Abstract
The cognitive dysmetria theory suggests a disconnectivity between the dorsolateral prefrontal cortex, thalami and vermis to explain the pathophysiology of schizophrenia. This study investigated the metabolic integrity of this neurologic circuit in patients with schizophrenia using proton magnetic resonance spectroscopy (H-MRS). Twenty-two patients with schizophrenia and twelve control subjects were studied. Metabolites concentrations were evaluated by a single-voxel technique in the prefrontal cortex, thalami and vermis. To our knowledge, this is the first H-MRS experience with concomitant evaluation of these regions in schizophrenic patients. We found no significant statistical difference in N-AA, Cho and Cr absolute concentrations and N-AA/Cho, N-AA/Cr and Cho/Cr ratios between the schizophrenic patients and control group. At the vermis, we found a constant spectrum with low levels of N-AA and higher levels of Cho and Cr. Our experience does not clearly support or refute the cognitive dysmetria theory. The consistency of metabolic findings in the cerebellar vermis could represent an important datum, highlighting the specificity of metabolic and functional activity in this region.
Keywords: schizophrenia, magnetic resonance imaging, proton magnetic resonance spectroscopy, cognitive dysmetria, cerebellum
Introduction
In schizophrenia, an alteration of the “cortico-cerebellar-thalamic-cortical circuit” (CCTCC) could be responsible for the failure of those neurological processes which allow reception, use and expression of information and experience. The lack of coordination of these mental processes has been called “cognitive dysmetria”1. Andreasen et al. developed this pathogenetic model suggesting a connection between the left dorsolateral prefrontal cortex, thalami and cerebellar vermis1-3. In particular, she evaluated the correlation of regional cerebral blood flow (r-CBF) by positron emission tomography (PET) with anatomical magnetic resonance (MRI) in patients with schizophrenia and control groups13. The aim of our study was to investigate the regional concentrations of the main cerebral metabolites in schizophrenic patients across the prefrontal cortex, thalami and cerebellar vermis using proton magnetic resonance spectroscopy (1H-MRS).1H-MRS provides a non-invasive measure of the biochemistry of the brain and detects chemicals containing hydrogen: N-acetylaspartate (NAA), choline-containing compounds (Cho) and Creatine (Cr).
Materials and Methods
Subjects
Twenty-two patients of both sexes (mean age: 37.4 years; median age: 35.5 years) with a diagnosis of schizophrenia after a structured clinical interview for DSM-IV Axis I Disorders (SCID) and a 12 subject volunteer control group (sex and age-matched) were prospectively studied. All subjects were right-handed. All the schizophrenic patients received stabilized doses of atypical antipsychotic medication. The mean number of years of illness was seven. None of the patients with schizophrenia had a history of head trauma, neurological, major mood or anxiety disorders, alcohol or substance abuse. None of the control subjects had a history of substantial medical illness, head trauma, psychiatric disorders, alcohol or substance abuse. All the control subjects received a structural clinical interview for DSM-III-R-Non-Patient Edition by experienced interviewers to exclude any psychiatric disorders. Written informed consent was obtained from all patients and control subjects.
Clinical Assessment
All patients met DSM-IV TR criteria for schizophrenia diagnosis and the psychopa-thology was assessed by PANSS (positive and negative symptoms scale). This scale was used to summarize psychopathology in three dimensions: negative, positive and disorganized. Clinical assessment, MR imaging and H-MR spectroscopy examinations were performed within the same week.
Cranial MR Imaging
MRI and H-MRS were performed on a Siemens 1.5-T Magnetom Vision imaging system using a standard quadrature head coil. Brain MR images were acquired to identify any cerebral pathology defined in the exclusion criteria and to position the MR spectroscopy volume. Conventional MRI included: 1) axial Fast spin-echo (FSE) T2-weighted images (TR= 3800 ms, TE=98 ms, NEX=2, echo-train length=11, ma-trix=190×256, section thickness= 5 mm); 2) sagittal 3D gradient-echo (mpr-GE) T1-weighted images (TR=9.7 ms, TE=4 ms, FA=12, matrix= 200×256, slab thickness= 160 mm, eff.thick-ness=1.25 mm, number partitions=128,); 3) Half-Fourier single shot fast SE (HASTE) images on three-spatial planes (TR=10.9 ms, TE= 87 ms, FA 154, matrix=240×256, thickness= 5 mm).
H-MR Spectroscopy
Single-voxel 1H-MR spectroscopy examinations were conducted in the same session with conventional cranial MR imaging. For1H-MRS, a single-voxel SE sequence (TR=1365 ms, TE=135 ms) was used. It is a double-echo sequence with excitation of three orthogonal slices (one 90° pulse, two 180° pulses). Water suppression was performed via Gaussian pulse by a frequency-selective 90° pulse which flips the longitudinal magnetization of water in the xy plane before the excitation pulse. The1H-MRS voxel size was 4 ml3. Voxel placement was performed by the same neuroradiologist.
Left dorsolateral prefrontal cortex, both tha-lami and cerebellar vermis were studied (Figure 1). In all subjects, post-processing of the raw1H-MRSI data was performed as described previously4. Resonance intensity values of metabolites were assessed using a combination of Xunspecl software (Philips Medical Systems, Andover, MA, USA) and free software developed at the Montreal Neurological Institute. Concentrations of Cho (3.21-3.23 ppm frequency), Cr (3.01-3.03 ppm) and N-AA (2.01-2.03 ppm) were evaluated.
Figure 1.
Axial, coronal and sagittal HASTE images showing the MR spectroscopy voxel placement.
N-AA/Cho, N-AA/Cr and Cho/Cr ratios were calculated. Study-specific phantom tests testing the reproducibility of1H-MR spectroscopy measurements were performed every week. We used a spherical phantom containing 0.1 molar solution consisting of lithium lactate and sodium acetate.
Statistical Analysis
A one-way multivariate analysis of covari-ance was performed to investigate the different concentrations for each metabolite (N-AA, Cho, Cr) in patients with schizophrenia and in the control group. Three dependent variables were used: N-AA/Cho, N-AA/Cr and Cho/Cr: group was the independent variable, age the covariate. Preliminary assumption testing was conducted to check for normality, linearity, univariate and multivariate outliers, homogeneity of variance-covariance matrices and mul-ticollinearity, with no serious violations noted. For univariate analysis, a Bonferroni adjusted alpha level of .017 was applied.
Results
Conventional MRI examination showed no brain abnormalities in either the schizophrenic or control group.
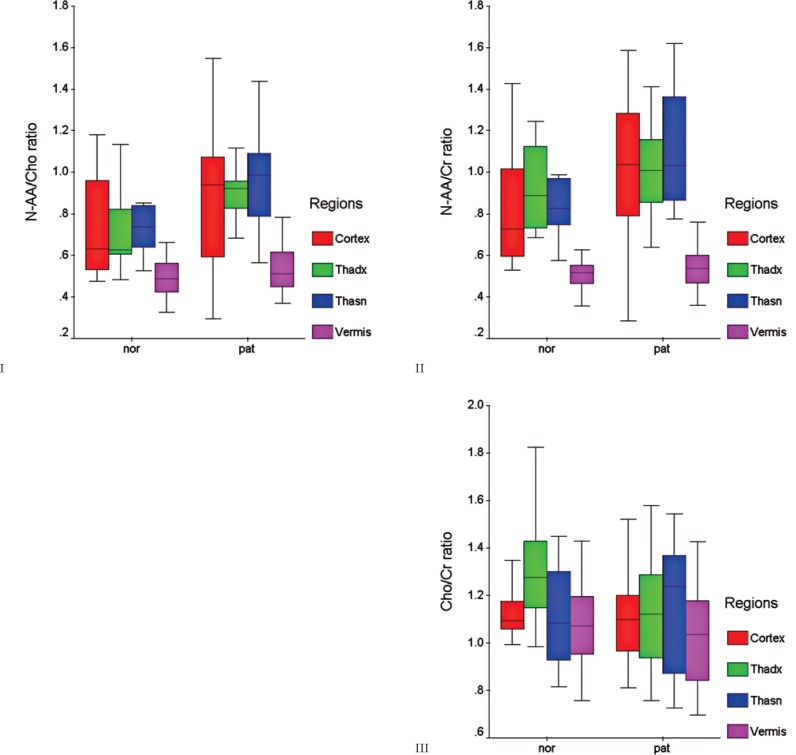
1H-MRS showed a reduced NAA concentration in 14/22 schizophrenic patients (63.63%) in the left dorsolateral prefrontal cortex, in 17/22 (77.2%) in the right thalamus and in 11/22 (50%) in the left thalamus. In the control group, NAA peak reduction was demonstrated in 9/12 subjects (75%) in the left dorsolateral prefrontal cortex and right thalamus. In the left thalamus, NAA concentration was reduced in 10/12 subjects (83.3%). In the cerebellar vermis, we found a consistent spectrum with low levels of NAA and higher concentrations of both Cho and Cr, with no significant difference between schizophrenic and normal groups (Figure 2). There was no significant statistical difference in NAA/Cho, NAA/Cr and Cho/Cr ratios between the pathologic and control groups (Table 1- Figure 3: charts I-II-III).
Figure 2.

Proton MR spectrum from a cerebellar vermis voxel: 1) N-AA peak at 2.01-2.03 ppm frequency. 2) Cr peak at 3.01-3.03 ppm frequency. 3) Cho peak at 3.21-3.23 ppm frequency.
Table 1.
Metabolite concentration mean ratios in four cerebral regions of the patients and control subjects.
| Metabolite concentration mean ratios (± SD) | |||
|---|---|---|---|
| Region | Cho/Cr | N-AA/Cr | N-AA/Cho |
| Right Thalami | |||
| Patients (n = 22) | 1.14 ±0.21 | 1.01 ± 0.21 | 0.91 ± 0.19 |
| Controls (n = 12) | 1.32 ± 0.27 | 0.93 ± 0.20 | 0.72 ± 0.20 |
| Significance (uv) | p = 0.049 | p = 0.353 | p = 0.022 |
| Significance (mv) | p = 0.050 | ||
| Left Talami | |||
| Patients (n = 22) | 1.16 ± 0.27 | 1.10 ±0.26 | 0.97 ± 0.24 |
| Controls (n = 12) | 1.12 ± 0.21 | 0.91 ±0.27 | 0.85 ± 0.35 |
| Significance (uv) | p = 0.237 | p = 0.040 | p = 0.382 |
| Significance (mv) | p = 0.159 | ||
| Prefrontal Cortex | |||
| Patients (n = 22) | 1.11 ±0.20 | 1.00 ±0.34 | 0.91 ± 0.32 |
| Controls (n = 12) | 1.12 ± 0.11 | 0.82 ± 0.32 | 0.73 ± 0.27 |
| Significance (uv) | p = 0.540 | p = 0.284 | p = 0.187 |
| Significance (mv) | p = 0.610 | ||
| Cerebellar Vermis | |||
| Patients (n = 22) | 1.04 ±0.23 | 0.55 ±0.12 | 0.54 ± 0.12 |
| Controls (n = 12) | 1.07 ± 0.20 | 0.50 ±0.07 | 0.49 ± 0.10 |
| Significance (uv) | p = 0.955 | p = 0.311 | p = 0.369 |
| Significance (mv) | p = 0.701 | ||
uv: univariate — mv: multivariate
Figure 3.
Charts I-II-III. The box-plot graphs demonstrate the distribution of N-AA/Cho, N-AA/Cr and Cho/Cr ratios around the median values of control subjects (nor) and patients (pat).
With regards to the clinical assessment, there was a significant correlation between metabolic ratios and different psychopatho-logical dimensions of schizophrenia assessed by PANSS. Specifically, the NAA/Cho ratio was significantly lower in the right thalamus (p=0.006) and left thalamus (p=0.007) in the positive dimension of schizophrenia. In the dorsolateral frontal cortex, both NAA/Cho (p=0.001) and NAA/Cr (p=0.003) ratios were lower in the positive dimension group.
Discussion
Previous1H-MRS studies showed conflicting results regarding changes in brain metabolites, such as N-acetylaspartate (NAA), in structures involved in schizophrenia5-15. Several authors reported a decrease in NAA in some cerebral regions, like the thalamus, frontal lobes, hippocampus or corpus callosum in schizophrenic patients5-14. A reduction in NAA concentration is thought to represent a loss of neurons and/or axons, as well as neuronal or axonal dysfunction or damage. Other studies to date have failed to detect any difference in metabolic concentrations between schizophrenic and control subjects7,12,15. Furthermore, only a few articles focused on the role of the cerebellar vermis7,16,17.
The specificity of our experience is the concomitant in vivo evaluation by single voxel1H-MRS of the following four regions: left dorsolateral prefrontal cortex, both thalami and cerebellar vermis. The present study explored the neural circuitry according the pathogenetic hypothesis of cognitive dysmetria, investigated by Andreasen et al.2. Their investigation was based on PET functional studies integrated with morphological MM showing, in schizophrenic patients, r-CBF abnormalities in cortical, cerebellar and thalamic regions. According to these results, the cognitive dysmetria theory was proposed to explain the pathophysiology of schizophrenia. A cortico-cerebellar-thalamic-cortical circuit (CCTCC) alteration was suggested1-3.
The main finding in our study is that there was no overall difference in NAA/Cho, NAA/ Cr and Cho/Cr ratios between the patients and control group in the explored regions mentioned above. In particular, we noted a statistically significant interindividual variability of the main brain metabolite peaks in the supraten-torial regions (frontal cortex and thalami), in contrast to a consistent spectrum in the cerebellar vermis. In all subjects, we obtained a cer-ebellar spectrum featuring higher Cho and Cr levels compared to lower NAA concentration.
This evidence of a relative cerebellar NAA decrease is well known18-20. However, the consistency of the finding could be an important datum in our series, highlighting the specificity of metabolic and functional activity of the cerebellum. In particular, the consistent spectrum obtained at the cerebellar vermis could reflect the absolute metabolic stability of this region.
The lack of global significance in our experience between the schizophrenic patients and the control group is consistent with literature data reporting normal1H-MRS spectral analysis in schizophrenia. However, we noted a statistically significant correlation between metabolic ratios and different psychopathological dimensions of schizophrenia. In the positive dimension group, the NAA/Cho ratio was significantly lower in both thalamic regions. Moreover, in this group of schizophrenic patients, both NAA/Cho and NAA/Cr ratios were lower in the dorsolateral frontal cortex. These findings could suggest a more serious involvement of both thalami and prefrontal cortex in the pathogenesis of positive symptoms of schizophrenia.
A possible limitation of our findings is the technical acquisition of spectral data. Spectroscopic voxels typically contain varying mixtures of gray and white matter (partial volume effect), which often complicates interpretation of the metabolic changes. Furthermore, with regard to technical precision, we used the absolute consistency of cerebellar spectral data as a further quality control of our acquisition system.
Another possible limitation of our investigation is the medical therapy. All patients in our sample received stabilized doses of atypical neuroleptics and this might have resulted in changes in locoregional metabolism. Nevertheless, some authors reported there was no relationship between the metabolites, duration of illness and antipsychotic treatment15. More recently, however, other reports demonstrated a relationship between antipsychotic treatment and H-MRS metabolite value. Szulk et al.21 observed a significantly decreased Glx/Cr ratio in the temporal lobe and a trend for an increase in the NAA/Cr ratio in the thalamus in a schizophrenic population after antipsychotic treatment, studied with H-MRS with short echo-time.
H-MRS with short echo-time, especially using high field (3-4T) MR equipment has the advantage of measuring other metabolites like glutathione (GSH), glutamine (Glx), GABA and glutamate (Glu), likely to be involved in the pathogenesis of schizophrenia. Many experiences reported a decrease of Glu concentration in the medial frontal region and an increase in Glx and GABA concentrations in patients with schizophrenia compared with healthy individuals22-24. Interestingly, both Glu and Glx levels in the frontal region decrease progressively with age in patients with schizophrenia, which could suggest a progressive loss of synaptic activity22.
In conclusion, the findings of our study neither support nor refute the cognitive dysmetria theory. The connectivity between frontal cortex, thalamus and cerebellum could be better investigated using other techniques. Recently, for example, diffusion tensor tractography with MRI was used to evaluate the white matter connectivity between cerebellum and thalamus in a group of schizophrenic patients and in a control group25,26.
References
- 1.Crespo-Facorro B Paradiso S Andreasen NC et al. Recalling word lists reveals “Cognitive Dysmetria” in schizophrenia: a positron emission tomography study. Am J Psychiatry. 1999; 156 (3): 386–392. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen NC O'Leary DS Cizadlo T et al. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Psychology. 1996; 93: 9985–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JJ Mohamed S Andreasen NC et al. Regional neural dysfunctions in chronic schizophrenia studied with positron emission tomography. Am J Psychiatry. 2000; 157 (4): 542–548. [DOI] [PubMed] [Google Scholar]
- 4.Marino S Zei E Battaglini N et al. Acute metabolic brain changes following traumatic brain injury and their relevance to clinical severity and outcome. J Neurol Neursurg Psychiatry. 2007; 78: 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasrallah HA Skinner TE Schmalbrock P et al. Proton magnetic resonance spectroscopy (1H MRS) of the hippocampal formation in schizophrenia: a pilot study. Br J Psychiatry. 1994; 165: 481–485. [DOI] [PubMed] [Google Scholar]
- 6.Deicken RF Zhou L Corwin F et al. Decreased left frontal lobe N-acetylaspartate in schizophrenia. Am J Psychiatry. 1997; 154 (5): 688–690. [DOI] [PubMed] [Google Scholar]
- 7.Omori M Pearce J Komoroski RA et al. In vitro 1H-magnetic resonance spectroscopy of postmortem brains with schizophrenia. Biol Psychiatry. 1997; 42 (5): 359–366. [DOI] [PubMed] [Google Scholar]
- 8.Bertolino A Callicott JH Elman I et al. Regionally specific neuronal pathology in untreatedpatients with schizophrenia: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry. 1998; 43 (9): 641–648. [DOI] [PubMed] [Google Scholar]
- 9.Omori M Murata T Kimura H et al. Thalamic abnormalities in patients with schizophrenia revealed by proton magnetic resonance spectroscopy. Psychiatry Res. 2000; 98 (3): 155–162. [DOI] [PubMed] [Google Scholar]
- 10.Deicken RF Johnson C Pegues M. Proton magnetic resonance spectroscopy of the human brain in schizophrenia. Rev Neurosci. 2000; 11 (2–3): 147–158. [DOI] [PubMed] [Google Scholar]
- 11.Wood SJ Berger G Velakoulis D et al. Proton Magnetic Resonance Spectroscopy in first episode psychosis and ultra high-risk individuals. Schizophr Bull (Bp). 2003; 29 (4): 831–843. [DOI] [PubMed] [Google Scholar]
- 12.Sigmundsson T Maier M Toone BK et al. Frontal lobe N-acetylaspartate correlates with psychopathology in schizophrenia: a proton magnetic resonance spectroscopy study. Schizophr Res. 2003; 64 (1): 63–71. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y Obata T Sassa T et al. Quantitative magnetic resonance spectroscopy of schizophrenia: relationship between decreased N-acetylaspartate and frontal lobe dysfunction. Psychiatry Clin Neurosci. 2006; 60: 365–372. [DOI] [PubMed] [Google Scholar]
- 14.Aydin K Ucok A Cakir S Quantitative proton MR spectroscopy findings in the corpus callosum of patients with schizophrenia suggest callosal disconnection. Am J Neuroradiol. 2007. 28: 1968–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delammillieure P Constans JM Fernandez J et al. Proton Magnetic Resonance Spectroscopy (1H-MRS) in schizophrenia: investigation of the right and left hippocampus, thalamus, and prefrontal cortex. Schizophr Bull (Bp). 2002; 28 (2): 329–339. [DOI] [PubMed] [Google Scholar]
- 16.Deicken RF Feiwell R Schuff N et al. Evidence for altered cerebellar vermis neuronal integrity in schizophrenia. Psychiatry Res. 2001; 107: 125–134. [DOI] [PubMed] [Google Scholar]
- 17.Ende G Hubrich P Walter S et al. Further evidence for altered cerebellar neuronal integrity in schizophrenia. Am J Psychiatry. 2005; 162 (4): 790–792. [DOI] [PubMed] [Google Scholar]
- 18.Costa MO Lacerda MT Garcia Otaduy MC et al. Proton magnetic resonance spectroscopy: normal findings in the cerebellar hemisphere in childhood. Pediatr Radiol. 2002; 32 (11): 787–792. [DOI] [PubMed] [Google Scholar]
- 19.Aragao F Mourao M Mendonça R et al. Neurospectroscopy - A pictorial essay and review of how to obtain and intepret MR spectroscopy. In: Proceedings 39th Annual Meeting. Boston: American Society of Neuroradiology; 2002: 484. [Google Scholar]
- 20.Healy MP McKnight T Priest D et al. MR spectroscopic analysis of metabolites in different regions of normal brains. In: Proceedings 39th Annual Meeting. Boston: American Society of Neuroradiology; 2002. [Google Scholar]
- 21.Szulc A Galinska B Tarasow E et al. Proton magnetic resonance spectroscopy study of brain metabolite changes after antipsychotic treatment. Pharmacopsychiatry. 2011; 44 (4): 148–157. [DOI] [PubMed] [Google Scholar]
- 22.Marsman A van den Heuvel MP Klomp DW et al. Glutamate in schizophrenia: a focused review and meta-analysis of (1)H-MRS studies. Schizophr Bull. 2013; 39 (1): 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kegeles LS Mao X Stanford AD et al. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012; 69 (5): 449–459. [DOI] [PubMed] [Google Scholar]
- 24.Klär AA Ballmaier M Leopold K et al. Interaction of hippocampal volume and N-acetylaspartate concentration deficits in schizophrenia: a combined MRI and 1H-MRS study. Neuroimage. 2010; 53 (1): 51–57. [DOI] [PubMed] [Google Scholar]
- 25.Steel RM Bastin ME McConnell S et al. Diffusion tensor imaging (DTI) and proton magnetic resonance spectroscopy (1H MRS) in schizophrenic subjects and normal controls. Psychiatry Res. 2001; 106 (3): 161–170. [DOI] [PubMed] [Google Scholar]
- 26.Magnotta VA Adix ML Caprahan A et al. Investigating connectivity between the cerebellum and thalamus in schizophrenia using diffusion tensor tractography: a pilot study. Psychiatry Res. 2008; 163: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]