Abstract
Background
An anterior cruciate ligament (ACL) injury greatly increases the risk for premature knee osteoarthritis (OA). Improved diagnosis and staging of early disease are needed to develop strategies to delay or prevent disabling OA.
Purpose
Novel magnetic resonance imaging (MRI) ultrashort echo time (UTE)–T2* mapping was evaluated against clinical metrics of cartilage health in cross-sectional and longitudinal studies of human participants before and after ACL reconstruction (ACLR) to show reversible deep subsurface cartilage and meniscus matrix changes.
Study Design
Cohort study (diagnosis/prognosis); Level of evidence, 2.
Methods
Forty-two participants (31 undergoing anatomic ACLR; 11 uninjured) underwent 3-T MRI inclusive of a sequence capturing short and ultrashort T2 signals. An arthroscopic examination of the medial meniscus was performed, and modified Outerbridge grades were assigned to the central and posterior medial femoral condyle (cMFC and pMFC, respectively) of ACL-reconstructed patients. Two years after ACLR, 16 patients underwent the same 3-T MRI. UTE-T2* maps were generated for the posterior medial meniscus (pMM), cMFC, pMFC, and medial tibial plateau (MTP). Cross-sectional evaluations of UTE-T2* and arthroscopic data along with longitudinal analyses of UTE-T2* changes were performed.
Results
Arthroscopic grades showed that 74% (23/31) of ACL-reconstructed patients had intact cMFC cartilage (Outerbridge grade 0 and 1) and that 90% (28/31) were Outerbridge grade 0 to 2. UTE-T2* values in deep cMFC and pMFC cartilage varied significantly with injury status and arthroscopic grade (Outerbridge grade 0–2: n = 39; P = .03 and .04, respectively). Pairwise comparisons showed UTE-T2* differences between uninjured controls (n = 11) and patients with arthroscopic Outerbridge grade 0 for the cMFC (n = 12; P = .01) and arthroscopic Outerbridge grade 1 for the pMFC (n = 11; P = .01) only and not individually between arthroscopic Outerbridge grade 0, 1, and 2 of ACL-reconstructed patients (P > .05). Before ACLR, UTE-T2* values of deep cMFC and pMFC cartilage of ACL-reconstructed patients were a respective 43% and 46% higher than those of uninjured controls (14.1 ± 5.5 vs 9.9 ± 2.3 milliseconds [cMFC] and 17.4 ± 7.0 vs 11.9 ± 2.4 milliseconds [pMFC], respectively; P = .02 for both). In longitudinal analyses, preoperative elevations in UTE-T2* values in deep pMFC cartilage and the pMM in those with clinically intact menisci decreased to levels similar to those in uninjured controls (P = .02 and .005, respectively), suggestive of healing. No decrease in UTE-T2* values for the MFC and new elevation in UTE-T2* values for the submeniscus MTP were observed in those with meniscus tears.
Conclusion
This study shows that novel UTE-T2* mapping demonstrates changes in cartilage deep tissue health according to joint injury status as well as a potential for articular cartilage and menisci to heal deep tissue injuries. Further clinical studies of UTE-T2* mapping are needed to determine if it can be used to identify joints at risk for rapid degeneration and to monitor effects of new treatments to delay or prevent the development of OA.
Keywords: anterior cruciate ligament tear, ACL reconstruction, osteoarthritis, MRI, quantitative MRI, UTE-T2*, UTE-T2*, mapping, articular cartilage, joint injury, posttraumatic OA
Osteoarthritis (OA) is a major public health issue for which disease-modifying treatments are needed. An anterior cruciate ligament (ACL) tear is a common and substantial joint injury that elevates the risk for meniscus dysfunction, cartilage loss, and accelerated development of knee OA.10,20,24 However, the cause of premature OA after an ACL rupture and repair is unclear. While altered biomechanics and loading patterns after ACL tears have been implicated in OA pathogenesis, injuries to the articular cartilage, subchondral bone, and menisci at the time of an ACL tear may also play a role.25,32 Improved clinical methods to delineate the early changes in articular cartilage and menisci occurring after an ACL injury and reconstruction are important in understanding OA pathogenesis and in the development of disease-modifying treatments.
Cartilage injury after an ACL tear is assumed based on the heightened risk of OA but cannot be appreciated by arthroscopic surface imaging or conventional morphological magnetic resonance imaging (MRI) in cartilage retaining intact articular surfaces. Visible cartilage lesions shortly after ACL injury have been predominantly to the lateral compartment in articular cartilage overlying bone bruises that are characteristic of impact injury.18,25 However, longitudinal MRI studies show increasing cartilage loss and volume changes in the medial femoral condyle (MFC) that were not apparent on baseline MRI scans of the injury.15,25 This indicates a clinical need for improved understanding of cartilage and meniscus changes in the medial compartment after ACL injuries as well as quantitative metrics to identify the presence, persistence, and progression of subsurface injuries leading to these clinically significant findings.
Arthroscopic probing, when performed, can show softening, which is considered a marker of early cartilage damage and degeneration. Laboratory methods such as histology, analysis of matrix composition, and evaluation of chondrocyte viability and metabolism show that substantial cartilage damage and degeneration can be present in cartilage appearing normal on surface inspection, including arthroscopic surgery.5,7,23,28 While basic and clinical studies indicate that progressive cartilage loss may not be reversible once the articular surface is compromised, numerous studies show a potential for chondroprotective therapies ranging from cytoprotective strategies, growth factor treatments, anticatabolic treatments and alteration of mechanical loading through physical therapy, weight reduction, or surgery to reverse pathological changes in cartilage retaining intact articular surfaces.2,5,17,21,28
Quantitative MRI shows the potential for noninvasive diagnosis and staging of subsurface matrix changes in cartilage retaining intact articular surfaces.2,4,7,8 These advanced MRI techniques include the more established methods of dGEMRIC (delayed gadolinium-enhanced MRI of cartilage), T1ρ, and T2 mapping.2,4,8 The dGEMRIC technique is sensitive to differences in proteoglycan content, while T1ρ provides some indication to proteoglycan status.2,22 T2 relaxation time, a quantitative measure of collagen orientation and tissue hydration,6,8,11,16,22 has been shown to be elevated in damaged cartilage and in cartilage of ACL-injured knees.12,19,25 Because of low sensitivity to short T2 values, standard T2 mapping, however, is limited in its ability to assess the deep layer of articular cartilage.30
Injuries to the deep layer of articular cartilage directly overlying the subchondral bone have been shown to occur in laboratory studies after impact loading at energies insufficient to fracture the articular surface.5,29 Because arthroscopic surgery is invasive and limited to a subjective evaluation of softening of the intact articular surfaces of cartilage, improving the MRI evaluation of cartilage deep tissue may increase sensitivity to cartilage damage and early degeneration after ACL injuries. Ultrashort echo time (UTE) imaging is sensitive to short T2 signals (T2 <10 milliseconds) and has the potential to assess articular cartilage in the deep layers.13
Previous ex vivo studies on human cartilage explants have shown that novel UTE-enhanced T2* (UTE-T2*) mapping reflects collagen structural integrity and degeneration in cartilage and menisci as determined by polarized microscopy.30,32 In vivo human studies have shown UTE-T2* mapping to be reproducible in the clinical setting.31 Recently, a cross-sectional clinical study of ACL-injured patients showed evidence that UTE-T2* mapping was effective in identifying subsurface injuries in menisci that were found to be clinically intact by conventional MRI and arthroscopic surgery.32
This study was conducted to test the hypotheses that (1) UTE-T2* mapping is sensitive to deep subsurface matrix changes in articular cartilage after ACL tears and tracks the cartilage disease state and that (2) clinical UTE-T2* mapping is capable of showing longitudinal changes in articular cartilage and menisci after anatomic ACL reconstruction (ACLR).
MATERIALS AND METHODS
In accordance with institutional review board–approved protocols, 42 human participants were recruited and provided informed consent for participation. The participants consisted of 31 patients with ACL tears clinically indicated for ACLR (mean age, 29.8 ± 9.5 years [range, 18–51 years]; mean body mass index [BMI], 28.4 ± 6.2 kg/m2 [range, 19.6–46.6 kg/m2]; 17 female/14 male; 13 right/18 left knees) and 11 uninjured controls (mean age, 27.8 ± 4.4 years [range, 23–37 years]; mean BMI, 25.0 ± 4.4 kg/m2 [range, 20.4–33.9 kg/m2]; 5 female/6 male) with no knee pain, no known or suspected knee pathological changes, and intact cartilage by 3-T MRI. The ACL-injured patients were recruited after a clinical decision was made to proceed with ACLR. Preinjury physical activities were not considered for recruitment criteria. The recruited participants did not have other pathological changes requiring operative intervention beyond a meniscus tear during the index procedure. All ACL-reconstructed patients underwent an arthroscopic examination and anatomic ACLR performed by 1 of 3 surgeons (C.R.C.—18 patients, R.V.W.—9 patients, F.H.F.—4 patients). For ACL-reconstructed patients, research 3-T MRI, acquiring sequences approved for National Institutes of Health (NIH) R01 AR052784 (principal investigator: C.R.C.), was performed within 2 weeks before ACLR. Only the affected knee of ACL-reconstructed patients was imaged. Image data from 24 of 31 patients had previously been included in a cross-sectional study32 examining only meniscus UTE-T2* values after ACL injuries. Among uninjured controls, 3-T MRI data sets from acquisition 2 of a 3-acquisition repeatability study31 were used for this work. New segmentation and image processing were performed on all data sets reported in this study. Sixteen of the ACL-reconstructed patients (mean age, 28 ± 9 years; mean BMI, 28 ± 7 kg/m2; 10 female; 5 right knees) underwent the same research 3-T MRI before and 2 years after surgery. The mean time between surgery and 2-year follow-up imaging was 25.7 ± 1.6 months (range, 23.0–30.9 months).
Arthroscopic Assessment Methods
Targeted examinations of the central and posterior weight-bearing regions of the MFC and the medial meniscus were performed by trained orthopaedic surgeons (C.R.C.—18 exams, R.V.W.—9 exams, and Orthopedic Sports Fellow/C.R.C.—4 exams) at the time of ACLR. The midsagittal plane of the MFC and the central and posterior weightbearing zones were defined by visual landmarks including the top of the notch and the borders of the MFC with the knee at 90° of flexion. Modified Outerbridge grades (0 = surface intact and firm; 1 = surface intact and soft; 2 = surface not intact, partial-thickness injury involving <50% of the depth; 3 = surface not intact, partial-thickness injury involving >50% of the depth; 4 = full-thickness injury) were assigned to these regions of interest (ROIs) and recorded for analysis. The medial meniscus was visualized and probed during arthroscopic examination to determine whether a tear was present.
MRI Methods
Participants were imaged using a 3-T MRI scanner (Magnetom Trio Tim, Siemens Medical Solutions, Erlangen, Germany) and an 8-channel knee coil (In Vivo Inc, Gaines-ville, Florida, USA). The 3-dimensional (3-D) acquisition-weighted stack of spirals imaging technique26,31 was used on all participants. Eleven echo images, including a UTE of 0.6 milliseconds (other echo times = 1, 2, 3, 4, 5, 7, 10, 20, 30, and 40 milliseconds), were acquired with a field of view of 140 mm and matrix size of 256. The scan time for each echo image was 1.92 minutes, adding to a total scan time of 22 minutes for all 11 acquisitions. Images were acquired with 2-mm section thickness, 60 slices, 24 in-plane spirals, 11.52-millisecond spiral readout time, 5-μs data sampling interval, and flip angle/repetition time of 30°/80 milliseconds. The 3-D imaging volume was centered on the tibiofemoral joint with sagittal orientation.
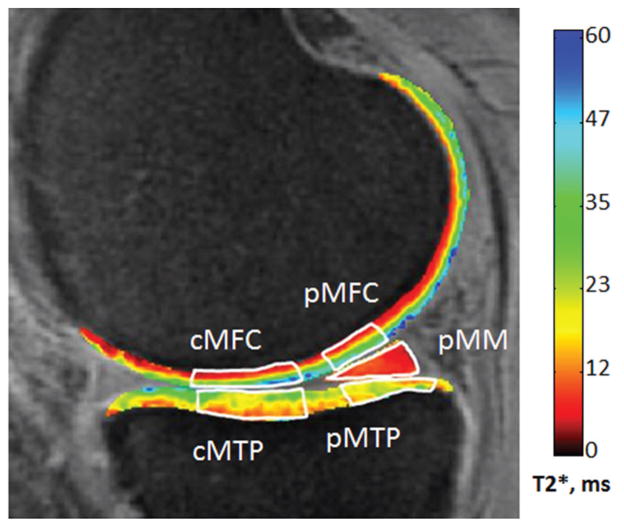
UTE-T2* maps were generated from the 11 echo images with a monoexponential pixel-by-pixel T2-fit routine using MRIMapper software (Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA, and Massachusetts Institute of Technology, Cambridge, Massachusetts, USA) running on a MATLAB platform (The MathWorks, Natick, Massachusetts, USA). To facilitate image registration, echo images were linearly interpolated to a matrix size of 512 (effective pixel size, 273 μm) before T2 curve fitting. Rigid in-plane image registration was applied to interpolated images to reduce patient motion-induced spatial offsets between successive echo images. Five ROIs were manually segmented from single sections to the center of the MFC: central and posterior MFC (cMFC and pMFC, respectively), central and posterior (submeniscus) medial tibial plateau (cMTP and pMTP, respectively), and posterior medial meniscus (pMM) (Figure 1). Zonal cartilage differences were assessed by further segmenting cartilage ROIs into superficial and deep halves as previously described.8 Mean UTE-T2* values for each superficial and deep cartilage ROI and meniscus region were recorded for analysis. A previous examination of this technique indicated that mean UTE-T2* quantitation errors associated with superficial and deep cartilage ROIs range from 6% to 16% for intersession precision repeatability and 0.80 to 0.98 for interclass correlation of intraobserver segmentation reproducibility.31
Figure 1.
Five regions of interest were evaluated in this work (white outlines). The central and posterior medial femoral condyle (cMFC and pMFC, respectively), central and posterior (submeniscus) medial tibial plateau (cMTP and pMTP, respectively), and posterior medial meniscus (pMM). Zonal cartilage differences were assessed by further segmenting cartilage regions of interest into superficial and deep halves.
Three-dimensional double-echo steady-state (DESS) images, acquired in the same manner as those of the NIH-sponsored Osteoarthritis Initiative sequence (www.oai.ucsf.edu) and acquired in the same research MRI sessions as UTE-T2* images, were independently evaluated by a musculoskeletal radiologist (B.H.D.). The DESS images were qualitatively evaluated for meniscus tear status and cartilage morphological characteristics of the cMFC, pMFC, cMTP, and pMTP using 3-D software (Osirix, Pixmeo, Geneva, Switzerland), which enabled a 3-plane reformat of the primary sagittal acquired data set. The radiologist was blinded to UTE-T2* values and arthroscopic Outerbridge grades and meniscus assessments.
Statistical Methods
The nonparametric Kruskal-Wallis test (KWT) was used to assess UTE-T2* differences across groups of participants with different degrees of pathological lesions; post hoc tests adjusted for multiple comparisons and evaluated pairwise differences. Two-tailed t tests were used to assess cross-sectional differences in UTE-T2* values between ACL-reconstructed and asymptomatic participants. The paired nonparametric Wilcoxon signed-rank test (WSRT) was used to assess longitudinal changes between presurgery and 2-year time points in UTE-T2* values of cartilage and menisci of ACL-injured patients after ACLR. All statistical analyses were performed with Excel (Microsoft, Redmond, Washington, USA) and SPSS (IBM Corp, Armonk, New York, USA). Statistical significance was accepted for P <.05.
RESULTS
Arthroscopic Assessment
The distribution of Outerbridge grades determined by arthroscopic assessment in the cMFC of the 31 ACL-reconstructed patients included in this study was as follows: grade 0, n = 12; grade 1, n = 11; grade 2, n = 5; grade 3, n = 0; and grade 4, n = 2; 1 patient was noted as having “fibrocartilage,” as shown in Table 1. In the pMFC, the distribution of Outerbridge grades was as follows: grade 0, n = 10; grade 1, n = 11; grade 2, n = 7; grade 3, n = 0; grade 4, n = 2; and fibrocartilage, n = 1. For the 16 patients included in the 2-year longitudinal analysis, the distribution of Outerbridge grades for the cMFC was as follows: grade 0, n = 7; grade 1, n = 4; grade 2, n = 4; grade 3, n = 0; grade 4, n = 0; and fibrocartilage, n = 1; the distribution for the pMFC was as follows: grade 0, n = 6; grade 1, n = 5; grade 2, n = 4; grade 3, n = 0; grade 4, n = 0; and fibrocartilage, n = 1.
TABLE 1.
Patient Demographics, MMT Status, Outerbridge Grade, and UTE-T2* Values for ACL-Reconstructed Cohorta
| Patient | Sex | Age, y | BMI, kg/m2 | MMT Status | Outerbridge Grade
|
UTE-T2*, ms
|
||
|---|---|---|---|---|---|---|---|---|
| cMFCb | pMFC | Deep cMFC | Deep pMFC | |||||
| 1 | F | 23 | 21.3 | No tear | 0 | 0 | 16 | 19 |
| 2 | M | 22 | 20.9 | No tear | 0 | 0 | 18 | 16 |
| 3 | F | 19 | 46.6 | No tear | 0 | 1 | 18 | 28 |
| 4 | F | 32 | 32.7 | No tear | 0 | 0 | 16 | 21 |
| 5 | M | 19 | 27.9 | No tear | 0 | 0 | 13 | 22 |
| 6 | M | 48 | 27.7 | No tear | 0 | 0 | 14 | 13 |
| 7 | F | 30 | 24 | Torn | 0 | 0 | 14 | 17 |
| 8 | F | 27 | 26.6 | Torn | 0 | 0 | 28 | 19 |
| 9 | M | 21 | 26.9 | Torn | 0 | 2 | 14 | 8 |
| 10 | F | 37 | 26.9 | Torn | 0 | 0 | 9 | 10 |
| 11 | F | 18 | 21.1 | Torn | 0 | 0 | 12 | 13 |
| 12 | M | 25 | 29.2 | Torn | 0 | 0 | 11 | 17 |
| 13 | F | 32 | 27.5 | No tear | 1 | 1 | 16 | 24 |
| 14 | M | 35 | 28.4 | No tear | 1 | 1 | 7 | 9 |
| 15 | M | 36 | 32.6 | No tear | 1 | 1 | 11 | 11 |
| 16 | F | 33 | 26.9 | No tear | 1 | 1 | 18 | 24 |
| 17 | F | 40 | 19.6 | No tear | 1 | 2 | 9 | 11 |
| 18 | M | 21 | 24.3 | No tear | 1 | 1 | 13 | 16 |
| 19 | M | 25 | 37.8 | Torn | 1 | 2 | 25 | 32 |
| 20 | M | 47 | 28.8 | Torn | 1 | 1 | 9 | 11 |
| 21 | F | 33 | 43.4 | Torn | 1 | 1 | 21 | 33 |
| 22 | M | 34 | 29.6 | Torn | 1 | 1 | 12 | 20 |
| 23 | F | 23 | 26.5 | Torn | 1 | 1 | 9 | 18 |
| 24 | F | 32 | 24.1 | No tear | 2 | 1 | 23 | 25 |
| 25 | M | 51 | 26.7 | No tear | 2 | 2 | 8 | 11 |
| 26 | M | 22 | 36.7 | No tear | 2 | 2 | 12 | 16 |
| 27 | F | 18 | 23.4 | Torn | 2 | 2 | 14 | 27 |
| 28 | F | 22 | 24.7 | Torn | 2 | 2 | 5 | 8 |
| 29 | F | 45 | 33.7 | Torn | 4 | 4 | 14 | 13 |
| 30 | M | 34 | 28.2 | Torn | 4 | 4 | —c | —c |
| 31 | F | 20 | 25.9 | No tear | Fibro | Fibro | 22 | 18 |
ACL, anterior cruciate ligament; BMI, body mass index; cMFC, central medial femoral condyle; F, female; fibro, fibrocartilage; M, male; MMT, medial meniscus tear; pMFC, posterior medial femoral condyle; UTE, ultrashort echo time.
The ACL-reconstructed patients are ordered by Outerbridge grade in the cMFC.
Tissue is too thin in the region of interest.
UTE-T2* Values by Cartilage Status
When binned according to the following groups, uninjured controls, ACLR with Outerbridge grade 0, ACLR with Outerbridge grade 1, and ACLR with Outerbridge grade 2, UTE-T2* values in deep cMFC and pMFC cartilage varied significantly with the degree of the pathological lesion (n = 39; KWT, P = .03 and .04, respectively). There was insufficient cartilage UTE-T2* data from participants with Outerbridge grades other than 0 to 2 for inclusion in these analyses: Outerbridge grade 3 (n = 0), Outerbridge grade 4 (n = 2), and “fibrocartilage” (n = 1) (Table 1). Among ACL-reconstructed patients with Outerbridge grade 0, 1, and 2, UTE-T2* values did not distinguish between the different degrees of arthroscopic degeneration. Mean superficial and deep UTE-T2* values were not found to vary between ACL-reconstructed patients with Outerbridge grade 0, 1, or 2 for the cMFC (n = 12, 11, and 5, respectively; KWT, P > .05) or the pMFC (n = 10, 11, and 7, respectively; KWT, P > .05).
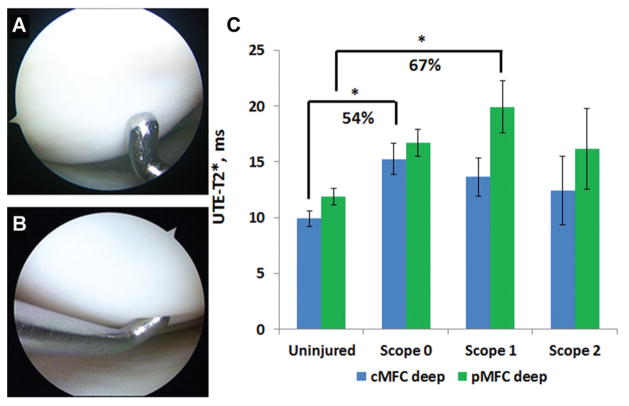
Among participants with intact articular surfaces of the MFC (ie, 3 groups: ACL-reconstructed patients with Outer-bridge grade 0 and 1 and uninjured controls), deep UTE-T2* values were found to discriminate between groups based on cartilage and injury status. Specifically, in deep cMFC cartilage, UTE-T2* values varied significantly between uninjured controls (n = 11), ACL-injured patients with Outerbridge grade 0 (“firm cartilage”; n = 12), and ACL-injured patients with Outerbridge grade 1 (“soft cartilage”; n = 11) (KWT, P = .01). In addition, post hoc pairwise comparison showed that UTE-T2* values in deep cMFC cartilage of ACL-reconstructed patients with firm cartilage (Outerbridge grade 0; n = 12) were elevated 54% compared with those of uninjured controls (15.3 ± 4.8 vs 9.9 ± 2.3 milliseconds, respectively; P = .01). In deep pMFC cartilage, UTE-T2* values also varied significantly with cartilage status (uninjured controls vs grade 0 vs grade 1; KWT, P = .01). Post hoc pairwise comparison found that UTE-T2* values in deep pMFC cartilage of ACL-reconstructed patients with softened but intact cartilage (Outerbridge grade 1; n = 11) were elevated 67% compared with those of uninjured controls (19.9 ± 7.7 vs 11.9 ± 2.4 milliseconds, respectively; P = .01) (Figure 2C). Post hoc comparisons showed no differences in UTE-T2* values between softened but intact deep cMFC cartilage (Outerbridge grade 1; 13.6 ± 6.5 milliseconds) and the other groups (P > .05). Similarly, no difference was detected between intact and firm deep pMFC cartilage (Outerbridge grade 0; 16.7 ± 3.8 milliseconds) and any other group (P > .05). Among ACL-reconstructed patients, UTE-T2* values for Outerbridge grade 0 cartilage did not differ from those for Outerbridge grade 1 cartilage (P > .05). Post hoc comparison of UTE-T2* values in intact and firm cartilage (Outerbridge grade 0) and UTE-T2* values in intact but softened cartilage (Outerbridge grade 1) of ACL-injured patients did not find a difference in deep cartilage of either the cMFC or pMFC (P > .05).
Figure 2.
Ultrashort echo time (UTE)–T2* in deep articular cartilage varies with the degree of arthroscopic softening among participants with intact articular surfaces (Kruskal-Wallis test, P = .01). (A) Arthroscopic image of the medial femoral condyle (MFC) of an anterior cruciate ligament (ACL)–reconstructed patient with firm and intact cartilage (Outerbridge grade 0). (B) The MFC of an ACL-reconstructed patient with intact and softened cartilage (Outerbridge grade 1). (C) ACL-reconstructed patients with intact and firm cartilage (grade 0) demonstrate significantly higher UTE-T2* values in deep central MFC cartilage (P = .01) compared with uninjured controls. In the posterior MFC, deep cartilage of ACL-reconstructed patients with softened but intact cartilage (grade 1) is significantly elevated compared with that of uninjured controls (P = .01). Error bars indicate standard error of the mean.
Mean UTE-T2* Values of Articular Cartilage and Menisci
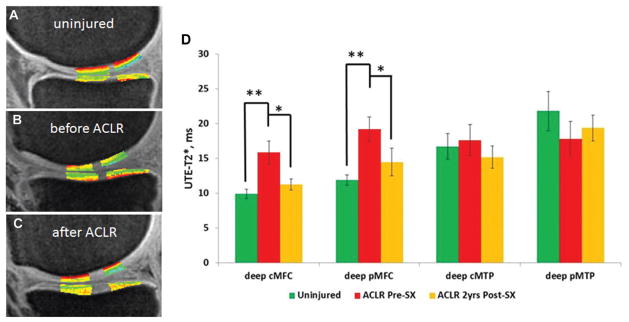
Before ACLR, mean UTE-T2* values of articular cartilage for all ACL-reconstructed patients (n = 31) in the deep cMFC and deep pMFC regions were 43% and 46% higher than those of uninjured controls (n = 11) (14.1 ± 5.5 vs 9.9 ± 2.3 milliseconds [cMFC] and 17.4 ± 7.0 vs 11.9 ± 2.4 milliseconds [pMFC], respectively; 2-tailed t test, P = .02 for both). There were no differences in UTE-T2* values of deep cMTP and deep pMTP cartilage (2-tailed t test, P = .47 and .68, respectively) before ACLR. For superficial articular cartilage, UTE-T2* values were not found to differ between ACL-reconstructed patients and uninjured controls in any region tested (2-tailed t test, P > .41). Within the subcohort of 16 ACL-reconstructed patients providing longitudinal 2-year UTE-T2* data, mean UTE-T2* values of deep cMFC and deep pMFC cartilage were similarly elevated compared with those in uninjured controls. For this subcohort, the mean UTE-T2* value in deep cMFC cartilage was 60% higher than that of uninjured controls (15.9 ± 6.5 vs 9.9 ± 2.3 milliseconds, respectively; 2-tailed t test, P = .007), and the mean UTE-T2* value in deep pMFC cartilage was 61% higher (19.2 ± 7.1 vs 11.9 ± 2.4 milliseconds, respectively; 2-tailed t test, P = .003) (Figure 3D).
Figure 3.
Example ultrashort echo time (UTE)–T2* maps from (A) an uninjured control participant, (B) an anterior cruciate ligament (ACL)–reconstructed patient before surgery, and (C) the same patient 2 years after ACL reconstruction (ACLR). (D) UTE-T2* mapping suggests that injuries to deep cartilage and menisci of ACL-injured patients resolve over 2 years after ACLR. Before surgery, ACL-reconstructed patients demonstrated significantly elevated UTE-T2* values in the deep central medial femoral condyle (cMFC) and deep posterior MFC (pMFC) (P = .007 and .003, respectively) compared with uninjured controls. Two years after ACLR, UTE-T2* values decreased to levels consistent with those of asymptomatic controls in all compartments. UTE-T2* values for the deep cMFC and pMFC fell a respective 29% and 24% (P = .02 for both). Error bars indicate standard error of the mean. *P <.05. **P <.01.
For the medial meniscus, we previously reported preoperative mean UTE-T2* values for 24 of the 31 ACL-reconstructed patients and 10 of the 11 uninjured controls reported here.32 Consistent with that prior analysis, the preoperative mean UTE-T2* value for the pMM in this larger ACLR cohort was elevated compared with that in the current cohort of uninjured controls. The mean UTE-T2* value in the pMM of all ACL-reconstructed patients was 52% higher than that of the uninjured controls (14.9 ± 4.5 vs 9.8 ± 1.4 milliseconds, respectively; 2-tailed t test, P = .001). Within the subcohort of 16 ACL-reconstructed patients providing longitudinal 2-year UTE-T2* data, the mean UTE-T2* value of the pMM was 49% higher than that of the uninjured controls (14.6 ± 4.1 vs 9.8 ± 1.4 milliseconds, respectively; 2-tailed t test, P = .001).
Longitudinal UTE-T2* Changes in Articular Cartilage Over 2 Years
A qualitative evaluation of cartilage morphological characteristics by a musculoskeletal radiologist showed that articular surfaces found to be intact before ACLR remained intact through 2-year follow-up of the ROIs evaluated by UTE-T2* mapping. A qualitative evaluation of UTE-T2* maps of ACL-reconstructed patients showed an absence of a strongly laminar appearance of the MFC in 11 of 16 (69%) participants. At 2 years after ACLR, UTE-T2* maps showed a resumption of a more laminar appearance of the MFC in 10 of 11 patients without a laminar appearance at preoperative MRI. Overall, a laminar appearance was observed in 15 of 16 (94%) ACL-reconstructed patients at 2-year follow-up. This finding compares favorably with the distribution in uninjured controls, where UTE-T2* maps demonstrated a laminar appearance of the MFC in 11 of 11 (100%) participants.
Significant longitudinal UTE-T2* changes were detected in the medial compartment’s articular cartilage of ACL-reconstructed patients at 2-year follow-up (Figure 3D). The 16 ACL-reconstructed patients demonstrated 29% and 24% decreases in mean UTE-T2* values for deep articular cartilage of the cMFC and pMFC, respectively, and a 19% decrease in mean UTE-T2* values for the pMM (cMFC: mean drop of 4.6 milliseconds, from 15.9 ± 6.5 to 11.3 ± 3.3 milliseconds [WSRT, P = .02]; pFMC: mean drop of 4.7 milliseconds, from 19.2 ± 7.1 to 14.5 ± 8.1 milliseconds [WSRT, P = .02]; pMM: mean drop of 2.8 milliseconds, from 14.6 ± 4.1 to 11.8 ± 3.5 milliseconds [WSRT, P = .005]). No significant longitudinal UTE-T2* changes in the cMTP or pMTP, superficial or deep, were observed (WSRT, P > .12). No significant UTE-T2* changes in superficial cMFC or superficial pMFC cartilage were detected over the 2 years in ACL-reconstructed patients (WSRT, P > .11). At 2-year follow-up, UTE-T2* values measured in ACL-reconstructed patients did not demonstrate significant differences compared with UTE-T2* levels measured in uninjured controls in any region examined (2-tailed t test, P > .1).
Meniscus Status and Longitudinal UTE-T2* Values of Articular Cartilage
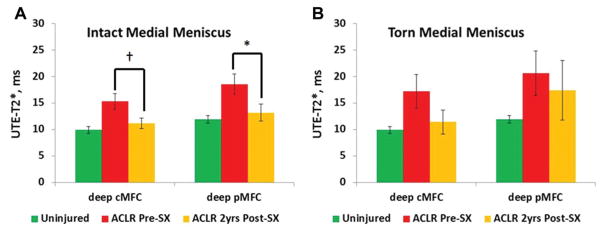
At ACLR, 5 of 16 ACL-reconstructed patients in the longitudinal cohort were found to have tears to the pMM by arthroscopic examination, 2 of whom underwent partial medial meniscectomy and 3 underwent meniscus preservation consisting of either repair or rasping. The change in UTE-T2* values of articular cartilage at 2 years after ACLR varied based on meniscus status at the time of surgery (Figure 4). In longitudinal ACL-reconstructed patients with intact medial menisci, preoperative UTE-T2* values in the deep cMFC were 54% higher than those in uninjured controls (15.3 ± 5.2 vs 9.9 ± 2.3 milliseconds, respectively; Mann-Whitney U test, P = .01), and UTE-T2* values in the deep pMFC were 56% higher (18.5 ± 6.3 vs 11.9 ± 2.4 milliseconds, respectively; MWUT, P = .02). Two years after ACLR, UTE-T2* values in deep cMFC cartilage in ACL-reconstructed patients with intact medial menisci (n = 11) showed a trend toward a decrease compared with preoperative values (from 15.3 ± 5.2 [before ACLR] to 11.2 ± 3.3 milliseconds [2 years after ACLR]; WSRT, P = .07), while UTE-T2* values of the deep pMFC decreased significantly (from 18.5 ± 6.3 [before ACLR] to 13.2 ± 5.2 milliseconds [2 years after ACLR]; WSRT, P = .01) to levels comparable with those of uninjured controls (KWT, P = .7). UTE-T2* values in the deep cMTP, deep pMTP, and superficial pMTP in participants with intact medial menisci showed no significant change over 2 years (P > .18), while UTE-T2* values of the superficial cMTP decreased 25% (from 29.7 ± 7.9 [before ACLR] to 22.4 ± 6.2 milliseconds [2 years after ACLR]; WSRT, P = .03).
Figure 4.
Change in ultrashort echo time (UTE)–T2* values of articular cartilage at 2 years after anterior cruciate ligament reconstruction (ACLR) varied based on meniscus status at the time of surgery. (A) Mean UTE-T2* values decreased 29% for deep posterior medial femoral condyle (pMFC) cartilage (P = .01) and showed a trend toward a decrease for deep central MFC (cMFC) cartilage (P = .07) 2 years after ACLR in joints with intact medial menisci (n = 11). (B) In ACL-reconstructed joints with torn medial menisci, no significant change in the mean UTE-T2* value of the MFC was detected (n = 5; P = .14 and .5 for deep cMFC and deep pMFC, respectively). Error bars indicate standard error of the mean. *P <.05. †Denotes a trend.
In contrast to the intact meniscus group, no significant decrease in UTE-T2* values was observed for deep cMFC cartilage (n = 5; WSRT, P = .14) or deep pMFC cartilage (n = 5; WSRT, P = .5) 2 years after ACLR in patients with torn medial menisci. While no differences had been observed at the time of surgery between UTE-T2* values of MTP cartilage of ACL-reconstructed patients with torn medial menisci and uninjured controls, a 62% increase in UTE-T2* values for the deep pMTP (from 12.2 ± 8.8 to 19.8 ± 6.5 milliseconds; WSRT, P = .04) was observed within this group 2 years after ACLR.
Longitudinal UTE-T2* Changes in the Meniscus Over 2 Years
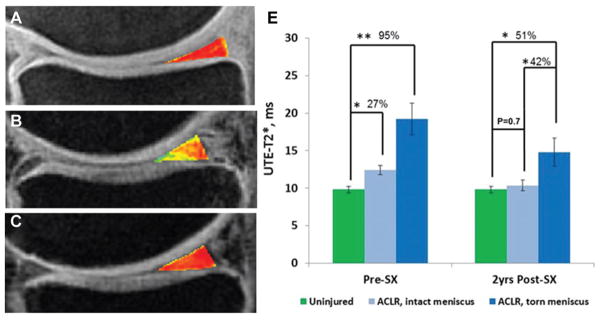
We previously reported pre-ACLR UTE-T2* data in the meniscus in 25 participants,32 inclusive of the 16 ACL-reconstructed patients in the longitudinal arm of the current study. Among the 16 participants in the longitudinal arm of this work, before ACLR, UTE-T2* values in the meniscus were elevated compared with those of uninjured controls and increased with increasing meniscus injuries (3 groups: uninjured controls, intact menisci, and torn menisci) (KWT, P <.0001) (Figure 5). The mean UTE-T2* value of the intact pMM of ACL-reconstructed patients (n = 11) was 27% higher than that of uninjured controls (n = 11) (12.5 ± 2.0 vs 9.8 ± 1.4 milliseconds, respectively; post hoc pairwise, P = .02), and the mean UTE-T2* value of the torn pMM in ACL-reconstructed patients (n = 5) was found to be 95% higher than that in uninjured controls (19.2 ± 3.8 vs 9.8 ± 1.4 milliseconds, respectively; post hoc pairwise, P < .0001) (Figure 5). Before ACLR, UTE-T2* values in torn menisci were not found to differ from those in intact menisci (post hoc pairwise, P > .14).
Figure 5.
Example ultrashort echo time (UTE)–T2* maps of the posterior medial menisci from (A) an uninjured control participant, (B) an anterior cruciate ligament (ACL)–reconstructed patient with an intact medial meniscus before surgery, and (C) the same patient 2 years after ACL reconstruction (ACLR). (D) UTE-T2* mapping demonstrates subsurface meniscus matrix changes in intact menisci of patients with ACL injuries. Before ACLR, UTE-T2* values in intact medial menisci of ACL-injured patients were 27% higher than those seen in uninjured controls (P = .02). Over 2 years after joint stabilization surgery, UTE-T2* values in intact menisci of ACL-reconstructed joints fell 17% (P = .03) to levels that did not differ from those of asymptomatic controls (P = .7). As expected, UTE-T2* values in torn menisci before surgery were significantly higher (95%) than those of uninjured controls (P <.0001). Two years after joint stabilization surgery, UTE-T2* values in torn menisci of ACL-reconstructed patients continued to demonstrate significant elevations compared with those in both asymptomatic controls (P = .02) and also intact menisci of ACL-reconstructed joints (P = .05). Error bars indicate standard error of the mean. *P <.05. **P <.01.
Within initially intact medial menisci of ACL-reconstructed patients, UTE-T2* values decreased 17% over 2 years (pMM: drop of 2.1 milliseconds [from 12.5 ± 2.0 to 10.4 ± 2.4 milliseconds]; WSRT, P = .03) to levels that did not differ from those measured in uninjured controls (MWUT, P = .7) (Figure 5). UTE-T2* values measured in torn medial menisci 2 years after ACLR, however, showed continued elevations compared with those of uninjured controls (n = 5 and 11, respectively; 14.8 ± 4.0 vs 9.8 ± 1.4 milliseconds, respectively; KST post hoc pairwise, P = .02) and were also elevated compared with those of ACL-reconstructed patients with intact medial menisci (n = 5 and 11, respectively; 14.8 ± 4.0 vs 10.4 ± 2.4 milliseconds, respectively; KWT post hoc pairwise, P = .05). Two-year MRI showed a new tear to the medial meniscus in 1 patient, whose medial meniscus was morphologically intact at the time of ACLR. The mean UTE-T2* value in this meniscus increased from 10 milliseconds before ACLR to 13 milliseconds 2 years after ACLR.
DISCUSSION
The current approach to the clinical treatment of OA is the palliation of symptoms arising from late-stage disease. Early-stage disease is clinically silent in that structural changes typically precede clinical signs and symptoms of pain, deformity, functional limitations, and disability. However, it is at these earliest stages when the articular surface and matrix remain intact and when pathological changes have the highest likelihood of reversal. Current clinical methods to detect subsurface damage are limited to arthroscopic probing for tissue softening, which is subjective and invasive. To support a paradigm shift toward the prevention of premature OA, new noninvasive methods to identify cartilage at risk before irreversible tissue disruption and loss are necessary.9
In this study, novel UTE-T2* mapping was shown to be sensitive to cross-sectional and longitudinal deep tissue matrix changes in the medial meniscus, MFC, and MTP after an ACL tear and reconstruction. Quantitative UTE-T2* mapping was found to vary with cartilage status as determined by injury status, morphological MRI, and arthroscopic grading, which are the current clinical standards for the assessment of articular cartilage. The information provided by UTE-T2* mapping was strongest in discriminating between cartilage and menisci of uninjured controls and those of ACL-reconstructed patients, where UTE-T2* values were significantly elevated even in ACL-reconstructed patients with arthroscopically normal articular cartilage and menisci. In this study in which 94% of ACL-reconstructed patients were arthroscopically Outer-bridge grade 0 to 2, UTE-T2* values of cartilage deep tissue were not significantly different between arthroscopic grades 0, 1, and 2. To fall within these arthroscopic grades, cartilage had either an intact surface or showed damage limited to superficial tissue. These findings highlight that a quantitative cross-sectional MRI technique optimized to evaluate cartilage deep tissue likely measures different aspects of articular cartilage than arthroscopic surface imaging and subjective tactile probing. While limited by small numbers and the absence of histopathology findings for the deep tissues evaluated, these data suggest that UTE-T2* mapping may be more sensitive to cartilage deep tissue matrix structures and changes than arthroscopic surgery.
The longitudinal data provide additional support for the possibility of increased sensitivity of UTE-T2* mapping to occult, subclinical deep cartilage and meniscus injuries. It is well established that a meniscus tear increases the risk of knee OA both in isolation and in combination with an ACL tear.20,24,27 This study showed that initially elevated UTE-T2* values in deep cartilage and medial menisci in patients without concomitant meniscus injuries significantly decreased 2 years after ACLR to levels consistent with those in uninjured controls. In contrast, no significant decrease in UTE-T2* values for the MFC and new elevation in UTE-T2* values in the submeniscus cartilage of the MTP were observed 2 years after ACLR in the higher OA risk category of those with combined ACL and meniscus tears. These findings are consistent with those of longer term outcome studies of ACLR, showing that joints without concomitant meniscus and cartilage injuries have a substantially lower risk for the subsequent development of premature OA.20,24,27
The finding that the initial elevation in UTE-T2* values in the MFC and pMM resolved over the first 2 years after ACLR in those without clinically evident medial meniscus tears has several interesting implications. First, the data point to injury sustained at the time of the ACL tear as the likely cause of the initial UTE-T2* changes in deep tissue. Second, a subsequent reduction of UTE-T2* signals in the lower OA risk group with intact menisci and restoration of a more lamellar pattern suggest that articular cartilage has some capacity to heal deep tissue injuries. Finally, the 2-year longitudinal findings of no significant decrease in UTE-T2* values for the MFC and new elevation in UTE-T2* values for the submeniscus cartilage of the MTP in the higher OA risk category with clinically evident meniscus tears at the time of ACLR are consistent with the hypothesis that an elevation in UTE-T2* is a sign of deep cartilage pathology.
While cartilage injuries to the lateral compartment corresponding to areas of bony contusion have been noted to be prevalent after ACL tears, damage to the MFC has been less obvious in cross-sectional studies.18,25 However, longitudinal data provide evidence for occult deep cartilage injuries and progressive pathological lesions to the medial compartment. Recently, Potter et al25 completed a prospective longitudinal cohort study of 42 knees with isolated ACL tears and showed MRI evidence of cartilage loss acutely after ACL tears in 66.7% of the lateral femoral condyles and 88.1% of the lateral tibial plateaus examined. In contrast, just 10% of the MFCs that were assessed showed cartilage loss on baseline structural MRI scans obtained at the time of injury. Of note, however, the adjusted risk for cartilage loss in the MFC doubled by 1 year and was 19 times baseline by years 7 to 11 after ACL tears. Another study by Frobell and colleagues15 similarly showed an increase in MFC volume, potentially indicative of cartilage swelling, the first year after an ACL tear. Of high clinical relevance, Potter et al25 noted that each increase in MFC cartilage loss reflected by a higher Outerbridge grade resulted in a significant reduction in subjective outcome measures. These longitudinal data suggest that elevations in UTE-T2* values of deep cartilage of the MFC of ACL-reconstructed patients observed in this study indicate matrix damage to the MFC that has escaped prior detection by structural MRI and routine arthroscopic examinations.
Several limitations should be considered in interpreting this study. First, arthroscopic assessments were available only for knees undergoing ACLR because it was not feasible to perform an arthroscopic examination on uninjured, asymptomatic participants and joints. Therefore, cartilage status was inferred to be healthy in this group based on history and MRI. Cartilage and menisci in knees sustaining ACL injuries cannot be considered normal, and indeed, even arthroscopically firm and intact cartilage (Outerbridge grade 0) from ACL-injured knees was found to show elevated UTE-T2* values compared with uninjured controls in this study. The use of uninjured controls as the best available approximation for healthy cartilage and menisci highlights the general difficulty in validating the clinical utility of a new metric that may be more sensitive than the current clinical standards of MRI-detected morphological changes and arthroscopic surgery. Furthermore, data from a relatively small number of participants were available for longitudinal analysis. Longer follow-ups with more participants are needed to determine the clinical relevance and predictive value of persistent elevations in UTE-T2* values in the development of clinical OA.
It is important to note that the T2* measurement reported in this work, UTE-enhanced T2* mapping, is not a pure measure of T2* in the UTE regimen. Instead, the UTE-T2* metric used here is calculated from a monoexponential fit routine and represents a weighted combination of all T2* decay components with measurable signals at echo times ranging from 0.6 milliseconds and 40 milliseconds. Notably, 7 of the echo images included in the T2* fit were acquired with echo times in the short echo time range (1–10 milliseconds). The degree to which measurements of purely short, or purely ultrashort, T2* signal relaxation aid in the detection of preclinical alterations to cartilage and meniscus matrices remains to be evaluated by sequences designed to specifically target those echo time regimes. However, the addition of short and ultrashort echo times in the T2* measurement presented here enhances sensitivity to short T2 signals emanating from deep articular cartilage and menisci that are not well evaluated by standard T2 and T1ρ.19,30
Other quantitative MRI metrics of cartilage health include the measurement of cartilage thickness and volume changes. While gross cartilage thinning and loss reflect progressive disease, thickness and volume changes provide an ambiguous picture of cartilage health in early OA and acutely after ACL injuries. Increases in cartilage thickness after ACL disruption have been shown to be the result of both proteoglycan synthesis1 and cartilage swelling presumably due to proteoglycan loss.14 Regionally, varying degrees of cartilage thinning have also been observed 3 to 30 months after ACL injuries, but the risk for cartilage loss appears to be mediated by concomitant meniscus injuries.3,14 Consequently, quantitative metrics reflective of cartilage matrix health such as UTE-T2* mapping are needed to assist in the interpretation of thickness and volume changes in pre-OA knees after ACLR and in early OA.
CONCLUSION
Through cross-sectional and longitudinal evaluations, this study shows both a potential for intact articular surfaces of cartilage to heal deep tissue injuries and the diagnostic potential of UTE-T2* mapping, a new noninvasive quantitative MRI metric, to track clinical disease states. The data further suggest that this metric may, in some instances, reflect deep tissue matrix changes not well appreciated by the current clinical standards of arthroscopic surgery and morphological MRI. Furthermore, a return of both qualitative and quantitative UTE-T2* values to those of uninjured controls 2 years after ACLR suggests healing. These findings also demonstrate the sensitivity of UTE-T2* mapping to matrix changes over a relatively short period of time, during which morphological MRI remained constant. This type of noninvasive quantitative imaging metric for the early detection of cartilage and meniscus damage at potentially reversible stages is critically important to the development of new strategies to prevent or delay the onset of OA.
Acknowledgments
The authors acknowledge James J. Irrgang, PT, PhD, ATC, FAPTA (Department of Orthopaedic Surgery, University of Pittsburgh), and Tim D. Oury, MD, PhD (Department of Pathology, University of Pittsburgh).
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was funded by the National Institutes of Health (R01 AR052784; principal investigator: C.R.C.). Y.Q. has a patent on the acquisition-weighted stack of spirals imaging method.
References
- 1.Adams ME, Brandt KD. Hypertrophic repair of canine articular cartilage in osteoarthritis after anterior cruciate ligament transection. J Rheumatol. 1991;18(3):428–435. [PubMed] [Google Scholar]
- 2.Akella SV, Regatte RR, Gougoutas AJ, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med. 2001;46(3):419–423. doi: 10.1002/mrm.1208. [DOI] [PubMed] [Google Scholar]
- 3.Amin S, Guermazi A, Lavalley MP, et al. Complete anterior cruciate ligament tear and the risk for cartilage loss and progression of symptoms in men and women with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(8):897–902. doi: 10.1016/j.joca.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41(5):857–865. doi: 10.1002/(sici)1522-2594(199905)41:5<857::aid-mrm1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Bear DM, Szczodry M, Kramer S, Coyle CH, Smolinski P, Chu CR. Optical coherence tomography detection of subclinical traumatic cartilage injury. J Orthop Trauma. 2010;24(9):577–582. doi: 10.1097/BOT.0b013e3181f17a3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carballido-Gamio J, Joseph GB, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T2 knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: a texture approach. Magn Reson Med. 2011;65(4):1184–1194. doi: 10.1002/mrm.22693. [DOI] [PubMed] [Google Scholar]
- 7.Chu CR, Izzo NJ, Irrgang JJ, Ferretti M, Studer RK. Clinical diagnosis of potentially treatable early articular cartilage degeneration using optical coherence tomography. J Biomed Opt. 2007;12(5):051703. doi: 10.1117/1.2789674. [DOI] [PubMed] [Google Scholar]
- 8.Chu CR, Williams A, Tolliver D, Kwoh CK, Bruno S, 3rd, Irrgang JJ. Clinical optical coherence tomography of early articular cartilage degeneration in patients with degenerative meniscal tears. Arthritis Rheum. 2010;62(5):1412–1420. doi: 10.1002/art.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu CR, Williams AA, Coyle CH, Bowers ME. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res Ther. 2012;14(3):212. doi: 10.1186/ar3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient: a prospective outcome study. Am J Sports Med. 1994;22(5):632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 11.Dardzinski BJ, Mosher TJ, Li S, Van Slyke MA, Smith MB. Spatial variation of T2 in human articular cartilage. Radiology. 1997;205(2):546–550. doi: 10.1148/radiology.205.2.9356643. [DOI] [PubMed] [Google Scholar]
- 12.David-Vaudey E, Ghosh S, Ries M, Majumdar S. T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging. 2004;22(5):673–682. doi: 10.1016/j.mri.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 13.Du J, Takahashi AM, Chung CB. Ultrashort TE spectroscopic imaging (UTESI): application to the imaging of short T2 relaxation tissues in the musculoskeletal system. J Magn Reson Imaging. 2009;29(2):412–421. doi: 10.1002/jmri.21465. [DOI] [PubMed] [Google Scholar]
- 14.Frobell RB. Change in cartilage thickness, posttraumatic bone marrow lesions, and joint fluid volumes after acute ACL disruption: a two-year prospective MRI study of sixty-one subjects. J Bone Joint Surg Am. 2011;93(12):1096–1103. doi: 10.2106/JBJS.J.00929. [DOI] [PubMed] [Google Scholar]
- 15.Frobell RB, Le Graverand MP, Buck R, et al. The acutely ACL injured knee assessed by MRI: changes in joint fluid, bone marrow lesions, and cartilage during the first year. Osteoarthritis Cartilage. 2009;17(2):161–167. doi: 10.1016/j.joca.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin DW, Wadghiri YZ, Zhu H, Vinton CJ, Smith ED, Dunn JF. Macroscopic structure of articular cartilage of the tibial plateau: influence of a characteristic matrix architecture on MRI appearance. AJR Am J Roentgenol. 2004;182(2):311–318. doi: 10.2214/ajr.182.2.1820311. [DOI] [PubMed] [Google Scholar]
- 17.Hurtig M, Chubinskaya S, Dickey J, Rueger D. BMP-7 protects against progression of cartilage degeneration after impact injury. J Orthop Res. 2009;27(5):602–611. doi: 10.1002/jor.20787. [DOI] [PubMed] [Google Scholar]
- 18.Johnson DL, Urban WP, Jr, Caborn DN, Vanarthos WJ, Carlson CS. Articular cartilage changes seen with magnetic resonance imaging-detected bone bruises associated with acute anterior cruciate ligament rupture. Am J Sports Med. 1998;26(3):409–414. doi: 10.1177/03635465980260031101. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Kuo D, Theologis A, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2. Initial experience with 1-year follow-up. Radiology. 2011;258(2):505–514. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 21.Martin JA, McCabe D, Walter M, Buckwalter JA, McKinley TO. N-acetylcysteine inhibits post-impact chondrocyte death in osteochondral explants. J Bone Joint Surg Am. 2009;91(8):1890–1897. doi: 10.2106/JBJS.H.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menezes NM, Gray ML, Hartke JR, Burstein D. T2 and T1rho MRI in articular cartilage systems. Magn Reson Med. 2004;51(3):503–509. doi: 10.1002/mrm.10710. [DOI] [PubMed] [Google Scholar]
- 23.Nelson F, Billinghurst RC, Pidoux I, et al. Early post-traumatic osteoarthritis-like changes in human articular cartilage following rupture of the anterior cruciate ligament. Osteoarthritis Cartilage. 2006;14(2):114–119. doi: 10.1016/j.joca.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37(7):1434–1443. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 25.Potter HG, Jain SK, Ma Y, Black BR, Fung S, Lyman S. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med. 2012;40(2):276–285. doi: 10.1177/0363546511423380. [DOI] [PubMed] [Google Scholar]
- 26.Qian Y, Boada FE. Acquisition-weighted stack of spirals for fast high-resolution three-dimensional ultra-short echo time MR imaging. Magn Reson Med. 2008;60(1):135–145. doi: 10.1002/mrm.21620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery: five- to fifteen-year evaluations. Am J Sports Med. 2000;28(4):446–452. doi: 10.1177/03635465000280040201. [DOI] [PubMed] [Google Scholar]
- 28.Shikhman AR, Amiel D, D’Lima D, et al. Chondroprotective activity of N-acetylglucosamine in rabbits with experimental osteoarthritis. Ann Rheum Dis. 2005;64(1):89–94. doi: 10.1136/ard.2003.019406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szczodry M, Coyle CH, Kramer SJ, Smolinski P, Chu CR. Progressive chondrocyte death after impact injury indicates a need for chondroprotective therapy. Am J Sports Med. 2009;37(12):2318–2322. doi: 10.1177/0363546509348840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams A, Qian Y, Bear D, Chu CR. Assessing degeneration of human articular cartilage with ultra-short echo time (UTE) T2* mapping. Osteoarthritis Cartilage. 2010;18(4):539–546. doi: 10.1016/j.joca.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams A, Qian Y, Chu CR. UTE-T2* mapping of human articular cartilage in vivo: a repeatability assessment. Osteoarthritis Cartilage. 2011;19(1):84–88. doi: 10.1016/j.joca.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams A, Qian Y, Golla S, Chu CR. UTE-T2* mapping detects subclinical meniscus injury after anterior cruciate ligament tear. Osteoarthritis Cartilage. 2012;20(6):486–494. doi: 10.1016/j.joca.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]