ABSTRACT
A 68-year-old male presented with blurred vision in both eyes. Ophthalmoscopy revealed bilateral prominent disc swelling and vitritis. No systematic neurological symptoms were observed. Magnetic resonance imaging revealed bilateral meningeal enhancement of the optic nerve. Small cell carcinoma was found, and antibodies against collapsing response-mediating protein-5 (CRMP-5) were detected in the serum. Ophthalmological manifestations disappeared during a decrease in tumour size with treatment for the malignancy. This case report describes this rare case of anti-CRMP-5 antibody–positive paraneoplastic perioptic neuritis without neurological symptoms, showing that prompt diagnosis and timely treatment of the underlying tumour are crucial to prevent increased levels of autoantibodies and irreversible damage to the nervous system.
KEYWORDS: Anti-CRMP-5 antibody, paraneoplastic optic neuropathy, small cell lung carcinoma
Introduction
Paraneoplastic optic neuropathy (PON) is a rare syndrome that can cause acute or subacute, bilateral, painless, progressive visual deterioration or visual loss characterised by optic disc swelling and vitritis.1,2 Most PON patients exhibit many neurological symptoms, including ophthalmoplegia, cerebellar ataxia, seizures, dementia, and various sensory and/or motor abnormalities.1,3–5 The various neurological symptoms are believed to be caused by an antitumour immune response3,6 associated with an increased level of autoantibodies against collapsing response-mediating protein-5 (CRMP-5),4,5,7–9 which is expressed in the optic nerve, neurons of the central and peripheral nervous systems, oligodendrocytes, the cytoplasm of small cell lung carcinoma (SCLC) cells, or thymomas.5,6,9–11 Only a few studies have reported anti-CRMP-5 antibody–positive PON cases that presented with optic neuropathy as the only clinical symptom. Magnetic resonance imaging (MRI) of these cases has been reported as normal,8,12 or involving unilateral optic nerve involvement.7 In the following case report, we describe an anti-CRMP-5 antibody–positive PON patient without neurological symptoms who presented with bilateral perioptic neuritis.
Case report
A 68-year-old male former smoker who smoked 40 cigarettes daily for 38 years was admitted to a hospital, complaining of painless blurred vision in both eyes starting a few weeks before his visit. Because ophthalmoscopy revealed that both eyes had pale swollen discs, he was transferred to our hospital. Initial neuro-ophthalmic testing showed a corrected visual acuity of 20/20 in his right eye and 20/25 in his left eye. He did not suffer from headaches or eye pain. The pupillary reactions were normal, and no relative afferent pupillary defect was observed. The patient’s ocular movements were normal. Systemic neurological symptoms, including sensorimotor deficits examined by a neurologist, were absent. Anterior segment features were normal, except that cataracts were apparent. Ophthalmoscopy and B-mode ultrasonography revealed prominent disc swelling and vitritis in both eyes (Figure 1A, B). Humphrey visual field testing showed that the blind spots were markedly enlarged, especially in the left eye, and that non-specific peripheral scotoma was present in both eyes (Figure 2A). Optical coherence tomography (OCT) revealed marked swelling of the optic disks but no sign of choroidal thickening or serous detachment of the retina. Fluorescein angiography indicated significant disc leakage (Figure 1C), but no arterial attenuation and no phlebitis were observed. Gadolinium-enhanced T1-weighted brain MRI showed bilateral meningeal enhancement of the optic nerve and protrusion of the optic disc into the vitreous cavity, especially in the left eye (Figure 3A, B). Magnetic resonance venography and angiography were unremarkable. Blood tests revealed no sign of diabetes and a normal range of serum angiotensin-converting enzyme (ACE). Treponemal antibodies were negative.
Figure 1.
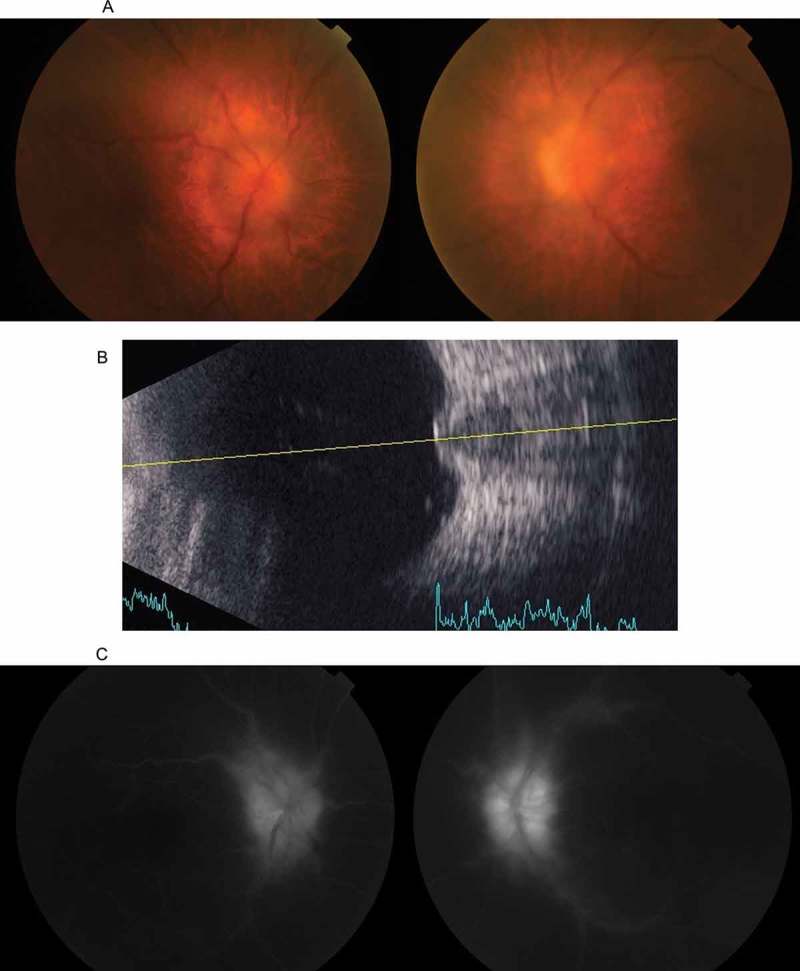
(A) A colour fundus photograph taken at the initial presentation, showing bilateral optic disc swelling. The haze is attributable to vitritis. (B) A B-mode ultrasonograph of the left eye, showing marked protrusion of the optic disc into the vitreous cavity. (C) Fluorescein angiography showing leakage from the optic disc.
Figure 2.
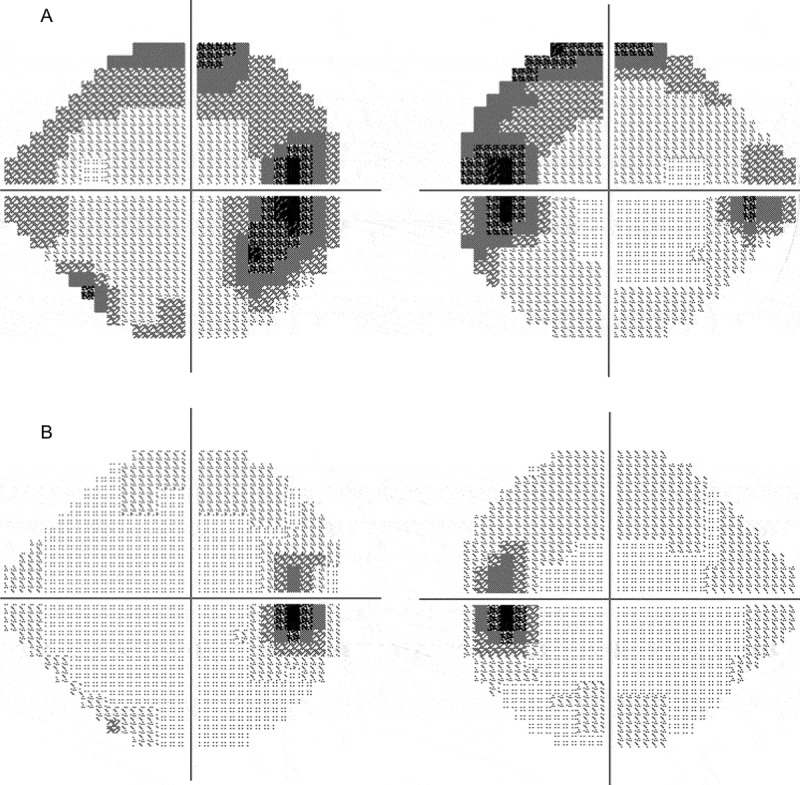
Humphrey visual fields test results before (A) and after (B) the treatment against the underlying malignancy. An enlarged Mariotte blind spot with non-specific scotoma is evident in both eyes in (A), which is not present in (B).
Figure 3.
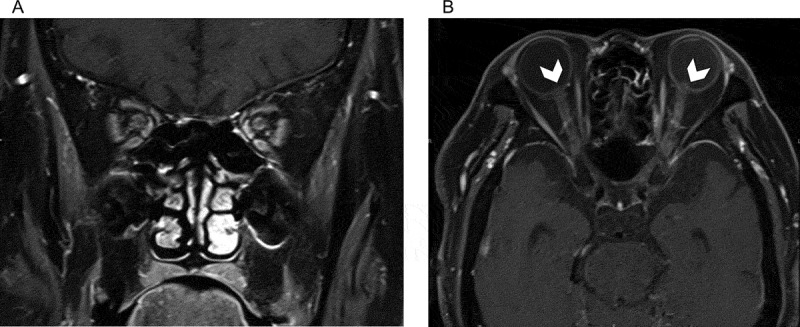
Gadolinium-enhanced T1-weighted coronal (A) and axial (B) magnetic resonance images. Enhancement of the bilateral optic nerve sheaths is evident in (A) and (B). Protrusion of the optic disc into the vitreous cavity (arrowheads) is also observed in (B).
A cerebrospinal fluid (CSF) test was performed. The opening pressure was normal. Pleocytosis (40 cells/mm3, 98% monocytes), a mildly elevated protein level (64 mg/dL; normal: 15–40 mg/dL), and a normal glucose level (62 mg/dL; normal: 50–70 mg/dL) were evident. Repeated CSF examinations revealed no malignancy in the cytology.
Whole-body computed tomography (CT) revealed a 19 × 19 mm mass in the right upper lung lobe and enlargement of the ipsilateral lymph nodes. Antibodies against CRMP-5 were detected in the serum at a level of +++, qualitatively measured by the Cosmic Corporation Co., Ltd. (Tokyo, Japan) using an immune assay. No other anti-neuronal antibodies to recoverin, amphiphysin, Paraneoplastic antigen Ma2 (PNMA2), Ri, Yo, Hu, Sox-2, titin, zic4, Glutamic Acid Decarboxylase (GAD)65, or Tr were detected. On bronchoscopic lung biopsy, small cell lung cancer was diagnosed on pathological examination.
Whole-body fluorodeoxyglucose positron emission tomography indicated that the tumour was localised to the right upper lobe and ipsilateral lymph nodes (Figure 4). The patient received four treatments involving a combination of chemotherapy (cisplatin and etoposide) and intravenous corticosteroid (dexamethasone, 9.9 mg on day 1 and 6.6 mg on days 2 and 3), in addition to local radiotherapy (40 Gy/30 fractions). The optic disc swelling promptly improved after receiving corticosteroid. In proportion with the progress of treatment, the tumour size decreased, with reduced numbers of vitreous cells. Antibodies against CRMP-5 in the serum decreased to a level of +. One year later, the visual acuities of both eyes were 20/16. The optic nerve swelling, the blind spot enlargement, and the non-specific scotoma evident on visual field testing had all disappeared (Figure 2B).
Figure 4.
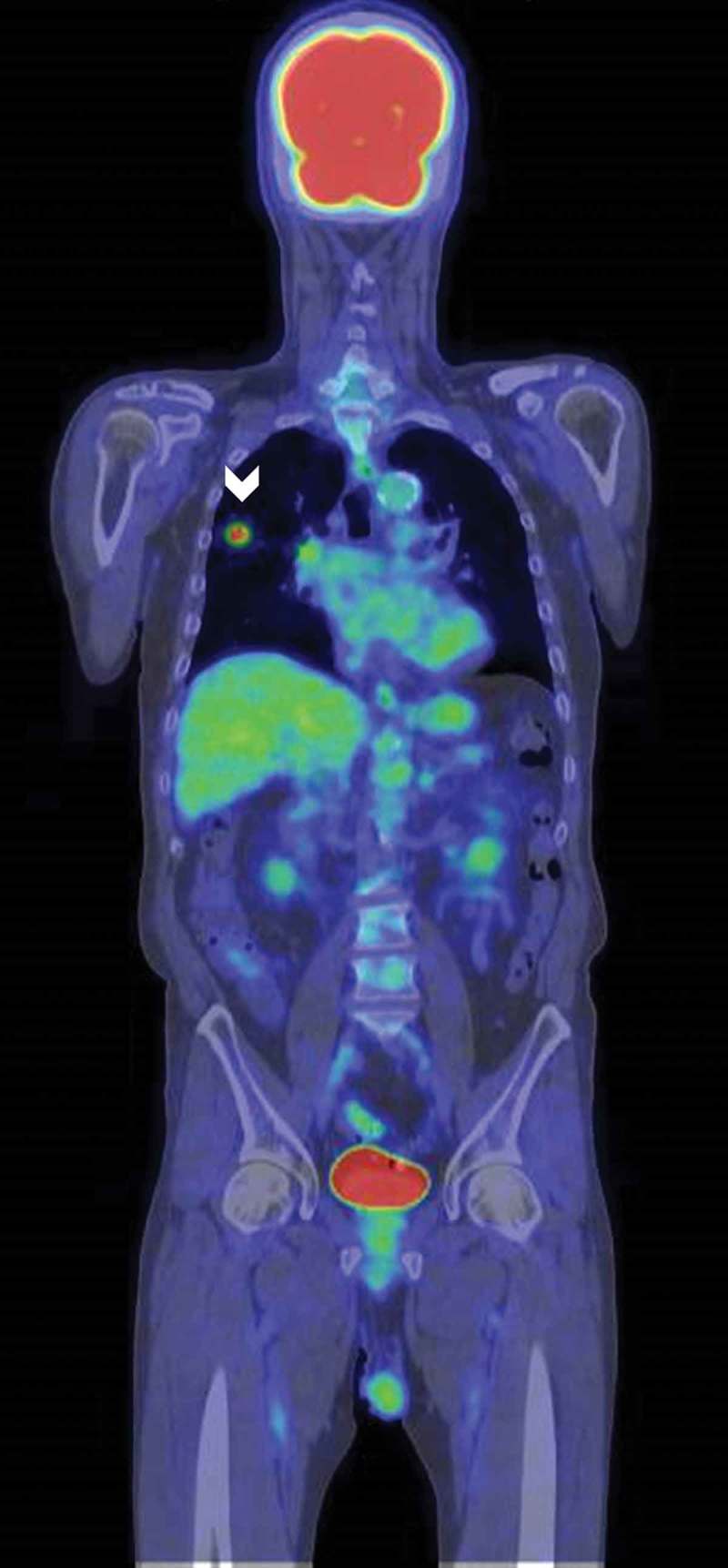
Whole-body fluorodeoxyglucose positron emission tomography. The tumour was detected in the right upper lung (arrowhead). No metastasis was detected.
Discussion
The anti-CRMP-5 antibody was first defined as an immunoglobulin G (IgG) autoantibody specific for CRMP-5,9 and is now thought to be the same autoantibody as anti-Crossveinless-2 (CV2).6,13,14 CRMP-5 is expressed in the optic nerve, neurons of the central and peripheral nervous systems, oligodendrocytes, and the cytoplasm of SCLC cells.5,6,10,11,13–15 It has been suggested that autoantibodies generated against the neoplastic CRMP-5 react as a host immune response to normal tissues, resulting in various neuronal disorders.6,10,16 In our case, bilateral meningeal enhancement of the optic nerve was evident on MRI, indicating that the main pathogenesis of the optic neuropathy was bilateral perioptic neuritis due to the involvement of oligodendrocytes around the optic nerve. This cause is consistent with preserved central visual acuity, prominent disc swelling, and prompt disappearance of disc swelling in response to the cancer treatment.
This case of PON had no neurological symptoms except optic neuropathy. To the best of our knowledge, only three anti-CRMP-5 antibody–positive PON cases with only optic neuropathy as a neurological symptom have been previously reported.7,8,12 The visual prognoses of these cases and MRI findings varied. One case7 suffered bilateral deterioration in light perception, and mild enhancement and enlargement were observed only in the right optic nerve on MRI. The other two cases presented normal MRI findings, with mild visual acuity improvement in one case12 and full recovery of visual acuity but substantial remaining visual field defects in the other case.8 Compared with these cases, recovery of our patient involved normal visual acuity and a normal visual field. It is possible that a weak antibody-antigen reaction or low-level antibody production (titre was not measured), and/or the main target of the autoantibody (in our case, perioptic nerve sheaths), explains both the initial mild visual deterioration and the positive visual outcome.
PON is difficult to diagnose because the disease is rare, especially in the absence of multiple neurological symptoms. Sagittal sinus thrombosis, Harada disease, sarcoidosis, intracranial hypertension, diabetes, or syphilis can be present as a differential diagnosis. However, sagittal sinus thrombosis was unlikely based on the MRI results. Harada disease was unlikely without choroidal thickening and without serous detachment of the retina revealed by OCT.17 Sarcoidosis was unlikely because of a normal level of ACE and because the pathology of the lymph nodes lacked typical signs of ophthalmic manifestations. Diabetes and syphilis were unlikely based on the blood test results.
Because PON is thought to be caused by an immune reaction, it is treated by immune suppression using systemic corticosteroids, local injection of triamcinolone acetonide, or intravenous immunoglobulin, in addition to treatment of the underlying cancer.6,12,18–20 The visual acuity of our case improved after receiving only chemotherapy and radiotherapy. It was possible that additional immune suppression was not necessary because the amount of antibody decreased with a decrease of the tumour size. It has been previously reported that the mechanism responsible for the ocular manifestations depended on the activity of the underlying malignancy, which involved detection of multiple autoantibodies.16 It is therefore recommended that prompt diagnosis and timely treatment of the underlying tumour to prevent an increase in autoantibody levels and irreversible damage to the nervous system.
Funding Statement
H.S. is supported by JSPS KAKENHI Grant number JP 16K11319.
Funding
H.S. is supported by JSPS KAKENHI Grant number JP 16K11319.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- [1].Gordon LK. Paraneoplastic syndromes in neuro-ophthalmology. J Neuroophthalmol 2015;35:306–314. [DOI] [PubMed] [Google Scholar]
- [2].Chan JW. Paraneoplastic retinopathies and optic neuropathies. Surv Ophthalmol 2003;48:12–38. [DOI] [PubMed] [Google Scholar]
- [3].Calvert PC. A CR(I)MP in the optic nerve: recognition and implications of paraneoplastic optic neuropathy. J Neuroophthalmol 2006;26:165–167. [DOI] [PubMed] [Google Scholar]
- [4].Thambisetty MR, Scherzer CR, Yu Z, Lennon VA, Newman NJ.. Paraneoplastic optic neuropathy and cerebellar ataxia with small cell carcinoma of the lung. J Neuroophthalmol 2001;21:164–167. [DOI] [PubMed] [Google Scholar]
- [5].Cross SA, Salomao DR, Parisi JE, Kryzer TJ, Bradley EA, Mines JA, Lam BL, Lennon VA.. Paraneoplastic autoimmune optic neuritis with retinitis defined by CRMP-5-IgG. Ann Neurol 2003;54:38–50. [DOI] [PubMed] [Google Scholar]
- [6].Rahimy E, Sarraf D.. Paraneoplastic and non-paraneoplastic retinopathy and optic neuropathy: evaluation and management. Surv Ophthalmol 2013;58:430–458. [DOI] [PubMed] [Google Scholar]
- [7].Sheorajpanday R, Slabbynck H, Van De Sompel W, Galdermans D, Neetens I, De Deyn PP.. Small cell lung carcinoma presenting as collapsin response-mediating protein (CRMP)-5 paraneoplastic optic neuropathy. J Neuroophthalmol 2006;26:168–172. [DOI] [PubMed] [Google Scholar]
- [8].Margolin E, Flint A, Trobe JD.. High-titer collapsin response-mediating protein-associated (CRMP-5) paraneoplastic optic neuropathy and vitritis as the only clinical manifestations in a patient with small cell lung carcinoma. J Neuroophthalmol 2008;28:17–22. [DOI] [PubMed] [Google Scholar]
- [9].Yu Z, Kryzer TJ, Griesmann GE, Kim K, Benarroch EE, Lennon VA.. CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann Neurol 2001;49:146–154. [PubMed] [Google Scholar]
- [10].Honnorat J, Antoine JC.. Paraneoplastic neurological syndromes. Orphanet J Rare Dis 2007;2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Adamus G, Brown L, Schiffman J, Iannaccone A.. Diversity in autoimmunity against retinal, neuronal, and axonal antigens in acquired neuro-retinopathy. J Ophthalmic Inflamm Infect 2011;1:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Arés-Luque A, García-Tuñón LA, Saiz A, Cabezas BC, Hernández-Echebarría LE, Franco M, Toribio A, Tejada J, Graus F.. Isolated paraneoplastic optic neuropathy associated with small-cell lung cancer and anti-CV2 antibodies. J Neurol 2007;254:1131–1132. [DOI] [PubMed] [Google Scholar]
- [13].Honnorat J, Antoine JC, Derrington E, Aguera M, Belin MF. Antibodies to a subpopulation of glial cells and a 66 kD developmental protein in patients with paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatr 1996;61:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].de la Sayette V, Bertran F, Honnorat J, Schaeffer S, Iglesias S, Defer G.. Paraneoplastic cerebellar syndrome and optic neuritis with anti-CV2 antibodies: clinical response to excision of the primary tumor. Arch Neurol 1998;55:405–408. [DOI] [PubMed] [Google Scholar]
- [15].Yu Z, Kryzer TJ, Griesmann GE, Kim K, Benarroch EE, Lennon VA.. CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann Neurol 2001;49:146–154. [PubMed] [Google Scholar]
- [16].Saito M, Saito W, Kanda A, Ohguro H, Ishida S.. A case of paraneoplastic optic neuropathy and outer retinitis positive for autoantibodies against collapsin response mediator protein-5, recoverin, and α-enolase. BMC Ophthalmol 2014;14:article 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].O’Keefe GA, Rao NA.. Vogt-Koyanagi-Harada disease. Surv Ophthalmol 2016. doi: 10.1016/j.survophthal.2016.05.002. [DOI] [PubMed] [Google Scholar]
- [18].Pulido J, Cross SA, Lennon VA, Pulido J, Swanson D, Muench M, Lachance DH.. Bilateral autoimmune optic neuritis and vitreitis related to CRMP-5-IgG: intravitreal triamcinolone acetonide therapy of four eyes. Eye (Lond) 2008;22:1191–1193. [DOI] [PubMed] [Google Scholar]
- [19].Luiz JE, Lee AG, Keltner JL, Thirkill CE, Lai EC.. Paraneoplastic optic neuropathy and autoantibody production in small-cell carcinoma of the lung. J Neuroophthalmol 1998;18:178–181. [PubMed] [Google Scholar]
- [20].Slamovits TL, Posner JB, Reidy DL, Thirkill CE, Keltner JL.. Pancreatic neuroendocrine paraneoplastic optic neuropathy: confirmation with antibody to optic nerve and hepatic metastasis. J Neuroophthalmol 2013;33:21–25. [DOI] [PubMed] [Google Scholar]


