. The cost-effectiveness of prenatal detection for congenital heart disease using telemedicine screening. J Telemed Telecare. 2013;19:190–196. doi: 10.1258/jtt.2012.120418. [DOI] [PubMed] [Google Scholar]
- 23.Papatheodorou SI, Evangelou E, Makrydimas G, Ioannidis JP. First-trimester ductus venosus screening for cardiac defects: a meta-analysis. BJOG. 2011;118:1438–1445. doi: 10.1111/j.1471-0528.2011.03029.x. [DOI] [PubMed] [Google Scholar]
- 24.Cardiac screening examination of the fetus: guidelines for performing the ‘basic’ and ‘extended basic’ cardiac scan. Ultrasound Obstet Gynecol. 2006;27:107–113. doi: 10.1002/uog.2677. [DOI] [PubMed] [Google Scholar]
- 25.Committee Opinion No 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol. 2012;120:1532–1534. doi: 10.1097/01.AOG.0000423819.85283.f4. [DOI] [PubMed] [Google Scholar]
- 26.Benn P, Cuckle H, Pergament E. Non-invasive prenatal testing for aneuploidy: current status and future prospects. Ultrasound Obstet Gynecol. 2013;42:15–33. doi: 10.1002/uog.12513. [DOI] [PubMed] [Google Scholar]
- 27.Bernard LS, Ramos GA, Fines V, Hull AD. Reducing the cost of detection of congenital heart disease in fetuses of women with pregestational diabetes mellitus. Ultrasound Obstet Gynecol. 2009;33:676–682. doi: 10.1002/uog.6302. [DOI] [PubMed] [Google Scholar]
- 28.Tegnander E, Eik-Nes SH. The examiner’s ultrasound experience has a significant impact on the detection rate of congenital heart defects at the second-trimester fetal examination. Ultrasound Obstet Gynecol. 2006;28:8–14. doi: 10.1002/uog.2804. [DOI] [PubMed] [Google Scholar]
- 29.McBrien A, Sands A, Craig B, Dornan J, Casey F. Impact of a regional training program in fetal echocardiography for sonographers on the antenatal detection of major congenital heart disease. Ultrasound Obstet Gynecol. 2010;36:279–284. doi: 10.1002/uog.7616. [DOI] [PubMed] [Google Scholar]
- 30.Friedberg MK, Silverman NH, Moon-Grady AJ, Tong E, Nourse J, Sorenson B, Lee J, Hornberger LK. Prenatal detection of congenital heart disease. J Pediatr. 2009;155:26–31. e1. doi: 10.1016/j.jpeds.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 31.Jegatheeswaran A, Oliveira C, Batsos C, Moon-Grady AJ, Silverman NH, Hornberger LK, Coyte P, Friedberg MK. Costs of prenatal detection of congenital heart disease. Am J Cardiol. 2011;108:1808–1814. doi: 10.1016/j.amjcard.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 32.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8:165–178. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]
- 33.Gekas J, Durand A, Bujold E, Vallée M, Forest JC, Rousseau F, Reinharz D. Cost-effectiveness and accuracy of prenatal Down syndrome screening strategies: should the combined test continue to be widely used? Am J Obstet Gynecol. 2011;204:175, e1–e8. doi: 10.1016/j.ajog.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham GC, Tompkinison DG. Cost and effectiveness of the California triple marker prenatal screening program. Genet Med. 1999;1:199–206. doi: 10.1097/00125817-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Hoogendoorn M, Evers SM, Schielen PC, van Genugten ML, de Wit GA, Ament AJ. Costs and effects of prenatal screening methods for Down syndrome and neural tube defects. Community Genet. 2008;11:359–367. doi: 10.1159/000133308. [DOI] [PubMed] [Google Scholar]
- 36.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDer-mott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 37.Copel JA, Tan AS, Kleinman CS. Does a prenatal diagnosis of congenital heart disease alter short-term outcome? Ultrasound Obstet Gynecol. 1997;10:237–241. doi: 10.1046/j.1469-0705.1997.10040237.x. [DOI] [PubMed] [Google Scholar]
- 38.Yount LE, Mahle WT. Economic analysis of palivizumab in infants with congenital heart disease. Pediatrics. 2004;114:1606–1611. doi: 10.1542/peds.2004-0224. [DOI] [PubMed] [Google Scholar]
- 39.Ganiats TG. Justifying prenatal screening and genetic amniocentesis programs by cost-effectiveness analyses: a re-evaluation. Med Decis Making. 1996;16:45–50. doi: 10.1177/0272989X9601600112. [DOI] [PubMed] [Google Scholar]
- 40.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 41.Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erick-son JD. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation. 2001;103:2376–2381. doi: 10.1161/01.cir.103.19.2376. [DOI] [PubMed] [Google Scholar]
- 42.Botto LD, Correa A, Erickson JD. Racial and temporal variations in the prevalence of heart defects. Pediatrics. 2001;107:E32. doi: 10.1542/peds.107.3.e32. [DOI] [PubMed] [Google Scholar]
- 43.Garne E, Stoll C, Clementi M. Evaluation of prenatal diagnosis of congenital heart diseases by ultrasound: experience from 20 European registries. Ultrasound Obstet Gynecol. 2001;17:386–391. doi: 10.1046/j.1469-0705.2001.00385.x. [DOI] [PubMed] [Google Scholar]
- 44.Nikkila A, Bjorkhem G, Kallen B. Prenatal diagnosis of congenital heart defects – a population based study. Acta Paediatr. 2007;96:49–52. doi: 10.1111/j.1651-2227.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- 45.Sklansky MS, Berman DP, Pruetz JD, Chang RK. Prenatal screening for major congenital heart disease: superiority of outflow tracts over the 4-chamber view. J Ultrasound Med. 2009;28:889–899. doi: 10.7863/jum.2009.28.7.889. [DOI] [PubMed] [Google Scholar]
- 46.Acherman RJ, Evans WN, Luna CF, Rollins R, Kip KT, Collazos JC, Restrepo H, Adasheck J, Iriye BK, Roberts D, Sacks AJ. Prenatal detection of congenital heart disease in southern Nevada: the need for universal fetal cardiac evaluation. J Ultrasound Med. 2007;26:1715–1719. doi: 10.7863/jum.2007.26.12.1715. quiz 20–21. [DOI] [PubMed] [Google Scholar]
- 47.Vergani P, Mariani S, Ghidini A, Schiavina R, Cavallone M, Locatelli A, Strobelt N, Cerruti P. Screening for congenital heart disease with the four-chamber view of the fetal heart. Am J Obstet Gynecol. 1992;167:1000–1003. doi: 10.1016/s0002-9378(12)80027-5. [DOI] [PubMed] [Google Scholar]
- 48.Achiron R, Glaser J, Gelernter I, Hegesh J, Yagel S. Extended fetal echocardiographic examination for detecting cardiac malformations in low risk pregnancies. BMJ. 1992;304:671–674. doi: 10.1136/bmj.304.6828.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogge G, Gaglioti P, Maccanti S, Faggiano F, Todros T. Prenatal screening for congenital heart disease with four-chamber and outflow-tract views: a multicenter study. Ultrasound Obstet Gynecol. 2006;28:779–784. doi: 10.1002/uog.3830. [DOI] [PubMed] [Google Scholar]
- 50.Carvalho JS, Mavrides E, Shinebourne EA, Campbell S, Thilaganathan B. Improving the effectiveness of routine prenatal screening for major congenital heart defects. Heart. 2002;88:387–391. doi: 10.1136/heart.88.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedman AM, Phoon CK, Fishman S, Seubert DE, Timor-Tritsch IE, Schwartz N. The utility of fetal echocardiography after an unremarkable anatomy scan. Obstet Gynecol. 2011;118:921–927. doi: 10.1097/AOG.0b013e31822e1264. [DOI] [PubMed] [Google Scholar]
- 52.Starikov RS, Bsat FA, Knee AB, Tsirka AE, Paris Y, Markenson GR. Utility of fetal echocardiography after normal cardiac imaging fndings on detailed fetal anatomic ultrasonography. J Ultrasound Med. 2009;28:603–608. doi: 10.7863/jum.2009.28.5.603. [DOI] [PubMed] [Google Scholar]
- 53.Muller PR, James A, Feldman K, Herlong JR. Utility of fetal echocardiogram in high-risk patients. Aust N Z J Obstet Gynaecol. 2005;45:117–121. doi: 10.1111/j.1479-828X.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- 54.Mavrides E, Cobian-Sanchez F, Tekay A, Moscoso G, Campbell S, Thilaganathan B, Carvalho JS. Limitations of using first-trimester nuchal translucency measurement in routine screening for major congenital heart defects. Ultrasound Obstet Gynecol. 2001;17:106–110. doi: 10.1046/j.1469-0705.2001.00342.x. [DOI] [PubMed] [Google Scholar]
- 55.Meyer-Wittkopf M, Cooper S, Sholler G. Correlation between fetal cardiac diagnosis by obstetric and pediatric cardiologist sonographers and comparison with postnatal findings. Ultrasound Obstet Gynecol. 2001;17:392–397. doi: 10.1046/j.1469-0705.2001.00381.x. [DOI] [PubMed] [Google Scholar]
- 56.Physician Fee Schedule. Baltimore, MD: Centers for Medicare and Medicaid Services; www.cms.gov/physician-fee-schedule/search/. (last updated 17 April 2014) [Google Scholar]