Abstract
This report describes cerebral aneurysmal rupture with asymmetry of ipsilateral dural sinus hypoplasia using quantitative color-coded I-Flow cerebral venography to identify the venous pressure gradient. We suggest the pressure gradient may be a potential factor in cerebral aneurysmal rupture. We used I-Flow quantitative cerebral venography to measure the venous pressure gradient during acute cerebral aneurysmal rupture and post embolization in a 67-year-old woman who presented with clinical symptoms of left third nerve palsy for several days with mild headache initially without subarachnoid hemorrhage. We encountered a high venous pressure gradient of severe ipsilateral dural sinus hypoplasia during acute rupture of a posterior communicating aneurysm. Venous dural sinus asymmetry has been considered a congenital benign and non-pathological condition. However, this case may present severe hypoplasia of the dural sinus with potential pressure gradient in some unusual condition. A high venous pressure gradient may be another factor in cerebral aneurysmal rupture.
Keywords: asymmetry, venous sinus, aneurysm, rupture, subarachnoid hemorrhage
Introduction
Varied factors may be related to the etiology of cerebral aneurysmal rupture. Although asymmetry of the cerebral venous sinus is a potential factor, yet venous pressure measurement has not been described with aneurysmal rupture1,2. This report describes an acute aneurysmal rupture with severe hypoplasia of the ipsilateral dural sinus. We used I-Flow quantitative cerebral venography to identify the high venous pressure gradient as being a possible factor of cerebral aneurysm rupture.
Case Report
A 67-year-old woman presented with left third nerve palsy for several days. She had a long history of poorly controlled hypertension. Initial CT showed no evidence of subarachnoid (SAH) or other intracranial hemorrhage. However, contrast-enhanced CT did disclose a posterior communicating aneurysm.
She was then referred for cerebral angiography and endovascular therapy since it was a non-ruptured aneurysm. Her clinical GCS was 15 and the same as on the angiography table initially. Cerebral angiography again confirmed a 6.5×8.5×5.2 mm wide-necked lobulated aneurysm at the left posterior communicating artery. Ipsilateral atresia of the left transverse sinus and hypoplasia of the left sigmoid sinus and left jugular vein were also noted. However, bilateral venous flows were equal and symmetrical by I-Flow color-coded quantitative measurements. She suddenly lapsed into a coma awaiting preparation of coiling and consent from her family.
Dyna CT was performed from angiography and massive SAH was discovered. Stent-as-sisted coil embolization was then performed with satisfactory aneurysmal embolization at the end of the procedure. I-Flow color-coded venous flow was measured again, venous flow was slower than before embolization possibly due to increased intracranial pressure with massive SAH. Again, the bilateral venous flows were very similar and equal except slower. She recovered very well after embolization of the aneurysm with a GCS of about 14 when she was discharged a week later.
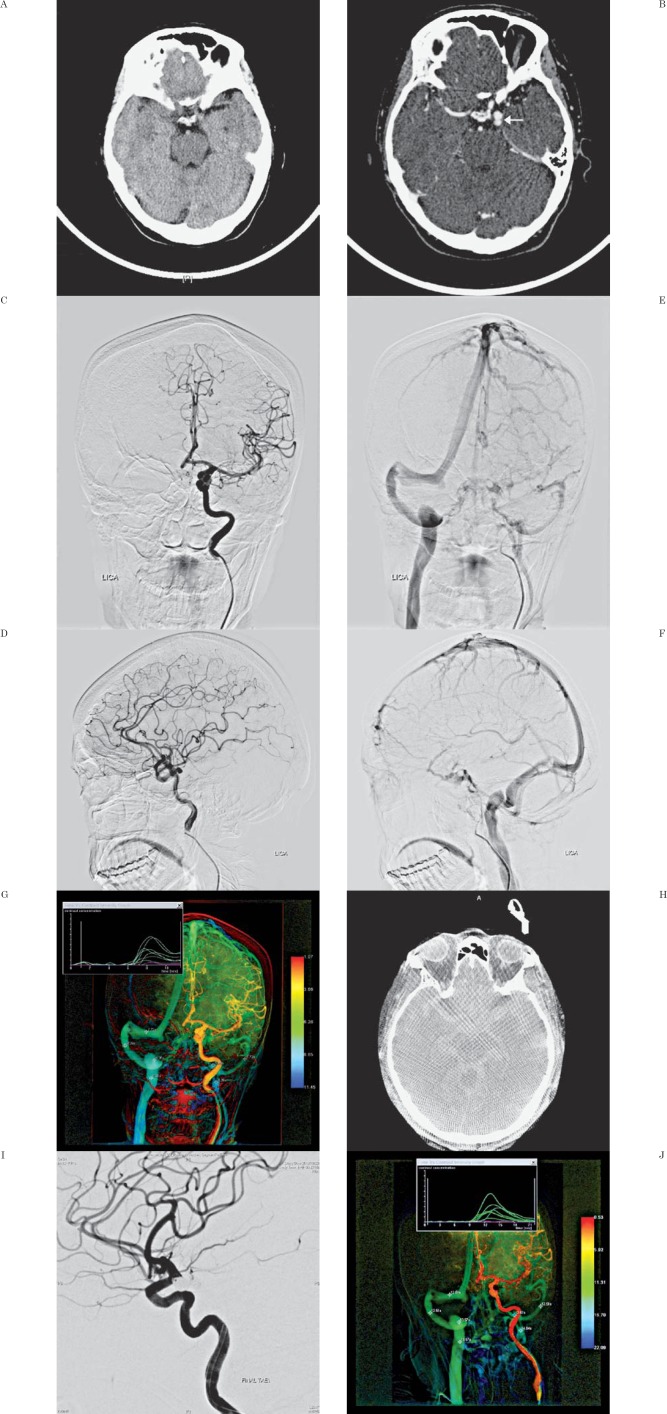
Figure 1.
A) Non-contrast head CT showed no evidence of subarachnoid or any intracranial hemorrhage. B) Contrast-enhanced CT showed a lobulated leftposterior communicating artery (PCA) aneurysm. C, D) Frontal and lateral views of left carotid angiography confirmed a lobulated left PCA aneurysm. E, F) Venous phase showed atresia of the left transverse sinus and severe hypoplasia of the left sigmoid sinus. G) Color-coded quantitative (CCQ) cerebral venography showed symmetrical and similar flow of the bilateral sigmoid sinus (7.72s) jugular bulb (8.26s) and jugular vein (8.26s). H) Dyna CT showed extensive subarachnoid hemorrhage. I) Post embolization angiography showed successful embolization of the left PCA aneurysm. J) Repeat post embolization CCQ cerebral venography showed much slower but similar venous flow again (12.51s, 13.57s and 14.64s) respectively.
Discussion
The spectrum of cerebral aneurysmal rupture includes highly variable factors including age, gender, genetics, site size, configuration, flow profile, wall stress and so on2-8. However, the implications of these factors in clinical practice may be very inconsistent7-15,16. Recently, we encountered an interesting finding of cerebral aneurysmal rupture which may be related to asymmetry of the dural sinus especially in female populations1,2,17,18. With the improvement in imaging techniques and software, we are now able to measure venous pressure gradient quantitatively from the venous blood flow rate and vessel diameter by phase contrast MRI or color-coded quantitative cerebral angiography. By correlating with phase contrast MRI and color-coded quantitative cerebral venography, similar and symmetrical venous flow rates may indicate higher venous pressure on the hypoplasia side of the venous system2. From our preliminary data, aneurysmal rupture may be related to this phenomenon and the female gender has a higher incidence of asymmetry of the dural sinus, especially atresia2.
Our current case should be classified as an unruptured cerebral aneurysm when this patient was admitted to hospital for evaluation of therapeutic options.
Initial cerebral angiography disclosed a lobu-lated aneurysm and atresia of the left transverse sinus and severe hypoplasia of the left sigmoid sinus. These findings had alerted us the need to inform the patient of the risk of potential aneurysmal rupture in our experience even though she presented with no rupture. During a conversation with the family and awaiting analysis and planning therapeutic options, she suddenly suffered a massive subarachnoid hemorrhage with no clinical change until aneurysm rupture.
Quantitative cerebral venography performed at rupture showed symmetrical and similar venous flow rates on both sides of the sigmoid sinuses and jugular vein. With flow dynamics, smaller diameter vessels require higher pressure to achieve the same flow as dominant and larger vessels. Thus, a pressure gradient may occur during aneurysmal rupture. The pressure gradient remained rather similar but slower even after successful embolization of the ruptured posterior communicating aneurysm15. Slower cerebral blood flow is a normal reaction to massive subarachnoid hemorrhage.
Our case illustrate an unusual presentation of the pressure gradient of cerebral venous flow associated with acute rupture of a cerebral aneurysm. Certainly, a lobulated aneurysm is an important factor for aneurysm rupture. However, the venous pressure gradient with asymmetry of the dural sinus may be considered a potential risk factor. This case report might provide another consideration and factor to manage unruptured cerebral aneurysms2.
References
- 1.Tsai FY Nguyen B Lin WC et al. Endovascular procedures for cerebrovenous disorders. Acta Neurochir Suppl. 2008; 101: 83–86. [DOI] [PubMed] [Google Scholar]
- 2.Tsai FY Yen A Guo WY et al. Venous hypertension and cerebral aneurysm rupture. Neuroradiol J. 2011: 24: 133–144. [DOI] [PubMed] [Google Scholar]
- 3.Dell S. Asymptomatic cerebral aneurysm: assessment of its risk of rupture. Neurosurgery. 1982; 10 (2): 162–166. [PubMed] [Google Scholar]
- 4.Rinkel GJE Djibuti M Algra A et al. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998; 29: 251–256. [DOI] [PubMed] [Google Scholar]
- 5.Friedman JA Piepgras DG Pichelmann MA et al. Small cerebral aneurysms presenting with symptoms other than rupture. Neurology. 2001; 57: 1212–1216. [DOI] [PubMed] [Google Scholar]
- 6.Vindlacheruvu RR Mendelow AD Mitchell P. Risk-benefit analysis of the treatment of unruptured intracranial aneurysms. J Neurol Neurosurg Psychiatry. 2005; 76: 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ventikos Y Holland EC Bowker TJ et al. Computational modelling for cerebral aneurysms: risk evaluation and interventional planning. Br J Radiol. 2009; 82 (1): S62–s71. [DOI] [PubMed] [Google Scholar]
- 8.Rahman M Smietana J Hauck E et al. Size ratio correlates with intracranial aneurysm rupture status: a prospective study. Stroke. 2010; 41: 916–920. [DOI] [PubMed] [Google Scholar]
- 9.Wermer MJH van der Schaaf IC Algra A et al. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke. 2007; 38: 1404–1410. [DOI] [PubMed] [Google Scholar]
- 10.Sato K Yoshimoto Y. Risk profile of intracranial aneurysms: rupture rate is not constant after formation. Stroke. 2011; 42: 3376–3381. [DOI] [PubMed] [Google Scholar]
- 11.Lua G Huanga L Zhanga XL et al. Influence of hemodynamic factors on rupture of intracranial aneurysms: patient-specific 3D mirror aneurysms model computational fluid dynamics simulation. Am J Neuroradiol. 2011; 32: 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlak MHM Rinkel GJE Greebe P et al. Trigger factors and their attributable risk for rupture of intracranial aneurysms: a case-crossover study. Stroke. 2011: 42: 1878–1882. [DOI] [PubMed] [Google Scholar]
- 13.Xiang JP Tremmel M Kolega J et al. Newtonian viscosity model could overestimate wall shear stress in intracranial aneurysm domes and underestimate rupture risk. J Neurointervent Surg. 2012; 4: 351–357. [DOI] [PubMed] [Google Scholar]
- 14.Lazzaro MA Ouyang B Chen M. The role of circle of Willis anomalies in cerebral aneurysm rupture. J Neurointervent Surg. 2012; 4: 22–26. [DOI] [PubMed] [Google Scholar]
- 15.Lin N Cahill KS Frerichs KU et al. Treatment of ruptured and unruptured cerebral aneurysms in the USA: a paradigm shift. J Neurointervent Surg. 2012; 4: 182–189. [DOI] [PubMed] [Google Scholar]
- 16.Liu XM Rinkel GJE. Aneurysm and clinical characteristics as risk factors for intracerebral hematoma from aneurysmal rupture. J Neurol. 2011; 258: 862–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai FY Wang AM Matovich VB et al. MR staging of acute dural sinus thrombosis: correlation with venous pressure measurements and implications for treatment and prognosis. Am J Neuroradiol. 1995; 16: 1021–1029. [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai FY Kostanian VJ Rivera MB et al. Cerebral venous congestion as indication for thrombolytic treatment. Cardiovasclntervent Radiol. 2007; 30 (4): 675–687. [DOI] [PubMed] [Google Scholar]