Abstract
Purpose
Sleep disruption and shift work have been associated with cancer risk, but epidemiologic evidence for prostate cancer remains limited. We aimed to prospectively investigate the association between midlife sleep- and circadian-related parameters and later prostate cancer risk and mortality in a population-based cohort of Finnish twins.
Methods
Data were drawn from the Older Finnish Twin Cohort and included 11,370 twins followed from 1981 to 2012. Over the study period, 602 incident cases of prostate cancer and 110 deaths from prostate cancer occurred. Cox regression was used to evaluate associations between midlife sleep duration, sleep quality, chronotype, and shift work with prostate cancer risk and prostate cancer-specific mortality. Within-pair co-twin analyses were employed to account for potential familial confounding.
Results
Compared to “definite morning” types, “somewhat evening” types had a significantly increased risk of prostate cancer (HR 1.3; 95% CI: 1.1, 1.6). Chronotype significantly modified the relationship between shift work and prostate cancer risk (p-interaction < 0.001). We found no significant association between sleep duration, sleep quality, or shift work and prostate cancer risk in the overall analyses and no significant association between any sleep- or circadian-related parameter and risk in co-twin analyses. Neither sleep- nor circadian-related parameters were significantly associated with prostate cancer-specific mortality.
Conclusions
The association between sleep disruption, chronotype, and shift work with prostate cancer risk and mortality has never before been studied in a prospective study of male twins. Our findings suggest that chronotype may be associated with prostate cancer risk and modify the association between shift work and prostate cancer risk. Future studies of circadian disruption and prostate cancer should account for this individual-level characteristic.
INTRODUCTION
The circadian system plays a critical role in synchronizing genetic, physiologic, and behavioral rhythms in the body. Sleep disruption and shift work may desynchronize this system, resulting in adverse health outcomes via a variety of biologically plausible mechanisms [1]. While the impact of sleep disruption and shift work on cancer risk is attracting increased research attention, most of the epidemiologic literature to date has focused on breast cancer risk [2, 3] and evidence for prostate cancer remains sparse [4].
Some studies suggest that shorter sleep duration and increased sleep disruption may be associated with increased prostate cancer risk [5–8], although the evidence remains inconsistent [9, 10]. Evidence for an association between shift work and prostate cancer also remains inconclusive. Recent case-control [11–13] and cohort [14, 15] studies have reported associations between rotating and night shift work and prostate cancer risk, while some cohort studies have reported no association [5, 16, 17]. Individual-level characteristics that may modify the association between working time and cancer risk have been largely understudied, which has potentially contributed to the heterogeneous findings across studies. Chronotype, characterized by an individual’s preference for morning or evening activity, may influence adaptability to various work schedules and thereby act as a unique marker of susceptibility to sleep and circadian disruption [18]. In fact, men with earlier (morning) chronotypes experience higher sleep and circadian misalignment during night shifts than do later (evening) chronotypes, while later (evening) chronotypes experience higher sleep and circadian misalignment during morning shifts than do earlier (morning) chronotypes [18]. Such findings highlight the importance of looking beyond the cancer risk associated with working or sleeping in a particular time window to a more personalized examination of the risk associated with working in a time window that is not compatible with one’s diurnal preference. Investigators have therefore recently begun to incorporate measures of chronotype into studies of breast [19–21] and prostate [12] cancer, as well as studies of shift work and melatonin levels [22, 23].
We aimed to investigate the influence of midlife sleep duration, sleep quality, chronotype, and shift work on prostate cancer risk later in life among men in the Older Finnish Twin Cohort, a population-based cohort of twins with 30 years of follow-up data. In addition to examining the risk of prostate cancer diagnosis, we explored the risk of prostate cancer-specific mortality, which reflects the most clinically aggressive disease. We further explored potential interactions between chronotype and shift work. These associations have never before been examined in a prospective study of male twins – a setting that allows for the application of powerful analytic methods to control for potential familial confounding (genetics and shared early environment). We hypothesized that shorter sleep duration, poorer sleep quality, and misalignment of chronotype and work type would be associated with an increased prostate cancer risk.
MATERIAL AND METHODS
Study population
This study was nested within the Older Finnish Twin Cohort, consisting of all Finnish same-sex twin pairs born before 1958 with both co-twins alive in 1975. Twin pairs were selected from the Central Population Registry of Finland in 1974, and twin zygosity was determined by a validated questionnaire shown to accurately classify >93% of twin pairs as monozygotic (MZ) or dizygotic (DZ) [24]. Questionnaires were mailed to participants in 1981 with a response rate of 84%. They contained questions on sleep patterns and chronotype in addition to comprehensive questions on socio-demographic, occupational, psychosocial, health, and lifestyle factors. The present study includes the 11,370 male twin individuals who responded to the 1981 questionnaire, were free of prostate cancer at that time, and who had data on at least one sleep- (sleep duration, sleep quality) or circadian-related exposure (chronotype, shift work). This study population includes 2,580 monozygotic (MZ) and 5,716 dizygotic (DZ) twins from pairs in which both brothers met the inclusion criteria. In addition, there were 456 MZ and 1,312 DZ twins without their co-twin and 1,306 twins of uncertain zygosity included in the study. The mean age (± standard deviation) of the participants at the time of study entry was 40.0 years (± 12.1).
The study was approved by the ethical committee of the Hjelt Institute, Faculty of Medicine, University of Helsinki. Permission for linkage of the cancer registry data was provided by the National Institute for Health and Welfare, Helsinki, Finland. Informed consent was obtained from all individuals.
Exposure assessment
Given the long latency of prostate cancer, we were interested in the influence of midlife sleep- and circadian-related exposures on prostate cancer risk and mortality later in life. Information on sleep duration and quality, chronotype, and shift work was obtained in the 1981 questionnaire. Sleep duration was obtained by asking: “How many hours do you usually sleep per 24 hours?” (9 response categories: ≤6, 6.5, 7, 7.5, 8, 8.5, 9, 9.5, and ≥10 hours). Sleep duration data were sparse at extreme values and so were collapsed into 3 categories: <7, 7–8, and >8 hours. The sleep quality question was: “Do you usually sleep well?” (5 response categories: “well,” “fairly well,” “fairly poorly,” “poorly,” and “cannot say”). Sleep quality data were similarly collapsed into 3 categories: well, fairly well, and fairly poorly/poorly. “Cannot say” responses were incorporated into a missing data category for this variable. The question assessing chronotype (“Will you try to estimate to what extent you are a morning or an evening person?”) was similar to that asked in the Horne and Østberg morningness-eveningness questionnaire (MEQ) [25]. The response categories for chronotype included: “I am clearly ‘a morning person’ (morning spry and evening sleepy),” “I am to some extent ‘a morning person,’” “I am to some extent ‘an evening person’ (morning sleepy and evening spry),” “I am clearly ‘an evening person’”). We classified chronotype data into 4 categories: definite morning type, somewhat morning type, somewhat evening type, and definite evening type. Data on shift work were obtained by assessing the respondent’s current or latest work type and were classified into 4 categories: fixed days, fixed nights, rotating shift, and not recently working. Rotating shift work refers to work that rotated through morning, evening, or night shifts in either a 2-shift or 3-shift pattern.
Outcome ascertainment
Data on prostate cancer incidence (ICD code 185) was obtained through record linkage to the Finnish Cancer Registry, where 100% of registered cases are histologically verified. Data on prostate cancer-specific mortality came from the cause-of-death register at Statistics Finland. All of those who died from prostate cancer had a diagnosis prior to death in the Finnish Cancer Registry. Data on emigration and vital status were obtained through linkage to the Population Register Center of Finland. Data from all registries were linked to Finnish Twin Cohort data using unique personal identity codes assigned to every permanent resident of Finland.
Statistical analysis
Descriptive analyses were performed to characterize the study population and examine differences across chronotypes, with means and standard deviations presented for continuous variables and counts and percentages for categorical variables (Table 1). For each sleep- and circadian-related exposure, Cox proportional hazard models were used to estimate age-adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) for the outcomes of prostate cancer diagnosis and prostate cancer-specific mortality. Age was the underlying time metameter. Each subject’s date of entry was defined as his exact age when the 1981 questionnaire was returned. Participants were followed prospectively through December 31, 2012 for the occurrence of prostate cancer, death, or emigration. Log-log plots of survival curves of the sleep- and circadian-related exposure categories were used to verify that the curves were parallel and the proportional-hazards assumption was not violated. Due to the dependent nature of this sample of twin pairs, standard errors and CIs were adjusted for possible within-pair correlations using robust variance estimators.
Table 1.
Baseline Characteristics by Chronotype, Older Finnish Twin Cohort, 1981
| Overall | Chronotype | ||||
|---|---|---|---|---|---|
|
| |||||
| n = 11,227a | Definite morning (n=3,159; 28.1%) | Somewhat Morning (n=3,275; 29.2%) | Somewhat Evening (n=3,676; 32.7%) | Definite evening (n=1,117; 10.0%) | |
|
| |||||
| Mean (SD) | |||||
|
| |||||
| Age | 40.0 (12.1) | 45.1 (13.4) | 39.9 (12.0) | 36.5 (11.0) | 37.7 (11.6) |
| BMI (kg/m2) | 24.5 (3.1) | 25.1 (3.2) | 24.5 (3.0) | 24.1 (3.0) | 24.1 (3.1) |
|
|
|||||
| N (%) | |||||
|
|
|||||
| Education: | |||||
| <6 years | 245 (2.2) | 116 (3.7) | 55 (1.7) | 54 (1.5) | 20 (1.8) |
| 6 years | 4,280 (38.1) | 1,469 (46.5) | 1,281 (39.1) | 1,205 (32.8) | 325 (29.1) |
| Middle School | 4,618 (41.1) | 1,075 (34.0) | 1,393 (42.5) | 1,670 (45.4) | 480 (43.0) |
| High school or more | 1,103 (9.8) | 205 (6.5) | 291 (8.9) | 440 (12.0) | 167 (15.0) |
| Missing | 981 (8.7) | 294 (9.3) | 255 (7.8) | 307 (8.4) | 125 (11.2) |
| Physical Activity | |||||
| Sedentary | 1,460 (13.0) | 399 (12.6) | 402 (12.3) | 476 (12.9) | 183 (16.4) |
| Occasional exerciser | 7,859 (70.0) | 2,330 (73.8) | 2,279 (69.6) | 2,507 (68.2) | 743 (66.5) |
| Conditioning exerciserb | 1,908 (17.0) | 430 (13.6) | 594 (18.1) | 693 (18.9) | 191 (17.1) |
| Employed | 9,163 (81.6) | 2,394 (76.5) | 2,786 (85.5) | 3,124 (85.5) | 859 (77.4) |
| Social Class | |||||
| Upper white collar | 875 (7.8) | 217 (6.9) | 255 (7.8) | 292 (7.9) | 111 (9.9) |
| Lower white collar | 1,938 (17.3) | 571 (18.1) | 594 (18.1) | 590 (16.1) | 183 (16.4) |
| Skilled worker | 4,747 (42.3) | 1,345 (42.6) | 1,402 (42.8) | 1,586 (43.1) | 414 (37.1) |
| Unskilled worker | 1,001 (8.9) | 340 (10.8) | 278 (8.5) | 279 (7.6) | 104 (9.3) |
| Farmer | 931 (8.3) | 285 (9.0) | 308 (9.4) | 277 (7.5) | 61 (5.5) |
| Other/unknown | 722 (6.4) | 92 (2.9) | 176 (5.4) | 337 (9.2) | 117 (10.5) |
| Missing | 1,013 (9.0) | 309 (9.8) | 262 (8.0) | 315 (8.6) | 127 (11.4) |
| Smoking status | |||||
| Never | 3,334 (29.7) | 914 (28.9) | 1,089 (33.3) | 1,091 (29.7) | 240 (21.5) |
| Occasional | 434 (3.9) | 139 (4.4) | 118 (3.6) | 135 (3.7) | 42 (3.8) |
| Former | 3,062 (27.3) | 992 (31.4) | 897 (27.4) | 917 (24.9) | 256 (22.9) |
| Current | 4,164 (37.1) | 1,025 (32.4) | 1,113 (34.0) | 1,467 (39.9) | 559 (50.0) |
| Missing | 233 (2.1) | 89 (2.8) | 58 (1.8) | 66 (1.8) | 20 (1.8) |
| Heavy alcohol usec | 4,684 (41.7) | 1,164 (37.7) | 1,300 (40.3) | 1,681 (46.3) | 539 (49.0) |
| Use of hypnotic agents and/or tranquilizers | |||||
| Never | 9,405 (83.8) | 2,473 (78.3) | 2,815 (86.0) | 3,198 (87.0) | 919 (82.3) |
| <10 days/year | 227 (2.0) | 63 (2.0) | 55 (1.7) | 78 (2.1) | 31 (2.8) |
| 10–59 days/year | 145 (1.3) | 54 (1.7) | 29 (0.9) | 39 (1.1) | 23 (2.1) |
| 60–180 days/year | 52 (0.5) | 14 (0.4) | 16 (0.5) | 14 (0.4) | 8 (0.7) |
| 180+ days/year | 104 (0.9) | 28 (0.9) | 26 (0.8) | 30 (0.8) | 20 (1.8) |
| Missing | 1,294 (11.5) | 527 (16.7) | 334 (10.2) | 317 (8.6) | 116 (10.4) |
| Snoring | |||||
| Never | 1,677 (14.9) | 397 (12.6) | 490 (15.0) | 615 (16.7) | 175 (15.7) |
| Sometimes | 6,232 (55.5) | 1,728 (54.7) | 1,874 (57.2) | 2,046 (55.7) | 584 (52.3) |
| Often | 1,667 (14.8) | 515 (16.3) | 468 (14.3) | 517 (14.1) | 167 (15.0) |
| Nearly always | 733 (6.5) | 295 (9.3) | 178 (5.4) | 195 (5.3) | 65 (5.8) |
| Missing | 918 (8.2) | 224 (7.1) | 265 (8.1) | 303 (8.2) | 126 (11.3) |
| Shift work | |||||
| Day | 9,218 (82.1) | 2,676 (84.7) | 2,763 (84.4) | 2,939 (80.0) | 840 (75.2) |
| Night | 94 (0.8) | 18 (0.6) | 17 (0.5) | 33 (0.9) | 26 (2.3) |
| Rotating | 1,771 (15.8) | 410 (13.0) | 461 (14.1) | 671 (18.3) | 229 (20.5) |
| Not recently working | 40 (0.4) | 11 (0.3) | 9 (0.3) | 9 (0.2) | 11 (1.0) |
| Missing | 104 (0.9) | 44 (1.4) | 25 (0.8) | 24 (0.7) | 11 (1.0) |
| Sleep Duration | |||||
| <7 hours | 1,744 (15.5) | 518 (16.4) | 439 (13.4) | 514 (14.0) | 273 (24.4) |
| 7–8 hours | 7,771 (69.2) | 2,121 (67.1) | 2,371 (72.4) | 2,612 (71.1) | 667 (59.7) |
| >8 hours | 1,653 (14.7) | 502 (15.9) | 441 (13.5) | 539 (14.7) | 171 (15.3) |
| Missing | 59 (0.5) | 18 (0.6) | 24 (0.7) | 11 (0.3) | 6 (0.5) |
| Sleep Quality | |||||
| Well | 4,385 (39.1) | 1,300 (41.2) | 1,304 (39.8) | 1,409 (38.3) | 372 (33.3) |
| Fairly well | 4,816 (42.9) | 1,282 (40.5) | 1,466 (44.8) | 1,635 (44.5) | 433 (38.8) |
| Fairly poorly/poorly | 950 (8.5) | 266 (8.4) | 226 (6.9) | 292 (7.9) | 166 (14.9) |
| Missing | 1,076 (9.6) | 311 (9.8) | 279 (8.5) | 340 (9.3) | 146 (13.1) |
BMI, body mass index, calculated from self-reported height and weight; MET, metabolic equivalents.
Number of subjects with data on chronotype
Conditioning exerciser refers to those reporting exercising at least 6 times per month for a mean duration for at least 30 minutes and with a mean intensity corresponding to at least vigorous walking to jogging.
Heavy alcohol use refers to reported consumption of >5 bottles of beer, 1 bottle of wine, or 4 drinks (≥18 mL of spirits) on the same occasion at least once a month during the preceding year.
To assess the association between chronotype and shift work with prostate cancer risk and prostate cancer-specific mortality, we conducted Cox proportional hazards regression, mutually adjusting for chronotype and shift work. Cox regression was also conducted to evaluate the association between sleep duration and quality and prostate cancer risk and mortality, mutually adjusting for sleep duration and quality. Our final multivariable models were adjusted for potential confounding variables, based on subject-matter knowledge: education (<6 years, 6 years, middle school, high school or more), BMI (kg/m2), physical activity (sedentary, occasional exerciser, conditioning exerciser), social class (upper white collar, lower white collar, skilled worker, unskilled worker, farmer, other), smoking status (never, occasional, former, current), alcohol use (deciles of number of drinks per week, with one standard drink defined as 12 grams of alcohol), snoring (never, sometimes, often, nearly always), and zygosity (MZ, DZ, XZ). Construction of a polychoric correlation matrix for these predictor variables showed all correlations to be generally low; only 4 correlations were greater than 0.2. The highest inter-correlation was between smoking status and education (r=0.42). Missing values of categorical variables were handled by creating a missing data indicator for inclusion in the models, and missing values of continuous variables were imputed using the mean value for that variable. We had complete data on participant age from registry linkage. Only 78 men (0.69%) were missing data on BMI and required mean imputation for this variable.
We also examined whether chronotype modified the association between work type and prostate cancer risk by creating product terms between chronotype and work type categories and estimating likelihood ratio tests. We similarly explored interactions between chronotype and sleep duration or quality.
We further performed co-twin analyses to assess the association between sleep- and circadian-related exposures and prostate cancer risk within twin pairs discordant for prostate cancer. These Cox models were stratified on twin pairs, allowing each twin pair to have its own baseline hazard. This serves as a powerful approach to account for potential familial confounding (genetics and shared family environment) when assessing twins discordant for sleep- and circadian-related exposures and prostate cancer outcomes. All statistical analyses were performed using Stata version 13.1 (Stata Corporation, College Station, TX, USA).
RESULTS
Over a median of 30 years of follow-up, 11,370 men contributed 289,714 person-years at risk for prostate cancer. During this time, 602 incident cases of prostate cancer and 110 deaths from prostate cancer occurred. The mean age (± standard deviation) of participants at the time of prostate cancer diagnosis was 69.9 years (± 8.9).
Baseline characteristics of participants are displayed in Table 1 by chronotype. The chronotype distribution in this study population was as follows: 28% definite morning type, 29% somewhat morning type, 33% somewhat evening type, and 10% definite evening type. Definite morning types were older, less educated, and had a higher BMI than other chronotypes. Definite evening types were more likely to report shorter sleep duration and poorer sleep quality, and be a current smoker, heavy drinker, and night or rotating-shift worker compared to those in other chronotype categories.
In analysis of the overall population, somewhat evening types had a 1.3-fold higher risk of prostate cancer compared to definite morning types (HR 1.3; 95% CI: 1.1, 1.6) in multivariable models (Table 2). No other chronotype was significantly associated with prostate cancer risk. In analyses conducted within twin pairs discordant for chronotype and prostate cancer outcome, we found no association between chronotype and prostate cancer risk (somewhat morning types [HR 1.1; 95% CI: 0.7, 1.6), somewhat evening types [HR 1.0; 95% CI 0.6, 1.5], definite evening types [HR 1.0; 95% CI, 0.5, 1.9]; reference = definite morning types).
Table 2.
Chronotype, Shift Work, Sleep Duration, Sleep Quality, and Prostate Cancer Risk (HR and 95% CI), Older Finnish Twin Cohort, 1981–2012
| Person-years | Prostate Cancer Incidence | Prostate Cancer-Specific Mortality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| No. events | Age-adjusted | Fully-adjusteda | No. events | Age-adjusted | Fully-adjusteda | ||||||
| Chronotypeb | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| Definite morning | 74,691 | 208 | 1.00 (ref) | 1.00 (ref) | 40 | 1.00 (ref) | 1.00 (ref) | ||||
| Somewhat morning | 85,266 | 167 | 1.0 | 0.8, 1.3 | 1.0 | 0.8, 1.2 | 36 | 1.2 | 0.8, 1.9 | 1.2 | 0.8, 1.9 |
| Somewhat evening | 98,030 | 181 | 1.4 | 1.1, 1.7 | 1.3 | 1.1, 1.6 | 31 | 1.3 | 0.8, 2.0 | 1.2 | 0.8, 2.0 |
| Definite evening | 28,723 | 39 | 0.9 | 0.7, 1.3 | 0.9 | 0.6, 1.2 | 3 | 0.4 | 0.1, 1.3 | 0.4 | 0.1, 1.3 |
| Missing | 3,005 | 7 | 0.9 | 0.4, 2.1 | 0.9 | 0.4, 2.0 | 0 | ||||
| Shift workbc | |||||||||||
| Day | 237,642 | 509 | 1.00 (ref) | 1.00 (ref) | 94 | 1.00 (ref) | 1.00 (ref) | ||||
| Night | 2,395 | 2 | 0.5 | 0.1, 2.0 | 0.5 | 0.1, 1.9 | 0 | ||||
| Rotating | 46,463 | 80 | 1.0 | 0.8, 1.2 | 1.0 | 0.7, 1.2 | 11 | 0.8 | 0.4, 1.5 | 0.7 | 0.3, 1.5 |
| Missing | 2,055 | 11 | 1.0 | 0.6, 1.8 | 1.1 | 0.6, 2.1 | 5 | 2.5 | 1.1, 6.0 | 2.6 | 1.1, 6.3 |
| Sleep durationd | |||||||||||
| <7 hours | 43,103 | 99 | 1.00 (ref) | 1.00 (ref) | 18 | 1.00 (ref) | 1.00 (ref) | ||||
| 7–8 hours | 204,336 | 405 | 0.9 | 0.7, 1.1 | 0.9 | 0.7, 1.2 | 68 | 0.9 | 0.5, 1.5 | 0.9 | 0.5, 1.5 |
| >8 hours | 40,728 | 92 | 0.9 | 0.7, 1.3 | 1.0 | 0.7, 1.3 | 24 | 1.4 | 0.8, 2.6 | 1.5 | 0.8, 2.8 |
| Missing | 1,547 | 6 | 1.4 | 0.6, 3.1 | 1.5 | 0.7, 3.4 | 0 | ||||
| Sleep qualityd | |||||||||||
| Well | 118,129 | 217 | 1.00 (ref) | 1.00 (ref) | 34 | 1.00 (ref) | 1.00 (ref) | ||||
| Fairly well | 124,028 | 281 | 1.0 | 0.8, 1.1 | 1.0 | 0.8, 1.1 | 55 | 1.2 | 0.8, 1.9 | 1.3 | 0.8, 1.9 |
| Fairly poorly/poorly | 21,262 | 61 | 0.9 | 0.7, 1.2 | 0.9 | 0.7, 1.2 | 13 | 1.2 | 0.6, 2.4 | 1.2 | 0.6, 2.4 |
| Missing | 26,294 | 43 | 0.9 | 0.6, 1.2 | 0.6 | 0.2, 1.4 | 8 | 1.1 | 0.5, 2.3 | 0.5 | 0.2, 1.1 |
Fully adjusted for the following covariates: age, education, BMI, physical activity, social class, smoking status, alcohol use, snoring, and zygosity.
Chronotype and shift work were run together in one model. Age-adjusted and fully-adjusted models both mutually adjusted for chronotype and shift work.
No prostate cancer cases within the “not recently working” shift work category (not displayed).
Sleep duration and quality were run together in a separate model. Age-adjusted and fully-adjusted models both mutually adjusted for sleep duration and sleep quality.
No significant associations between sleep duration, sleep quality, or shift work and prostate cancer risk were found in the overall (Table 2) or within-pair co-twin analyses (data not shown). In addition, we found no significant associations between any sleep- or circadian-related parameter and prostate cancer-specific mortality (Table 2). Estimates were unchanged when models for shift work and chronotype were also adjusted for sleep duration and quality (data not shown).
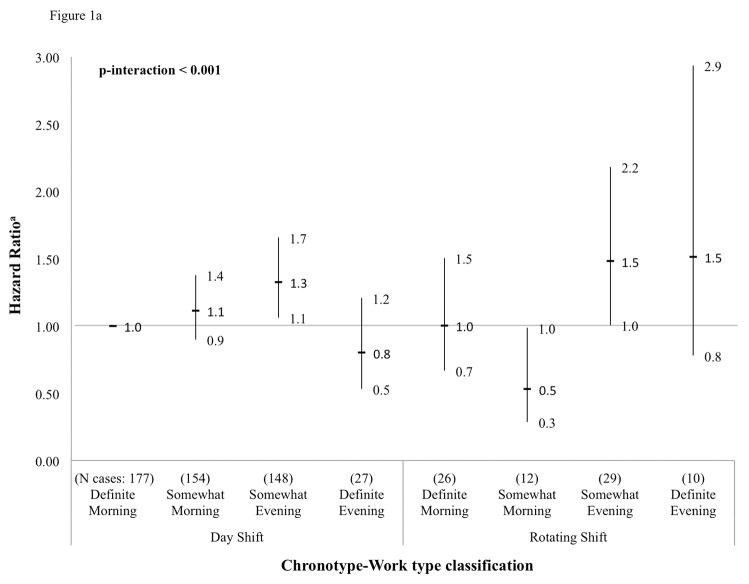
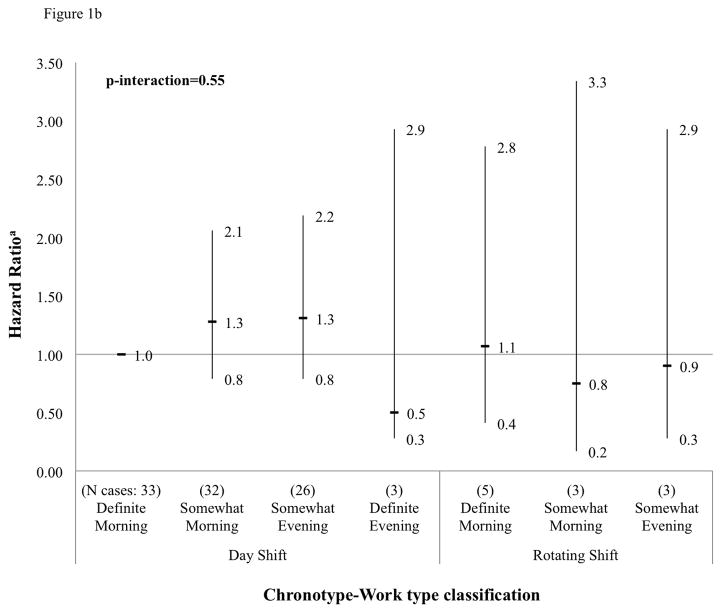
We found a highly significant interaction between chronotype and work type for the outcome of prostate cancer incidence in the overall study population (p-interaction < 0.001) (Figure 1a). Somewhat evening type day workers were at a 1.3-fold higher risk of prostate cancer compared to definite morning type day workers (HR 1.3; 95% CI: 1.1, 1.7). Also at a suggestively increased risk were somewhat morning type day workers (HR 1.1; 95% CI: 0.9, 1.4), somewhat evening type rotating-shift workers (HR 1.5; 95% CI: 1.0, 2.2), and definite evening type rotating-shift workers (HR 1.5; 95% CI: 0.8, 2.9). At a suggestively decreased risk of prostate cancer were somewhat morning type rotating-shift workers (HR 0.5; 95% CI: 0.3, 1.0). Examination of an interaction between chronotype and night work was precluded by a limited number of prostate cases among night-workers (n=2). Chronotype by work type interactions were not significant for the outcome of prostate cancer-specific mortality, although power was lower for these analyses (p-interaction=0.55) (Figure 1b). Magnitudes of risk for the outcome of mortality were comparable to those obtained from incidence analyses, but no significant associations were detected perhaps due to low case counts in cross-tabulated categories. In addition, no significant interactions between chronotype and sleep duration or quality were found (data not shown).
Figure 1.
Figure 1a. Cross-tabulation between chronotype and work type, and prostate cancer risk (HR and 95% CI), Older Finnish Twin Cohort, 1981–2012 (583 cases)
aHazard ratios for product terms between chronotype and work type categories. Reference = definite morning type in day work.
bNote: Examination of an interaction between chronotype and night work was precluded by a limited number of prostate cancer cases among night workers (n=2).
Figure 1b. Cross-tabulation between chronotype and work type, and prostate cancer-specific mortality (HR and 95% CI), Older Finnish Twin Cohort, 1981–2012 (105 cases)
aHazard ratios for product terms between chronotype and work type categories. Reference = definite morning type in day work.
bNote: Examination of an interaction between chronotype and night work was precluded by a limited number of prostate cancer-specific death cases among night workers and no cases among definite evening type rotating shift workers.
DISCUSSION
In this prospective, population-based cohort study of Finnish male twins, we found that somewhat evening types were at a significantly higher risk of prostate cancer than definite morning types. We also found a significant interaction between chronotype and shift work. Compared to definite morning types in day work, we observed a significantly increased risk of prostate cancer among somewhat evening types in rotating-shift and day work as well as a decreased risk among somewhat morning types in rotating-shift work. Sleep duration, sleep quality, and shift work were not significantly associated with prostate cancer risk in the overall or co-twin analyses, and no significant association was found between any sleep- or circadian-related exposure and prostate cancer-specific mortality. Altogether, our data support the hypothesis that chronotype may interact with work type to influence prostate cancer risk, but, similar to some prior studies [9, 10], they do not support the hypothesis that sleep duration or sleep quality are associated with prostate cancer risk.
Chronotype has been evaluated in one prior study of shift work and prostate cancer risk [12]. Similar to the findings of this study [12], our analyses conducted within the overall study population revealed that somewhat evening types were at a significantly higher risk for prostate cancer than definite morning types. However, we found that this association disappeared when the relationship between chronotype and prostate cancer risk was analyzed within twin pairs discordant for chronotype and prostate cancer outcome. This result might suggest that the significant association observed in the overall study population could have been driven by an unaccounted for shared genetic or shared environment factor, and the possibility of a factor influencing both chronotype and prostate cancer could be further explored. An earlier report from the Finnish Twin Cohort estimated that genetic factors account for approximately half of the inter-individual variability in diurnal type, with the remainder accounted for by non-shared environmental factors [26], while the heritability of liability to prostate cancer is also estimated to be about 50% [27]. Alternatively, this may be a chance finding due to the smaller numbers and thus lower power of the discordant twin pair analysis.
Previous evidence suggests that rotating shift workers may be particularly susceptible to circadian disruption as their biologic clock is frequently at odds with substantially displaced bouts of activity over the 24-hour time span [28]. Our findings did not consistently align with this suggestion: rotating-shift work was associated with a significantly increased risk of prostate cancer among somewhat evening types, but with a significantly decreased risk among somewhat morning types.
We further assessed chronotype as an effect modifier of the relationship between work type and prostate cancer risk. We hypothesized that the inconsistent or null findings of former studies of shift work and prostate cancer risk [5, 16, 17] may be partially rooted in a lack of consideration of chronotype as an important modifier of this association. Indeed, qualitative effect modification was noted in our study, whereby the direction of prostate cancer risk associated with each work type differed across chronotypes.
It has been proposed that shift work may increase prostate cancer risk through mechanisms of sleep reduction/disruption, circadian disruption, and/or light-induced suppression of melatonin secretion [4, 29]. Recent evidence suggests that chronotype may modify the degree of sleep and melatonin disruption that accompanies various shift patterns [18, 22, 23]. It is biologically plausible that chronotype may modify the association between shift work and cancer along several of these proposed pathways. Later chronotypes have a later subjective, internal night and exhibit a later peak in melatonin compared to earlier chronotypes [30]. Day work is expected to coincide, at least partially, with the biological night of later chronotypes and therefore may be more disruptive to patterns of sleep, circadian rhythm, and melatonin secretion among these individuals. In line with this hypothesis, we found that somewhat evening types in day work were at a significantly higher risk of prostate cancer than definite morning types in day work. In contrast, definite evening types in day work were not found to be at an increased risk, despite likely engaging in activity during periods that have even greater overlap with their biologic night. However, there were a small number of participants in this strata and this finding may be due to a lack of power or chance.
In the present study, we found no significant association between reported sleep duration or quality and prostate cancer incidence or prostate cancer-specific mortality. Few prior studies have evaluated associations between sleep and prostate cancer, and the epidemiologic evidence that does exist has been inconsistent. Some studies examining this association have reported shorter, more disrupted sleep to be associated with an increased risk of total prostate cancer [6, 7], advanced or metastatic prostate cancer [6, 7], and prostate cancer mortality [5] in healthy baseline populations. However, two recent prospective cohort studies of baseline healthy populations of men in Sweden [9] and the U.S. [10] similarly found no association between sleep duration or quality and risk of prostate cancer (total, advanced, or lethal).
Important strengths of our study include its prospective design, long duration of follow-up, population-based sample, complete and reliable outcome data obtained through registry linkage, high questionnaire response rate (84%), detailed questionnaire data on socio-demographic, lifestyle, and sleep factors, and use of within-family analyses with a twin-co-twin design to explore the associations of interest while controlling for potential confounding by familial factors (genetics and shared early environment).
However, several limitations should be noted. First, chronotype was assessed with a single question rather than a series of questions, as in the Horne and Østberg MEQ [25]. However, it has been shown that answers to a similar self-classification of diurnal preference question are highly correlated with chronotype classifications derived from more comprehensive validated questionnaires [31]. In addition, a broad definition of shift work measured at a single time point prohibited exploration of detailed shift systems, duration of shift work, or shift intensity. Further, while we did not have data on family history of prostate cancer, the unique nature of this twin population permitted within-pair co-twin analysis – a powerful approach to account for confounding by both genetics and shared early environment. Although we adjusted for a variety of potential confounders, it is not possible to rule out residual confounding by an unobserved risk factor uniquely related to the exposures of interest. Finally, we cannot rule out the possibility that the incidence findings might be partially explained by prostate-specific antigen (PSA) testing. It is unlikely that PSA testing would vary by chronotype or sleep behavior, although there could be an association between work type and PSA testing. We would expect that the low-cost and universal health care available in Finland allowed for widespread access to healthcare across all socioeconomic groups in our study population. Moreover, routine PSA testing among asymptomatic men was not common in Finland during the study period [32], and prostate cases in Finland tend to be more aggressive at diagnosis than in the U.S. [33, 34]. Cases are thus expected to be clinically relevant. We further examined risk associated with prostate cancer-specific mortality, which reflects the most aggressive prostate tumors. The relative risks for mortality were not consistently stronger or weaker than those obtained for incidence, and thus do not suggest confounding due to PSA testing.
CONCLUSION
In this prospective, population-based cohort study of Finnish male twins, we found some suggestion that chronotype may be associated with prostate cancer risk and may modify the association between shift work and prostate cancer risk. Future studies exploring the impact of circadian disruption on prostate cancer risk should account for this individual-level characteristic. The possibility of a shared genetic or environmental factor influencing both chronotype and prostate cancer should also be explored.
Acknowledgments
Grants: JK is supported by the Academy of Finland (Grants 265240 & 263278). SCM and BAD are supported by the National Cancer Institute at the National Institutes of Health Training Grant NIH T32 CA09001.
We are grateful to Kauko Heikkilä, Ph.Lic for assistance in database management.
Funding: JK is supported by the Academy of Finland (Grants 265240 & 263278). SCM is supported by the National Cancer Institute at the National Institutes of Health Training Grant NIH T32 CA09001.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Archer SN, Oster H. How sleep and wakefulness influence circadian rhythmicity: effects of insufficient and mistimed sleep on the animal and human transcriptome. [published online ahead of print 2015] J Sleep Res. doi: 10.1111/jsr.12307. [DOI] [PubMed] [Google Scholar]
- 2.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93(20):1557–1562. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 3.Hansen J. Increased breast cancer risk among women who work predominantly at night. Epidemiology. 2001;12(1):74–77. doi: 10.1097/00001648-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Sigurdardottir LG, Valdimarsdottir UA, Fall K, et al. Circadian disruption, sleep loss, and prostate cancer risk: A systematic review of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1002–1011. doi: 10.1158/1055-9965.EPI-12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gapstur SM, Diver WR, Stevens VL, Carter BD, Teras LR, Jacobs EJ. Work schedule, sleep duration, insomnia, and risk of fatal prostate cancer. Am J Prev Med. 2014;46(3S1):S26–S33. doi: 10.1016/j.amepre.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 6.Kakizaki M, Inoue K, Kuriyama S, et al. Sleep duration and the risk of prostate cancer: The Ohsaki Cohort Study. Br J Cancer. 2008;99:176–178. doi: 10.1038/sj.bjc.6604425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sigurdardottir LG, Valdimarsdottir UA, Mucci LA, et al. Sleep disruption among older men and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(5):872–879. doi: 10.1158/1055-9965.EPI-12-1227-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sigurdardottir LG, Markt SC, Rider JR, et al. Urinary melatonin levels, sleep disruption, and risk of prostate cancer in elderly men. Eur Urol. 2015;67(2):191–194. doi: 10.1016/j.eururo.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markt SC, Grotta A, Nyren O, et al. Insufficient sleep and risk of prostate cancer in a large Swedish cohort. Sleep. 2015;38(9):1405–10. doi: 10.5665/sleep.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markt SC, Flynn-Evans EE, Valdimarsdottir U, et al. Sleep duration and disruption and prostate cancer risk: A 23-year prospective study. CEBP. 2015 doi: 10.1158/1055-9965.EPI-14-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conlon M, Lightfoot N, Kreiger N. Rotating shift work and risk of prostate cancer. Epidemiology. 2007;18(1):182–183. doi: 10.1097/01.ede.0000249519.33978.31. [DOI] [PubMed] [Google Scholar]
- 12.Papantoniou K, Castaño-Vinyals G, Espinosa A, et al. Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. [published online ahead of print December 20, 2014] Int J Cancer. doi: 10.1002/ijc.29400. [DOI] [PubMed] [Google Scholar]
- 13.Parent MÉ, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. Am J Epidemiol. 2012;176(9):751–759. doi: 10.1093/aje/kws318. [DOI] [PubMed] [Google Scholar]
- 14.Kubo T, Ozasa K, Mikami K, et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: Findings from the Japan Collaborative Cohort Study. Am J Epidemiol. 2006;164(6):549–555. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- 15.Kubo T, Oyama I, Nakamura T, et al. Industry-based retrospective cohort study of the risk of prostate cancer among rotating-shift workers. Int J Urol. 2011;18:206–211. doi: 10.1111/j.1442-2042.2010.02714.x. [DOI] [PubMed] [Google Scholar]
- 16.Schwartzbaum J, Ahlbom A, Feychting M. Cohort study of cancer risk among male and female shift workers. Scand J Work Environ Health. 2007;33(5):336–343. doi: 10.5271/sjweh.1150. [DOI] [PubMed] [Google Scholar]
- 17.Yong M, Blettner M, Emrich K, Nasterlack M, Oberlinner C, Hammer GP. A retrospective cohort study of shift work and risk of cancer-specific mortality in German male chemical workers. Scand J Work Environ Health. 2014;40(5):502–510. doi: 10.5271/sjweh.3438. [DOI] [PubMed] [Google Scholar]
- 18.Juda M, Vetter C, Roenneberg T. Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. J Biol Rhythms. 2013;28(2):141–151. doi: 10.1177/0748730412475042. [DOI] [PubMed] [Google Scholar]
- 19.Fritschi L, Erren TC, Glass DC, et al. The association between different night shiftwork factors and breast cancer: A case-control study. Br J Cancer. 2013;109(9):2472–2480. doi: 10.1038/bjc.2013.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen J, Lassen CF. Nested case-control study of night shift work and breast cancer risk among women in the Danish military. Occup Environ Med. 2012;69(8):551–556. doi: 10.1136/oemed-2011-100240. [DOI] [PubMed] [Google Scholar]
- 21.Ramin C, Devore EE, Pierre-Paul J, Duffy JF, Hankinson SE, Schernhammer ES. Chronotype and breast cancer risk in a cohort of US nurses. Chronobiol Int. 2013;30(9):1181–1186. doi: 10.3109/07420528.2013.809359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatti P, Mirick DK, Davis S. The impact of chronotype on melatonin levels among shift workers. Occup Environ Med. 2014;71(3):195–200. doi: 10.1136/oemed-2013-101730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung M, Tranmer J, Hung E, Korsiak J, Day AG, Aronson KJ. Shift work, chronotype, and melatonin patterns among female hospital employees on day and night shifts. CEBP. 2016;25:830–838. doi: 10.1158/1055-9965.EPI-15-1178. [DOI] [PubMed] [Google Scholar]
- 24.Sarna S, Kaprio J, Sistonen P, Koskenvuo M. Diagnosis of twin zygosity by mailed questionnaire. Hum Hered. 1978;28(4):241–254. doi: 10.1159/000152964. [DOI] [PubMed] [Google Scholar]
- 25.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 26.Koskenvuo M, Hublin C, Partinen M, Heikkila K, Kaprio J. Heritability of diurnal type: A nationwide study of 8753 adult twin pairs. J Sleep Res. 2007;16(2):156–162. doi: 10.1111/j.1365-2869.2007.00580.x. [DOI] [PubMed] [Google Scholar]
- 27.Hjelmborg JB, Scheike T, Holst K, et al. The heritability of prostate cancer in the Nordic Twin Study of Cancer. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2303–2311. doi: 10.1158/1055-9965.EPI-13-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juda M, Vetter C, Roenneberg T. The Munich ChronoType Questionnaire for Shift-Workers (MCTQShift) J Biol Rhythms. 2013;28(2):130–140. doi: 10.1177/0748730412475041. [DOI] [PubMed] [Google Scholar]
- 29.Haus EL, Smolensky MH. Shift work and cancer risk: Potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev. 2013;17(4):273–284. doi: 10.1016/j.smrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura S, Hida A, Aritake S, et al. Validity of the Japanese version of the Munich ChronoType Questionnaire. Chronobiol Int. 2014;31(7):845–850. doi: 10.3109/07420528.2014.914035. [DOI] [PubMed] [Google Scholar]
- 31.Roenneberg T, Kuehnle T, Juda M, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11(6):429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Pogodin-Hannolainen D, Juusela H, Tammela TLJ, et al. Prostate cancer screening: A survey of attitudes and practices among Finnish physicians in 1999 and 2007. J Med Scre. 2011;18:46–49. doi: 10.1258/jms.2010.010090. [DOI] [PubMed] [Google Scholar]
- 33.Andriole GL, Crawford ED, Grubb RL, Buys SS. Mortality Results from a Randomized Prostate-Cancer Screening Trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mäkinen T, Tammela TLJ, Hakama M, et al. Tumor characteristics in a population-based prostate cancer screening trial with prostate-specific antigen. Clin Cancer Res. 2003;9:2435–2439. [PubMed] [Google Scholar]