Abstract
Significance: Soluble guanylate cyclase (sGC) is an intracellular enzyme that plays a primary role in sensing nitric oxide (NO) and transducing its multiple signaling effects in mammals.
Recent Advances: The chaperone heat shock protein 90 (hsp90) associates with signaling proteins in cells, including sGC, where it helps to drive heme insertion into the sGC-β1 subunit. This allows sGC-β1 to associate with a partner sGC-α1 subunit and mature into an NO-responsive active form.
Critical Issues: In this article, we review evidence to date regarding the mechanisms that modulate sGC activity by a pathway where binding of hsp90 or sGC agonist to heme-free sGC dictates the assembly and fate of an active sGC heterodimer, both by NO and heme-dependent or heme-independent pathways.
Future Directions: We discuss some therapeutic implications of the NO-sGC-hsp90 nexus and its potential as a marker of inflammatory disease. Antioxid. Redox Signal. 26, 182–190.
Keywords: : cGMP, S-nitrosation, nitrosylation, signal transduction, desensitization
Introduction
The active mammalian or invertebrate soluble guanylate cyclase (sGC) is a heterodimer made up of similar α and β subunits that each contain N-terminal regulatory, middle dimerization, and C-terminal catalytic domains (12, 20, 88). A metal cofactor (iron protoporphyrin IX, heme) binds only in the regulatory domain of the beta subunit and is critical for sGC function because it enables NO to bind and activate the enzyme (12, 20, 88). NO activates the sGC heterodimer by binding to its heme group, which causes protein structural changes that enable sGC to catalyze conversion of GTP to cGMP and mediates many of the biological actions of NO (11, 22, 45). This constitutes the NO-sGC-cGMP signaling pathway, which maintains cardiovascular health (37, 45, 76). Accordingly, the pathogenesis of several diseases appears linked to insufficient sGC activity (37, 45, 76).
Heat shock protein 90 (Hsp90) is a ubiquitously expressed ATP-dependent chaperone that helps to fold, stabilize, or modify the functions of select client proteins (42, 46). Hsp90 functions through its subdomain molecular motions and its inherent ATPase activity to help control client protein maturation, trafficking, and lifetime in cells (14, 62, 82). The molecular-level impacts of hsp90 on various client proteins are just beginning to be elucidated (38, 59, 64, 74). Hsp90 is known to associate with several heme proteins and the ascribed outcomes include assisting in protein maturation, stabilization, function, or activity and in shaping enzyme product distribution (Table 1) (50). Hsp90 was first reported to associate with sGC in 2003 (90), and subsequent cell and molecular studies identified possible regions of protein interaction (58) and the possible functional roles for the interaction (increase in the stabilization and activity of sGC) (2, 3, 24, 25, 67, 95), but did not explore how these outcomes may come about. In this review, we discuss recent work that reveals how hsp90, heme, NO, pharmacologic sGC agonists, and related cellular processes may govern sGC function in unexpected new ways.
Table 1.
Known Hsp90 Chaperone Heme–Protein Interactions in Eukaryotes
| Protein | Role of heme | Associated Hsp90 chaperone, possible role |
|---|---|---|
| Eukaryotic initiation factor 2-alpha (elF2) kinase HRI (heme-regulated inhibitor) | Repress kinase activity, signal transduction | Amplify/enable function |
| NO synthase 1, 2, and 3 (NOS) | Catalytic O2 activation | Aid heme insertion, boost activity of heme-replete enzyme, stabilize protein |
| Cytochrome P450 2B1 | Catalytic O2 activation | GRP94 (?), enable heme reconstitution |
| NADPH oxidase 1, 2, and 5 | Catalytic O2 reduction | Boost activity and influence enzyme products |
| Soluble guanylate cyclase | Gas sensing signal transduction | Boost activity and increase enzyme lifetime |
Eukaryotic heme synthesis and hsp90-driven cytosolic heme insertions
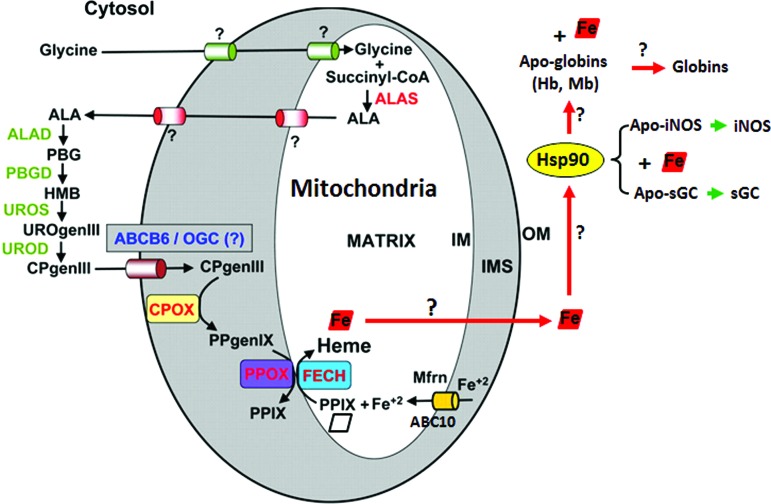
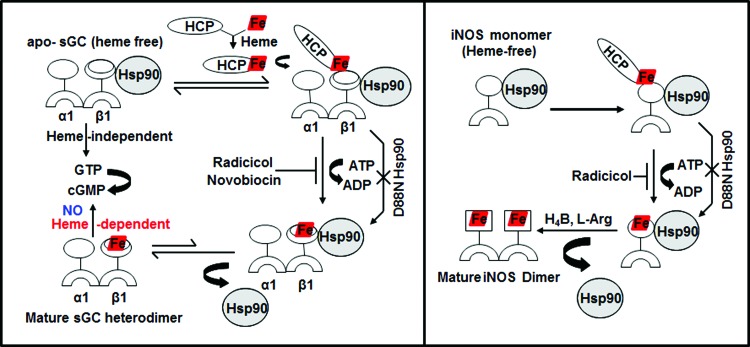
Heme proteins are involved in a remarkable array of biological functions, including cell energetics, oxygen transport and storage, numerous enzymatic transformations, cell signaling, and host defense (60, 61). In all cases, the heme cofactor is essential for function, but because free heme is reactive, its production is tightly regulated (83). The specific steps of heme biosynthesis are well known and take place in both the cytosolic and mitochondrial compartments of a cell (13, 79, 92), with the final three steps occurring in the mitochondria (Fig. 1). Contrastingly, little is known about how heme is transported out of mitochondria or how heme insertion occurs in soluble proteins in the cytosol (17, 73). However, recent studies from our group (23, 25) as well as pioneering studies from Osawa's group (6) have shown specific involvement of chaperon hsp90 in cytosolic heme insertion into soluble proteins. Osawa and colleagues showed for the first time that hsp90 is required for cellular heme insertion into neuronal NO synthase (nNOS). Later, Ghosh et al. uncovered a role for hsp90 in inducible NOS (iNOS) heme insertion (23). In the latter case, hsp90 was shown to primarily associate with an apo-iNOS monomer in cells, and then found to drive heme insertion into the apoenzyme by an ATP-dependent process, after which hsp90 interaction with the heme-replete mature iNOS fell apart. Recently, it was found that heme insertion into the β subunit of sGC is also hsp90 dependent (25). Given that sGC structural homologs (H-NOX) and NOS have markedly different protein structures and heme environments (10, 48), these findings hint that hsp90 may play a broader role in heme protein maturation than was previously realized. In the sGC studies, hsp90 associated primarily with the heme-free form of sGC-β1 in cells, but the association fell apart once heme became inserted. Although the association did not require hsp90 to have an intact ATPase activity, as judged from results using hsp90 inhibitors, radicicol or novobiocin, or by using an ATPase-defective hsp90 mutant (D88N), an intact ATPase activity was essential to actually drive heme insertion into the apo-sGCβ1. Thus, the model suggested for hsp90 function in sGC maturation mimics the model proposed for driving heme insertion into apo-iNOS (Fig. 2). These similarities imply that hsp90 may operate through a common mechanism to target and stabilize heme-free forms of client heme proteins, and then enable their maturation by driving heme insertion in an ATP-dependent process.
FIG. 1.
The pathway for heme biosynthesis and indeterminate trafficking of heme in the cytosol. Heme is bioactive and its synthesis is tightly regulated. The final steps of heme biosynthesis occur inside the mitochondria, following which it is effluxed out into the cytosol to be incorporated in heme proteins that mature and function outside the mitochondria. Adapted and modified from Severance and Hamza Chem Rev 109: 4596–4616, 2009. ALA, delta-aminolevulinic acid; ALAD, delta-aminolevulinic acid dehydratase; ALAS, delta-aminolevulinic acid synthase; CPgenIII, coproporphyrinogen III; CPOX, coproporphyrinogen oxidase; FECH, ferrochelatase; Hb, hemoglobin; HMB, hydroxymethylbilane; IM, inner membrane; IMS, intermembrane space; Mb, myoglobin; Mfrn, mitoferrin; OGC, oxoglutarate carrier; OM, outer membrane; PBG, porphobilinogen; PBGD, porphobilinogen deaminase; PPgenIX, protoporphyrinogen IX; PPIX, protoporphyrin IX; PPOX, protoporphyrinogen oxidase; UROD, uroporphyrinogen decarboxylase; UROgenIII, uroporphyrinogen III; UROS, uroporphyrinogen synthase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 2.
Similarities in hsp90-apoprotein interactions with respect to heme-deplete/replete states of sGC-β1 and iNOS. Hsp90 binds to apo-iNOS/sGC-β1 (heme-free) and uses its ATPase activity to help drive heme insertion into the two proteins. Hsp90 then dissociates from the mature heme-replete iNOS/sGC proteins, whose catalysis can now be activated. This process can be blocked by hsp90 inhibitors, radicicol or novobiocin, and is antagonized by the ATPase-defective D88N-hsp90. HCP indicates an unknown heme carrier protein. sGC, soluble guanylate cyclase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Relevance of apo-sGC/NOS-hsp90 interactions in cells
sGC is a key enzyme of the NO signaling pathway and is a therapeutic target in cardiopulmonary disease, with several sGC agonists currently in clinical development (29, 54, 69, 75–77, 80). NO produced locally from NOS enzymes acts as a messenger that crosses cell membranes, binding to sGC heme, thereby promoting the synthesis of the second messenger, cGMP, which in turn produces vasorelaxation in smooth muscle and participates in a number of downstream signaling pathways (22, 45). Impaired NO and cGMP signaling has been implicated in the pathogenesis of a number of cardiovascular diseases ranging from pulmonary arterial hypertension (PAH) to cardiac hypertrophy and heart failure (9, 40, 47). Studies suggest that the interaction between apo-sGC or apo-NOS and hsp90 in cells is vital for heme insertion into both proteins (23, 25), which in turn is required for their maturation as biologically functional enzymes. Thus, any endogenous or pharmacologic disruption of their hsp90 interactions would be expected to antagonize their heme insertion and limit their maturation and biological function. In fact, disruption or dysregulation of their hsp90 binding would likely have a synergistic impact on any cellular processes that depend on NO signaling because it would diminish both production of the signal molecule (NO) by NOS enzymes and the capacity of the sGC to respond to NO (to make cyclic GMP). Thus, heme insertion in NOS and sGC is of dual significance in the NO signaling cascades. Currently, it is unknown if any of the hsp90 inhibitors being developed for cancer therapy (85) also impact maturation of sGC or iNOS by inhibiting their heme insertions, but this possibility should be considered. In addition, future work stands to explore if the interactions of hsp90 with apo-sGC or apo-iNOS might be manipulated in a positive way to ensure that sufficient or greater cGMP production occurs to restore proper functioning of biological NO signaling cascades in disease states.
NO-triggered heme insertion into sGC-β1, mutually exclusive hsp90 interaction versus sGC-α1β1 heterodimer formation, and a novel activation pathway for the sGC activator, BAY 60-2770
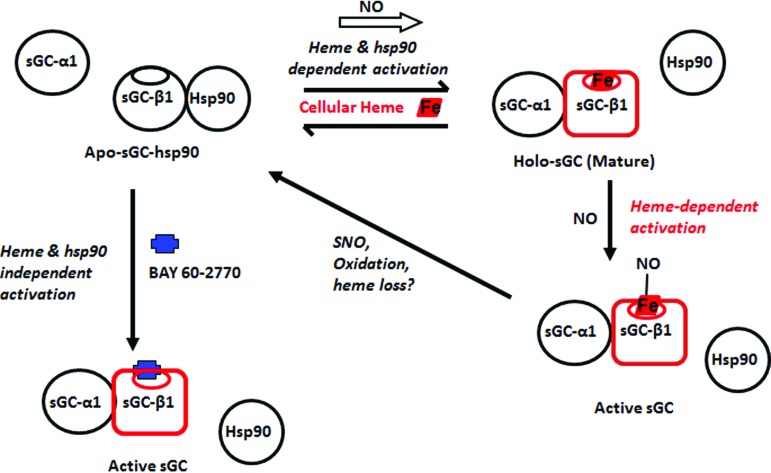
A recent study revealed that exposing cultured cells to a low dose of NO triggered hsp90-assisted heme insertion into the population of apo-sGC-β1 that existed in the cells (24) (Fig. 3). This occurred in cells that naturally express sGC and in cells transfected to transiently express sGC. The NO effect was relatively rapid, becoming complete within 2 min of exposure, and more slowly reversed with time if the NO exposure was continued. Remarkably, the rapid heme insertion into apo-sGC-β1 was linked to a rapid and reversible swapping of its protein binding partners, that is, from its initial hsp90 partner to its sGC-α1 subunit partner, thus forming a mature sGC-α1β1 heterodimer (Fig. 3). All these NO effects required that hsp90 had an active ATPase activity and that sufficient cellular heme was available and was insertable into the apo-sGC-β1 (i.e., an sGC-β1 heme binding mutant expressed in the cells did not alter its protein associations in response to NO). Overall, the study suggested that a dynamic and previously overlooked interplay may exist between NO, cellular heme, sGC, and hsp90, which can quickly increase the population of mature sGC heterodimers in cells when they encounter exposure to NO.
FIG. 3.
sGC NO response, maturation, and exclusive interaction of sGC-β1 with hsp90 versus sGC-α1. An equilibrium exists in cells between an hsp90-bound apo-sGC-β1 and a heme-replete sGC-β1 that is associated with sGC-α1. NO can rapidly shift this equilibrium to the right when cell heme levels are sufficient and hsp90 is active. NO can then bind to the heme in the sGC heterodimer and activate catalysis (lower right). Further NO exposure may cause S-nitrosation of sGC-β1 and heme oxidation/loss and thereby desensitize sGC toward NO and promote its hsp90 reassociation. Binding of the heme-independent activator, BAY 60-2770 (blue), to the apo-sGC-hsp90 species can occur independent of active hsp90 and cellular heme, and this triggers the same changes in sGC-β1 structure and protein interactions that are needed to activate its catalysis. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Within the same study, the pharmacologic sGC activator, BAY 60-2770, was found to mimic all the changes in sGC-β1 protein associations and consequent increase in sGC heterodimer formation that took place when the cells were given NO (Fig. 3). However, in contrast to the NO effect, the ability of BAY 60-2770 to do so did not require an intact hsp90 ATPase activity, nor that cellular heme be available or be insertable into the apo-sGC-β1 (24). These differences make sense if the BAY 60-2770 acts through its binding within the heme pocket of the apo-sGC-β1, which is where activators of this structural class are known to bind (77). Crystal structures of BAY 60-2770 bound to an sGC protein homolog (Nostoc enzyme) show that it binds in the protein's heme pocket and causes discreet changes in the protein structure that are thought to mimic the structural changes that take place when NO binds to heme-replete sGC-β1 when it is in complex as an sGC-α1β1 heterodimer (24, 39). These structural changes within sGC-β1 are thought in turn to cause a cascade of structural changes within the sGC heterodimer that lead to activation of enzyme catalysis (20, 89). In this context, it is remarkable to imagine that BAY 60-2770 binding within the heme pocket of the apo-sGC-β1, when it is in complex with hsp90, may cause similar, or perhaps distinct, structural changes within the β1 subunit that lead it to dissociate from hsp90 and to associate instead with sGC-α1. Although untested, this activation process by BAY 60-2770 would appear to differ from how it may activate an sGC heterodimer, where it must presumably bind to a heme-free sGC-β1 subunit that is already complexed with an sGC-α1 partner instead of hsp90. These different possibilities should be further explored and perhaps could also be investigated from a pharmacore development viewpoint, given that oxidatively damaged forms of sGC that respond to BAY 60-2770 appear to accumulate during several common inflammatory diseases (78).
Molecular aspects of the hsp90-sGC-β1 interaction
Early studies that employed antibody pulldown or affinity tag capture methods to study the hsp90-sGC interaction in mammalian cells reported that their association involved the M domain of hsp90 and two regions within sGC-β1, one in its per-arnt-sim (PAS) domain (amino acids 204–244) and another in its coiled-coil domain (amino acids 379–408) (58). Subsequent studies showed that both of these structural regions in sGC-β1 enable sGC-α1β1 heterodimerization (65, 97). Thus, in retrospect, the early studies also support the concept that sGC-β1 binding to hsp90 or to sGC-α1 may be mutually exclusive and have a reciprocal relationship.
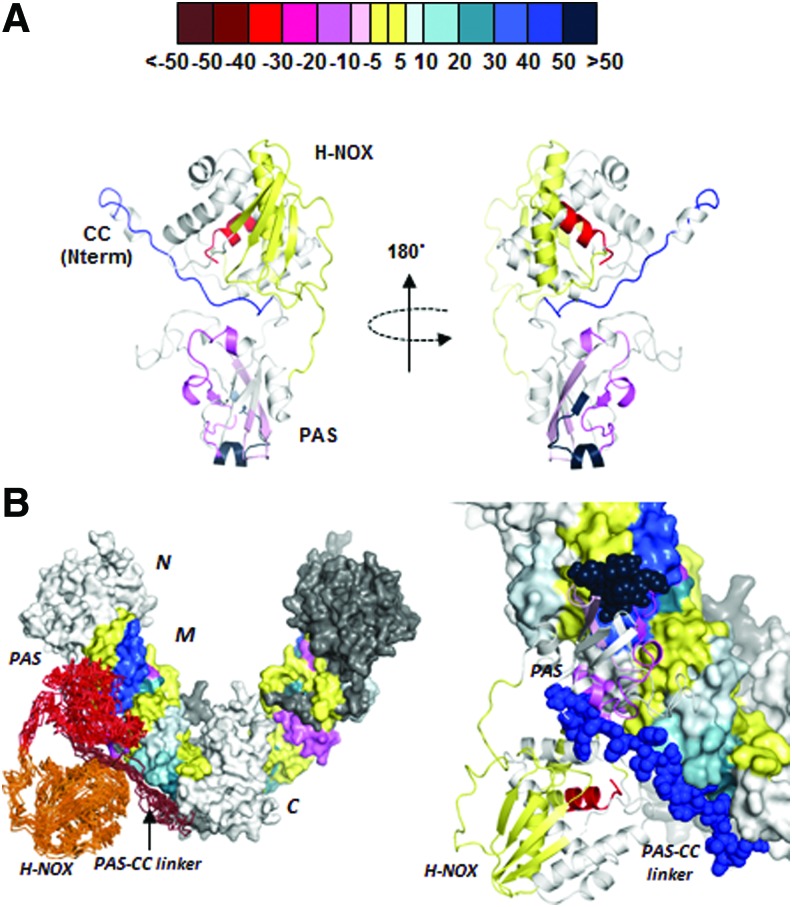
A more recent study (67) used fluorescence polarization and hydrogen–deuterium exchange mass spectrometry to investigate the binding interactions between purified hsp90, sGC-β1, and some of their individual domains. The results suggest that the hsp90 M domain interacts primarily with two regions in apo-sGC-β1 that are located in the middle and C-terminal end of its PAS domain (Fig. 4). Based on a structural modeling and docking analysis (Fig. 4), the authors proposed that the interactions could create a sort of bidentate structural scaffold that (i) prevents apo-sGC-β1 from interacting with its partner sGC-α1 subunit and (ii) could facilitate structural change in the apo-sGC-β1 heme domain that may allow heme insertion. Because hsp90 can bind heme directly (41), it could also conceivably participate in furnishing heme to the apo-sGC-β1 during the maturation process. In these ways, hsp90 actions during sGC maturation would mimic how it influences protein associations and small ligand binding events in other hsp90 client proteins, particularly in clients that contain a PAS domain, including the aryl hydrocarbon receptor and HIF1α (44, 53, 87). Going forward, it will be important to test if the hsp90-apo-sGC-β1 interactions identified in the biophysical studies with purified proteins actually facilitate sGC maturation in an intact cell system. In addition, because cochaperone proteins are likely to aid the hsp90-driven heme insertion into sGC-β1, understanding their identities and participation would create a more comprehensive picture of how sGC maturation is regulated.
FIG. 4.
Structural changes within apo-sGC-β11-358 upon its complex formation with the hsp90 M domain and a model for the possible complex. Hydrogen–deuterium exchange mass spectrometry was used to analyze the hsp90 M domain and sGC-β11-358 proteins alone and in complex with one another. (A) Ribbon diagram of the H-NOX and PAS domains of apo-sGC-β11-358 (modeled based on the Nostoc HNOX structure) indicating protein regions that become more exposed and have greater proton exchange (pink to red), more protected and have less proton exchange (blue to violet), or do not change (yellow) upon complex formation with the hsp90 M domain. Values in the color bar indicate the percentage difference in the total exchangeable protons for the peptides when the sGC protein was in complex with hsp90 M versus when it was alone. (B) Computer-assisted docking of the apo-sGC-β11-358 onto the hsp90 dimer, built from data obtained with hydrogen–deuterium exchange mass spectrometry and protein data bank information. Adapted from Ref. (26). PAS, per-arnt-sim. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
sGC-β1 heme binding is also sensitive to the redox state of the heme, with the affinity of sGC-β1 being greater toward ferrous heme than toward ferric (19). Given that sGC is thought to lose the oxidized ferric heme under high oxidant stress (19), it is vital to determine the mechanisms involved. For example, Might hsp90 play a role in aiding ferric heme loss from its sGC-β1 client protein? Might hsp90 hold the ferric heme transiently, only to transfer it back to apo-sGC-β1 as ferrous heme if the redox state of the cell returns to normal conditions? These questions may be addressed in future studies. At this point, the studies raise the possibility that the apo-sGCβ1-hsp90 interactions facilitate a dynamic heme transfer among the participating protein components, which in turn might be influenced by factors, including sGC protein modifications or changes in the intracellular and heme redox states, perhaps in a manner similar to what has been found to occur in other systems (63).
Impact of NO on heme protein maturation and its relevance to diseases
In cells, NO can affect heme availability and heme insertion in various ways. NO is known to cause a prolonged and significant loss of intracellular iron in cells once iNOS is expressed (36, 93). Moreover, NO is known to directly inhibit the mitochondrial enzyme, ferrochelatase, which is responsible for iron insertion into the newly synthesized porphyrin (21). Waheed et al. found that NO exposure blocks heme insertion in a number of apoprotein targets expressed in mammalian cells, including NOS enzymes, catalase, cytochrome P450, and hemoglobin (91), thus suggesting that NO can act as a global inhibitor of heme insertion. Manifestations could possibly occur in any disease characterized by chronically elevated NO levels, for example, in the acute malarial anemia prevalent among children of Sub-Saharan Africa (1, 33) or in pulmonary inflammatory diseases such as asthma (26, 35). As sGC is abundantly expressed in human airway smooth muscle cells, it is logical to predict that high airway NO levels may inhibit sGC heme insertion, resulting in increased amounts of heme-free sGC in a diseased state such as asthma. This may result in desensitization of sGC toward its natural activator NO, and further studies should aim to address these possibilities.
S-nitrosation of sGC and its relevance to NO desensitization or sGC deactivation
During prolonged NO exposure, sGC ultimately decreases its cGMP output by a process known as NO desensitization (55, 72). One potential mechanism of desensitization is through the S-nitrosation of Cys residues that are located in close proximity to the heme (4, 15, 43, 49, 68). S-nitrosation (SNO) is an NO-dependent post-translational modification that may directly alter protein structure and function (8, 28, 52). In sGC, it is thought to occur when NO, in addition to binding to the heme cofactor, ultimately modifies free thiols in the protein. The full-length sGC-β1 contains 34 cysteine residues (18), but only three of these (C78, C122, and C174) in sGC-β1 have been characterized within a minimum functional ligand-binding heme domain of sGC (amino acids 1-194) to study cysteine S-nitrosation (32). Using the biotin switch method, activity assays, and infrared spectroscopy, Sayed et. al (68), Frenhoff et. al (16), and Liu et. al (43) demonstrated that C78 and C122 on the β1 subunit are the key residues for sGC desensitization via S-nitrosation. One report (16) suggested that SNO modification of sGC cysteine residues (Cys 78 and Cys 122) actually involves the sGC heme moiety participating in the redox reaction, resulting in a deactivated ferric sGC that cannot respond toward NO.
A recent study (24) suggests an additional way that SNO modifications of sGC-β1 might be associated with NO desensitization: The buildup of SNO-sGC in cells exposed to NO was found to correlate with the kinetics of hsp90 association with sGC-β1 and a concomitant loss of the sGC-α1β1 heterodimer population. This suggests that the SNO modification(s) might alter the sGC-β1 in ways that weaken the heterodimer interaction, thus leading to NO desensitization. It also raises questions regarding the role of hsp90 association with the SNO-sGC-β1 postactivation, for example, Is it a natural process that helps to protect sGC-β1 from degradation (56)? Does it facilitate denitrosation? Is it accompanied by any hsp90-driven changes in sGC-β1 heme occupancy as described above (30, 51)? Besides the mechanisms of desensitization that possibly involve SNO modification of sGC-β1, additional mechanisms include (i) ferrous heme oxidation to ferric (27, 71, 96) and (ii) direct heme nitrosylation by NO (86).
Regarding the natural activation/deactivation cycle that sGC engages in during signal transduction, previous studies show that there are different time frames for deactivation, and it is still unclear if simple NO dissociation from the sGC heme directly leads to deactivation. In vitro deactivation of sGC enzyme occurs within minutes after NO dissociation (7, 34), whereas in vivo cell sGC activity can deflect within seconds after it is activated by NO (5), suggesting that additional factors are present in cells to speed sGC deactivation. Whether fast deactivation involves allosteric mechanisms, protein partners, or even SNO modifications are all intriguing possibilities that need to be addressed.
Heme-free or desensitized sGC in cells/tissues and potential therapeutic importance of sGC activators
Increased oxidant stress that occurs under inflammatory conditions (81) is thought to cause oxidation and loss of the sGC heme, thus creating a population of sGC that is insensitive to NO (19). This has increased interest toward developing drug candidates that can activate sGC independent of NO or its heme (37, 76, 78) and has piqued interest in the cellular mechanisms that may control the heme content, protein associations, and activity of sGC. Indeed, it is becoming evident that sGC can exist as a blend of heme-containing and heme-free populations in cells even under normal culture conditions (24, 25), and growing evidence suggests that this equilibrium shifts to favor the heme-free form under pathophysiological conditions characterized by increased oxidant stress (78, 84). The presence of heme-free sGC in a variety of transformed and primary cell types (24, 25) may reflect the original finding by Ignarro and colleagues who first purified sGC from bovine lung in a heme-deficient form (31, 57) and also support results of Roy et al. (66) that showed heme-free sGC normally exists in cells. Under these circumstances, activators of soluble guanylyl cyclase that can modulate sGC activity in a selective manner either by targeting the heme-containing (BAY 41-2272) or heme-free form (BAY 58-2770 or BAY 60-2770) of the enzyme possess considerable therapeutic potential (70, 76, 77). Such sGC agonists are of current interest as treatment for cardiovascular and cardiopulmonary diseases.
Molecular mechanisms that underlie pharmacological activation of the heme-free sGC appear to involve the triggering of sGC heterodimerization, which occurs, for example, in response to BAY 60-2770 insertion into the sGC-β1 subunit (Fig. 3). Thus, although the relative importance of this process would depend on the relative abundance of the apo-sGCβ1-hsp90 complex in cells, it may or may not need to involve hsp90 for an effective drug response (24, 25). Regarding the heme-dependent sGC activators, BAY 41-2272 is a structural analog to the commercial drug, Adempas or Riociguat (BAY 63-2521), which was recently approved by the FDA (54) to treat pulmonary hypertension in humans and has since been approved in Europe with expected worldwide approval. The success of this heme-dependent sGC activator in treating pulmonary hypertension (PAH) patients may be attributed to the low NO levels (94) that prevail in the diseased pulmonary arteries. This, coupled with the fact that Adempas works well in synergy with low NO levels (40, 94), makes it an effective drug in PAH. In contrast, diseases that are characterized by chronic high NO levels (26) may induce an increase in the heme-free sGC-β1. Under these conditions, heme-independent sGC agonists such as BAY 58-2667 or BAY 60-2770 can become more valuable because of their ability to selectively activate the oxidized/heme-free form of sGC (70, 77). However, since the natural state of sGC may be a blend of heme-containing and heme-free states, perhaps the most effective therapy might have both types of sGC drugs used in combination, with the relative ratio reflective of the individual and the extent of disease progression.
Heme-free sGC and sGC-hsp90 interactions as markers in cardiopulmonary diseases
Studies (24, 25) suggest that observing hsp90-sGCβ1 association in cells may itself indicate that heme-free sGC-β1 is present. The concept that the sGCβ1-hsp90 interaction is mutually exclusive with sGC-α1β1 heterodimerization (24) means it could be a marker of sGC status and activity. A predominant sGC heterodimer may be indicative of healthy condition, whereas a weak heterodimer with a strong sGCβ1-hsp90 component would indicate a diseased state. This paradigm may have potential applications in the pathogenesis of diseased states in organs where sGC is present (for example, lung, brain, or intestine) and its vasodilatory function holds biological relevance. The effects of high oxidant stress or of high NO can possibly be followed as markers or fingerprints in clinical diagnosis to detect the extent of a cardiopulmonary disease. For example, finding a lower amount of sGC-α1β1 heterodimer with a relatively increased amount of sGCβ1-hsp90 complex, perhaps coupled to SNO modification of the sGC-β1 subunit, would imply dysfunctional sGC and may indicate the severity of the disease and allow subsequent monitoring of treatment response. These concepts could help achieve better clinical diagnosis and outcomes across a wide spectrum of diseases.
Abbreviations Used
- H-NOX
heme nitric oxide/oxygen
- Hsp90
heat shock protein 90
- NO
nitric oxide
- NOS
nitric oxide synthase
- PAH
pulmonary arterial hypertension
- PAS
per-arnt-sim
- sGC
soluble guanylate cyclase
- SNO
S-nitrosation
Acknowledgments
Dr. D.J.S. is supported by NIH grants, HL081064 and GM097041, and Bayer grants for target award.
References
- 1.Anstey NM, Granger DL, Hassanali MY, Mwaikambo ED, Duffy PE, and Weinberg JB. Nitric oxide, malaria, and anemia: inverse relationship between nitric oxide production and hemoglobin concentration in asymptomatic, malaria-exposed children. Am J Trop Med Hyg 61: 249–252, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Antonova G, Lichtenbeld H, Xia T, Chatterjee A, Dimitropoulou C, and Catravas JD. Functional significance of hsp90 complexes with NOS and sGC in endothelial cells. Clin Hemorheol Microcirc 37: 19–35, 2007 [PubMed] [Google Scholar]
- 3.Antonova GN, Snead CM, Antonov AS, Dimitropoulou C, Venema RC, and Catravas JD. Nitric oxide preconditioning regulates endothelial monolayer integrity via the heat shock protein 90-soluble guanylate cyclase pathway. Am J Physiol Heart Circ Physiol 292: H893–H903, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Baskaran P, Heckler EJ, van den Akker F, and Beuve A. Identification of residues in the heme domain of soluble guanylyl cyclase that are important for basal and stimulated catalytic activity. PLoS One 6: e26976, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellamy TC. and Garthwaite J. Sub-second kinetics of the nitric oxide receptor, soluble guanylyl cyclase, in intact cerebellar cells. J Biol Chem 276: 4287–4292, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Billecke SS, Draganov DI, Morishima Y, Murphy PJM, Dunbar AY, Pratt WB, and Osawa Y. The role of hsp90 in heme-dependent activation of apo-neuronal nitric-oxide synthase. J Biol Chem 279: 30252–30258, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Brandish PE, Buechler W, and Marletta MA. Regeneration of the ferrous heme of soluble guanylate cyclase from the nitric oxide complex: acceleration by thiols and oxyhemoglobin. Biochemistry 37: 16898–16907, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Broillet MC. S-nitrosylation of proteins. Cell Mol Life Sci 55: 1036–1042, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buys E. and Sips P. New insights into the role of soluble guanylate cyclase in blood pressure regulation. Curr Opin Nephrol Hypertens 23: 135–142, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crane BR, Arvai AS, Ghosh DK, Wu C, Getzoff ED, Stuehr DJ, and Tainer JA. Structure of nitric oxide synthase oxygenase dimer with pterin and substrate. Science 279: 2121–2126, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Derbyshire ER. and Marletta MA. Biochemistry of soluble guanylate cyclase. Handb Exp Pharmacol 191: 17–31, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Derbyshire ER. and Marletta MA. Structure and regulation of soluble guanylate cyclase. Annu Rev Biochem 81: 533–559, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Dumont ME, Cardillo TS, Hayes MK, and Sherman F. Role of cytochrome c heme lyase in mitochondrial import and accumulation of cytochrome c in Saccharomyces cerevisiae. Mol Cell Biol 11: 5487–5496, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faou P. and Hoogenraad NJ. Tom34: a cytosolic cochaperone of the Hsp90/Hsp70 protein complex involved in mitochondrial protein import. Biochim Biophys Acta 1823: 348–357, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Fernhoff NB, Derbyshire ER, and Marletta MA. A nitric oxide/cysteine interaction mediates the activation of soluble guanylate cyclase. Proc Natl Acad Sci USA 106: 21602–21607, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernhoff NB, Derbyshire ER, Underbakke ES, and Marletta MA. Heme-assisted S-nitrosation desensitizes ferric soluble guanylate cyclase to nitric oxide. J Biol Chem 287: 43053–43062, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleming MD. and Hamza I. Mitochondrial heme: an exit strategy at last. J Clin Invest 122: 4328–4330, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friebe A, Wedel B, Harteneck C, Foerster J, Schultz G, and Koesling D. Functions of conserved cysteines of soluble guanylyl cyclase. Biochemistry 36: 1194–1198, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Fritz BG, Hu X, Brailey JL, Berry RE, Walker FA, and Montfort WR. Oxidation and loss of heme in soluble guanylyl cyclase from Manduca sexta. Biochemistry 50: 5813–5815, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fritz BG, Roberts SA, Ahmed A, Breci L, Li W, Weichsel A, Brailey JL, Wysocki VH, Tama F, and Montfort WR. Molecular model of a soluble guanylyl cyclase fragment determined by small-angle X-ray scattering and chemical cross-linking. Biochemistry 52: 1568–1582, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furukawa T, Kohno H, Tokunaga R, and Taketani S. Nitric oxide-mediated inactivation of mammalian ferrochelatase in vivo and in vitro: possible involvement of the iron-sulphur cluster of the enzyme. Biochem J 310: 533–538, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garthwaite J. New insight into the functioning of nitric oxide-receptive guanylyl cyclase: physiological and pharmacological implications. Mol Cell Biochem 334: 221–232, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Ghosh A, Chawla-Sarkar M, and Stuehr DJ. Hsp90 interacts with inducible NO synthase client protein in its heme-free state and then drives heme insertion by an ATP-dependent process. FASEB J 25: 2049–2060, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh A, Stasch JP, Papapetropoulos A, and Stuehr DJ. Nitric oxide and heat shock protein 90 activate soluble guanylate cyclase by driving rapid change in its subunit interactions and heme content. J Biol Chem 289: 15259–15271, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh A. and Stuehr DJ. Soluble guanylyl cyclase requires heat shock protein 90 for heme insertion during maturation of the NO-active enzyme. Proc Natl Acad Sci USA 109: 12998–3003, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh S. and Erzurum SC. Nitric oxide metabolism in asthma pathophysiology. Biochim Biophys Acta 1810: 1008–1016, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gladwin MT. Deconstructing endothelial dysfunction: soluble guanylyl cyclase oxidation and the NO resistance syndrome. J Clin Invest 116: 2330–2332, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Handy DE. and Loscalzo J. Nitric oxide and posttranslational modification of the vascular proteome: S-nitrosation of reactive thiols. Arterioscler Thromb Vasc Biol 26: 1207–1214, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Hobbs AJ. and Moncada S. Antiplatelet properties of a novel, non-NO-based soluble guanylate cyclase activator, BAY 41-2272. Vascul Pharmacol 40: 149–154, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann LS, Schmidt PM, Keim Y, Schaefer S, Schmidt HH, and Stasch JP. Distinct molecular requirements for activation or stabilization of soluble guanylyl cyclase upon haem oxidation-induced degradation. Br J Pharmacol 157: 781–795, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ignarro LJ, Degnan JN, Baricos WH, Kadowitz PJ, and Wolin MS. Activation of purified guanylate cyclase by nitric oxide requires heme. Comparison of heme-deficient, heme-reconstituted and heme-containing forms of soluble enzyme from bovine lung. Biochim Biophys Acta 718: 49–59, 1982 [DOI] [PubMed] [Google Scholar]
- 32.Karow DS, Pan D, Davis JH, Behrends S, Mathies RA, and Marletta MA. Characterization of functional heme domains from soluble guanylate cyclase. Biochemistry 44: 16266–16274, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller CC, Kremsner PG, Hittner JB, Misukonis MA, Weinberg JB, and Perkins DJ. Elevated nitric oxide production in children with malarial anemia: hemozoin-induced nitric oxide synthase type 2 transcripts and nitric oxide in blood mononuclear cells. Infect Immun 72: 4868–4873, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kharitonov VG, Sharma VS, Magde D, and Koesling D. Kinetics of nitric oxide dissociation from five- and six-coordinate nitrosyl hemes and heme proteins, including soluble guanylate cyclase. Biochemistry 36: 6814–6818, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Khatri SB, Hammel J, Kavuru MS, Erzurum SC, and Dweik RA. Temporal association of nitric oxide levels and airflow in asthma after whole lung allergen challenge. J Appl Physiol 95: 436–440, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Kim YM, Bergonia HA, Muller C, Pitt BR, Watkins WD, and Lancaster JR., Jr. Loss and degradation of enzyme-bound heme induced by cellular nitric oxide synthesis. J Biol Chem 270: 5710–5713, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Kots AY, Bian K, and Murad F. Nitric oxide and cyclic GMP signaling pathway as a focus for drug development. Curr Med Chem 18: 3299–3305, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Krukenberg KA, Street TO, Lavery LA, and Agard DA. Conformational dynamics of the molecular chaperone Hsp90. Q Rev Biophys 44: 229–255, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar V, Martin F, Hahn MG, Schaefer M, Stamler JS, Stasch JP, and van den Akker F. Insights into BAY 60-2770 activation and S-nitrosylation-dependent desensitization of soluble guanylyl cyclase via crystal structures of homologous nostoc H-NOX domain complexes. Biochemistry 52: 3601–3608, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai YC, Potoka KC, Champion HC, Mora AL, Gladwin MT. Pulmonary arterial hypertension: the clinical syndrome. Circ Res 115: 115–130, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JM, Lee WH, Kay HY, Kim ES, Moon A, and Kim SG. Hemin, an iron-binding porphyrin, inhibits HIF-1α induction through its binding with heat shock protein 90. Int J Cancer 130: 716–727, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Li J. and Buchner J. Structure, function and regulation of the hsp90 machinery. Biomed J 36: 106–117, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Liu L, Wang D, Xu H, Mi M, and Li Z. The process of S-nitrosation in sGC β1(1-194) revealed by infrared spectroscopy. J Mol Struct 1089: 102–106, 2015 [Google Scholar]
- 44.Liu YV, Baek JH, Zhang H, Diez R, Cole RN, and Semenza GL. RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol Cell 25: 207–217, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, and Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev 52: 375–414, 2000 [PubMed] [Google Scholar]
- 46.Makhnevych T. and Houry WA. The role of Hsp90 in protein complex assembly. Biochim Biophys Acta 1823: 674–682, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, and Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol 60: 1455–1469, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Martin F, Baskaran P, Ma X, Dunten PW, Schaefer M, Stasch JP, Beuve A, and van den Akker F. Structure of cinaciguat (BAY 58-2667) bound to Nostoc H-NOX domain reveals insights into heme-mimetic activation of the soluble guanylyl cyclase. J Biol Chem 285: 22651–22657, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mayer B, Kleschyov AL, Stessel H, Russwurm M, Munzel T, Koesling D, and Schmidt K. Inactivation of soluble guanylate cyclase by stoichiometric S-nitrosation. Mol Pharmacol 75: 886–891, 2009 [DOI] [PubMed] [Google Scholar]
- 50.McClellan AJ, Xia Y, Deutschbauer AM, Davis RW, Gerstein M, and Frydman J. Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approaches. Cell 131: 121–135, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Meurer S, Pioch S, Pabst T, Opitz N, Schmidt PM, Beckhaus T, Wagner K, Matt S, Gegenbauer K, Geschka S, Karas M, Stasch JP, and Schmidt HH, and Müller-Esterl W. Nitric oxide-independent vasodilator rescues heme-oxidized soluble guanylate cyclase from proteasomal degradation. Circ Res 105: 33–41, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Miersch S. and Mutus B. Protein S-nitrosation: biochemistry and characterization of protein thiol-NO interactions as cellular signals. Clin Biochem 38: 777–791, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Minet E, Mottet D, Michel G, Roland I, Raes M, Remacle J, and Michiels C. Hypoxia-induced activation of HIF-1: role of HIF-1alpha-Hsp90 interaction. FEBS Lett 460: 251–256, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Mittendorf J, Weigand S, Alonso-Alija C, Bischoff E, Feurer A, Gerisch M, Kern A, Knorr A, Lang D, Muenter K, Radtke M, Schirok H, Schlemmer KH, Stahl E, Straub A, Wunder F, and Stasch JP. Discovery of riociguat (BAY 63–2521): a potent, oral stimulator of soluble guanylate cyclase for the treatment of pulmonary hypertension. ChemMedChem 4: 853–865, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Mulsch A, Busse R, and Bassenge E. Desensitization of guanylate cyclase in nitrate tolerance does not impair endothelium-dependent responses. Eur J Pharmacol 158: 191–198, 1988 [DOI] [PubMed] [Google Scholar]
- 56.Nedvetsky PI, Meurer S, Opitz N, Nedvetskaya TY, Müller H, and Schmidt HH. Heat shock protein 90 regulates stabilization rather than activation of soluble guanylate cyclase. FEBS Lett 582: 327–331, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Ohlstein EH, Wood KS, and Ignarro LJ. Purification and properties of heme-deficient hepatic soluble guanylate cyclase: effects of heme and other factors on enzyme activation by NO, NO-heme, and protoporphyrin IX. Arch Biochem Biophys 218: 187–198, 1982 [DOI] [PubMed] [Google Scholar]
- 58.Papapetropoulos A, Zhou Z, Gerassimou C, Yetik G, Venema RC, Roussos C, Sessa WC, and Catravas JD. Interaction between the 90-kDa heat shock protein and soluble guanylyl cyclase: physiological significance and mapping of the domains mediating binding. Mol Pharmacol 68: 1133–1141, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Park SJ, Kostic M, and Dyson HJ. Dynamic interaction of Hsp90 with its client protein p53. J Mol Biol 411: 158–173, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ponka P. Cell biology of heme. Am J Med Sci 318: 241–256, 1999 [DOI] [PubMed] [Google Scholar]
- 61.Ponka P. Tissue-specific regulation of iron metabolism and heme synthesis: distinct control mechanisms in erythroid cells. Blood 89: 1–25, 1997 [PubMed] [Google Scholar]
- 62.Prodromou C. The ‘active life’ of Hsp90 complexes. Biochim Biophys Acta 1823: 614–623, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ragsdale SW. and Li Yi. Thiol/disulfide redox switches in the regulation of heme binding to proteins. Antioxid Redox Signal 14: 1039–1047, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ratzke C, Berkemeier F, and Hugel T. Heat shock protein 90's mechanochemical cycle is dominated by thermal fluctuations. Proc Natl Acad Sci USA 109: 161–166, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rothkegel C, Schmidt PM, Atkins DJ, Hoffmann LS, Schmidt HH, Schröder H, and Stasch JP. Dimerization region of soluble guanylate cyclase characterized by bimolecular fluorescence complementation in vivo. Mol Pharmacol 72: 1181–1190, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Roy B, Mo E, Vernon J, and Garthwaite J. Probing the presence of the ligand binding haem in cellular nitric oxide receptors. Br J Pharmacol 153: 1495–1504, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sarkar A, Dai Y, Haque MM, Seeger F, Ghosh A, Garcin ED, Montfort WR, Hazen SL, Misra S, and Stuehr DJ. Heat shock protein 90 associates with the per-arnt-sim domain of heme-free soluble guanylate cyclase: implications for enzyme maturation. J Biol Chem 290: 21615–21628, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sayed N, Baskaran P, Ma X, van den Akker F, and Beuve A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc Natl Acad Sci USA 104: 12312–12317, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schindler U, Strobel H, Schönafinger K, Linz W, Löhn M, Martorana PA, Rütten H, Schindler PW, Busch AE, Sohn M, Töpfer A, Pistorius A, Jannek C, and Mülsch A. Biochemistry and pharmacology of novel anthranilic acid derivatives activating heme-oxidized soluble guanylyl cyclase. Mol Pharmacol 69: 1260–1268, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Schmidt HH, Schmidt PM, and Stasch JP. NO- and haem-independent soluble guanylate cyclase activators. Handb Exp Pharmacol 191: 309–339, 2009 [DOI] [PubMed] [Google Scholar]
- 71.Schrammel A, Behrends S, Schmidt K, Koesling D, and Mayer B. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol Pharmacol 50: 1–5, 1996 [PubMed] [Google Scholar]
- 72.Schroder H, Leitman DC, Bennett BM, Waldman SA, and Murad F. Glyceryl trinitrate-induced desensitization of guanylate cyclase in cultured rat lung fibroblasts. J Pharmacol Exp Ther 245: 413–418, 1988 [PubMed] [Google Scholar]
- 73.Severance S. and Hamza I. Trafficking of heme and porphyrins in metazoa. Chem Rev 109: 4596–4616, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Southworth DR. and Agard DA. Client-loading conformation of the Hsp90 molecular chaperone revealed in the cryo-EM structure of the human Hsp90:Hop complex. Mol Cell 42: 771–781, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stasch JP, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, Minuth T, Perzborn E, Schramm M, and Straub A. Pharmacological actions of a novel NO-independent guanylyl cyclase stimulator, BAY 41–8543: in vitro studies. Br J Pharmacol 135: 333–343, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stasch JP, Pacher P, and Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123: 2263–2273, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stasch JP, Schmidt P, Alonso-Alija C, Apeler H, Dembowsky K, Haerter M, Heil M, Minuth T, Perzborn E, Pleiss U, Schramm M, Schroeder W, Schröder H, Stahl E, Steinke W, and Wunder F. NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br J Pharmacol 136: 773–783, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, Ak HS, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Müller H, Turgay Y, Rothkegel C, Tersteegen A, Kemp-Harper B, Müller-Esterl W, and Schmidt HH. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 116: 2552–2561, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steiner H, Kispal G, Zollner A, Haid A, Neupert W, and Lill R. Heme binding to a conserved Cys-Pro-Val motif is crucial for the catalytic function of mitochondrial heme lyases. J Biol Chem 271: 32605–32611, 1996 [DOI] [PubMed] [Google Scholar]
- 80.Straub A, Stasch JP, Alonso-Alija C, Benet-Buchholz J, Ducke B, Feurer A, and Fürstner C. NO-independent stimulators of soluble guanylate cyclase. Bioorg Med Chem Lett 11: 781–784, 2001 [DOI] [PubMed] [Google Scholar]
- 81.Tabima DM, Frizzell S, and Gladwin MT. Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radic Biol Med 52: 1970–1986, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taipale M, Jarosz DF, and Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 11: 515–528, 2010 [DOI] [PubMed] [Google Scholar]
- 83.Taketani S. Aquisition, mobilization and utilization of cellular iron and heme: endless findings and growing evidence of tight regulation. Tohoku J Exp Med 205: 297–318, 2005 [DOI] [PubMed] [Google Scholar]
- 84.Torregrossa AC, Aranke M, and Bryan NS. Nitric oxide and geriatrics: implications in diagnostics and treatment of the elderly. J Geriatr Cardiol 8: 230–242, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trepel J, Mollapour M, Giaccone G, and Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 10: 537–549, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsai AL, Berka V, Sharina I, and Martin E. Dynamic ligand exchange in soluble guanylyl cyclase (sGC): implications for sGC regulation and desensitization. J Biol Chem 286: 43182–43192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsuji N, Fukuda K, Nagata Y, Okada H, Haga A, Hatakeyama S, Yoshida S, Okamoto T, Hosaka M, Sekine K, Ohtaka K, Yamamoto S, Otaka M, Grave E, and Itoh H. The activation mechanism of the aryl hydrocarbon receptor (AhR) by molecular chaperone HSP90. FEBS Open Bio 4: 796–803, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Underbakke ES, Iavarone AT, and Marletta MA. Higher-order interactions bridge the nitric oxide receptor and catalytic domains of soluble guanylate cyclase. Proc Natl Acad Sci USA 110: 6777–6782, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Underbakke ES, Iavarone AT, Chalmers MJ, Pascal BD, Novick S, Griffin PR, and Marletta MA. Nitric oxide-induced conformational changes in soluble guanylate cyclase. Structure 22: 602–611, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Venema RC, Venema VJ, Ju H, Harris MB, Snead C, Jilling T, Dimitropoulou C, Maragoudakis ME, and Catravas JD. Novel complexes of guanylate cyclase with heat shock protein 90 and nitric oxide synthase. Am J Physiol Heart Circ Physiol 285: H669–H678, 2003 [DOI] [PubMed] [Google Scholar]
- 91.Waheed SM, Ghosh A, Chakravarti R, Biswas A, Haque MM, Panda K, and Stuehr DJ. Nitric oxide blocks cellular heme insertion into a broad range of heme proteins. Free Radic Biol Med 48: 1548–1558, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang X, Dumont ME, and Sherman F. Sequence requirements for mitochondrial import of yeast cytochrome c. J Biol Chem 271: 6594–6604, 1996 [PubMed] [Google Scholar]
- 93.Weiss G, Werner-Felmayer G, Werner ER, Grunewald K, Watcher H, and Hentze MW. Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J Exp Med 180: 969–976, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilkins MR. Pulmonary hypertension: the science behind the disease spectrum. Eur Respir Rev 21: 19–26, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yetik-Anacak G, Xia T, Dimitropoulou C, Venema RC, and Catravas JD. Effects of hsp90 binding inhibitors on sGC-mediated vascular relaxation. Am J Physiol Heart Circ Physiol 291: H260–H268, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Zhao Y, Brandish PE, Di Valentin M, Schelvis JP, Babcock GT, and Marletta MA. Inhibition of soluble guanylate cyclase by ODQ. Biochemistry 39: 10848–10854, 2000 [DOI] [PubMed] [Google Scholar]
- 97.Zhou Z, Gross S, Roussos C, Meurer S, Müller-Esterl W, and Papapetropoulos A. Structural and functional characterization of the dimerization region of soluble guanylyl cyclase. J Biol Chem 279: 24935–24943, 2004 [DOI] [PubMed] [Google Scholar]