Abstract
Background: Loss of control (LOC) eating in youth is associated with excess body weight and adiposity. After adjusting for fat mass, youth with LOC eating have higher blood pressure and higher low-density lipoprotein cholesterol compared to youth without LOC eating. Increased inflammation may account for this relationship, although few data have examined this hypothesis. Therefore, this study explored the association between LOC eating and high-sensitivity C-reactive protein (hsCRP), a marker of inflammation.
Methods: We investigated hsCRP concentrations in relation to LOC eating in a convenience sample of 194 youth (age 14.3 ± 2.1 years; 63.9% female; BMI-z 1.64 ± 1.06). The presence of LOC eating in the past month was assessed by the Eating Disorder Examination interview. Serum hsCRP was measured by enzyme-linked immunosorbent assay. Adiposity was measured by air displacement plethysmography or dual-energy x-ray absorptiometry. We compared hsCRP in those with and without LOC eating in analyses accounting for sex, adiposity, height, depressive symptoms, and eating psychopathology.
Results: Youth with LOC eating had significantly greater hsCRP than youth without LOC eating (p = 0.02), after accounting for all covariates. The number of LOC eating episodes in the past month was positively associated with hsCRP (p = 0.01). The relationship between LOC eating and hsCRP was not mediated by depressive symptoms or eating psychopathology (ps > 0.05).
Conclusions: Youth with disinhibited eating may manifest increased chronic inflammation. Those with LOC eating may be an important subgroup at risk for adverse health outcomes associated with both chronic inflammation and obesity. Future research should examine whether hsCRP concentrations mediate the relationship between LOC eating and its association with cardiometabolic risk.
Keywords: : loss of control eating, binge eating, inflammatory markers, inflammation, high-sensitivity C-reactive protein
Introduction
Obesity is associated with persistent low-grade chronic inflammation.1 In individuals with obesity, secretion of inflammatory cytokines is increased and secretion of adiponectin is decreased, promoting a low-grade chronic proinflammatory state.1 This proinflammatory state is associated with several obesity-related diseases, such as type 2 diabetes (T2D) and cardiovascular disease.1,2 C-reactive protein (CRP) is a protein secreted in the liver in response to increases in inflammatory cytokines.3 Although CRP is an acute phase reactant that rises dramatically in response to acute inflammation,3 among those who are not acutely ill, variation in CRP concentration as measured by high-sensitivity CRP (hsCRP) has also been shown to be a clinically useful marker of subclinical, low-grade chronic inflammation.1,4 CRP concentration has been positively associated with obesity, metabolic syndrome, and T2D in adults,5 as well as elevated systolic and diastolic blood pressure in youth.6 Prospectively, and independent of BMI, CRP concentration has been shown to predict several negative health outcomes, such as the development of hypertension7 and T2D8 in adults. Such data suggest that chronic inflammation may play a causal role in the development of obesity-related comorbidities, including aspects of the metabolic syndrome and cardiovascular disease.9–12 Addressing chronic inflammation may reduce cardiometabolic risk and prevent the development of negative health outcomes.9,13
Binge eating disorder is also highly associated with obesity and its negative physiological consequences, such as insulin resistance and dyslipidemia.14–16 Although the association between binge eating and obesity-related comorbid conditions may be, in large part, explained by the increased weight of those with binge eating,14,17,18 some preliminary data suggest that even independent of adiposity, adults with obesity and binge eating disorder have worse metabolic and inflammatory profiles than adults with obesity, but without the disorder.19
Although youth typically do not meet full criteria for binge eating disorder, loss of control (LOC) eating, the subjective experience of being unable to stop eating regardless of the amount of food consumed, is commonly reported by children and adolescents.20 For youth, it appears that the subjective experience of LOC eating is a more salient indicator of aberrant eating than episode size.21,22 LOC eating is associated with excess body weight and adiposity,23 and reports of LOC eating increases the risk of excess adiposity gain during childhood,24 as well as incidence of overweight and obesity in adolescents and young adults.25 After adjusting for fat mass, youth who report LOC eating have higher blood pressure and higher low-density lipoprotein cholesterol compared to their peers who do not report LOC eating.26 Moreover, those with LOC eating are five times more likely to develop metabolic syndrome at 5-year follow-up, with changes in BMI only partially explaining this association.27 However, it is unknown if youth with LOC eating have elevated inflammatory factors, which may contribute to the worsening metabolic components and increased cardiometabolic risk observed.
We therefore examined the relationship between LOC eating and hsCRP concentration in a sample of children and adolescents. Based on findings that adults with obesity and binge eating disorder have worse metabolic and inflammatory profiles compared to their counterparts with obesity, but without the disorder,19 and that youth with reported LOC eating have worse metabolic characteristics than those without LOC eating,26 we hypothesized that children and adolescents who reported LOC eating would have higher serum concentrations of hsCRP than youth without LOC, after adjusting for adiposity. In addition, given that past research has shown a bidirectional link between psychopathology and inflammation,28–30 and that youth with LOC eating report more psychopathology than youth without LOC eating,23,31,32 we examined whether depressive symptoms or eating-related psychopathology mediated the relationship between LOC eating and hsCRP concentration.
Materials and Methods
Participants
A cross-sectional secondary data analysis was carried out using a convenience sample of 194 children and adolescents assembled from prior studies approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The studies adhered to the ethical standards of the Helsinki Declaration. Participants who completed the Eating Disorder Examination (EDE) and had serum hsCRP concentrations measured within a 4-month window were included. All studies were enriched by design for subjects who are overweight (BMI ≥85th to <95th percentile for age and sex) or obese (BMI ≥95th percentile) according to the CDC growth standards.33 Exclusion criteria were pregnancy, major medical or psychiatric illnesses, and use of medication known to affect weight or eating behavior. Subjects had participated in either a nonintervention protocol (ClinicalTrials.gov ID: NCT00631644, n = 7; NCT00320177, n = 31; NCT00001522, n = 11; NCT00001195, n = 20) or an intervention protocol (NCT00263536, n = 51, a pilot trial to prevent weight gain in children and adolescents who are overweight or obese; NCT00001723, n = 74, a treatment trial for non-Hispanic Black and White adolescents with obesity and at least one weight-related comorbidity). Only baseline data were used from the two intervention protocols, while either baseline or follow-up data were used from the four nonintervention protocols. If a participant had data available at multiple time points, then the earliest available time point was used for analysis. Written consent and assent were provided by parents and children, respectively.
Procedure
For all studies, participants and a parent or guardian were seen at the NIH Hatfield Clinical Research Center. Anthropometric measurements and a blood draw were collected after an overnight fast and before breakfast was consumed. Participants completed the following assessments:
Body mass index
Triplicate measures of height were collected by stadiometer and weight was measured by calibrated scale to the nearest 0.1 kg. BMI (kg/m2) was calculated using weight and average height. BMI-z score was calculated adjusting for age and sex according to the CDC growth standards.33
Body composition
Body fat mass (kg) was measured using dual-energy x-ray absorptiometry (DXA) or air displacement plethysmography (Bod Pod; Life Measurement, Inc., Concord, CA). DXA measurements were taken using a calibrated Hologic QDR Discovery instrument (Hologic, Bedford, MA). Both DXA34,35 and air displacement plethysmography36 have been validated as measures of body composition. Data were adjusted to account for known differences between the Bod Pod and DXA.37
Pubertal status
Pubertal status was categorized using Tanner staging38 based on physical examination by an endocrinologist or nurse practitioner. Breast development in girls was assessed by inspection and palpation and assigned according to the five stages of Tanner.39 If stage was discordant between right and left testes/breasts, the higher Tanner stage was assigned. For boys, testicular volume (mL) was measured by using a set of orchidometer beads as standards according to Prader40 and assigned to one of following five stages: Tanner Stage 1 (testes ≤3 mL), Tanner Stage 2 (testes 4–9 mL), Tanner Stage 3 (testes 10–15 mL), Tanner Stage 4 (testes 16–24 mL), or Tanner Stage 5 (testes ≥25 mL).
LOC eating and eating-related psychopathology
Participants completed the EDE version 12.041 or the child version of the EDE.42 The two versions have been effectively combined in prior studies.43 The number of LOC eating episodes was recorded and LOC eating was deemed present if participants endorsed at least one episode of LOC eating in the past 28 days. The EDE global score, which represents the average of four subscales assessing restraint, and eating, shape, and weight concerns, was used as a measure of eating-related psychopathology.41 The EDE has shown excellent interrater reliability and discriminant validity in pediatric samples.23,43
Children's depression inventory
The Children's Depression Inventory (CDI)44 is a 21-item self-report measure assessing depressive symptoms in the past 2 weeks, with greater scores indicating higher pathology. The CDI is a reliable and valid measurement of depressive symptoms in youth,45 including acceptable internal consistency and test–retest reliability in both clinical46 and community samples.47
Serum hsCRP
Fasting serum hsCRP (mg/L) was measured by enzyme-linked immunosorbent assay at the NIH Clinical Research Center Department of Laboratory Medicine, using a Cobas 6000 Analyzer (Roche Diagnostics, Indianapolis, IN).
Statistical Analysis
All statistical analyses were conducted using IBM SPSS Statistics 23 (Armonk, NY). All data were screened for outliers and normality. For hsCRP concentration, one outlier was recoded to the next highest value. Group differences were assessed using t-tests and Chi-square analyses as appropriate. HsCRP concentration, number of LOC eating episodes in the past 28 days, and CDI scores were log transformed to achieve normality.
The following covariates were considered: study (coded as treatment vs. nontreatment), sex (coded as male or female), race (coded as non-Hispanic White or other), fat mass (kg), height (cm), and Tanner stage. As only sex and fat mass significantly contributed to any model, all models included sex and fat mass as covariates. In addition, height was included as a covariate to adjust body fat mass measurements for height (akin to calculating weight for height as done for the BMI). All tests were two tailed, and differences were considered significant when p-values were ≤.05.
One-way analysis of covariance (ANCOVA) was used to compare the hsCRP concentration of participants with and without LOC eating in the past 28 days. For hsCRP concentrations, unadjusted mean and standard deviation values are provided, as well as adjusted mean and standard error values, which have been adjusted for sex, fat mass, and height. A multiple linear regression model was conducted to examine if LOC eating frequency was associated with hsCRP concentration, adjusting for sex, fat mass, and height.
To examine whether psychopathology explained the relationship between LOC eating and hsCRP concentrations, ANCOVAs were repeated with depressive symptoms and eating-related psychopathology as additional covariates. In addition, four mediation models were conducted using Preacher and Hayes Indirect Mediation macro for SPSS.48 Mediation models examined whether (1) depressive symptoms mediated the relationship between the presence of LOC eating and hsCRP concentrations, (2) depressive symptoms mediated the relationship between the frequency of LOC eating and hsCRP concentrations, (3) eating-related psychopathology mediated the relationship between the presence of LOC eating and hsCRP concentrations, and (4) eating-related psychopathology mediated the relationship between the frequency of LOC eating and hsCRP concentrations. Bootstrapping with 1000 resamples was used to estimate the 95% confidence interval (CI) for indirect effects in each mediation model.
Results
Participants were 194 children and adolescents between the ages of 8 and 18 years (M = 14.28, SD = 2.10). The majority of participants were overweight (14.4%) or obese (57.2%). Seventy-five (38.7%) participants reported at least one episode of LOC eating in the past 28 days. Among those with LOC eating, 36 (48.0%) reported subjectively large LOC eating episodes only, 17 participants (8.8%) reported both subjectively and objectively large LOC eating episodes, and 22 participants (11.3%) reported only objectively large LOC eating episodes in the past 28 days. Based on the number of objectively large LOC episodes in the past 28 days, 10 participants met criteria for binge eating disorder. Among all youth who reported LOC eating, the number of reported episodes in the past 28 days ranged from 1 to 58 (M = 4.87, SD = 8.12). On average, the time between the EDE interview and the hsCRP blood draw was 13.33 days (SD = 24.96). Participant characteristics based on LOC eating status are shown in Table 1. Youth reporting LOC eating were slightly younger, were more likely to be female, had significantly greater BMI-z score, CDI and EDE global scores, and greater hsCRP (not adjusted for covariates) than those who did not report LOC eating.
Table 1.
Participant Characteristics
| LOC eating (n = 75) | No LOC eating (n = 119) | p | |
|---|---|---|---|
| Age in years, M (SD) | 13.55 (2.27) | 14.73 (1.85) | <0.001 |
| Sex, n (%) | 0.03 | ||
| Male | 20 (26.7%) | 50 (42.0%) | |
| Female | 55 (73.3%) | 69 (58.0%) | |
| Race, n (%) | 0.76 | ||
| Non-Hispanic White | 28 (37.3%) | 47 (39.5%) | |
| Non-Hispanic Black | 32 (42.7%) | 58 (48.7%) | |
| Hispanic | 6 (8.0%) | 3 (2.5%) | |
| Other/unknown | 9 (12.0%) | 11 (9.2%) | |
| BMI-z score, M (SD) | 1.91 (0.80) | 1.46 (1.17) | 0.002 |
| Fat Mass (kg), M (SD) | 35.61 (19.03) | 31.00 (21.01) | 0.14 |
| Pubertal statusa, n (%) | 0.26 | ||
| Prepuberty | 4 (5.3%) | 6 (5.0%) | |
| Early/midpuberty | 24 (32.0%) | 27 (22.7%) | |
| Late puberty | 37 (49.3%) | 73 (61.3%) | |
| Not available | 10 (13.3%) | 13 (10.9%) | |
| Height (cm), M (SD) | 159.98 (9.50) | 164.69 (10.60) | 0.003 |
| hsCRP (mg/L), M (SD) | 4.34 (5.57) | 3.68 (6.86) | 0.004 |
| CDI, M (SD) | 7.61 (5.32) | 4.78 (4.46) | <0.001 |
| EDE global score, M (SD) | 1.28 (0.80) | 0.56 (0.60) | <0.001 |
Defined as prepuberty (Tanner Stage 1), early/midpuberty (Tanner Stage 2–3), and late puberty (Tanner Stage 4–5).
CDI, Children's Depression Inventory; EDE, Eating Disorder Examination; hsCRP, high-sensitivity C-reactive protein; LOC, loss of control.
HsCRP and LOC Eating
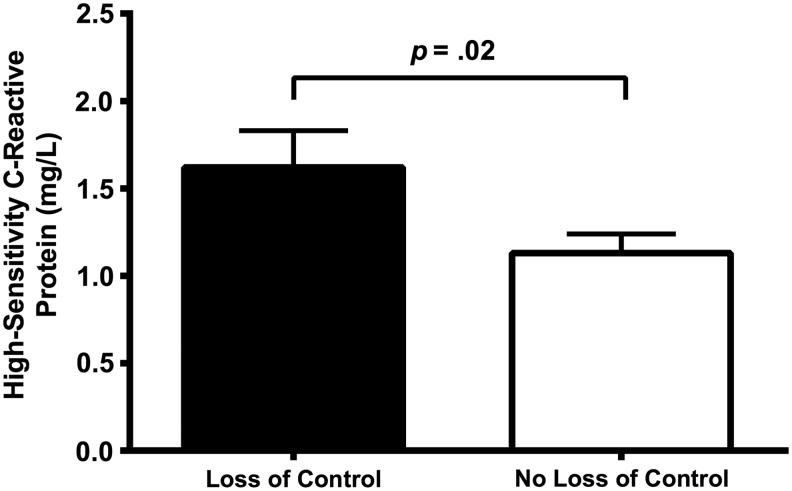
Adjusting for covariates, presence of LOC eating was significantly associated with hsCRP concentration [F(1,168) = 5.51, p = 0.02, η2p = 0.03; Figure 1; Table 2], such that youth who reported LOC eating had higher hsCRP concentration [unadjusted: M = 4.34, SD = 5.57; adjusted M ± SE interval: (1.44 ± 1.83)] than youth who did not report LOC eating [unadjusted: M = 3.68, SD = 6.86; adjusted M ± SE interval: (1.03 ± 1.24)]. The number of LOC eating episodes was significantly associated with hsCRP concentration, such that, an increase in the frequency of LOC eating episodes was associated with an increase in hsCRP [b = 0.25, t(168) = 2.49, p = 0.01].
Figure 1.
High-sensitivity C-reactive protein (HsCRP) concentrations by loss of control (LOC) eating status. HsCRP concentrations in youth with LOC eating were significantly greater than hsCRP concentrations in youth without LOC eating; p = 0.02. Data from analysis of covariance, adjusted for sex, fat mass (kg), and height (cm). Bars represent adjusted mean ± standard error of the mean for youth who reported LOC eating and youth who did not report LOC eating.
Table 2.
Analysis of Covariance for High-Sensitivity C-Reactive Protein by Loss of Control Presence
| df | F | η2p | p | |
|---|---|---|---|---|
| Sex | 1 | 10.21 | 0.06 | 0.002 |
| Total fat mass | 1 | 254.29 | 0.59 | <0.001 |
| Height | 1 | 9.64 | 0.05 | 0.002 |
| LOC presence | 1 | 5.51 | 0.03 | 0.02 |
| Error | 168 |
HsCRP, LOC Eating, and Psychopathology
Including depressive symptoms and eating-related psychopathology in the model (Table 3), LOC eating status remained significantly related to hsCRP concentration [F(1,144) = 4.92, p = 0.03]. Neither depressive symptoms [F(1,144) = 0.10, p = 0.75] nor eating-related psychopathology [F(1, 144) = 0.04, p = 0.84] was significantly related to hsCRP concentration. Furthermore, depressive symptoms did not significantly mediate the relationship between the presence of LOC eating and hsCRP concentration (95% CI: −0.11 to 0.08) or the relationship between the frequency of LOC eating and hsCRP (95% CI: −0.05 to 0.08). Similarly, eating-related psychopathology did not significantly mediate the relationship between the presence of LOC eating and hsCRP concentration (95% CI: −0.14 to 0.22) or the relationship between the frequency of LOC eating and hsCRP (95% CI: −0.14 to 0.14).
Table 3.
Analysis of Covariance for High-Sensitivity C-Reactive Protein by Loss of Control Presence with Psychopathology
| df | F | η2p | p | |
|---|---|---|---|---|
| Sex | 1 | 11.24 | 0.07 | 0.001 |
| Total fat mass | 1 | 141.35 | 0.50 | <0.001 |
| Height | 1 | 9.18 | 0.06 | 0.003 |
| CDI | 1 | 0.10 | 0.001 | 0.75 |
| EDE global score | 1 | 0.04 | <0.001 | 0.84 |
| LOC presence | 1 | 4.92 | 0.03 | 0.03 |
| Error | 144 |
Discussion
Consistent with previous findings in adults with binge eating disorder,19 we found that youth with LOC eating had significantly higher inflammation, as measured by hsCRP, than those without LOC, even after adjusting for differences in fat mass. Notably, these findings were independent of other factors that might contribute to elevated hsCRP. In past research, psychopathology (such as depression, stress, and anxiety) has been robustly associated with elevated inflammation.28–30 Consistent with prior studies,23,49,50 youth with LOC eating in this sample reported higher depressive symptoms and eating-related psychopathology than youth without LOC eating; however, depressive symptoms and eating-related psychopathology were subclinical across all participants. Therefore, the lack of association between psychopathology and LOC eating may have been due to the limited range of psychopathology in the sample. It is possible that a mediation effect would have been observed in a sample with clinically significant depressive symptoms or eating-related psychopathology. Alternatively, the relationship between LOC eating and hsCRP values may be mediated by other factors, such as diet quality.
Youth with reported LOC eating tend to consume meals comprising more calories from carbohydrates and fewer calories from protein, as well as more high-calorie dessert-type foods.51 Youth with LOC eating also appear to eat in response to negative mood. Indeed, state negative affect immediately before a laboratory test meal was associated with greater carbohydrate, snack, and dessert intake and less protein, fruit, and dairy intake in a sample of adolescents with LOC eating.52 Poor diet quality, in turn, has been associated with chronic inflammation.53–56 Therefore, diet quality and mood-induced eating may be potential mediators of the relationship between LOC eating and elevated hsCRP concentration.
Additional characteristics inherent to LOC eating episodes may also mediate the relationship between LOC eating and increased hsCRP. This is supported by our finding of a significant and positive association between LOC eating frequency and hsCRP, even after adjusting for sex, fat mass, and height. For example, individuals often report eating rapidly during binge eating episodes.57 Increased eating speed is associated with increased cardiometabolic risk58,59; therefore, it could be hypothesized that the rapid consumption of food during frequent LOC eating episodes may impact chronic inflammation. However, little is known about the association between such characteristics and chronic inflammation, thus future research is warranted.
Notably, the average unadjusted serum hsCRP in both groups was greater than 3 mg/L, which is the cut-off value for high cardiovascular risk.60 This is likely due to our sample, which was greatly enriched for overweight and obesity. In this study, the average sex/age-specific BMI was at the 95th percentile. Excess adiposity has been robustly associated with elevated hsCRP concentrations in both children and adults.61,62 Indeed, fat mass was strongly associated with elevated hsCRP concentration in all of this study's analyses. Recruitment of a cohort expected to have high hsCRP concentration may have allowed us to observe associations between LOC eating and hsCRP that might not be detectable among primarily nonoverweight cohorts that would be expected to have little elevation in inflammatory markers. However, given the strong association between fat mass and inflammatory markers, it is particularly notable that differences in youth with and without LOC eating were observed in a sample enriched for overweight and obesity.
Our findings suggest that chronic inflammation may be present in youth with disinhibited eating. This is important as it may be particularly vital to identify, and ultimately intervene with, youth to reduce cardiometabolic risk associated with chronic inflammation. Approximately 80% of adolescents with obesity will remain obese in adulthood.63 Youth who remain obese into adulthood are at increased risk for adulthood hypertension, T2D, elevated low-density lipoprotein cholesterol, reduced high-density lipoprotein cholesterol, elevated triglycerides, and increased intima-media thickness of the carotid artery.64 However, these disease processes may begin in childhood. For example, one study found that intima-media thickness—a marker for early atherosclerotic changes—is higher in children with obesity compared to healthy weight children.65 However, not all youth who are overweight develop negative health outcomes as adults; therefore, screening youth for chronic inflammation may help to identify high-risk youth for targeted interventions.1,66
Strengths of this study include the relatively large sample size, assessment of LOC eating using a structured clinical interview, and objective assessment of fat mass. Limitations include the convenience nature of the sample, which may limit generalizability. As our analyses were cross-sectional, the directionality of findings is unknown. In addition, LOC eating was assessed at one time point only. Approximately 30%–50% of youth report persistent LOC eating over time,67,68 thus it is unknown if the significant effects were driven by youth with persistent LOC eating. Moreover, although hsCRP has been shown to be a clinically useful marker of low-grade chronic inflammation,1 other markers of inflammation, such as proinflammatory and anti-inflammatory cytokines, were not examined.
Conclusions
In conclusion, elevated inflammation may begin to manifest in childhood and adolescence among individuals with disinhibited eating. Thus, youth with LOC eating may be a particularly important subgroup at high risk for negative health outcomes associated with both chronic inflammation and obesity. Interventions that successfully target disinhibited eating, or its underlying causes, in youth may reduce inflammation and improve health outcomes. Future research should replicate these findings and examine alternative potential mechanisms of the relationship between LOC eating and inflammatory markers, such as diet quality or eating speed. Most importantly, research should prospectively examine the relationship between LOC eating, inflammatory markers, and cardiometabolic health.
Funding
Funding for this study was provided by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, grant 1ZIAHD000641 with supplemental funding from NIMHD and OBSSR (to J.A.Y.), National Research Service Award 1F32HD056762 from the National Institute of Child Health and Human Development; and Uniformed Services University of the Health Sciences grant R072IC (to M.T.-K.). No funding sources had any role in the study design, collection, analysis, or interpretation of the data, writing the article, or the decision to submit the article for publication.
Author Disclosure Statement
The authors report no relevant competing interests. Dr. Yanovski has received grant support from Zafgen, Inc. for studies of patients with Prader–Willi syndrome and from Rhythm Pharmaceuticals for genetic sequencing studies of patients with early-onset obesity.
Dr. Yanovski is a Commissioned Officer in the U.S. Public Health Service (PHS). The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of USUHS, the U.S. Department of Defense, or the PHS.
References
- 1.Deboer MD. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: A need for screening tools to target interventions. Nutrition 2013;29:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bistrian B. Systemic response to inflammation. Nutr Rev 2007;65:170–172 [DOI] [PubMed] [Google Scholar]
- 3.Du Clos TW. Function of C-reactive protein. Ann Med 2000;32:274–278 [DOI] [PubMed] [Google Scholar]
- 4.Araujo JP, Lourenco P, Azevedo A, et al. . Prognostic value of high-sensitivity C-reactive protein in heart failure: A systematic review. J Card Fail 2009;15:256–266 [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Berges D, Consuegra-Sanchez L, Penafiel J, et al. . Metabolic and inflammatory profiles of biomarkers in obesity, metabolic syndrome, and diabetes in a Mediterranean population. DARIOS Inflammatory study. Rev Esp Cardiol (Engl Ed) 2014;67:624–631 [DOI] [PubMed] [Google Scholar]
- 6.Semiz S, Rota S, Ozdemir O, Ozdemir A, et al. . Are C-reactive protein and homocysteine cardiovascular risk factors in obese children and adolescents? Pediatr Int 2008;50:419–423 [DOI] [PubMed] [Google Scholar]
- 7.Sesso HD, Buring JE, Rifai N, et al. . C-Reactive protein and the risk of developing hypertension. JAMA 2003;290:2945–2951 [DOI] [PubMed] [Google Scholar]
- 8.Freeman DJ, Norrie J, Caslake MJ, et al. . C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes 2002;51:1596–1600 [DOI] [PubMed] [Google Scholar]
- 9.Esser N, Paquot N, Scheen AJ. Inflammatory markers and cardiometabolic diseases. Acta Clin Belg 2015;70:193–199 [DOI] [PubMed] [Google Scholar]
- 10.Libby P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr Rev 2007;65:140–146 [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: Scientific rationale for the cardiovascular inflammation reduction trial (CIRT). J Thromb Haemost 2009;7 Suppl 1:332–339 [DOI] [PubMed] [Google Scholar]
- 12.Itariu BK, Stulnig TM. Obesity, insulin resistance, and inflammaging. In: Rahman I, Bagchi D. (eds), Inflammation, Advancing Age and Nutrition: Research and Clinical Interventions. Academic Press: Waltham, MA, 2014, pp. 157–164 [Google Scholar]
- 13.Ridker PM. Moving beyond JUPITER: Will inhibiting inflammation reduce vascular event rates? Curr Atheroscler Rep 2013;15:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham TM, Massaro JM, Hoffmann U, et al. . Metabolic characterization of adults with binge eating in the general population: The Framingham Heart Study. Obesity (Silver Spring) 2014;22:2441–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson JI, Lalonde JK, Coit CE, et al. . Longitudinal study of the diagnosis of components of the metabolic syndrome in individuals with binge-eating disorder. Am J Clin Nutr 2010;91:1568–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klatzkin RR, Gaffney S, Cyrus K, et al. . Binge eating disorder and obesity: Preliminary evidence for distinct cardiovascular and psychological phenotypes. Physiol Behav 2015;142:20–27 [DOI] [PubMed] [Google Scholar]
- 17.Dingemans AE, Van Furth EF. Binge Eating Disorder psychopathology in normal weight and obese individuals. Int J Eat Disord 2012;45:135–138 [DOI] [PubMed] [Google Scholar]
- 18.Yanovski SZ. Binge eating disorder and obesity in 2003: Could treating an eating disorder have a positive effect on the obesity epidemic? Int J Eat Disord 2003;34 Suppl:S117–S120 [DOI] [PubMed] [Google Scholar]
- 19.Succurro E, Segura-Garcia C, Ruffo M, et al. . Obese Patients With a Binge Eating Disorder Have an Unfavorable Metabolic and Inflammatory Profile. Medicine (Baltimore) 2015;94:e2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanofsky-Kraff M, Marcus MD, Yanovski SZ, Yanovski JA. Loss of control eating disorder in children age 12 years and younger: Proposed research criteria. Eat Behav 2008;9:360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldschmidt AB, Jones M, Manwaring JL, et al. . The clinical significance of loss of control over eating in overweight adolescents. Int J Eat Disord 2008;41:153–158 [DOI] [PubMed] [Google Scholar]
- 22.Shomaker LB, Tanofsky-Kraff M, Elliott C, et al. . Salience of loss of control for pediatric binge episodes: Does size really matter? Int J Eat Disord 2010;43:707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanofsky-Kraff M, Yanovski SZ, Wilfley DE, et al. . Eating-disordered behaviors, body fat, and psychopathology in overweight and normal-weight children. J Consult Clin Psychol 2004;72:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanofsky-Kraff M, Cohen ML, Yanovski SZ, et al. . A prospective study of psychological predictors of body fat gain among children at high risk for adult obesity. Pediatrics 2006;117:1203–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonneville KR, Horton NJ, Micali N, et al. . Longitudinal associations between binge eating and overeating and adverse outcomes among adolescents and young adults: Does loss of control matter? JAMA Pediatr 2013;167:149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radin RM, Tanofsky-Kraff M, Shomaker LB, et al. . Metabolic characteristics of youth with loss of control eating. Eat Behav 2015;19:86–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanofsky-Kraff M, Shomaker LB, Stern EA, et al. . Children's binge eating and development of metabolic syndrome. Int J Obes (Lond) 2012;36:956–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anisman H, Merali Z. Cytokines, stress, and depressive illness. Brain Behav Immun 2002;16:513–524 [DOI] [PubMed] [Google Scholar]
- 29.Bankier B, Barajas J, Martinez-Rumayor A, Januzzi JL. Association between anxiety and C-reactive protein levels in stable coronary heart disease patients. Psychosomatics 2009;50:347–353 [DOI] [PubMed] [Google Scholar]
- 30.Dowlati Y, Herrmann N, Swardfager W, et al. . A meta-analysis of cytokines in major depression. Biol Psychiatry 2010;67:446–457 [DOI] [PubMed] [Google Scholar]
- 31.Elder KA, Paris M, Jr., Anez LM, Grilo CM. Loss of control over eating is associated with eating disorder psychopathology in a community sample of Latinas. Eat Behav 2008;9:501–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan CM, Yanovski SZ, Nguyen TT, et al. . Loss of control over eating, adiposity, and psychopathology in overweight children. Int J Eat Disord 2002;31:430–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuczmarski RJ, Ogden CL, Guo SS, et al. . 2000. CDC growth charts for the United States: Methods and development. Vital Health Stat 11 2002:1–190 [PubMed] [Google Scholar]
- 34.Rothney MP, Brychta RJ, Schaefer EV, et al. . Body composition measured by dual-energy X-ray absorptiometry half-body scans in obese adults. Obesity (Silver Spring) 2009;17:1281–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellis KJ. Human body composition: In vivo methods. Physiol Rev 2000;80:650–680 [DOI] [PubMed] [Google Scholar]
- 36.Silva DRP, Ribeiro AS, Pavao FH, et al. . Validity of the methods to assess body fat in children and adolescents using multi-compartment models as the reference method: A systematic review. Rev Assoc Med Bras 2013;59:475–486 [DOI] [PubMed] [Google Scholar]
- 37.Robotham DR, Schoeller DA, Mercado AB, et al. . Estimates of body fat in children by hologic QDR-2000 and QDR-4500A dual-energy X-ray absorptiometers compared with deuterium dilution. J Pediatr Gastroenterol Nutr 2006;42:331–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanner JM. Growth and endocrinology of the adolescent. In: Garner L. (ed), Endocrine and Genetic Diseases of Childhood. W. B. Saunders: Philadelphia, PA, 1969, pp. 19–60 [Google Scholar]
- 39.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanner JM. Growth and maturation during adolescence. Nutr Rev 1981;39:43–55 [DOI] [PubMed] [Google Scholar]
- 41.Fairburn CG, Cooper Z. The eating disorder examination (12th edition). In: Fairburn CG, Wilson GT. (eds), Binge Eating: Nature, Assessment, and Treatment. Guilford Press: New York, 1993, pp. 317–360 [Google Scholar]
- 42.Bryant-Waugh RJ, Cooper PJ, Taylor CL, Lask BD. The use of the eating disorder examination with children: A pilot study. Int J Eat Disord 1996;19:391–397 [DOI] [PubMed] [Google Scholar]
- 43.Glasofer DR, Tanofsky-Kraff M, Eddy KT, et al. . Binge eating in overweight treatment-seeking adolescents. J Pediatr Psychol 2007;32:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovacs M, Beck AT. An empirical-clinical approach toward a definition of childhood depression. In: Schulterbrandt JG, Raskin A. (eds), Depression in Childhood: Diagnosis, Treatment and Conceptual Models. Raven Press: New York, 1977, pp. 1–25 [Google Scholar]
- 45.Reynolds WM, Anderson G, Bartell N. Measuring depression in children: A multimethod assessment investigation. J Abnorm Child Psychol 1985;13:513–526 [DOI] [PubMed] [Google Scholar]
- 46.Fundudis T, Berney TP, Kolvin I, et al. . Reliability and validity of two self-rating scales in the assessment of childhood depression. Br J Psychiatry 1991;159:36–40 [PubMed] [Google Scholar]
- 47.Smucker MR, Craighead WE, Craighead LW, Green BJ. Normative and reliability data for the Children's Depressive Inventory. J Abnorm Child Psychol 1986;14:25–39 [DOI] [PubMed] [Google Scholar]
- 48.Selig JP, Preacher KJ. Mediation models for longitudinal data in developmental research. Res Hum Dev 2009;6:144–164 [Google Scholar]
- 49.Goossens L, Braet C, Van Vlierberghe L, Mels S. Loss of control over eating in overweight youngsters: The role of anxiety, depression and emotional eating. Eur Eat Disord Rev 2009;17:68–78 [DOI] [PubMed] [Google Scholar]
- 50.Goossens L, Soenens B, Braet C. Prevalence and characteristics of binge eating in an adolescent community sample. J Clin Child Adolesc Psychol 2009;38:342–353 [DOI] [PubMed] [Google Scholar]
- 51.Tanofsky-Kraff M, McDuffie JR, Yanovski SZ, et al. . Laboratory assessment of the food intake of children and adolescents with loss of control eating. Am J Clin Nutr 2009;89:738–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ranzenhofer LM, Hannallah L, Field SE, et al. . Pre-meal affective state and laboratory test meal intake in adolescent girls with loss of control eating. Appetite 2013;68:30–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.George SM, Neuhouser ML, Mayne ST, et al. . Postdiagnosis diet quality is inversely related to a biomarker of inflammation among breast cancer survivors. Cancer Epidemiol Biomarkers Prev 2010;19:2220–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akbaraly TN, Shipley MJ, Ferrie JE, et al. . Long-term adherence to healthy dietary guidelines and chronic inflammation in the prospective Whitehall II study. Am J Med 2015;128:152–160e154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boynton A, Neuhouser ML, Wener MH, et al. . Associations between healthy eating patterns and immune function or inflammation in overweight or obese postmenopausal women. Am J Clin Nutr 2007;86:1445–1455 [DOI] [PubMed] [Google Scholar]
- 56.Park KH, Zaichenko L, Peter P, et al. . Diet quality is associated with circulating C-reactive protein but not irisin levels in humans. Metabolism 2014;63:233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marcus MD, Wing RR, Hopkins J. Obese binge eaters: Affect, cognitions, and response to behavioral weight control. J Consult Clin Psychol 1988;56:433–439 [DOI] [PubMed] [Google Scholar]
- 58.Zhu B, Haruyama Y, Muto T, Yamazaki T. Association between eating speed and metabolic syndrome in a three-year population-based cohort study. J Epidemiol 2015;25:332–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee KS, Kim DH, Jang JS, et al. . Eating rate is associated with cardiometabolic risk factors in Korean adults. Nutr Metab Cardiovasc Dis 2013;23:635–641 [DOI] [PubMed] [Google Scholar]
- 60.Ridker PM. Cardiology patient page. C-reactive protein: A simple test to help predict risk of heart attack and stroke. Circulation 2003;108:e81–e85 [DOI] [PubMed] [Google Scholar]
- 61.Wen X, Pekkala S, Wang R, et al. . Does systemic low-grade inflammation associate with fat accumulation and distribution? A 7-year follow-up study with peripubertal girls. J Clin Endocrinol Metab 2014;99:1411–1419 [DOI] [PubMed] [Google Scholar]
- 62.Wu DM, Chu NF, Shen MH, Wang SC. Obesity, plasma high sensitivity C-reactive protein levels and insulin resistance status among school children in Taiwan. Clin Biochem 2006;39:810–815 [DOI] [PubMed] [Google Scholar]
- 63.Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: A systematic review and meta-analysis. Obes Rev 2016;17:95–107 [DOI] [PubMed] [Google Scholar]
- 64.Juonala M, Magnussen CG, Berenson GS, et al. . Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med 2011;365:1876–1885 [DOI] [PubMed] [Google Scholar]
- 65.Reinehr T, Kiess W, De Sousa G, et al. . Intima media thickness in childhood obesity: Relations to inflammatory marker, glucose metabolism, and blood pressure. Metabolism 2006;55:113–118 [DOI] [PubMed] [Google Scholar]
- 66.Bluher S, Schwarz P. Metabolically healthy obesity from childhood to adulthood—Does weight status alone matter? Metabolism 2014;63:1084–1092 [DOI] [PubMed] [Google Scholar]
- 67.Tanofsky-Kraff M, Shomaker LB, Olsen C, et al. . A prospective study of pediatric loss of control eating and psychological outcomes. J Abnorm Psychol 2011;120:108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hilbert A, Hartmann AS, Czaja J, Schoebi D. Natural course of preadolescent loss of control eating. J Abnorm Psychol 2013;122:684–693 [DOI] [PubMed] [Google Scholar]