Abstract
The course of events in ischemic strokes is normally seen from a point in which the penumbra is already in place. Since there is no known treatment for edema reduction, mainstream medicine focuses on re-opening the occluded vessel. Here we show that reducing the penumbra saves neuronal units from undergoing apoptosis.
Keywords: ischemic stroke, penumbra, ozone therapy
Introduction
The treatment and treatment outcome of ischemic brain strokes is still unsatisfactory. Even medical associations dismiss major progress in the current means of counteracting the disease1. The number of totally disabled patients after ischemic stroke has remained high over the decades at about 17% and without any sign of alleviation.
Another point is that mainstream medicine does not focus on the first event after stroke onset because the first stage of inflammation, the incoming penumbra or perifocal edema, is not identified as a target for intervention since no medication exists.
Physiology and pathophysiology of the neuronal unit
The neuronal unit is composed of neurons, astrocytes, and specialized endothelial cells and pericytes building the blood brain barrier (BBB). The microglial cells patrolling the tissue as guards belong to the wider environment. It is common knowledge that the brain metabolizes mainly glucose and it is taken for granted that neurons are a part of glucose users. In 1999 Magistretti et al. reported that neurons mainly metabolize lactate and are widely devoid of the glycolytic pathways2. In 2004 another publication by Pellerin and Magistretti investigated the topic further and proved the till then theoretical explanation3. The reason is that neurons are the racehorses of our brain needing high amounts of energy to do their work in firing and transmission of excitations. The exocytic release of neurotransmitters and their reuptake are highly energy-consuming processes. While astrocytes depend on glycolysis to create pyruvate and metabolize pyruvate via acetyl-CoA in the mitochondrial tricarbon cycle, neurons use lactate (lactate contains only one H-atom more). In glycolysis, phosphof-ructokinase-1, the enzyme creating fructose-1,6-diphosphate, is the limiting factor. This enzyme is the bottleneck in glycolysis. If neurons depended on the glycolysis to create ATP they could not work as high-speed transmitters and would be disabled in their firing capability. Nature found the solution in providing neurons with lactate enabling them to feed their mitochondria located close to the neuronal spines with 'fast food', lactate.
Even other glial cells, like microglial cells, react promptly in case of lesions in the neuronal tissue. After laser occlusion of a single capillary the microglial cells occupy the area in a time as short as seven minutes4.
Ischemic stroke penumbra
From the efficiency point of view, this division of metabolism is very effective under physiological conditions. But it creates an 'Achilles heel' in case of disturbance and inflammation. Any obstruction in oxygen supply leads to ATP depletion and within eight minutes to necrosis. Necrosis is induced in neurons by ATP deficiency close to zero, resulting in instant cell death5. This is why we find at least a scar in the location of the first impact of ischemic stroke in most cases.
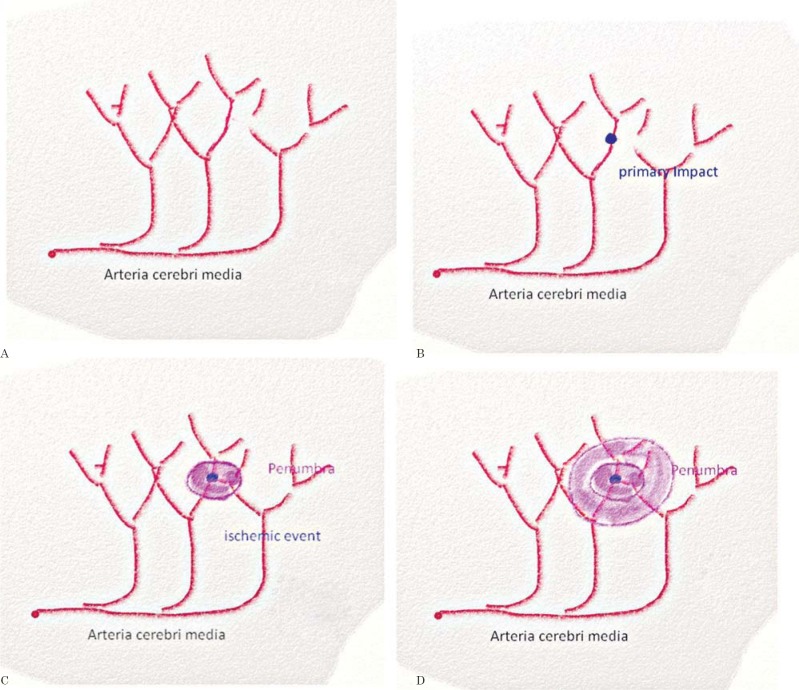
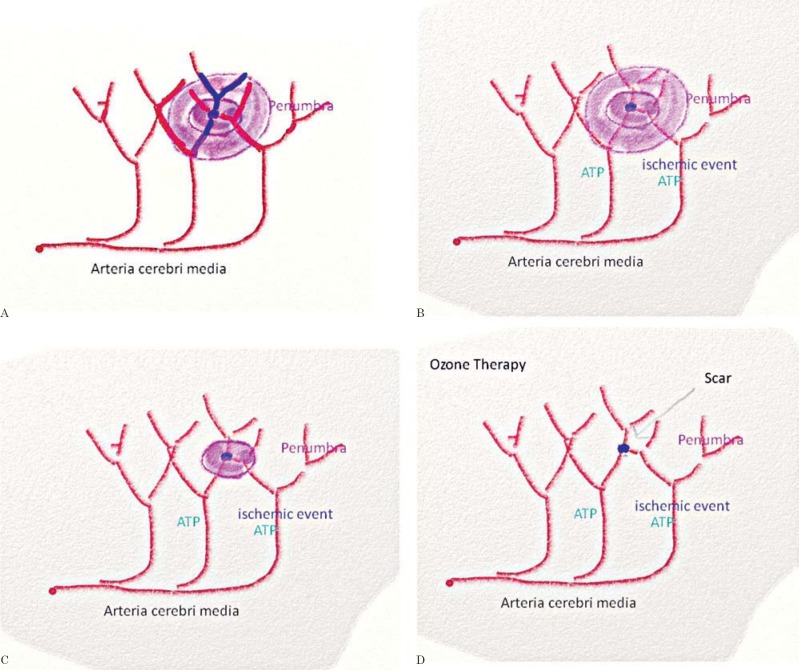
Neuronal death sends a wave of potassium into the neighborhood depolarizing the cells and creating the penumbra. In the penumbra zone cells can survive since the BBB is still intact and astrocytes and glial cells still have the means to create ATP by disabled but still functioning electron transport chains (ETCs). The relative resistance to ischemia is based on glycogen storage and use. In this area the cells will undergo apoptosis. This means that ETC complex IV still works providing the mitochondria with minimal ATP amounts enabling the cells to undergo controlled destruction, apoptosis6. The mitochondria still maintain a mitochondrial membrane potential (mΔΨ) as part of their limited survival ability. Within 24 to 48 hours the penumbra passes the stages of apoptosis. But within this time with the special means of ATP delivery we can revitalize the area (Figure 1A-D).
Figure 1.
A) Intact arteria cerebri media. B) Ischemic stroke and area of first impact. C) Widening of depolarized cells by potassium efflux. D) Penumbra.
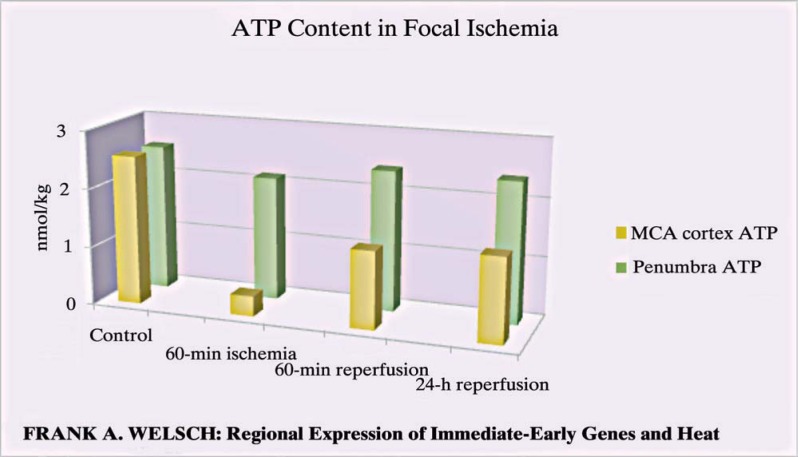
The ATP content (Figure 2) in the MCA area is close to zero after occlusion and never reaches the former level after reperfusion. The content does not equal zero since the astrocytes still produce ATP by their ETC. Here, in the middle cerebral artery (MCA) area the astrocytes will also develop apoptosis, since the effector cells, the neurons passed away. In the penumbra area we still find higher amounts of ATP for the same reason.
Figure 2.
Yellow bars show ATP deprivation after first impact, recovery of ATP content depends on astrocytes using glycolysis. Green bars show ATP in the penumbra area with ATP production continuing via ETC complex IV in astrocytes.
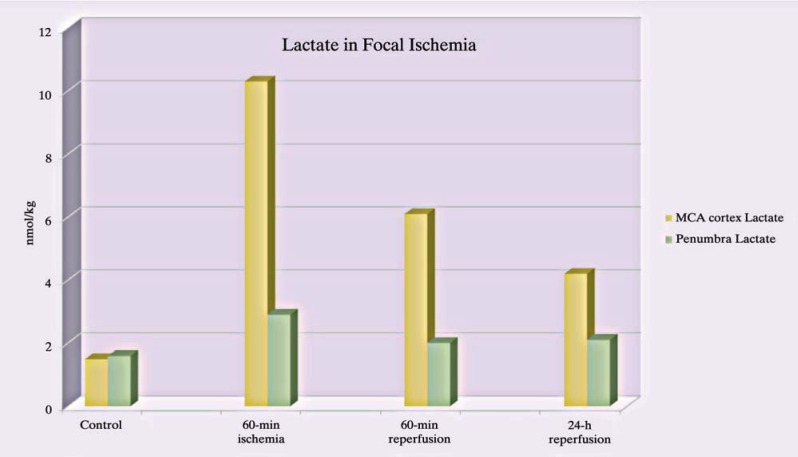
If we look at the lactate content in the MCA area and penumbra we will find a vital difference (Figure 3). The MCA area produced in an animal trial by occlusion of the MCA shows an overload of lactate5. This phenomenon mirrors the fact of surviving astrocytes with glycolysis and ETC partially functioning and the surplus of lactate results in the lack of surviving neurons which normally metabolizes the substrate. A larger portion of lactate is derived from the fact, that astrocytes themselves are no longer able to use pyruvate because of cell membrane depolarization despite the fact that there is no lack of oxygen in the penumbra area.
Figure 3.
Yellow bars show excessive lactate production after 60 min ischemia in the necrotic zone of neurons with still surviving astrocytes. Green bars show minor changes in the penumbra regarding lactate.
Penumbra water
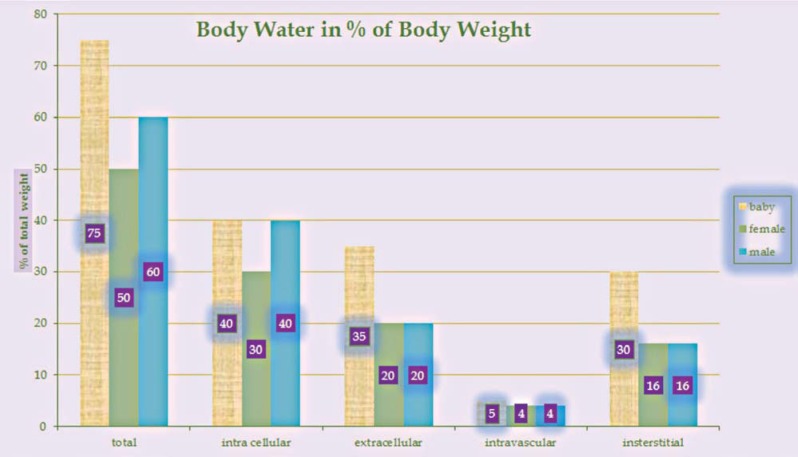
Researchers and clinicians consider penumbra edema a major problem. Still some clinicians infuse hyperbaric glucose or mannitol solutions to get rid of the water. If we look at the distribution of body water (Figure 4) we find that in males 40% of total body water is located intracellularly7. From here we can assume that the edema water mainly comes from the depolarized cells and not out of the vasa. We have good reason to assume so, since the BBB is widely intact, most of the water is stored (40%) inside cells, only 16% reside in the intercellular space, and edema occurs even after total occlusion of the MCA. So the conclusion is: in the first period after ischemia the edema water comes from the affected cells. Later, after the end of the ap-optotic pathways, the BBB will break down and then the edema becomes vasogenic. This view is shared by publications telling us that 'When vascular occlusion occurs, edema initially results in part from shifts in ions between the intracellular and extracellular compartments; this change leads to an accumulation of excess water within astrocytes and other cells, a change that in turn leads to swelling'8. On the other hand, we know by the function of aquapor-ins that these channels transport water without allowing protons to switch membrane side, here in the form of H3O+, and to pass their channel. In this way aquaporins do not disturb the membrane polarisation. The unsolved question is whether they are held in check by phosphorylation like the ATP-dependent K+ channel. In case of depolarization they would shed the water into the interstitium producing the penumbra. Otherwise the different water distribution in concert with the osmotic pressure of proteins would alter the normal arrangement9,10. By removing the edema water early after ischemic stroke physicians close the door to revitalizing the stunned astrocytes, glial cells, and neurons.
Figure 4.
Focusing on blue bars (male), 40% of the total body water is located intracellularly.
Timetable of ischemic stroke
Back to the beginning of ischemic stroke: in most cases only a small artery or some capillaries are involved. Occlusion of supplying arteries like the MCA leads to death. The penumbra emerges as described above and is still perfused by mainly intact capillaries of the BBB. Only the small spot of first impact does not show any blood flow. The penumbra expands until it comes to the point that the cells are able to supply themselves with enough ATP to run their ion channels to maintain their membrane potential. This struggle creates the outer borderline of the penumbra. In the penumbra area the BBB is still intact during the time of apoptosis and glucose and oxygen is delivered but not taken up by the cell due to depolarization of the cell membranes and the stunned metabolism. Only the small obstructed vessel shows no perfusion signs. To restart the metabolism of the stunned cells ATP is needed. Glycolysis starts with the input of two mol ATP (the first to create glucose-6-phosphate and the second to create fructose-1.6-diphosphate), and exactly these two mol of ATP per mol glucose are missing in the penumbra to kick start metabolism (Figure 5).
Figure 5.
Successive steps in ATP production. See text for explanation.
Conventional mainstream medical treatment
Let us now look at conventional treatment for brain strokes. Guidelines limit the use of rtPA to four hours after onset of brain stroke.
Approximately 7% of the victims reach a clinic within this therapeutic window. Even if the rtPA application reopens the occluded vessel the situation is unchanged. The latest publication records a clinical trial with rtPA and rtPA in combination with endovascular therapy11. The first trial was stopped because there was no advantage of the combined therapy. Another paper published in the same edition of NEJM demonstrated no advantage of endovascular therapy over rtPA treatment12. Depolarized cells in the penumbra are supplied by glucose and oxygen even without treatment or ambitions to reopen the single occluded vessel. But in the wake of depolarization they cannot start using the supply. Inflammation has started in the zone behind the occlusion and in the penumbra. The cellular environment becomes increasingly acidic with activation of metallo-proteases which in turn will use the newly incoming oxygen supply to create radicals after successful re-opening of the vessel. These radicals extend the till then limited tissue damage (undergoing apoptosis) into a larger area of necrotic debris or inflict bleeding resulting in a much more extended scarring. This phenomenon is well-documented and known as reperfusion injury. The therapeutic window of four hours tries to limit the damage caused by reperfusion injury. But this treatment does not restart the metabolism in the penumbra. Warnings have been published by the American Society of Intensive Medicine not to use this method at all1. Only a small percentage of patients treated with rtPA because of the therapeutic window seem to justify this treatment. And the evidence of induction of bleeding should be kept in mind. The end is that in most conventionally treated cases the area of first impact and the penumbra undergo scarring leading to extended loss of function and disability.
Basis for explanation of ozone therapy
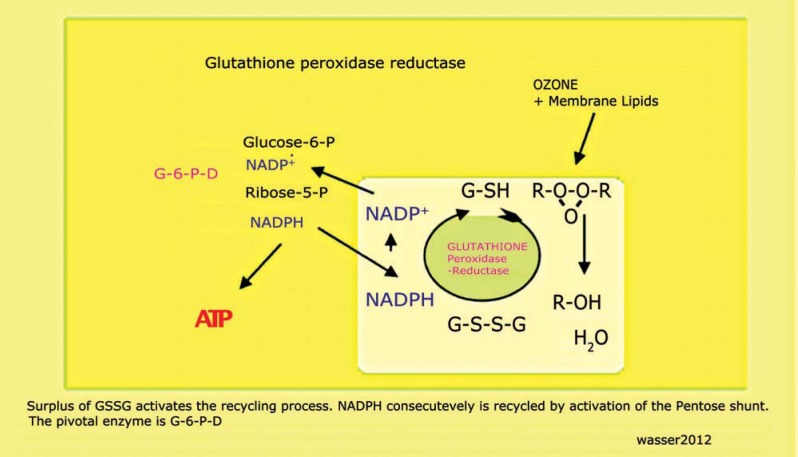
Ozone is simply a small molecule which has only one intention: the oxidation of organic chemical double bonds. Ozone gas cannot be produced enriched to a hundred percent with ozone. It always contains oxygen, normally in the range of up to 97%. Therefore the gas should not be directly injected into the veins or arteries since it will disappear in ms encountering the first cell membrane. Instead we use major auto-hemo-therapy (MAHT) to induce intracellular ATP stocks and use mainly the RBCs as transporters for ATP. RBCs supply and maintain the serum with a threshold of 1 μxmol/ml blood signal undisturbed metabolism13. This ability of RBCs to disperse ATP at the area of need is used for therapy.
Here the unsaturated fatty acids — mostly oleic acids — of the membrane phospholipids will react in ms with the ozone molecule to build ozonides. The presence of protecting enzymes inside and outside the cell dismantles the ozonides (= peroxides) into compounds no longer threatening to damage cellular structures. One of the main enzymes is glutathione peroxidase-reductase, a member of the glutathione peroxidase family, containing a selenium molecule inside the active center. The glutathione peroxidase-reductase defuses ozonides with glutathione as the substrate and proton donor into water, alcohols, and other compounds. In this reaction the reduced glutathione is transformed into the oxidized form. Since there are only limited amounts of glutathione per cell available — most of the compound is found in RBCs7 — the oxidized form must be regenerated to gain reduced glutathione to start or to be ready for the next round of detoxification.
By means of NADPH as proton donator, reduced glutathione is recycled. NADP+, the leftover of this reaction, changes the redox potential of the cell and ignites another round of regeneration. NADP+ is transformed into NADPH in the first two steps of the pentose shunt. The limiting enzyme is glucose-6-phosphate-dehy-drogenase (G-6-PD) which starts the pentose shunt. A lack of this enzyme more frequently found in Asia than in Europe disables the detoxification of radicals by glutathione peroxidase-reductase leading to hemolysis in the bottle or blood bag in case ozone gas is used. Of course there is no absolute G-6-PD deficiency since this would have devastating effects on all cellular functions.
RBCs control Yin and Yang
RBCs filled with ATP travel through the capillaries of the BBB sensing the low ATP content (< 1 μmol/ml full blood) and start giving away their ATP13 which is instantly used by the stunned cells. All of a sudden there is energy available to create glucose-6-phosphate and fructose-l, 6-diphosphate to restart glycolysis. With the first pyruvate available and the ion channels running in the cell membrane, re-polarisation of the double layer occurs, and ATP is generated by the ETC which did not lack oxygen but pyruvate or acetyl-CoA in the penumbra area.
ATP and effect on penumbra
The earlier the penumbra is resupplied with ATP, the more visible is the instant effect on the clinical picture. In the first hours after stroke onset one can see the paralysis resolving. And the effect lasts with no blowback. The edema shrinks and only the area of first impact, the area of necrosis and debris from decaying neurons will be visible in MRI as a minor scar (Figure 6D).
Figure 6.
A) The penumbra is still perfused as the BBB is still intact. B) The extent of scarring in conventional treatment. C) Shrinking penumbra after ATP delivery. D) Spot of scarring, identical to the first impact and necrosis after ozone therapy.
Outcome in a pilot study
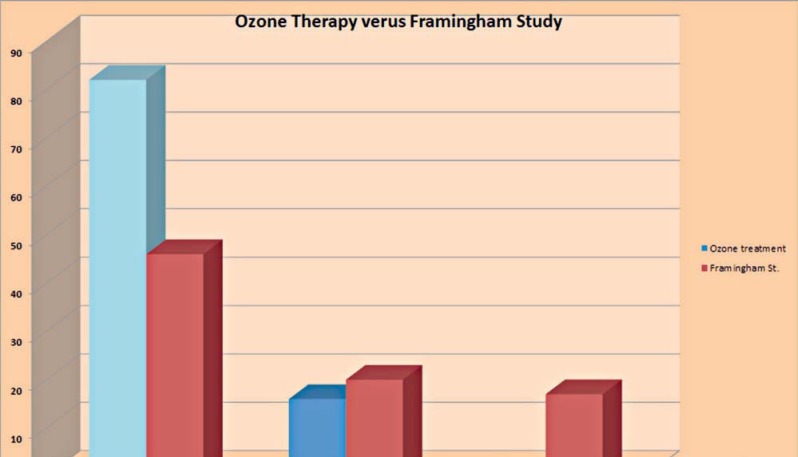
About 15 years ago I published 45 cases of ischemic brain strokes treated with extracorporeal administered ozone gas to full blood14. The outcome was judged by three stages: 1) full recovery, 2) disabled with loss of fine motor function, but able to control daily life, 3) fully disabled (Figure 7).
Figure 7.
Stage 1 after stroke, full recovery. Stage II, disabled but managing daily life activities. Stage III totally disabled and immobilized.
The Framingham Study shows over decades an outcome of 17% of the patients in stage 3 with no signs of decline even after the introduction of rtPA therapy. In the pilot study there was no patient enlisted in the stage 3 group. I am well aware that we cannot compare 45 patients with the high numbers of the Framing-ham Study, but if we can reduce the outcome in stage 3 by 5-10%, this would be a major step forward for the stroke victims to live a decent life after ischemic stroke.
Therapeutic methodology with MAHT
Ozone concentration and frequency of treatment: since ozone is not a medication with a dosage/efficacy ratio we must try to produce as much ATP inside cells, here RBCs, as possible to influence the perifocal edema via ATP delivery by RBCs. According to the SFDA in Beijing the highest concentration of ozone in oxygen gas is limited to 60 $$μmg/ml to prevent the overproduction of lysophospholipids which would interrupt membrane integrity. The effect would be hemolysis of blood cells or the necrosis of other cells. The application of MAHT is recommended with 60 $$μmg/ml (equivalent to 3% ozone in 97 % oxygen) in 50 ml of blood. The surplus of oxygen will stay in the supernatant and is not used for therapeutic intervention. This lack of oxygen use prevents reperfusion injury. The often used term oxygen-ozone therapy is therefore the wrong expression and does not match the biochemical reactions. In principle and with a look at the pathophysiology of the penumbra development one extracorporeal ozone treatment is sufficient. But since ischemic stroke patients are multimorbid six to ten treatments, once on the following days, are recommended.
Conclusion
Because of the described rationale, we believe that using the physiological potential of RBCs to supply suffering tissues with ATP we can influence the outcome after ischemic stroke. The therapeutic window is extended to up to 48 hours until irreversible damage occurs in the sense of extended scarring. This method is safe and without side-effects since we use only the glycolysis to induce higher ATP levels in RBCs. Of course a large randomized study is advisable to better evaluate this therapeutic proposal.
Acknowledgements
The author and Marco Leonardi, NRJ editor, duly acknowledge the great help of numerous Chinese hospitals, only to name the Liao Ning Stroke Treatment Centre, Sujiatun District, Shenyang, and ANSCHAN CHAND DA Hospital who showed us the largest number of cases treated with the techniques here described.
We also thank Luo Xiaoguang, Neurology Department, First Affiliated Hospital of China Medical University, director He Zhiyi, for the initiation of a controlled, double blinded clinical trial. And our thanks go to Pei Qi, who made all the necessary contacts.
References
- 1.Alexandrov AV Schellinger PD Saqqur M et al. Reperfusion and outcomes in penumbra vs. systemic tissue plasminogen activator clinical trials. Int J Stroke. 2011; 6 (2): 118–122. [DOI] [PubMed] [Google Scholar]
- 2.Magistretti PJ Pellerin L Rothman DL et al. Energy on demand. Science. 1999; 283 (5401): 496–497. [DOI] [PubMed] [Google Scholar]
- 3.Pellerin L Magistretti PJ. Neuroscience. Let there be (NADH) light. Science. 2004; 305 (5680): 50–52. [DOI] [PubMed] [Google Scholar]
- 4.Nimmerjahn A Kirchhoff F Helmchen F et al. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005; 308 (5726): 1314–1318. [DOI] [PubMed] [Google Scholar]
- 5.Welsh FA. Regional expression of immediate-early genes and heat-shock genes after cerebral ischemia. Ann N Y Acad Sci. 1994; 723: 318–327. [PubMed] [Google Scholar]
- 6.Kushnareva Y Newmeyer DD. Bioenergetics and cell death. Ann N Y Acad Sci. 2010; 1201: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wissenschaftliche Tabellen - Documenta Geigy. 7. Auflage. Diem K Lentner C eds. Stuttgart: Georg Thieme Verlag; 1975. [Google Scholar]
- 8.Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005; 352 (17): 1791–1798. [DOI] [PubMed] [Google Scholar]
- 9.Berendsen HJ. Bioinformatics. Reality simulation-Observe while it happens. Science. 2001; 2304–2305. [DOI] [PubMed]
- 10.de Groot BL Grubmüller H. Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF. Science. 2001; 294 (5550): 2353–2357. [DOI] [PubMed] [Google Scholar]
- 11.Broderick JP Palesch YY Demchuk AM et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013; 368 (10): 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciccone A Valvassori L Nichelatti M et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013; 368 (10): 904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rapaport E Fontaine J. Anticancer activities of adenine nucleotides in mice are mediated through expansion of erythrocyte ATP pools. Proc Natl Acad Sci U S A. 1989; 86 (5): 1662–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wasser GH. Zerebrale Durchblutungsstörungen. In: Ozon-Handbuch. Grundlagen. Prävention. Therapie. Beck EG Viebahn-Hänsler R, eds. Landsberg: Ecomed: 1955. p. V-6.3 1-V-6.3 12. [Google Scholar]