Abstract
The placenta is a key organ in programming the fetus for later disease. This review outlines eight of many structural and physiological features of the placenta which are associated with adult onset chronic disease. 1) Placental efficiency relates the placental mass to the fetal mass. Ratios at the extremes are related to cardiovascular disease risk later in life. 2) Placental shape predicts a large number of disease outcomes in adults but the regulators of placental shape are not known. 3) Non-human primate studies suggest that at about mid-gestation, the placenta becomes less plastic and less able to compensate for pathological stresses. 4) Recent studies suggest that lipids have an important role in regulating placental metabolism and thus the future health of offspring. 5) Placental inflammation affects nutrient transport to the fetus and programs for later disease. 6) Placental insufficiency leads to inadequate fetal growth and elevated risks for later life disease. 7) Maternal height, fat and muscle mass are important in combination with placental size and shape in predicting adult disease. 8) The placenta makes a host of hormones that influence fetal growth and are related to offspring disease. Unfortunately, our knowledge of placental growth and function lags far behind that of other organs. An investment in understanding placental growth and function will yield enormous benefits to human health because it is a key player in the origins of the most expensive and deadly chronic diseases that humans face.
INTRODUCTION
One of the most important findings gleaned from recent epidemiological research across the globe is that placental phenotype predicts post-natal disease in offspring. Scientist have long known that the fetus depends on the placenta to acquire its nutrients for growth. For the past twenty-five years it has been recognized that fetal growth itself is an independent predictor of adult-onset disease. Since the placenta regulates the flow of nutrients from the mother to the fetus, most placental biologists assume that it must also play a role in determining disease risk in the adult life of the offspring.
There is ever-increasing evidence that the host of biological mechanisms that regulate placental growth and development also serves as causative agents for programming of chronic disease[1]. This article introduces eight out of many placental features that are associated with the programming of the fetus for later disease and outlines remaining gaps in knowledge regarding the links between epidemiological associations and their biological causes.
1) Placental Size and Efficiency
There are a number of adult disease conditions that are associated with placental size such as heart failure[2] and hypertension[3]. However, placental size and mass are crude measures of function, hence additional insight into the efficacy of overall placental function can be learned by comparing placental weight to fetal weight. Thus, various combinations of placental and fetal growth patterns offer insight into the developmental mechanisms that lead to disease in later life well beyond the predictive value of either birthweight or placental weight alone. Consequently, the concept of “efficiency” of the placenta has become useful. Efficiency is defined as the ratio of placental weight to fetal weight at any given stage of gestation, but is usually applied at term [4]. By custom, Europeans tend to use the placental weight to fetal weight ratio to define efficiency whereas North Americans more often use the inverse. So if the placenta is 15% of the fetal weight, the fetus is therefore 6.7 times heavier than the placenta. Either way, efficiency indicates how much fetal mass has accumulated for every gram of placenta. For example, if two 3,000 gram term babies are born with placentas that weigh 450 grams and 900 grams respectively, the former placenta is deemed twice as efficient as the latter.
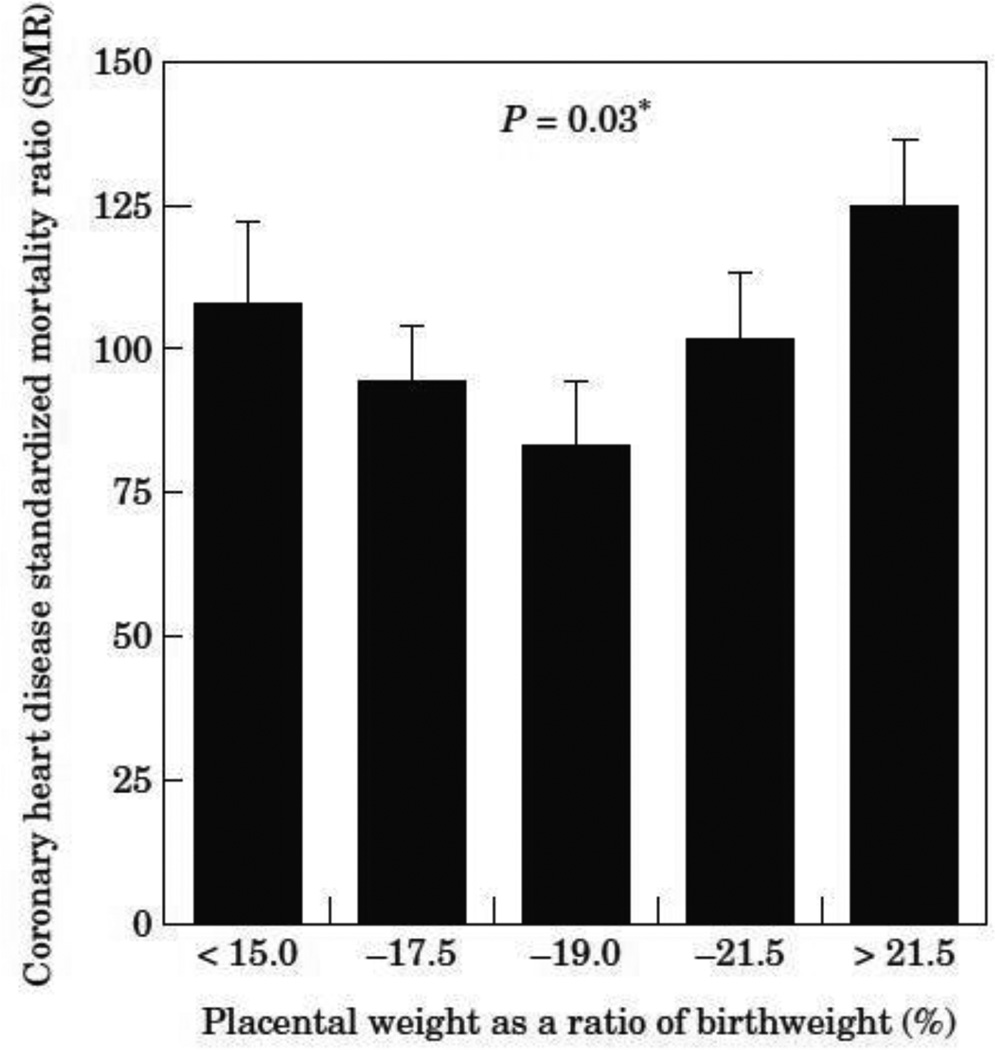
While the concept of efficiency has become a useful tool for biologists who study fetal programming it should be stated up front that it does not much insight into the regulation of placental or fetal growth. However, it does provide a measure of integrated placental function over gestation. Several studies have linked placental efficiency to disease outcomes in offspring. Martyn et al [5], showed that among men in Sheffield, UK, placental weight expressed as a percentage of fetal weight produces a “U” shaped curve. Figure 1 shows that the highest risks for coronary heart disease were found in pregnancies characterized by placentas that were less than 15% of birthweight, or were greater than 22% of the fetal weight [5]. Thus, both high efficiencies, at the 15% end of the scale, and low efficiencies at the 22% end of the scale, were associated with elevated risks for heart related deaths.
Figure 1.
The risk of coronary heart disease based on the weight ratio of placenta and fetus. [5]
The use of proxy markers of efficiency is also helpful, such as the ratio of a dimension or area of the delivered placenta to fetal weight. For example, the area of the delivered placenta as a percentage of birthweight was associated with a 2.5 fold risk for sudden cardiac death among women in the Helsinki Birth Cohort but not in men [6].
The concept of placental efficiency is an artificial construct which is influenced by complex biological factors that directly affect placental mass and underlie the transport function required for fetal growth. Nevertheless, placental efficiency is at best a crude marker of the environmental conditions in which a population of pregnant women was exposed. Recent data demonstrate that average placental efficiency within a population can change across time. In one Saudi population, the average placental weight increased by 120g in less than 10 years without a change in the average birthweight of ~3.25 kg [7]. Thus, on a population basis, the average placenta became less efficient for this group over a single decade.
The changing efficiency suggests that the growth patterns of placentas in a given population can be altered by the surrounding nutritional environment and perhaps other unknown biological factors that could affect women in the population. Other features of the environment such as shifts in the constituent microorganisms that constitute the maternal microbiome, might also play a role. Such variations in the maternal microbiome could also reflect dietary changes [8]. This aspect of placental biology needs further investigation. The determinants of placental efficiency will remain in the realm of speculation until the biological mechanisms that determine placental growth and related transport functions are found. This was nicely shown in a published discussion of fetal growth regulation [9].
Placental efficiency is also interesting in light of differences in growth strategies between male and female fetuses. Males tend to invest less in placental mass for the degree of fetal weight at birth and thus have more efficient placentas than do females who invest more in placental tissue for a given mass of fetus that accumulates at term [10, 11].
2) Placental Shape
If a placenta is not perfectly round at delivery, it can be described via its longest axis and a perpendicular short axis. These two axes, length and width, were measured on all delivered placentas in Helsinki hospitals for half a century and similar measurements were also recorded in The Netherlands, India and Saudi Arabia. These records now allow us to relate placental size and shape to fetal growth and disease conditions among adults (Table 1).
Table 1.
Diseases and their relationship to placental phenotype
| Stroke | placenta pathology; narrow placenta in preeclampsia)[5, 12] |
| Hypertension | placenta size and shape[3] |
| Hypertension | small placentas[3] |
| Metabolic Disease/Obesity | placenta surface area; U shaped risk[13] |
| Coronary Heart Disease | placenta size and shape, maternal phenotype[14] |
| Heart Failure | small placenta, short mother[2] |
| Sudden Cardiac Death | thin placenta (men); large placenta (women)[6] |
| Asthma | short length, placental dimensions[15] |
Although length and width generally correlate as expected [16], what is unexpected is that often one dimension, but not the other is associated with a particular disease outcome or condition. The specificity associated with the predictive value of disease provided by only a single placental axis has been previously reviewed [17] [18], but a few examples will suffice to make the point. In Riyadh, Saudi Arabia, the width of the delivered term placenta but not its length was associated with offspring birthweight and head circumference [16]. This effect was observed most strongly in short mothers. Birth weight increased by 125 g for every cm increase in placental width (95% confidence interval 88 – 162, p < 0.001), but only by 20 g per cm increase in each cm of placental length (95% CI, 13 to 53, p = 0.2). Thus placental growth in one placental dimension was more highly associated with fetal growth than the other. Similarly, in babies born to mothers in Helsinki who had preeclampsia, it was the width of the placenta, but not its length that was most highly associated with the severity of the disease [12]. In a separate study, short placental length but not width was associated with lifespan in 1200 men in the Helsinki Birth Cohort [19]. Changes in placental shape may also occur following a severe reduction in placental capacity; this is discussed in more detail in the “Placental Plasticity” section below based on studies in the non-human primate.
The differing biological roles of length and width in the placenta remain unexplained. If the placenta begins as a perfectly round structure, there is no length or width, just a single diameter. One would also expect that the placental axes would grow together and at the same rate so that the placenta would maintain a round shape over the whole of the gestational period. However, among some 6,000 deliveries, the average dimension of placentas in the Helsinki Birth Cohort was about 2.6 ± SD 2.0 cm longer than wide (range, 0–21 cm) (unpublished). Because differences in dimension appear to have biological meaning, many questions arise. Are the growth rates of the axes independently regulated? If so, what are the biological mechanisms that drive growth in different directions and why is an asymmetrical placenta more likely to predict disparate factors like the rate of fetal growth, male lifespan and offspring disease? These questions are ripe for study.
Finding strategies to study the regulation of placental shape is not easy. However, we offer two suggestions for low hanging fruit. 1) Serial measurements of placental size, shape and mass across the whole of gestation. This can be done in a non-invasive study using state of the art ultrasonography. 2) There are animal models where nutritional or restriction of blood flow patterns are associated with changes in shape. Using such models, we should be able to determine the temporal and spatial features that are associated with asymmetrical growth of the placenta. See the discussion of placental shape in the non-human primate study below.
3) Placental Plasticity
The degree to which the placenta is able to alter its growth trajectory according to the availability of nutrients has not been well studied. However, it has long been known that farmers have learned to vary the nutrition of ewes in order to deliver larger lambs at birth [20]. Farmers would reduce the nutrient intake of previously well-fed ewes in the first few weeks of pregnancy by placing the pregnant sheep on poor pasture to stimulate rapid placental growth and expansion of its vasculature. When returned to a lush pasture, the fetus would then grow to a larger size with the support from its newly enlarged placenta. There are parallels in the human.
Several species of non-human primates have two implantation sites that give rise to primary and secondary placental lobes that are connected by bridge vessels [21]; this arrangement occurs rarely in humans too. The accommodation of the primary lobe following functional loss of the secondary lobe following ligation of the bridge vessels provides clues to growth responses and limitations of placental adaptation. The responses to ligation of the bridge vessels has been studied in the rhesus monkey at 80 days (0.47 gestation) and at 110 days (0.67 gestation), where term is 167 days [22]. In these studies, near term fetal growth was nearly normal in the early ligation group but reduced in the older 110 day group, thus demonstrating early but not late gestation plasticity. In the early ligation group, the functional primary lobe was, on average, heavier and thicker than controls, having grown nearly twice as fast as normal in response to ligation. The rapid growth of the lobe was associated with a change in the length to width ratio compared to control or late ligation lobes (1.25 vs. 1.08). Compensatory growth was, thus, more rapid in one direction than the other.
Ligation at 0.67 gestation produced a very different primary lobe compared to lobes of control unligated placentas or late ligation placentas. In the late gestation ligation group, primary lobes grew at only half the normal rate, indicating a greatly diminished adaptive growth and not surprisingly, fetal growth was negatively impacted. These experiments demonstrate the obvious: fetal growth depends on an adequate placenta. But more importantly, the experiments demonstrate that the primate placenta loses its capacity for robust compensatory growth at some point early in the second half of gestation. The data further show that the shape of the placenta can be modified by changes in nutrient availability, supporting the idea that changes in shape within a specific population is influenced by the nutrition environment. The ligation experiments suggest that failure to mount a robust response to external insults can lead to placental insufficiency and fetal compromise.
4) Integration of Placental Signaling and Lipid Transport
A key function of the placenta is to transport maternal nutrients to the fetus, a function that can be dramatically altered through changes in signaling pathways. One might assume that signaling conditions which lead to suppression of transport systems would have detrimental effects on fetal growth and alter lifelong disease risks. The interactions among pathways within the placenta are known to respond to changing environments including maternal obesity, maternal nutrient delivery to the placenta, maternal cortisol levels and hypoxia. Studies by Jansson and colleagues [23] are examples of investigations into signaling pathway interactions that integrate the actions of several systems. Unfortunately, there are few studies that pursue this purpose in the placenta.
The interplay between signaling systems is important, highly complex and beyond the scope of this review. Over the past two decades, tremendous progress has been made in understanding the regulation of glucose and amino acid transport. These have been recently reviewed [24–27]. However, one class of molecule in the signaling field is just now receiving adequate attention: lipid molecules. Recent data show that maternal lipid profiles are important as determinants of fetal growth and thus as programming agents [28]. Lipids affect placental metabolism and are required for normal fetal growth. Deficits in supply of long chain polyunsaturated fatty acids (LCPUFA) due to an inadequate diet have pronounced detrimental effects on the maturing fetal organ systems. The brain and cardiovascular systems especially require substantial amounts of maternally acquired LCPUFA in late gestation for normal development [29, 30]. For the cardiovascular system, a deficient perinatal supply of LCPUFAs leads to hypertension in the adult [31, 32]. Thus, the developing fetal brain and cardiovascular systems depend heavily on the placental supply of LCPUFAs and omega-3-PUFAs.
In addition to their necessity for normal fetal development, fatty acids are critical for normal placental function. Fatty acids and LCPUFA in particular, are potent stimulators of the retinoid X receptor (RXR) and peroxisome proliferator activated receptor (PPAR) nuclear pathways [33, 34], two pathways that are indispensable for normal placentation and trophoblast function [35]. LCPUFA influence the antioxidant capacity of the placenta via nuclear factor erythroid type 2 (NRF2 / NF-E2), a master regulator of antioxidant gene expression. Roles for LCPUFA in anti-inflammatory pathways include direct stimulation of mediators produced via COX2 [36, 37] and being ligands for GPR120 which suppresses inflammation [38]. These additional roles also have implications for fetal development especially in pregnancies complicated by oxidative stress, inflammation and gestational diabetes [39–41]. Maternal supplementation with LCPUFA has beneficial effects [42], but may not ameliorate poor fetal outcomes in high-risk pregnancies [43], suggesting these outcomes are due to dysfunctions placental transport rather than disruptions in maternal supplies. Low fetal LCPUFA levels will likely become more common as the prevalence of maternal obesity and diabetes continues to rise [44]. The role of placental lipoprotein lipases in releasing fatty acids has been nicely documented [45] and an interaction between unsaturated fatty acids and amino acid transport has been clearly demonstrated [46]. However, the exact mechanisms by which lipids are transported and stored have yet to be determined. Data from our laboratory suggest that the cytotrophoblast is as important as the syncytiotrophoblast in regulating lipid storage [47]. Thus, there may be many additional lipid based signaling pathways within the tissues of the placenta that are yet to be discovered.
5) Placental Inflammation
Placental inflammation can arise from several causes. The most well-known is in response to an invading infectious agent. In addition, it is becoming increasingly evident that abnormal maternal metabolic status can stimulate inflammatory processes in the placenta without an infectious agent being present. Radaelli et al., showed that among the 400 plus gene transcripts modified in women with insulin dependent glucose control in the 3rd trimester, stress-activated and inflammatory response genes represented some 18% of regulated genes [48]. Interleukins, leptin, and tumor necrosis factor-alpha receptors were upregulated and their downstream molecular adaptors were prominently changed. Changes in genes regulating extracellular matrix components and angiogenic processes underlie structural reorganization of the placenta in diabetic women. In a comparison study, Enquobahrie et al., reported similar trends but with additional pathways and clusters that suggest a host of changes that go beyond inflammation [49]. These studies illustrate the high degree to which maternal metabolic disease is influential in determining placental form and function.
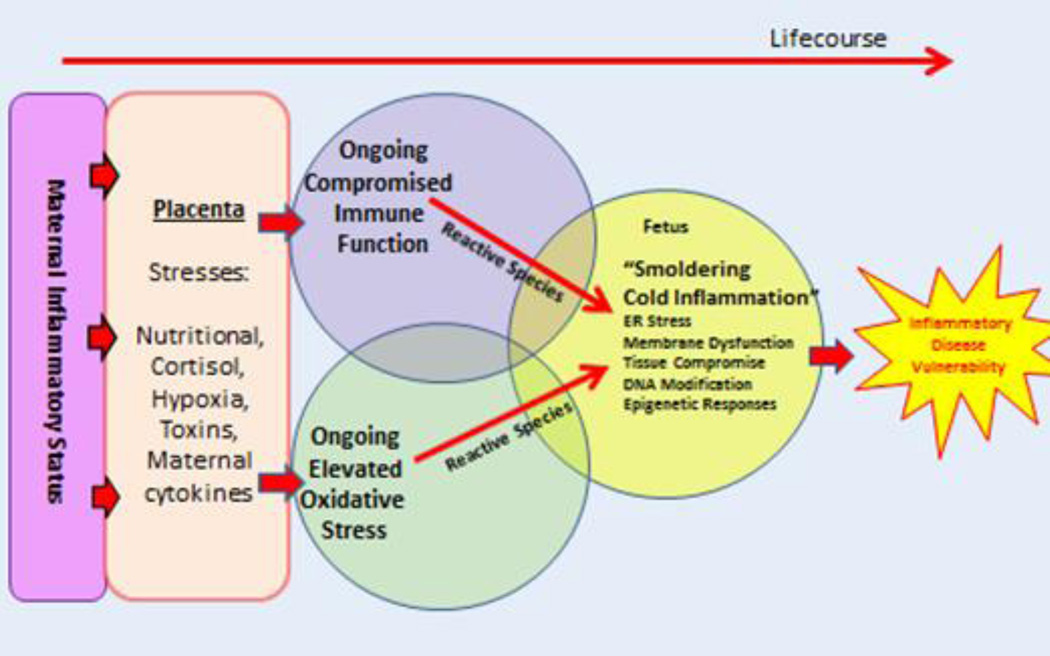
Women with low muscle mass, who themselves often had low birthweight, have low rates of protein turn-over [50, 51] and abnormal placental amino acid transport properties [51]. In addition, thinness in pregnant women is associated with a pro-inflammatory gene expression pattern with increased levels of interferon gamma and associated pathways but not with granulocyte or monocyte infiltration [52]. Thus, such placentas are not inflamed using the classical definition of “hot” inflammation that is characterized by immune cell infiltration. It appears that the field of placentology would benefit from new definitions of inflammation that would indicate the various levels of inflammatory responses that represent different physiological, pathological and gene expression states. The need to recognize different presentations of inflammation has been at least superficially addressed in the field of cancer [53]. We speculate that when placentas have “smoldering” inflammation, various fetal functions are affected that lead to fetal programming and enduring pro-inflammatory processes that last well into postnatal life. Figure 2 illustrates how hypothetical increases in inflammation and oxidative stressors in the womb could work in tandem to cause lifelong pro-inflammatory states in the fetus. The field of placentology is ripe for new, broadly agreed upon definitions of inflammation based not only on histological markers but gene expression patterns as well.
Figure 2.
The primary elements that lead to a pro-inflammatory state in both fetal and postnatal life. Beginning left, pro-inflammatory molecules affect placental inflammatory gene expression depending on the physiological status of the mother. A pro-inflammatory placenta leads to compromised immune function in the fetus along with elevated levels of oxidative stress. The outcome is a fetus that suffers “smoldering, cold inflammation” that may persist and make it more likely that the offspring will be more vulnerable for adult-onset chronic disease.
6) Placental Insufficiency
Placental vascular insufficiency can stem from an anatomically inadequate placenta, poor vascular development, or chronically decreased uterine or umbilical blood flow, all of which lead to inadequate oxygen and substrate flow to the fetus. The negative consequences of placental insufficiency are multifactorial and include, but are not limited to diminished substrate delivery leading to fetal growth restriction. Increased oxidative stress, increased cytokine production and associated inflammation, altered endocrine status and changes in epigenetic signaling may further compound the long term programming effects of placental insufficiency. The importance of robust placental blood flow and a functional vasculature in animal models of placental insufficiency are reviewed elsewhere [54, 55]. Decreased umbilical artery blood flow velocity is associated with smaller terminal villi [56] and reduced trophoblast and capillary volumes [57] which contribute to decreased nutrient supply to the fetus. In the face of decreased nutrient supply, the fetus will curb growth and redistribute cardiac output; even those organs thought to be somewhat “protected” may still be compromised and make other adaptations [58–60]. For more vulnerable organs such as the kidney, liver and pancreas, the programming effects of placental insufficiency on nephron number, hypertension, and metabolic disease have been well documented [61–66]. Yet, there are basic questions that remain unanswered. Under what circumstances do the vascular elements of the placenta fail to grow and expand as gestation proceeds? What are the processes that lead to the appropriate microvascular development required to maintain the low resistance status of the placenta? Is it possible to stimulate new growth in undergrown placentas to reverse the condition of placental insufficiency?
7) Maternal Phenotype
While birthweight remains a reliable indicator of fetal growth and future disease risk, additional associations with adult-onset disease have been discovered. Among people in the Helsinki Birth Cohort, phenotypic features reflecting maternal body composition in combination with placental phenotypic features are now known to be important predictors of long term disease risk. This is especially true for cardiovascular disease. The primary causes of cardiac death include coronary artery occlusion (myocardial infarction), heart failure and sudden cardiac death (ventricular fibrillation). In each case, placental phenotype is related to the risk for the condition. However, the predictive value of the placenta is further enhanced by knowing more about the body type of the mother. Particularly important are maternal body mass index, height alone or pelvic dimensions.
Three combinations of placental/maternal phenotype predict coronary disease among men, but not women in the Helsinki Birth Cohort. In all three, a low ponderal index of the newborn predicted the disease. One maternal-placental combination was in women who were below the median in height and two were in women above the median height with either a high or low body mass index. In the short women, the hazard ratio for coronary heart disease was 1.14 (95% confidence interval, C.I. 1.08–1.21, p=0.0001) for each cm difference in the length and width. In the tall women with a high body mass index, the ratio was 1.25 (C.I. 1.10–1.42, p=0.0007) per 40 cm2 decrease in surface area. In the tall mothers with a BMI that was below the median, the ratio was 1.07 (C.I. 1.02–1.13, p=0.01) per 1% increase in the placental weight/birthweight ratio. Interestingly, coronary heart disease in women was not predicted by placental growth in this cohort. In first born women only, a 1kg increase in birthweight was associated with a 25% lower risk for coronary disease (HR 0.75 C.I. 0.60–0.93, p=0.008).
In the Helsinki Birth Cohort, heart failure was associated with a small placental surface area. In people born to mothers of below median height and a placental area less than 225 cm2, the odds ratio for chronic heart failure was 2.3 (1.2–4.2, p=0.05), compared with people born with a placental area greater than 295 cm2. In the same cohort, sudden cardiac death was associated a thin placenta, the only known disease association with placental thinness. The hazard ratio was 1.47 (C.I. 1.11–1.93, p=0.006) for each g/cm2 decrease in thickness. These findings raise interesting questions. Does a woman’s body type affect the form and function of the placenta or do placentas of a particular phenotype affect maternal physiology in a specific way? At present, we know little about the determinants of placental growth and its interaction with the maternal body. This gap in knowledge presents an important opportunity for clever new approaches to placental biology.
8) Placental Hormone Production
Placental production of growth hormone (GH-V), human chorionic somatomammotropin (CSH), corticotropin-releasing hormone (pCRH), 11β-hydroxysteroid dehydrogenase type II (11β-HSD2) and thyroid hormone are all important for maintaining a normal pregnancy and supporting normal fetal growth. However, the degree to which these hormones cause programming effects in the offspring has not been well studied. Herein we suggest that IGF-1, glucocorticoids and thyroid hormone are linked to fetal growth and long term outcomes.
IGF1 and IGF2 are both important as growth factors in fetal and placental development. Both are synthesized in macrophages and endothelial cells throughout gestation (Hien et al., J Anat. 2009 Jul; 215(1): 60–68). The fetus relies on insulin-dependent IGF-1 secretion, which ensures a direct linkage between nutrient availability and growth [67]. This is in contrast to infants who switch to GH regulation to reach their adult growth potential. Maternal under- and over-nutrition result in obesity in adult offspring coexisting with hyperinsulinemia even after postnatal catch-up growth. IGF-I is an important regulator of growth and the somatotropic axis is significantly dysregulated by maternal malnutrition [68]. Treatment with IGF-I postnatally has been shown to decrease systolic blood pressures, insulin concentrations and retroperitoneal fat pads in fetal growth restricted (FGR) offspring [69]. Additionally, GH sensitivity is decreased in FGR offspring, further supporting that fetal growth and programming are associated with the anabolic actions of GH in response to a poor postnatal nutritional environment [70].
The IGF2 gene strongly influences placental growth; under conditions where placental size is reduced, such as fetal growth restriction, its placental expression is also reduced [71]. If IGF2 is deleted from fetal and placental tissues the mouse placenta has a reduced labyrinthine nutrient exchange area and a loss of glycogen cells in the junctional zone [72]. The H19 gene normally silences the IGF2 gene. When the H19 gene is deleted, the IGF2 gene is upregulated stimulating a selective increase in the labyrinthine zone which allows a greater area for nutrient exchange[72, 73]. The changes in placental growth related to changes in IGF2 gene expression are reflected in nutrient flux to the fetus and thus, fetal growth.
Epigenetic modification of the glucocorticoid system has been shown to be developmentally sensitive in several organ systems. Human studies have shown that elevated prenatal stress correlates with abnormal fetal brain development [74]. Glucocorticoids are required for tissue maturation and organ development, especially in lungs and heart. However, FGR as well as subsequent cardiovascular and metabolic alterations in the adult life can occur with supra-physiologic levels of glucocorticoids [75, 76]. Since cortisol is known to inhibit fetal growth, the placenta protects the fetus from exposure to even normal maternal levels of cortisol. To maintain lower physiologic levels of fetal cortisol, placental 11β-HSD2 acts to buffer these negative effects of cortisol by catalyzing reactions to promote the inactive 11-keto forms. 11β-HSD2 is expressed in cytotrophoblasts during the first trimester and in syncytiotrophoblast as gestation progresses [77]. Placental expression and activity of 11β-HSD2 are decreased in FGR, fetal hypoxia, chorioamnionitis, and maternal protein restriction [78]. Glucocorticoids have long been shown to be a major programming agent for adult disease. The placenta plays an integral role in coordinating both placental and maternal metabolic processes as well as optimizing the nutritional needs of a growing fetus, which all influence the downstream lifelong health consequences. However, the regulation of these processes and glucocorticoid systems within the placenta are still incompletely understood.
Maternal thyroid disease including both hyper- and hypothyroidism are associated with poor pregnancy outcomes such as FGR, preterm birth, and pre-eclampsia [79]. Thyroid hormone (TH) has long been known to be essential for normal fetal brain development and thus, severe maternal hypothyroidism from iodine deficiency is the most common preventable cause of mental retardation around the world [80, 81]. Studies in fetal sheep show TH also has a critical role in the maturation of the myocardium, decreasing cardiomyocyte endowment both when there is too much, or too little TH [82]. TH promotes secretion of placental hormones critical for pregnancy maintenance as early as implantation [83] and cytotrophoblast cells are responsive to TH by increasing syncytialization rate, thereby stimulating secretion of factors that promote placenta integrity [84, 85]. Despite the obvious role of TH in placental function, little is known about placental TH signaling at a cellular level. Given the known relationship between obesity and thyroid hormone status [86] and increasing rates of maternal obesity, it is imperative to understand placental thyroid signaling under both normal and obesogenic conditions.
The clear cut effects of these hormones on fetal and placental growth strongly suggest a role in fetal programming. However, except for IGF-1, cortisol and thyroid hormone, little is known about the role of other hormones in determining the long term health of offspring; both those produced by the placenta and those produced by the mother or fetus but who have actions on the placenta. The role of placental hormones in programming is one of the most neglected areas of research in reproductive biology.
CONCLUSIONS
The placenta is a key organ in programming the fetus for later disease. In recent years many aspects of placental phenotype and function are associated with adult-onset disease. It is now appropriate to consider several late onset diseases to be placental diseases because the associations are so powerful. The discovery that placental shape interacts with maternal body composition as predictors of later disease is especially profound and provides a sense of urgency for our efforts in understanding the biological factors that underlie the associations. There is a need for young scientists to develop high levels of expertise in this field at a cellular, but also at a systems level to fully appreciate the maternal-placental-fetal interactions. Without profound progress in the field, the origins of the chronic diseases that cause the highest rates of mortality worldwide will not be understood.
Acknowledgments
The authors thank Lisa Rhuman and Kim Rogers for their excellent office assistance.
REFERENCES
- 1.Barker DJ, Thornburg KL. The obstetric origins of health for a lifetime. Clinical obstetrics and gynecology. 2013;56:511–519. doi: 10.1097/GRF.0b013e31829cb9ca. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Gelow J, Thornburg K, Osmond C, Kajantie E, Eriksson JG. The early origins of chronic heart failure: impaired placental growth and initiation of insulin resistance in childhood. European journal of heart failure. 2010;12:819–825. doi: 10.1093/eurjhf/hfq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker DJ, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The surface area of the placenta and hypertension in the offspring in later life. The International journal of developmental biology. 2010;54:525–530. doi: 10.1387/ijdb.082760db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson ME, Ford SP. Comparative aspects of placental efficiency. Reproduction (Cambridge, England) Supplement. 2001;58:223–232. [PubMed] [Google Scholar]
- 5.Martyn CN, Barker DJ, Osmond C. Mothers' pelvic size, fetal growth, and death from stroke and coronary heart disease in men in the UK. Lancet. 1996;348:1264–1268. doi: 10.1016/s0140-6736(96)04257-2. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJ, Larsen G, Osmond C, Thornburg KL, Kajantie E, Eriksson JG. The placental origins of sudden cardiac death. Int J Epidemiol. 2012;41:1394–1399. doi: 10.1093/ije/dys116. [DOI] [PubMed] [Google Scholar]
- 7.Alwasel SH, Abotalib Z, Aljarallah JS, Osmond C, Alkharaz SM, Alhazza IM, et al. Secular increase in placental weight in Saudi Arabia. Placenta. 2011;32:391–394. doi: 10.1016/j.placenta.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aye IL, Rosario FJ, Powell TL, Jansson T. Reply to Carbillon: Fetal/placental weight ratio and placental function. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E261. doi: 10.1073/pnas.1521808113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gender medicine. 2007;4:19–30. doi: 10.1016/s1550-8579(07)80004-0. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. American journal of human biology : the official journal of the Human Biology Council. 2010;22:330–335. doi: 10.1002/ajhb.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kajantie E, Thornburg KL, Eriksson JG, Osmond C, Barker DJ. In preeclampsia, the placenta grows slowly along its minor axis. The International journal of developmental biology. 2010;54:469–473. doi: 10.1387/ijdb.082833ek. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson JG, Gelow J, Thornburg KL, Osmond C, Laakso M, Uusitupa M, et al. Long-term effects of placental growth on overweight and body composition. International journal of pediatrics. 2012;2012:324185. doi: 10.1155/2012/324185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eriksson JG, Kajantie E, Thornburg KL, Osmond C, Barker DJ. Mother's body size and placental size predict coronary heart disease in men. European heart journal. 2011;32:2297–2303. doi: 10.1093/eurheartj/ehr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barker DJ, Osmond C, Forsen TJ, Thornburg KL, Kajantie E, Eriksson JG. Foetal and childhood growth and asthma in adult life. Acta paediatrica. 2013;102:732–738. doi: 10.1111/apa.12257. [DOI] [PubMed] [Google Scholar]
- 16.Alwasel SH, Abotalib Z, Aljarallah JS, Osmond C, Al Omar SY, Harrath A, et al. The breadth of the placental surface but not the length is associated with body size at birth. Placenta. 2012;33:619–622. doi: 10.1016/j.placenta.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Burton GJ, Barker D, Moffett A, Thornburg K. The Placenta and Human Developmental Programming. Cambridge University Press; 2010. [Google Scholar]
- 18.Thornburg KL, Marshall N. The placenta is the center of the chronic disease universe. Am J Obstet Gynecol. 2015;213:S14–S20. doi: 10.1016/j.ajog.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barker DJ, Osmond C, Thornburg KL, Kajantie E, Eriksson JG. The lifespan of men and the shape of their placental surface at birth. Placenta. 2011;32:783–787. doi: 10.1016/j.placenta.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCrabb GJEA, Hasking BJ. Maternal undernutrition during mid-pregnancy in sheep; variable effects on placental growth. J Agricult Sci. 1992;118:127–132. [Google Scholar]
- 21.Novy MJ, Aubert ML, Kaplan SL, Grumbach MM. Regulation of placental growth and chorionic somatomammotropin in the rhesus monkey: effects of protein deprivation, fetal anencephaly, and placental vessel ligation. Am J Obstet Gynecol. 1981;140:552–562. doi: 10.1016/0002-9378(81)90232-5. [DOI] [PubMed] [Google Scholar]
- 22.Roberts VH, Rasanen JP, Novy MJ, Frias A, Louey S, Morgan TK, et al. Restriction of placental vasculature in a non-human primate: a unique model to study placental plasticity. Placenta. 2012;33:73–76. doi: 10.1016/j.placenta.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansson T, Powell TL. Role of placental nutrient sensing in developmental programming. Clin Obstet Gynecol. 2013;56:591–601. doi: 10.1097/GRF.0b013e3182993a2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moe AJ. Placental amino acid transport. Am J Physiol. 1995;268:C1321–C1331. doi: 10.1152/ajpcell.1995.268.6.C1321. [DOI] [PubMed] [Google Scholar]
- 25.Jansson T, Ekstrand Y, Bjorn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes. 2002;51:2214–2219. doi: 10.2337/diabetes.51.7.2214. [DOI] [PubMed] [Google Scholar]
- 26.Jansson T, Ylven K, Wennergren M, Powell TL. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta. 2002;23:392–399. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- 27.Illsley NP. Glucose transporters in the human placenta. Placenta. 2000;21:14–22. doi: 10.1053/plac.1999.0448. [DOI] [PubMed] [Google Scholar]
- 28.Kulkarni SR, Kumaran K, Rao SR, Chougule SD, Deokar TM, Bhalerao AJ, et al. Maternal lipids are as important as glucose for fetal growth: findings from the Pune Maternal Nutrition Study. Diabetes care. 2013;36:2706–2713. doi: 10.2337/dc12-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early human development. 1980;4:121–129. doi: 10.1016/0378-3782(80)90015-8. [DOI] [PubMed] [Google Scholar]
- 30.Innis SM. Essential fatty acid transfer and fetal development. Placenta. 2005;26(Suppl A):S70–S75. doi: 10.1016/j.placenta.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Weisinger HS, Armitage JA, Sinclair AJ, Vingrys AJ, Burns PL, Weisinger RS. Perinatal omega-3 fatty acid deficiency affects blood pressure later in life. Nature medicine. 2001;7:258–259. doi: 10.1038/85354. [DOI] [PubMed] [Google Scholar]
- 32.Armitage JA, Pearce AD, Sinclair AJ, Vingrys AJ, Weisinger RS, Weisinger HS. Increased blood pressure later in life may be associated with perinatal n-3 fatty acid deficiency. Lipids. 2003;38:459–464. doi: 10.1007/s11745-003-1084-y. [DOI] [PubMed] [Google Scholar]
- 33.Lengqvist J, Mata De Urquiza A, Bergman AC, Willson TM, Sjovall J, Perlmann T, et al. Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor alpha ligand-binding domain. Molecular & cellular proteomics : MCP. 2004;3:692–703. doi: 10.1074/mcp.M400003-MCP200. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki K, Takahashi K, Nishimaki-Mogami T, Kagechika H, Yamamoto M, Itabe H. Docosahexaenoic acid induces adipose differentiation-related protein through activation of retinoid x receptor in human choriocarcinoma BeWo cells. Biological & pharmaceutical bulletin. 2009;32:1177–1182. doi: 10.1248/bpb.32.1177. [DOI] [PubMed] [Google Scholar]
- 35.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Molecular cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 36.Gao L, Wang J, Sekhar KR, Yin H, Yared NF, Schneider SN, et al. Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. The Journal of biological chemistry. 2007;282:2529–2537. doi: 10.1074/jbc.M607622200. [DOI] [PubMed] [Google Scholar]
- 37.Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, et al. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nature chemical biology. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL, et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 2011;152:2456–2464. doi: 10.1210/en.2010-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brass E, Hanson E, O'Tierney-Ginn PF. Placental oleic acid uptake is lower in male offspring of obese women. Placenta. 2013;34:503–509. doi: 10.1016/j.placenta.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pagan A, Prieto-Sanchez MT, Blanco-Carnero JE, Gil-Sanchez A, Parrilla JJ, Demmelmair H, et al. Materno-fetal transfer of docosahexaenoic acid is impaired by gestational diabetes mellitus. American journal of physiology Endocrinology and metabolism. 2013;305:E826–E833. doi: 10.1152/ajpendo.00291.2013. [DOI] [PubMed] [Google Scholar]
- 42.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics. 2003;111:e39–e44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- 43.Horvath A, Koletzko B, Szajewska H. Effect of supplementation of women in high-risk pregnancies with long-chain polyunsaturated fatty acids on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. The British journal of nutrition. 2007;98:253–259. doi: 10.1017/S0007114507709078. [DOI] [PubMed] [Google Scholar]
- 44.Leddy MA, Power ML, Schulkin J. The impact of maternal obesity on maternal and fetal health. Reviews in obstetrics & gynecology. 2008;1:170–178. [PMC free article] [PubMed] [Google Scholar]
- 45.Magnusson-Olsson AL, Lager S, Jacobsson B, Jansson T, Powell TL. Effect of maternal triglycerides and free fatty acids on placental LPL in cultured primary trophoblast cells and in a case of maternal LPL deficiency. American journal of physiology Endocrinology and metabolism. 2007;293:E24–E30. doi: 10.1152/ajpendo.00571.2006. [DOI] [PubMed] [Google Scholar]
- 46.Lager S, Jansson T, Powell TL. Differential regulation of placental amino acid transport by saturated and unsaturated fatty acids. American journal of physiology Cell physiology. 2014;307:C738–C744. doi: 10.1152/ajpcell.00196.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolahi K, Louey S, Varlamov O, Thornburg K. Real-Time Tracking of BODIPY-C12 Long-Chain Fatty Acid in Human Term Placenta Reveals Unique Lipid Dynamics in Cytotrophoblast Cells. PloS one. 2016;11:e0153522. doi: 10.1371/journal.pone.0153522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radaelli T, Varastehpour A, Catalano P, Hauguel-de Mouzon S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes. 2003;52:2951–2958. doi: 10.2337/diabetes.52.12.2951. [DOI] [PubMed] [Google Scholar]
- 49.Enquobahrie DA, Williams MA, Qiu C, Meller M, Sorensen TK. Global placental gene expression in gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200:206, e1–e13. doi: 10.1016/j.ajog.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 50.Duggleby SL, Jackson AA. Relationship of maternal protein turnover and lean body mass during pregnancy and birth length. Clinical science. 2001;101:65–72. [PubMed] [Google Scholar]
- 51.Lewis RM, Greenwood SL, Cleal JK, Crozier SR, Verrall L, Inskip HM, et al. Maternal muscle mass may influence system A activity in human placenta. Placenta. 2010;31:418–422. doi: 10.1016/j.placenta.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 52.O'Tierney PF, Lewis RM, McWeeney SK, Hanson MA, Inskip HM, Morgan TK, et al. Immune response gene profiles in the term placenta depend upon maternal muscle mass. Reproductive sciences. 2012;19:1041–1056. doi: 10.1177/1933719112440051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calay ES, Hotamisligil GS. Turning off the inflammatory, but not the metabolic, flames. Nature medicine. 2013;19:265–267. doi: 10.1038/nm.3114. [DOI] [PubMed] [Google Scholar]
- 54.Reynolds LP, Caton JS, Redmer DA, Grazul-Bilska AT, Vonnahme KA, Borowicz PP, et al. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol. 2006;572:51–58. doi: 10.1113/jphysiol.2005.104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thornburg KL, Louey S. Uteroplacental circulation and fetal vascular function and development. Current vascular pharmacology. 2013;11:748–757. doi: 10.2174/1570161111311050012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Macara L, Kingdom JC, Kaufmann P, Kohnen G, Hair J, More IA, et al. Structural analysis of placental terminal villi from growth-restricted pregnancies with abnormal umbilical artery Doppler waveforms. Placenta. 1996;17:37–48. doi: 10.1016/s0143-4004(05)80642-3. [DOI] [PubMed] [Google Scholar]
- 57.Jackson MR, Walsh AJ, Morrow RJ, Mullen JB, Lye SJ, Ritchie JW. Reduced placental villous tree elaboration in small-for-gestational-age pregnancies: relationship with umbilical artery Doppler waveforms. Am J Obstet Gynecol. 1995;172:518–525. doi: 10.1016/0002-9378(95)90566-9. [DOI] [PubMed] [Google Scholar]
- 58.Barry JS, Davidsen ML, Limesand SW, Galan HL, Friedman JE, Regnault TR, et al. Developmental changes in ovine myocardial glucose transporters and insulin signaling following hyperthermia-induced intrauterine fetal growth restriction. Experimental biology and medicine (Maywood, NJ) 2006;231:566–575. doi: 10.1177/153537020623100511. [DOI] [PubMed] [Google Scholar]
- 59.Louey S, Jonker SS, Giraud GD, Thornburg KL. Placental insufficiency decreases cell cycle activity and terminal maturation in fetal sheep cardiomyocytes. J Physiol. 2007;580:639–648. doi: 10.1113/jphysiol.2006.122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsirka AE, Gruetzmacher EM, Kelley DE, Ritov VH, Devaskar SU, Lane RH. Myocardial gene expression of glucose transporter 1 and glucose transporter 4 in response to uteroplacental insufficiency in the rat. The Journal of endocrinology. 2001;169:373–380. doi: 10.1677/joe.0.1690373. [DOI] [PubMed] [Google Scholar]
- 61.Denisenko OLB, Louey S, Thornburg K, Bomsztyk K, Bagby S. Maternal malnutrition and placental insufficiency induce global downregulation of gene expression in fetal kidneys. J Dev Origins Health and Dis. 2011;2:124–133. doi: 10.1017/S2040174410000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Limesand SW, Rozance PJ, Macko AR, Anderson MJ, Kelly AC, Hay WW., Jr Reductions in insulin concentrations and beta-cell mass precede growth restriction in sheep fetuses with placental insufficiency. American journal of physiology Endocrinology and metabolism. 2013;304:E516–E523. doi: 10.1152/ajpendo.00435.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paixao AD, Alexander BT. How the kidney is impacted by the perinatal maternal environment to develop hypertension. Biology of reproduction. 2013;89:144. doi: 10.1095/biolreprod.113.111823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rozance PJ, Anderson M, Martinez M, Fahy A, Macko AR, Kailey J, et al. Placental insufficiency decreases pancreatic vascularity and disrupts hepatocyte growth factor signaling in the pancreatic islet endothelial cell in fetal sheep. Diabetes. 2015;64:555–564. doi: 10.2337/db14-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yates DT, Macko AR, Nearing M, Chen X, Rhoads RP, Limesand SW. Developmental programming in response to intrauterine growth restriction impairs myoblast function and skeletal muscle metabolism. Journal of pregnancy. 2012;2012:631038. doi: 10.1155/2012/631038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zohdi V, Moritz KM, Bubb KJ, Cock ML, Wreford N, Harding R, et al. Nephrogenesis and the renal renin-angiotensin system in fetal sheep: effects of intrauterine growth restriction during late gestation. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1267–R1273. doi: 10.1152/ajpregu.00119.2007. [DOI] [PubMed] [Google Scholar]
- 67.Gluckman PD, Pinal CS. Regulation of fetal growth by the somatotrophic axis. The Journal of nutrition. 2003;133:1741S–1746S. doi: 10.1093/jn/133.5.1741S. [DOI] [PubMed] [Google Scholar]
- 68.Smith T, Sloboda DM, Saffery R, Joo E, Vickers MH. Maternal nutritional history modulates the hepatic IGF-IGFBP axis in adult male rat offspring. Endocrine. 2014;46:70–82. doi: 10.1007/s12020-013-0034-8. [DOI] [PubMed] [Google Scholar]
- 69.Vickers MH, Ikenasio BA, Breier BH. IGF-I treatment reduces hyperphagia, obesity, and hypertension in metabolic disorders induced by fetal programming. Endocrinology. 2001;142:3964–3973. doi: 10.1210/endo.142.9.8390. [DOI] [PubMed] [Google Scholar]
- 70.Donzeau A, Bouhours-Nouet N, Fauchard M, Decrequy A, Mathieu E, Boux de Casson F, et al. Birth Weight Is Associated With the IGF-1 Response to GH in Children: Programming of the Anabolic Action of GH? The Journal of clinical endocrinology and metabolism. 2015;100:2972–2978. doi: 10.1210/jc.2015-1603. [DOI] [PubMed] [Google Scholar]
- 71.Fowden AL, Ward JW, Wooding FP, Forhead AJ, Constancia M. Programming placental nutrient transport capacity. J Physiol. 2006;572:5–15. doi: 10.1113/jphysiol.2005.104141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coan PM, Burton GJ, Ferguson-Smith AC. Imprinted genes in the placenta--a review. Placenta. 2005;26(Suppl A):S10–S20. doi: 10.1016/j.placenta.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 73.Chiao E, Fisher P, Crisponi L, Deiana M, Dragatsis I, Schlessinger D, et al. Overgrowth of a mouse model of the Simpson-Golabi-Behmel syndrome is independent of IGF signaling. Developmental biology. 2002;243:185–206. doi: 10.1006/dbio.2001.0554. [DOI] [PubMed] [Google Scholar]
- 74.Charil A, Laplante DP, Vaillancourt C, King S. Prenatal stress and brain development. Brain research reviews. 2010;65:56–79. doi: 10.1016/j.brainresrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 75.Goland RS, Jozak S, Warren WB, Conwell IM, Stark RI, Tropper PJ. Elevated levels of umbilical cord plasma corticotropin-releasing hormone in growth-retarded fetuses. The Journal of clinical endocrinology and metabolism. 1993;77:1174–1179. doi: 10.1210/jcem.77.5.8077309. [DOI] [PubMed] [Google Scholar]
- 76.Petraglia F, Florio P, Benedetto C, Gallo C, Woods RJ, Genazzani AR, et al. High levels of corticotropin-releasing factor (CRF) are inversely correlated with low levels of maternal CRF-binding protein in pregnant women with pregnancy-induced hypertension. The Journal of clinical endocrinology and metabolism. 1996;81:852–856. doi: 10.1210/jcem.81.2.8636316. [DOI] [PubMed] [Google Scholar]
- 77.Shams M, Kilby MD, Somerset DA, Howie AJ, Gupta A, Wood PJ, et al. 11Beta-hydroxysteroid dehydrogenase type 2 in human pregnancy and reduced expression in intrauterine growth restriction. Human reproduction (Oxford, England) 1998;13:799–804. doi: 10.1093/humrep/13.4.799. [DOI] [PubMed] [Google Scholar]
- 78.Seckl JR, Cleasby M, Nyirenda MJ. Glucocorticoids, 11beta-hydroxysteroid dehydrogenase, and fetal programming. Kidney international. 2000;57:1412–1417. doi: 10.1046/j.1523-1755.2000.00984.x. [DOI] [PubMed] [Google Scholar]
- 79.Casey BM, Leveno KJ. Thyroid disease in pregnancy. Obstet Gynecol. 2006;108:1283–1292. doi: 10.1097/01.AOG.0000244103.91597.c5. [DOI] [PubMed] [Google Scholar]
- 80.Hetzel BS. Eliminating iodine deficiency disorders--the role of the International Council in the global partnership. Bulletin of the World Health Organization. 2002;80:410–413. discussion 3–7. [PMC free article] [PubMed] [Google Scholar]
- 81.Forhead AJ, Fowden AL. Thyroid hormones in fetal growth and prepartum maturation. The Journal of endocrinology. 2014;221:R87–R103. doi: 10.1530/JOE-14-0025. [DOI] [PubMed] [Google Scholar]
- 82.Chattergoon NN, Giraud GD, Louey S, Stork P, Fowden AL, Thornburg KL. Thyroid hormone drives fetal cardiomyocyte maturation. FASEB J. 2012;26:397–408. doi: 10.1096/fj.10-179895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Colicchia M, Campagnolo L, Baldini E, Ulisse S, Valensise H, Moretti C. Molecular basis of thyrotropin and thyroid hormone action during implantation and early development. Human reproduction update. 2014;20:884–904. doi: 10.1093/humupd/dmu028. [DOI] [PubMed] [Google Scholar]
- 84.Barber KJ, Franklyn JA, McCabe CJ, Khanim FL, Bulmer JN, Whitley GS, et al. The in vitro effects of triiodothyronine on epidermal growth factor-induced trophoblast function. The Journal of clinical endocrinology and metabolism. 2005;90:1655–1661. doi: 10.1210/jc.2004-0785. [DOI] [PubMed] [Google Scholar]
- 85.Nishii H, Ashitaka Y, Maruo M, Mochizuki M. Studies on the effect of thyroid hormone and epidermal growth factor on the cultured human cytotrophoblast. Endocrinologia japonica. 1991;38:279–286. doi: 10.1507/endocrj1954.38.279. [DOI] [PubMed] [Google Scholar]
- 86.Michalaki MA, Vagenakis AG, Leonardou AS, Argentou MN, Habeos IG, Makri MG, et al. Thyroid function in humans with morbid obesity. Thyroid : official journal of the American Thyroid Association. 2006;16:73–78. doi: 10.1089/thy.2006.16.73. [DOI] [PubMed] [Google Scholar]