Abstract
A 14-year-old female post-transplant patient with a history of post-transplant lymphoproliferative disease/lymphoma presented with fever and lethargy. Computed tomography of the brain demonstrated a hypodense lesion with surrounding edema in the right periventricular region not seen on a routine study performed two weeks earlier. On magnetic resonance imaging (MRI) this lesion was mainly iso-intense to gray matter on T2-weighted (T2W) images and demonstrated peripheral contrast enhancement. Diffusion restriction was seen within most of the lesion including, but not limited to, its periphery. Lesion location and MRI characteristics, particularly on T2W and diffusion sequences, were suggestive of lymphoma. The patient's history of post-transplant lymphoproliferative disorder also supported this diagnosis. However, in view of the patient's immunocompromised state, rapid onset of symptoms, and recent normal CT scan of the brain, infection was also entertained. Biopsy revealed short branching hyphae consistent with aspergillosis. This case is interesting as the MRI restriction pattern and the patient's history were more suggestive of lymphoma, but in reality the lesion represented an evolving aspergillosis abscess. Biopsy was necessary to further proceed with appropriate medical management, which is significantly different for the two entities.
Keywords: CNS aspergillosis, brain abscess, post-transplant lymphoproliferative disease, fungal disease
Introduction
Invasive central nervous system aspergillosis is being seen with increased frequency, particularly with the growing number of immuno-suppressed patients1 In most cases invasive aspergillosis develops in the paranasal sinuses and lungs, and secondarily spreads hematog-enously to the brain1. The hyphae are angioin-vasive and obstruct intracerebral blood vessels leading to infarcts, which are commonly hemorrhagic2. The sterile infarct becomes septic when the fungus penetrates the blood vessel wall into the ischemic brain parenchyma causing a mixed inflammatory reaction and necrosis2. CNS aspergillosis frequently involves the basal nuclei, thalami and splenium of the corpus callosum1, and as a result of the hematogenous spread, aspergillus abscesses often occur at the gray-white matter junction. It is important to note that CNS aspergillosis can occur in many different forms including basilar meningitis, sino-orbital disease, carotid artery invasion, dural abscesses, massive hemorrhagic necrosis, granuloma formation, cerebritis, parenchymal abscesses, bland non-hemorrhagic infarcts, and mycotic aneurysms; of these, the two most common forms on pathology are massive hemorrhagic necrosis and abscess3. Given the multiple forms of aspergillosis, knowledge of various imaging presentations of fungal disease is imperative as diagnosis is essential for prompt initiation of medical therapy.
Case Report
A 14-year-old patient who was status post-intestinal transplant in 2005 secondary to gasto-schisis and necrotizing enterocolitis presented to the emergency department with lethargy, fever, headache, and ear pain. The patient had a history of rejection and post-transplant lymphoproliferative disease (PTLD) that were treated with steroids and infliximab (Remi-cade®). Initial physical examination was non-revealing and laboratory work demonstrated a decreased white blood cell count. In view of the patient's immunosuppressed status and her clinical presentation, there was suspicion for meningitis and a contrast-enhanced CT scan of the brain was ordered.
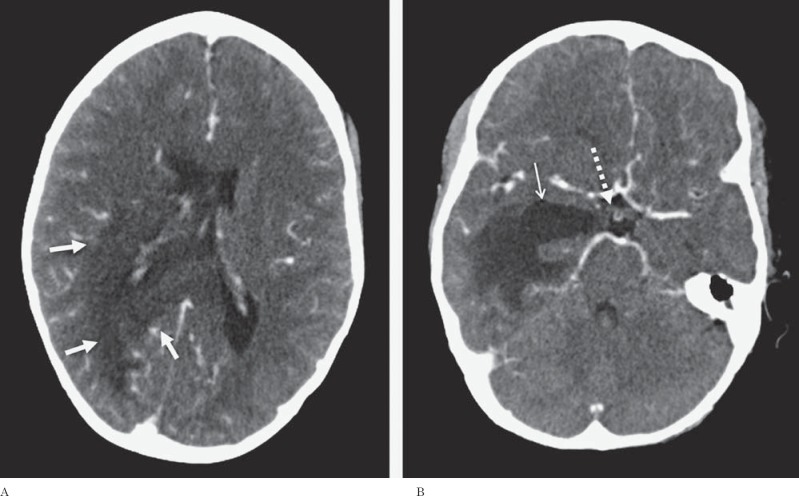
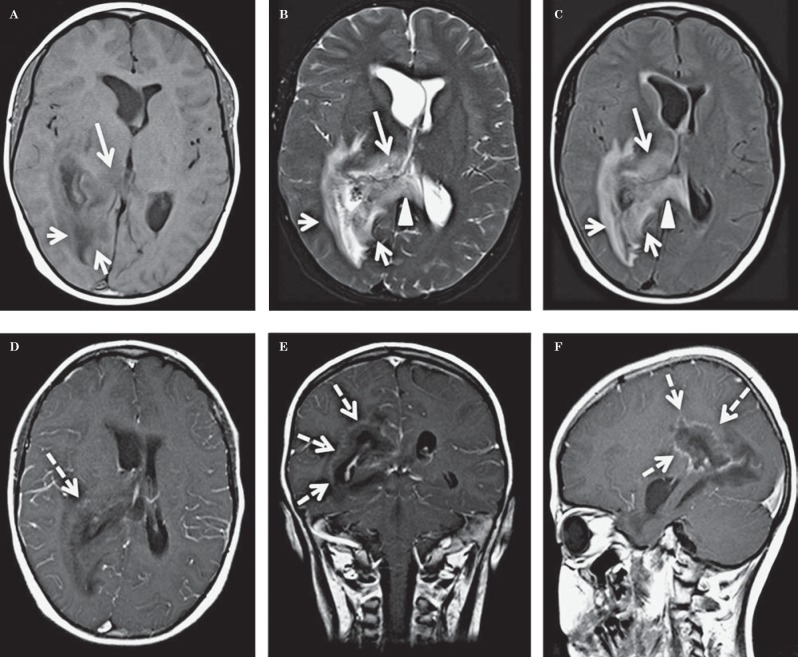
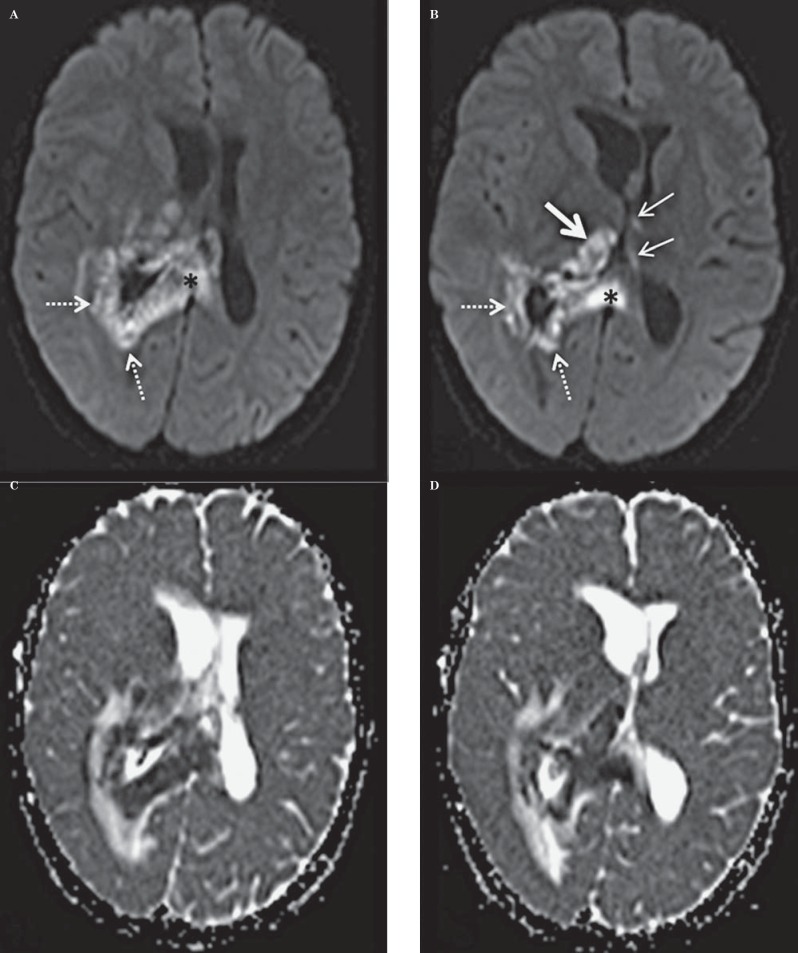
The CT demonstrated a large hypodense area surrounding the atrium and the temporal horn of the right lateral ventricle (Figure 1). This hy-podensity exerted local mass effect and a mild right to left midline shift and uncal herniation (Figure 1). The right lateral ventricle was also dilated. No obvious enhancement was seen on the contrast portion of the examination (Figure 1). A CT scan of the neck (which extended to the level of the lateral ventricles) performed two weeks earlier did not reveal any abnormality through the same level of the brain (Figure 2). A contrast-enhanced MRI of the brain revealed a lesion surrounding the atria of the right lateral ventricle and extending to the splenium of the corpus callosum. An abnormal signal also involved the right thalamus. The lesion was hy-pointense to gray matter on T1-weighted (T1W) images, isointense on T2-weighted (T2W) images, hyperintense on fluid-attenuated inversion recovery sequence (FLAIR) and demonstrated subtle peripheral enhancement on the post-contrast examination (Figure 3). Within the lesion there were focal areas of susceptibility artifacts on the GRE sequence likely representing minimal petechial hemorrhage (Figure 4). The lesion demonstrated an increased signal on diffusion weighted images (DWI) with corresponding decreased signal on the apparent diffusion coefficient (ADC) maps indicative of restricted diffusion (Figure 5). This restricted diffusion involved the bulk of the lesion, including its periphery and extended into the splenium of the corpus callosum and involved the thalami bilaterally right greater than left (Figure 5). The restricted diffusion extended beyond the area of low signal on GRE. Given the MRI findings, the primary differential diagnosis included a neoplastic process such as PTLD/ lymphoma (also supported by the patient's medical history) versus an infectious process (particularly in view of the rapid development of the lesion since the previous negative CT taken two weeks earlier). Imaging of the chest did not show any enlarged adenopathy or focal lesions.
Figure 1.
A 14-year-old post-transplant patient who presented with lethargy, fever, headache and ear pain. Axial contrast-enhanced CT images through the lateral ventricles (A) and uncus (B) demonstrate a large non enhancing hypodense lesion (solid white arrows) surrounding the atria and the temporal horn of the right lateral ventricle extending into the corpus callosum (A). The temporal horn of the right lateral ventricle is dilated (thin solid arrow) and there is uncal herniation (dashed arrow) (B). (Technique: KVp = 120; mAs = 270; slice thickness 3.00 mm; contrast: Optiray 320 total of 50 ml).
Figure 2.
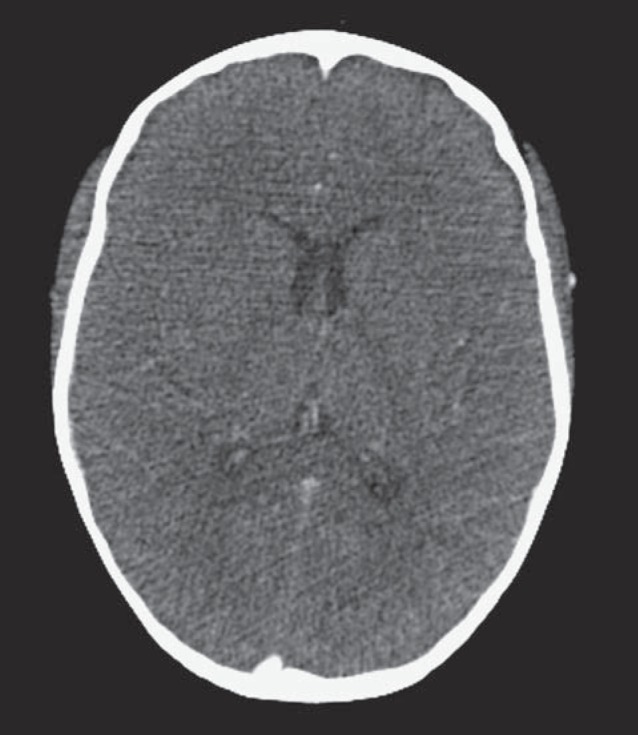
A 14-year-old post-transplant patient who presented with lethargy, fever, headache and ear pain. Axial contrast-enhanced CT scan through the ventricles at the same level as in Figure 1A performed 2.5 weeks prior to presentation did not reveal any abnormality. (Technique: KVp = 100; mAs = 72; slice thickness = 1.50 mm; contrast Optiray 320 total of 46 ml).
Figure 3.
A 14-year-old post-transplant patient who presented with lethargy, fever, headache and ear pain. MR images through the ventricles, at the same level as the CT images in Figure 1A demonstrate a lesion in the right peratrial region (small short arrows A-C) which is hypointense on T1W image, isointense on T2W image, and hyperintense on the FLAIR image. The lesion extends along the splenium of the corpus callosum (arrowheads B, C) and involves the right thalamus (long arrow A-C). Post-contrast images demonstrate subtle peripheral enhancement best seen on the coronal and sagittal images (dashed arrows D-F). A) Axial non-contrast spin echo T1W image [1.5 Tesla (TR/TE = 679 msec./8.4 ms) Slice Thickness = 5.00 mm]. B) Axial non-contrast fast spin echo T2W image [1.5 Tesla (TR/TE = 6130 ms/107 ms) Slice Thickness 5.00 mm]. C) Axial non-contrast FLAIR image [1.5 Tesla (TR/TE = 8000 ms/125 ms) slice thickness 5.00 mm]. D) Axial post-contrast spin echo T1W image [1.5 Tesla (TR/TE = 500 ms/17 ms) slice thickness 5.00 mm, 6 ml gadopentetate dimeglumine, Magnevist]. E) Coronal post-contrast spin echo T1W image [1.5 Tesla (TR/TE = 500 ms/17 ms) slice thickness 5.00 mm, 6 ml gadopentetate dimeglumine, Magnevist]. F) Sagittal post-contrast spin echo T1W image [1.5 Tesla (TR/TE = 608 ms/17 ms) slice thickness 5.00 mm, 6 ml gadopentetate dimeglumine, Magnevist].
Figure 4.
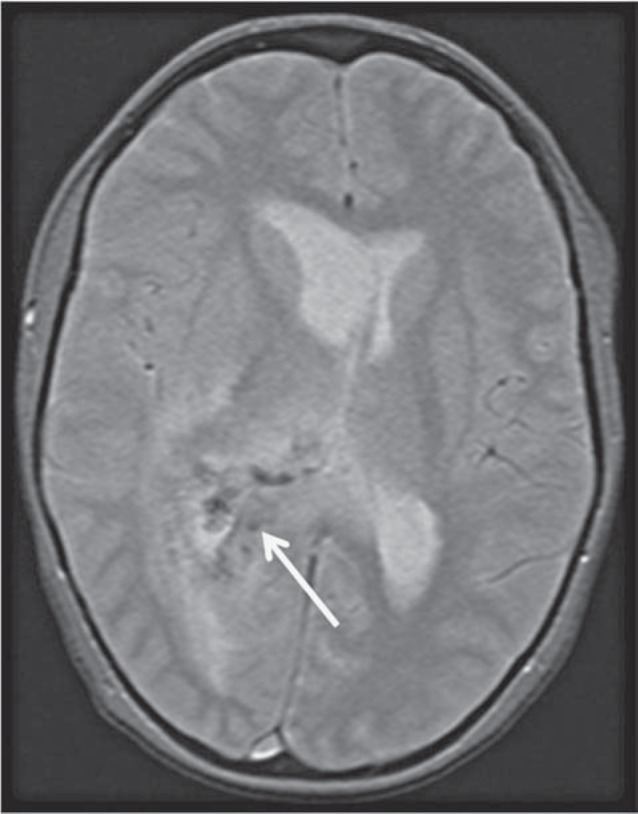
A 14-year-old post-transplant patient who presented with lethargy, fever, headache and ear pain: Axial noncontrast gradient echo T2W images [1.5 Tesla (TR/TE = 500 ms/10 ms) slice thickness 5.00 mm]. The gradient echo sequence demonstrates a susceptibility artifact in the lesion (white arrow).
Figure 5.
A 14-year-old post-transplant patient who presented with lethargy, fever, headache and ear pain. A.B) Axial DWI [1.5 Tesla (TR/TE = 5200 ms/107 ms) Slice thickness 5 mm]. C, D) Axial ADC maps corresponding to the DWI maps above [1.5 Tesla (TR/TE = 5200 ms/107 ms) Slice thickness 5 mm]. MR images through the ventricles, at the same level as the CT images in Figure 1A demonstrate restricted diffusion on DWI in the right periatrial region (dotted arrows A, B) with extension into the splenium of the corpus callosum (black asterisk A, B). Additional areas of restriction are noted in the right thalamus (solid large white arrow B) with much smaller areas in the left thalamus (thin white arrows B). C, D) The corresponding ADC maps demonstrating decreased ADC values.
The patient's symptoms rapidly progressed with development of left hemiparesis and increasing lethargy. She was unable to undergo a lumbar puncture because of the transfalcine and uncal herniation. As the medical management of PTLD and infectious diseases is significantly different, tissue sampling was needed to guide the appropriate therapy. The patient underwent an open biopsy of the right periatrial lesion as well as a right hemicraniec-tomy for brain decompression. Pathology demonstrated tissue characterized predominantly by necrosis with foci of haemorrhage and clusters of neutrophils as well as the presence of vascular thrombi (Figure 6), suggesting that there had been multiple infarcts of perforating vessels secondary to thrombosis. Periodic acid-schiff (PAS), Giemsa (GMS) and silver stains identified short branching hyphae consistent with aspergillosis as the responsible pathogen (Figure 7). Extensive neutrophilic infiltration was not yet present, indicating that an abscess had not yet completely formed. Lymphomatous cells could not be appreciated in the specimen, making lymphoma an unlikely cause. The patient was started on antifungal therapy including Voriconazole and Amphotericin B.
Figure 6.
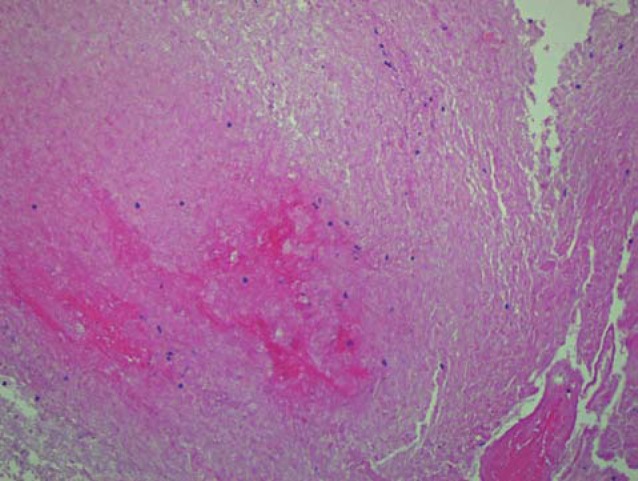
High power hematoxylin and eosin stain obtained on tissue following craniotomy and open biopsy demonstrates extensive brain necrosis and clusters of neutrophils.
Figure 7.
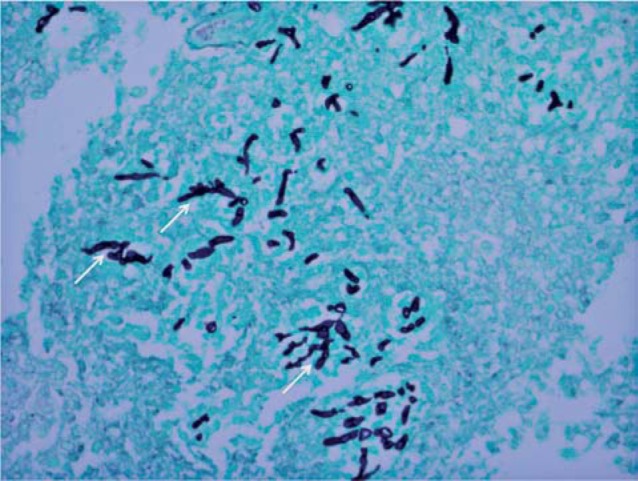
Silver stain (original magnification x 400) obtained on the biopsy specimen demonstrates the typical short branching hyphae seen with aspergillosis (thin white arrows).
Discussion
On MRI, the appearances of CNS aspergillosis may vary depending on the stage or spectrum of disease; while an aspergillosis abscess has certain classic features, the other forms (namely angioinvasion producing necrotizing vasculitis, thrombosis and secondary hemorrhages) may have overlapping features with other entities, particularly CNS lymphoma. A well-formed aspergillosis abscess classically has thick irregular walls with intracavitary projections. The wall and the intracavitary projections demonstrate restricted diffusion, whereas the core of the abscess typically does not restrict4, although a few cases have been reported to demonstrate core restriction5. The abscess is typically iso to low signal, predominantly peripherally, on T2W images consistent with accumulation of fungi containing iron, magnesium, and manganese, as well as blood breakdown products6. The iso to low signal on T2W images is, however, not specific for aspergillosis and may be seen in various infections as well as in neoplastic brain.
Typical imaging findings when aspergil-lus infection consists of necrotizing vasculitis, thrombosis and secondary hemorrhages include multiple focal lesions or a large confluent lesion with hyperintense signal on T2-weighted and FLAIR images with corresponding restricted diffusion on DWI sequences7,8. Hemorrhage is common and can be identified on both CT and susceptibility weighted MR images9. Subtle peripheral enhancement can be detected on post-contrast MRI scans1.
What is unique about our patient is that both the core and much of the periphery demonstrated restricted diffusion, as is classically seen in cerebral lymphoma. These imaging features likely represented a combination of various forms of CNS aspergillosis with infarcts accounting for central restricted diffusion, hemorrhages accounting for the low signal on GRE sequence, and peripheral restriction and low T2W signal suggesting a developing, but not yet completely formed abscess. This is consistent with the pathology report.
The main radiologic and clinical differential consideration in this case was CNS PTLD, which commonly presents with hemorrhage, necrosis and peripheral enhancement10. Most cases manifest as a solitary mass that typically affects the periventricular region10. The lesion is typically hypo or isointense to gray matter on T1W images. On T2W images, the lesion appears hypointense, presumably due to hyper-cellularity, although focal hyperintensity can be seen reflecting areas of necrosis10. There is usually intralesional restricted diffusion, with low signal intensity on the apparent diffusion coefficient map11.
Given that several forms of CNS aspergillosis exist and may be present at once, a constellation of MRI features can exist overlapping with imaging features of PTLD. As these two entities occur in a similar patient population, consideration has to be given to both entities but definite diagnosis may not be possible based on imaging alone. As a result, tissue diagnosis may be necessary, which was the case in this particular patient. CNS aspergillosis has a poor prognosis in immunocompromised patients with high mortality and morbidity12. As such, it is always important to include fungal infections in the differential diagnosis of brain lesions when dealing with transplant patients so that appropriate therapy can be instituted promptly.
Acknowledgements
Michael D. Noremberg, MD for neuropathology consultation; Prodipto Pal, MD for preparing the pathology images.
References
- 1.DeLone DR Goldstein RA Petermann G et al. Disseminated aspergillosis involving the brain: distribution and imaging characteristics. Am J Neuroradiol. 1999; 20: 1597–1604. [PMC free article] [PubMed] [Google Scholar]
- 2.Guermazi A Gluckman E Tabti B et al. Invasive central nervous system aspergillosis in bone marrow transplantation recipients: an overview. Eur Radiol. 2003; 13: 377–388. [DOI] [PubMed] [Google Scholar]
- 3.Kleinschmidt-DeMasters BK. Central nervous system aspergillosis: a 20-year retrospective series. Hum Pathol. 2002; 33 (1): 116–124. [DOI] [PubMed] [Google Scholar]
- 4.Luthra G Parihar A Nath K et al. Comparative evaluation of fungal, tubercular, and pyogenic brain abscesses with conventional and diffusion MR imaging and proton MR spectroscopy. Am J Neuroradiol. 2007: 28: 1332–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaviani P Schwartz RB Hedley-Whyte T et al. Diffusion-weighted imaging of fungal cerebral infection. Am J Neuroradiol. 2005; 26: 1115–1121. [PMC free article] [PubMed] [Google Scholar]
- 6.Yamada K Zoarski GH Rothman MI et al. An intracranial aspergilloma with low signal on T2-weighted images corresponding to iron accumulation. Neuroradiology, 2001; 43: 559–561. [DOI] [PubMed] [Google Scholar]
- 7.Jain KK Mittal SK Kumar S et al. Imaging features of central nervous system fungal infections. Neurol India. 2007; 55 (3): 241–250. [DOI] [PubMed] [Google Scholar]
- 8.Keyik B Edgüer T Hekimoğlu B. Conventional and diffusion-weighted MR imaging of cerebral aspergillosis. Diagn Interv Radiol. 2005; 11 (4): 199–201. [PubMed] [Google Scholar]
- 9.Mathur M Johnson CE Sze G. Fungal infections of the central nervous system. Neuroimaging Clin N Am. 2012; 22 (4): 609–632. [DOI] [PubMed] [Google Scholar]
- 10.Castellano-Sanchez AA Li S Qian J et al. Primary central nervous system posttransplant lymphoproliferative disorders. Am J Clin Pathol. 2004; 121 (2): 246–253. [DOI] [PubMed] [Google Scholar]
- 11.Sauter A Faul C Bitzer M et al. Imaging findings in immunosuppressed patients with Epstein Barr virus-related B cell malignant lymphoma. Am J Roentgenol. 2010; 194 (2): 141–149. [DOI] [PubMed] [Google Scholar]
- 12.Yamada K Shrier DA Rubio A et al. Imaging findings in intracranial aspergillosis. Acad Radiol. 2002; 9 (2): 163–171. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz KM Erickson BJ Lucchinetti C. (2006) Pattern of T2 hypointensity associated with ring-enhancing brain lesions can help to differentiate pathology. Neuroradiology. 2006; 48 (3): 143–149. [DOI] [PubMed] [Google Scholar]