Abstract
Background
Bordetella pertussis colonizes the human respiratory mucosa. Most studies on B. pertussis adherence have relied on cultured mammalian cells that lack key features present in differentiated human airway cells or on animal models that are not natural hosts of B. pertussis. The objectives of this work are to evaluate B. pertussis infection on highly differentiated human airway cells in vitro and to show the role of B. pertussis fimbriae in cell adherence.
Methods
Primary human airway epithelial (PHAE) cells from human bronchi and a human bronchial epithelial (HBE) cell line were grown in vitro under air-liquid interface conditions.
Results
PHAE and HBE cells infected with B. pertussis wild type strain revealed bacterial adherence to cell’s apical surface and bacterial induced cytoskeleton changes and cell detachment. Mutations in the major fimbrial subunits Fim2/3 or in the minor fimbrial adhesin subunit FimD affected B. pertussis adherence to predominantly HBE cells. This cell model recapitulates the morphologic features of the human airway infected by B. pertussis and confirms the role of fimbriae in B. pertussis adherence. Furthemore, HBE cells show that fimbrial subunits, and specifically FimD adhesin, are critical in B. pertussis adherence to airway cells.
Conclusions
The relevance of this model to study host-parasite interaction in pertussis lies in the striking physiologic and morphologic similarity between the PHAE and HBE cells and the human airway ciliated and goblet cells in vivo. These cells can proliferate in vitro, differentiate, and express the same genetic profile as human respiratory cells in vivo.
Keywords: Bordetella pertussis, fimbriae major subunit Fim2 or Fim3, minor subunit FimD, adherence
INTRODUCTION
Bordetella pertussis is a Gram-negative microorganism directly implicated in the causation of whooping cough or pertussis, a highly transmissible infection of the respiratory tract (1). Pertussis is associated with severe manifestations in susceptible infants, including pneumonia, seizures, encephalopathy, apnea, and death (2,3). Pertussis incidence decreased after the introduction of the whole-cell pertussis vaccine; however, pertussis infection rates have been increasing in the US since 1980 (4). In 1999, an estimated 48.5 million cases and 295,000 deaths occurred worldwide due to pertussis (5). Pertussis persists because neither vaccination nor natural infection induce long-lasting immunity (6). Epidemiological data, particularly in the US, suggest that waning immunity may be even more rapid following acellular pertussis vaccination, although valid head-to-head comparisons of whole-cell pertussis and acellular pertussis vaccines do not exist (7–9). In addition, waning immunity may be aggravated by pathogen adaptation and genetic variation (10).
B. pertussis attachment to the respiratory epithelium, the first step in the infection process, is mediated by a number of appendages, including filamentous hemagglutinin (FHA), pertactin, and fimbriae. These bacterial surface-located molecules facilitate B. pertussis colonization of the respiratory tract and establishment of the disease process. B. pertussis fimbriae have two serologic major subunits, Fim2 and Fim3, with a molecular mass of 22 KDa each. Phase variation-controlled fimbrial expression results in B. pertussis strains expressing one or more types of fimbrial major subunits (Fim2, Fim3, FimX) at a time (11). The gene cluster containing the biosynthetic genes for fimbriae also contain the genes necessary for expression of FHA (12). Based on crystallography analysis, B. pertussis fimbriae have a helical and polar structure (13). Fimbriae are expressed during human infection and are immunogenic (14). Both major and minor fimbrial subunits have binding properties implicated in cell adherence and their relative role in colonization of the respiratory tract has yet to be determined. FimD is a minor subunit protein located at the fimbrial tip, and similar to other fimbriae from Gram-negative bacteria, it functions as a highly specific adhesin to host surface receptors (15,16). The major and minor fimbrial subunits were implicated in fimbriae-mediated adherence of B. pertussis to laryngeal cells based on mutation analysis. The B316 B. pertussis mutant strain, which lacks all fimbrial subunits, adhered significantly less to laryngeal cells than even the B52 mutant strain, which does not express fim2 and fim3, indicating that the FimD minor fimbrial adhesin subunit is crucial for adherence of B. pertussis to laryngeal cells (17). Since mice are not the natural host for B. pertussis infection it is unclear if these findings are representative of fimbrial adherence in the human host.
Limited information is available on the mechanism of FimD adherence, the specific host cell receptors, or how it contributes to colonization of human respiratory mucosa. In humans, the upper and lower airways are protected by an epithelium that provides a physical barrier between inspired air and the underlying respiratory tract tissue. The epithelium generates a mucociliary movement that clears particulate material, including pathogenic bacteria, from the airway and keep them from reaching the lower lungs (18). Studies of B. pertussis and its adhesins have focused on cultured mammalian cells lacking most of the characteristics of human airway epithelial cells. Only studies in vitro and in animal models report that FimD may contribute to the colonization of the mouse respiratory tract (19). Primary human airway epithelial (PHAE) cells, derived from donor tracheal and bronchial tissue, are grown in culture under an air-liquid interface where they form an epithelium manifesting ciliated, goblet, and basal cells that morphologically mimic human airway epithelium in vivo (20). Furthermore, the transcriptional profile of PHAE cells closely resembles that of in vivo airway epithelia (21).
The objective of this work was to evaluate B. pertussis infection in vitro and as a proof-of-principle demonstrating the role of B. pertussis fimbriae in adherence to primary human airway epithelial cells. PHAE and HBE cells were infected with wild type B. pertussis and fimbrial mutants and bacterial-cell interaction were evaluated by microscopy and by cell-associated bacterial assays. This model showed that B. pertussis fimbrial adhesion is involved in adherence to human airway epithelial cells. The primary airway cell infection model is suitable to study host-parasite interactions involved in the pathogenesis of B. pertussis infection.
MATERIALS AND METHODS
Strains
B. pertussis Tohama I parental wild-type strain, B. pertussis BP536 strain (BP356, Fim2+, Fim3−, FimD+) and its fimbrial mutant derivatives, the major subunit fimbrial mutant (B52, fim2::SacI, fim3::kan; Fim2−, Fim3−, FimD+) and the fimbrial adhesion mutant (B316, fimB::kan; Fim2−, Fim3−, FimD−) were used (19,22). B. pertussis BP536 strain produces Fim2 and FimD and negligible amounts of Fim3 in vitro. Furthemore, this strain has the potential to express fim3 by a phase variation-mediated promoter-unlocking mechanism (11). B. pertussis strains grown on Bordet Gengou (BG) agar (BD, Franklin Lakes, NJ) for 5–7 days at 37°C with 5% CO2 were harvested and resuspended in 500µL PBS to reach an OD (A600nm) 1.0 or 1×107 colony forming units (CFU) per mL for cell infections.
Culture cells
Primary human airway epithelia (PHAE) cultures from human bronchi were obtained from the In Vitro Models and Cell Culture Core, Cystic Fibrosis Research Center, University of Iowa College of Medicine (http://www.medicine.uiowa.edu/genetherapy/Cell_Tissue/). The human bronchial tissue sample collection was approved by the University of Iowa Institutional Review Board (IRB-01-Biomedical) as described before (23,24). Also, all samples are anonymized. The isolation and processing of PHAE was previously described (20). This in vitro airway culture model manifests polarized epithelial cells with tight junctions and distinct apical and basolateral membranes. Cells were maintained at 37°C with 5% CO2. The basolateral surface of the PHAE culture was maintained with airway medium 2 (1:1 ratio of DMEM and Ham’s F-12 supplemented with 2% Ultroser G [BioSepra S.A., France]) while the apical surface was exposed to air.
An immortalized bronchial epithelial 16HBE14 (HBE) cell line was grown in 75 cm2 cell culture flasks with BEBM media (Lonza Corporation, Walkersville) at 37°C with 5% CO2 (25). When 80% confluence was obtained, HBE cells were trypsinized and resuspended in BEBM. Approximately 1.5×104 cells were seeded onto the apical side of Millicell filter inserts (Millipore, PIHA 012 50) that were pretreated with type VI human placental collagen (Sigma-Aldrich). The following day 500 µL of fresh Ultroser G serum substitute USG media (BioSepra S.A., France) was added basolaterally and cells were incubated in air-liquid interface at 37°C in 5% CO2 for 10–15 days with USG media changes every 2 days (26,27). Human cervical carcinoma HeLa cells (ATCC CCL-2.2) were cultured on filter inserts with Dulbecco’s minimal Eagle medium (Gibco) at 37°C with 5% CO2. The following day, 500 µL of fresh Ultroser G serum substitute USG media was added basolaterally and cells were incubated at 37°C in 5% CO2 for 10–15 days with USG media changes every 2 days to obtain polarized cell multilayers.
Cell viability assay
Viability of HBE cells infected with B. pertussis strains was evaluated by MTT (3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Molecular probes Vybrant® MTT Cell Proliferation Assay Kit, Grand Island, NY) at 3, 9, 12 and 24 hours after bacterial infection. The extent to which MTT is reduced to a purple formazan dye has been correlated to cell viability (28). Cleavage of MTT by bacteria did not influence this assay, as the bacteria did not affect the absorbance at 570 nm. Uninfected epithelial sheets were used as negative controls.
Adherence assays
Epithelial cells infected with 5µL of B. pertussis bacterial suspension (1×107 CFU per mL) in PBS were incubated at 37°C with 5% of CO2. After incubation (for 1 to 12h depending the assay), cells were washed three times with PBS to remove unbound bacteria. For quantitative analysis, cells were lysed with 100µL of 1% Triton X-100 in PBS for 10 min, serially diluted, and plated on Bordet Gengou agar plates for further CFU/mL counting. Quantitative analysis of adherence assays was performed in duplicate and repeated several times. For microscopy imaging, cells were fixed and processed for examination under transmission electron microscopy as described below.
Inhibition assays were conducted as conventional HBE-cell quantitative adherence assays
Cells were supplemented with putative adherence inhibitors, added immediately before infection. The inhibitors tested included purified Fim2/3 fimbrial antigen from B. pertussis (Sanofi Pasteur, Swiftwater, PA), purified FimD protein (GenScript, Piscataway, NJ), and purified LngA protein (GenScript, Piscataway, NJ).
Gentamicin invasion assays
Gentamicin assays to evaluate B. pertussis invasion of HBE cells followed conventional adherence assays (29). In brief, infected cells were washed 3 times after 9 h of incubation, and supplemented with BEBM media containing with 50 µg/mL gentamicin. Cells incubated at 37°C in 5% CO2 for 60 min were then washed 3 times with PBS followed by cells lysis with 100µL of 1% Triton X-100 in PBS for 10 min. Cell lysates were serially diluted and plated on Bordet Gengou agar plates for CFU counting.
Electron microscopy
Transmission electron microscopy was used to evaluate the adherence of B. pertussis to PHEA and HBE cells. Infected cells on air-liquid interface membranes were fixed serially with 2.5% glutaraldehyde and 1% osmium tetroxide and 1% uranyl acetate. Samples were dehydrated serially with increasing concentrations of ethanol (25%, 50%, 75%, 100%) before embedding with EPON812 (Electron microscopy sciences, Washington, PA) following standard procedures (30). Slice sections 0.5-mm-thick were created with a diamond knife and stained with toluidine blue for light microscopy examination before visualization under a transmission electron microscope (JEOL lOOC-ASID).
Samples to be evaluated by scanning electron microscope (SEM) were dehydrated, embedded, and mounted onto metallic stubs with double-sided carbon tape, sputter coated with a thin layer of metals (gold and palladium) and visualized. Evaluation of B. pertussis-infected cells by light microscopy (LM) required formalin fixation of millicell membranes containing cells followed by embedding in paraffin. Microtome sections from the paraffin block were stained with Giemsa, toluidine blue, or hematoxylin and eosin before visualization under the LM.
Production of mice antisera to Fim2, Fim3 and FimD B. pertussis
recombinant proteins Anti-Fim2, anti-Fim3 or anti-FimD mouse polyclonal antisera were obtained after peritoneal immunization of 7- to 8-week old BALB/c mice with 30 µg of either Fim2, Fim3 or FimD recombinant proteins mixed with incomplete Freund adjuvant. Each group of mice was immunized every two week for a total of three immunizations. Ten days after the last immunization, sera was collected from all mice and stored at −80°C for further experiments. These animal experiments were approved by the IACUC committee at Vanderbilt University School of Medicine.
Confocal Laser Scanning Microscopical (CLSM) Analyses
Bacterial samples were grown as described above, and analyzed by confocal laser scanning microscopy as previously described (31). Briefly, either fim2 and fimD-expressing wild type B. pertussis, or fimbrial mutants harboring inactivation of fim2, fim3, or fim2/3-fimD were grown onto BG plates for 3-days aerobically at 37°C. E. coli DH5α (as a negative control) were grown on LB agar plates overnight at 37°C. Cells were resuspended in phosphate buffered saline (pH 7.4) and collected by centrifugation and blocked with 0.1% bovine serum albumin for 1 hour before either the anti-Fim2 or anti-FimD antibodies were applied to cells, for one hour. Cells were washed with PBS three times before incubated with anti-mice IgG (conjugated to Alexa Fluor 488) for 30 minutes, washed, mounted and visualized with a Zeiss LSM 710. Images were analyzed in both widefield and confocal modalities at 630× magnification and micrographs were collected with Zen 2010 software. Micrographs shown are representative of three biological replicates.
Immunoblotting
Proteins present in whole cell lysates from fim2 and fimD-expressing B. pertussis wild type strain and fim2/3 and fim2/3-fimD mutants were separated by three independent electrophoresis on pre-cast 4–20% gradient SDS-PAGE gel (Bio-Rad, Hercules, CA) and transferred to three nitrocellulose membranes by Semi-dry Blotting Unit Electrophoresis System (Thermo Fisher Scientific, Waltham, MA). Each nitrocellulose membrane was blocked with 10% skim milk/PBS-T for one hour, washed three times with PBS-T, probed with either anti-Fim2, anti-Fim3, or anti-FimD mice sera in 2% skim milk/PBS-T for one hour, washed three times with PBS-T, probed by IRDye® 680LT goat anti-mouse IgG (LI-COR, Lincoln, NE) for one hour, washed three times with PBS-T, and scanned by the LI-COR Odyssey Infrared Imaging System at 700 nm channel. Media was used as negative control and recombinant purified Fim2, Fim3 or FimD were used as positive controls. Fim2, Fim3 and FimD recombinant proteins were ordered from GenScript, Piscataway, NJ.
Statistical analysis
Differences in adherence between wild-type and mutant B. pertussis strains were evaluated by ANOVA; p-values less than 0.05 were considered statistically significant. Adherence values were the mean of duplicate or triplicate samples. All experiments were performed at least in duplicate.
RESULTS
B. pertussis
adheres to both PHAE and HBE cell lines In this study we used PHAE cells to evaluate B. pertussis adherence, invasion, and cytoskeletal changes. PHAE cells form a pseudostratified epithelium comprised of ciliated, goblet and basal cells that morphologically mimic human airway epithelium in vivo (Figure 1, panels A and E).
Figure 1. Primary human airway epithelial (PHAE) cells infected with B. pertussis wild-type strain.
PHAE cells were infected with B. pertussis BP536 Tohama I wild-type strain for 6 h before processing for microscopy. Panels A and E, uninfected PHAE cells. Panels B, C, D, and F, cells infected with B. pertussis wild-type strain. Panels A, B, C and D are transmission electron-micrographs. Panels E and F are toluidine blue stained light micrographs. Arrows in panel B, C and D indicate B. pertussis. Arrows in panel F indicate cell detachment and cell flattening.
PHAE cells infected with fim2 and fimD-expressing B. pertussis wild type strain showed that bacteria not only adhered to the PHAE cell surfaces but also induced cytoskeleton changes. Several patterns were observed associated with B. pertussis adherence to cells. First, bacteria tended to adhere in clusters (Figure 1, panel B and D); they attached along the cilial tufts (Figure 1, panel B to D); the bacterial attachment to the cilial surface was intimate (Figure 1, panel C and D); and bacteria also adhered to non-cilia membrane projections (Figure 1, panel C). No invasion of PHAE cells by B. pertussis was observed. Cytoskeletal changes induced by B. pertussis on PHAE cells included effacement of cilia structures, flattening of cells, and cell detachment (Figure 1, panel F). These changes were more prominent when bacterial-cell infection time increased to 9, 12 or 24h (data not shown).
The HBE cell line, derived from goblet and basal cells, expressed mucus at the apical side but did not express ciliated epithelium (Figure 2, panel A). Bacteria adhered to the membrane surfaces (Figure 2, panels B and C) with some bacteria intimately attached to the membrane (Figure 2, panel C). B. pertussis invaded cells and replicated inside the cytoplasm (Figure 2, panel D). HBE cells infected with B. pertussis also underwent morphological changes including: rounding of the cell body and cell detachment from the cell multilayer. Cytoskeletal changes among infected cells were observed at 6h (Figure 2, panel F) and increased overtime. An inverse relationship was observed between the infection time and viability. HBE cell viability of infected cells was close to 100% between 3 to 6 h, when infection time increased from 9 to 12 h cell viability decreased to 50% (data not shown). Uninfected cells remained viable in culture for days. Accordingly, a time point of 6h infection assays was used to evaluate cells for adherence.
Figure 2. Human airway epithelial (HBE) cells infected with B. pertussis wild-type strain.
HBE cells were infected with B. pertussis BP536 Tohama I wild-type strain for 6 h before processing for microscopy. Panels A and E, are uninfected HBE control cells; panels B, C, D and F are cells infected with B. pertussis. Panels A, B, C and D are transmission electron-micrographs. Panels E and F are haematoxilin/eosin light micrographs. Arrows in panels B, C and D indicate B. pertussis. Arrow in panel F indicate cell detachment after B. pertussis infection.
B. pertussis fimbriae are involved in adherence to airway epithelial cells
To evaluate the role of B. pertussis fimbriae on adherence PHAE and HBE cells were used. We tested fim2 and fimD-expressing B. pertussis wild-type strain and its fimbrial mutant derivatives in in-vitro bacterial-cell association assays. These assays showed that fim2 and fimD-expressing B. pertussis adhered to both PHAE and HBE cells in a fimbriae-dependent manner. Both fim2/3 and fim2/3-fimD mutant strains adhered at lower levels to both PHAE and HBE cells compare to wild-type strain. Wild-type adherence to HBE cells was more than three logs higher than the fim2/3-fimD mutant strain (p<0.05). Fimbriae-mediated B. pertussis adherence to HBE cells seemed to depend more on FimD than on Fim2. Wild-type fim2 and fimD-expressing B. pertussis adhered to PHAE cells six-fold as much as the fim2/3-fimD mutant strain (p<0.05) (Figure 3, panel A). B. pertussis strains adhered to HeLa cells at lower levels and, in contrast to PHAE and HBE cells, the difference in adherence between wild type and the fim2/3-fimD mutant strain was only three-fold (Figure 3, panel A). To confirm expression of fimbrial proteins among B. pertussis wild type and mutant strains immunoblot analysis was conducted on SDS-PAGE separated protein transferred to nitrocellulose membranes using anti-Fim2, anti-Fim3, and anti-FimD antibodies. B. pertussis wild type strain produced Fim2 mayor subunit and FimD adhesin (Figure 3. panel B). The anti-Fim3 antibody recognizes the Fim3 recombinant protein positive control and it cross-reacts with Fim2 produced by B. pertussis Tohama and B536 strains (Data not shown). Fim2 and Fim3 subunits have a molecular mass of 22 kDa while the FimD protein was 40kDa. Neither fim2/3 nor fim2/3-fimD mutant strains expressed Fim2 or Fim3. Only fim2/3 mutant expressed FimD adhesin subunit (Figure 3. panel B).
Figure 3. Bacterial-cell association assays using epithelial cells infected with B. pertussis strains.
Panel A. Fimbriae-mediated B. pertussis adherence to epithelial cells. PHAE, HBE, and HeLa cells infected with B. pertussis BP536 Tohama I wild-type strain for 6h were used for quantitative adherence assays. Cell infections were conducted in duplicate. Number of colony forming units per mL (CFU/mL) results are shown (logarithmic scale). WT: B. pertussis BP536 wild-type strain (B. pertussis Tohama I derivative). Fim2/3: fim2/3 B. pertussis mutant; fim2/3-fimD: B. pertussis BP536 fim2/3-fimD mutant. The difference in adherence between wild-type and fim2/3-fimD mutant for each type of PHAE, HBE, and HeLa cells was statistically significant (p<0.05). The difference in adherence between wild type and the fim2/3 mutant was only significant with HBE and HeLa cells (p<0.05), not for PHAE cells. This figure is a representative experiment conducted in duplicate and repeated two to three times. Asterisks on B. pertussis fimbrial mutants represent statistically significant differences in adherence to cells when compared to B. pertussis wild type. Panel B. Immunoblots to evaluate expression of Fim2 and FimD proteins among B. pertussis wild-type strains (Tohama and BP536), B. pertussis fim2/3 mutant strain, or B. pertussis fim2/3-fimD mutant strain. Proteins are derived from whole cell lysates that after separation in an SDS-PAGE, were transferred to a membrane and evaluated with anti-Fim2 or anti-FimD mouse polyclonal antibodies. Arrow indicates the location of the protein of interest and the corresponding mass.
Major subunit Fim2 and adhesin subunit FimD proteins are exposed on B. pertussis surface
CLSM analysis of bacterial cells revealed that fim2 and fimD-expressing B. pertussis wild type strain secretes both FimD and Fim2 on its cell surface in abundance, a result that was not seen with a negative control sample containing E. coli DH5α (Figure 4). As expected, a fim2/3/D triple mutant does not have FimD nor Fim2 decorating its outer membrane. Interestingly, the fim2/3 double mutant produces FimD, but not Fim2 on its cell surface. These results indicate that the fim2/3/D loci are critical for deployment of FimD and Fim2 to the surface of B. pertussis, but that the fim2/3 loci are dispensible for arrangement of FimD on the outer membrane (Figure 4).
Figure 4. Fim2 and FimD proteins surface exposure by B. pertussis.
Confocal Laser Scanning Microscopical (CLSM) Analyses were conducted to evaluate the presence of Fim2 or FimD proteins on the surface of B. pertussis strains. Mouse anti-Fim2 polyclonal antibody or mouse anti-FimD polyclonal antibodies were used to label negative control E. coli DH5α, B. pertussis wild-type strain, B. pertussis fim2/3 mutant strain, or B. pertussis fim2/3-fimD mutant strain. Images were taken at 630× magnification and scale bars indicate 50 µm.
Wild-type B. pertussis attached to ciliated surfaces at apical regions on PHAE cell
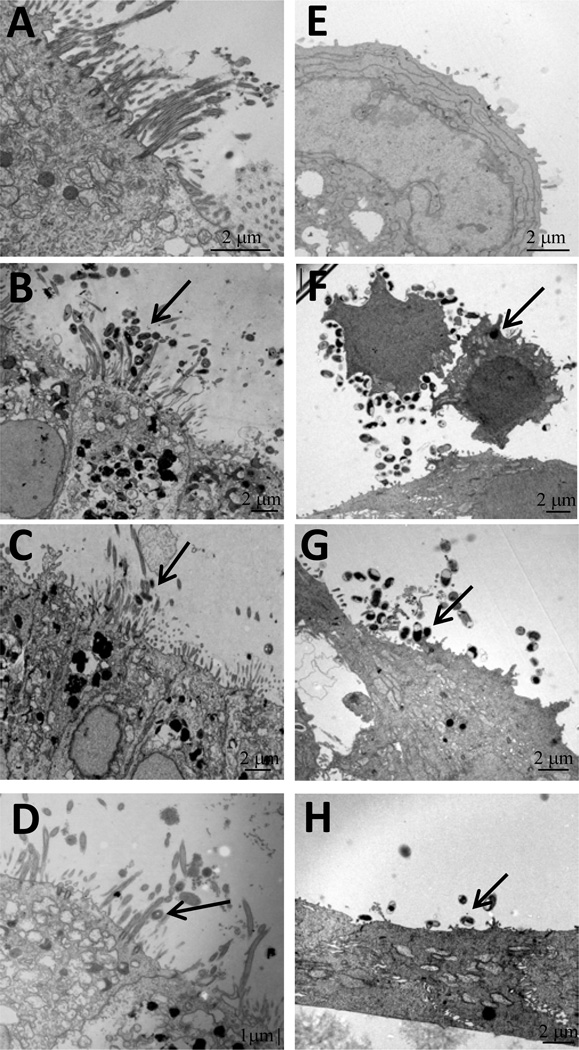
While some bacteria were observed binding to cilia or apical membrane surfaces individually, most bacteria were observed binding in clusters (Figure 5, panel B). The fim2/3 and fim2/3-fimD mutant strains were observed adhering to PHAE-cell cilia, although in lesser numbers as compared to the fim2 and fimD-expressing B. pertussis wild type strain (Figure 5, panels C and D). HBE cells infected with wild-type B. pertussis also showed bacteria binding in clusters to HBE cell membranes (Figure 5, panel F). The fim2/3 and fim2/3-fimD mutants adhered in lower numbers than wild-type B. pertussis (Figure 5, panels G and H). Gentamicin assays demonstrated marginal difference in invasion of HBE cells by wild-type, fim2/3 mutant, and fim2/3-fimD mutant B. pertussis strains (Data not shown).
Figure 5. PHAE and HBE cells infected with B. pertussis wild-type and fimbrial mutant strains.
HBE cells were infected for 6 h with B. pertussis wild-type strain, fim2/3 mutant, or fim2/3-fimD mutant strains before processing for transmission electron-microscopy as described in material and methods. Panels A to D show PHAE cells; panels E to H, HBE cells; panel A and E, uninfected cells; panels B and F, B. pertussis wild-type; panel C and G, B. pertussis fim2/3 mutant strain; panel D and H, B. pertussis fim2/3-fimD mutant strain.
Purified fimbrial subunit proteins compete with B. pertussis fimbriae for binding to HBE cells
To evaluate the specificity of B. pertussis fimbrial-mediated binding to human airway epithelial cells we compared HBE cells infected with fim2 and fimD-expressing B. pertussis in the presence of purified Fim2/3 and FimD proteins as competitors. Fim3 protein was used in combination with Fim2 to block any negligible amount of Fim3 being produced by the fim2- and fimD-expressing B. pertussis wild type strain. HBE cells were selected for these competition studies because they adhere in a fimbrial-dependent manner and at significantly higher levels compared with PHAE cells. Adherence of fim2 and fimD-expressing B. pertussis wild-type strain to HBE cells was inhibited by purified Fim2/3 in a dose-dependent manner (Figure 6, panel A). Ten µg/mL of purified Fim2/3 proteins were required to decrease binding of B. pertussis to HBE cells by more than 50%. The effect of purified FimD protein on B. pertussis competition for binding to HBE cells was more dramatic, as only 15 ng/mL were sufficient to decrease fim2 and fimD-expressing B. pertussis wild-type strain adherence to the level of the fim2/3-fimD mutant (Figure 6, panel B). Adherence of fimbrial mutant derivatives to HBE cells did not change at any concentration of the purified fimbrial subunit proteins, suggesting that fimbrial-dependent adherence was specific. We have used purified LngA – major fimbrial subunit of enterotoxigenic E. coli CS21 type IV pili – as a control. LngA shares no homology with Fim2/3 or FimD proteins at the amino acid level. No effect of LngA purified protein at 0 to 10 µg/mL was observed on adherence of wild-type B. pertussis to HBE cells. At the maximum concentration of 100 µg/mL, the adherence of B. pertussis to HBE cells decreased to ~30%, which was still higher than the adherence level reached by fimbrial mutants (Figure 6, panel C).
Figure 6. Bacterial-cell association assay using HBE cells infected with B. pertussis strains in the presence of fimbrial inhibitors.
HBE cells were infected with B. pertussis wild-type strain, fim2/3 mutant or fim2/3-fimD mutant in the presence of increasing concentration of purified B. pertussis fimbriae proteins Fim2/3 and FimD and enterotoxigenic E. coli CS21 fimbriae LngA protein, as control. Cell infections at each inhibitor concentration were conducted in duplicate. Difference in adherence to cells between wild type and fimbrial mutants was statistically significant (p<0.01) only in the absence or, in some cases, at low concentrations of Fim2/3 and FimD inhibitors. Differences in adherence between wild type and fimbrial mutant were always statistically significant (p<0.01) regardless the presence or absence of LngA inhibitor. This figure show representative experiments conducted in duplicate and repeated two to three times. Asterisk represents statistically significant differences in wild type adherence to cells with respect to fimbrial mutants.
Our results indicate that both Fim2/3 and FimD purified proteins compete for fimbrial-dependent B. pertussis adherence to HBE cells and support the hypothesis that both fimbrial major subunits and fimbrial tip adhesin FimD are involved in B. pertussis adherence to human airway epithelial cells.
DISCUSSION
Bordetella pertussis is an obligate human pathogen that resides in the respiratory tract during infection. Bacterial interactions with human respiratory epithelial cells constitute a key event in host colonization and in the pathogenesis of whooping cough, in which multiple host cell surface receptors and several B. pertussis factors are involved (32,33). Following infection of the upper airway, B. pertussis adheres to cilia via specific receptor-ligand interactions. Critical bacterial adhesion-mediating molecules include fimbriae, FHA, and pertactin, among others (34). In this study we report the use of PHAE and HBE cells in an in vitro model to evaluate B. pertussis-cell interactions. We show that the bacterial-PHAE cell interactions in vitro closely resemble those reported from respiratory tissue biopsies from infants that died from pertussis. B. pertussis adherence to HBE cells was in part mediated by fimbriae as B. pertussis fimbrial mutants adhered at lower levels compared with wild type.
Histopathology of lung tissue from infants who died from whooping cough shows that B. pertussis proliferates extracellularly and localizes at the cilia (35). B. pertussis invades cells, alters the morphology of airway cells, and induces a severe inflammatory response that is manifested with abundant neutrophils, necrotizing bronchiolitis, and intra-alveolar hemorrhage. In vitro studies have revealed that adherent B. pertussis were primarily localized close to the ciliary tuft (35–37). We show in great microscopic detail that B. pertussis has affinity to PHAE cell cilia and that adhering bacteria form clusters in close association with cilia and non-cilial apical surfaces. B. pertussis infection seems to alter PHAE cells cytoskeleton as columnar cells flatten and detach from the basal membrane surface. We also showed that B. pertussis adhere to the HBE cell surface and invade the cytoplasm of these cells. B. pertussis also alter HBE cytoskeleton and induce cell rounding and detachment. We believe that the PHAE cell model of B. pertussis infection reproduce the infection of human airway epithelia in vivo and they may be used to study the role of virulence factors in host-parasite interactions. Specifically, it may be used to study B. pertussis interactions with cilia and the diffence in interaction between ciliated and non-ciliated cells. The HBE cell model is a good model to study bacterial adherence, including fimbrial adherence to plasma membrane. It may also be used to evaluate B. pertussis factor in invasion and cytotoxicity.
Studies of B. pertussis interaction with host cells have largely involved murine, porcine, and rabbit animal models, none of which are the natural host for whooping cough, with possible exception of the baboon which was shown to closely reproduce the clinical feature of the B pertussis disease (38–41). In vitro studies on the role of B. pertussis fimbriae in adherence have relied on human-derived or animal-derived airway cell lines that are poorly differentiated and may not express the native surface receptors associated with cilial structures (17). Use of human respiratory epithelial cells obtained from bronchoscopy brushing specimens to evaluate B. pertussis infections in vitro was initially reported in 1983 (42). This study revealed that B. pertussis specifically bound to cilial structures and that FHA was involved in binding (43). The main limitations of those experiments were: respiratory cells could not replicate in vitro, a fresh bronchoscopy procedure was needed for each infection experiment, cells were not grown in an air-fluid interface platform and therefore the system was not replicating the infection process in vivo, and specific roles of B. pertussis fimbriae testing or other colonization factors were not evaluated.
Differentiated human-derived airway cells have not been used to study the role of B. pertussis fimbriae. We showed that fimbriae mediates, in part, wild-type B. pertussis adherence to PHAE cells and to HBE cell surfaces. Evidence for adhesin subunit FimD and major Fim subunits protein expression was evaluated by immune blot analysis and immuno-fluorescence. We showed that fim2/3 and fimD mutants adhere less well to PHAE and HBE cells than the fim2-expressing wild type counterpart. The fimbrial-associated binding to HBE cells was statistically significant as fim2-expressing wild type strain adhered to these cells 3 logs higher than the fim2/3- fimD mutant. The fimbrial-associated binding to PHAE cells was also statistically significant yet wild type adherence to PHAE cells was only 6-fold as much as the fim2/3- fimD mutant. The proportion of B. pertussis fimbrial-dependent binding to PHAE cells was lower than the same binding to HBE cells suggesting that B. pertussis binding to PHAE cells require critical adherence factors other than fimbriae, such as FHA. Also, HBE cells may express more membrane-associated fimbrial receptors than PHAE cells. Studies on the role of individual adherence factors including fimbriae, FHA and pertactin, using differentiated human airway cells, may reveal the importance that each factor may have on adherence to ciliated, goblet or basal human airway epithelial cells.
Since B. pertussis fimbrial-mediated binding to HBE cells was significantly higher than to PHAE cell, we choose HBE cells to evaluate specificity of fimbrial-mediated binding. Inhibition experiments using purified Fim2/3 major subunit or FimD adhesin subunit showed that these proteins competed with B. pertussis for binding to HBE cells. FimD protein was able to complete are a higher level with respect to Fim2/3 suggesting that fimbrial-mediated binding to HBE cells is predominantly mediated by FimD specific receptors. In our study we used fim2- and fimD-expressing B. pertussis wild type strains that do not express fim3. Further adherence experiments may need to be conducted to explore the role of alternative B. pertussis major fimbrial subunits such as Fim3 or FimX, to confirm that minor FimD subunit, rather than major subunits, plays a major role in cell adherence. These results are relevant because they demonstrate that B. pertussis fimbriae are critical in bacterial adherence to human airway. They also confirm previous reports on the role of B. pertussis fimbriae in adherence that were based on experiments using undifferentiated epithelial cell lines, mouse respiratory tissues and monocytes (15,17,19,44).
The PHAE and HBE cell adherence models are highly relevant because they reproduce the interactions between B. pertussis and respiratory cells. PHAE cells specifically demonstrate binding of bacteria to cilial structures previously reported to play a crucial role in the pathogenesis of B. pertussis respiratory infection (35). HBE cells also reproduce the cytoskeletal changes, leading to B. pertussis invasion of respiratory cells. This study also confirms the role of fimbriae in B. pertussis adherence to primary human respiratory cells and invites further research on the identification of specific adhesin receptors on respiratory epithelial surfaces.
B. pertussis fimbriae belong to the chaperon-usher fimbrial family that features major and minor subunits. In B. pertussis, Fim2 and Fim3 are two related but serologically distinct major subunits, both carrying the FimD minor adhesin subunit (19). B. pertussis fimbriae also mediated adherence to monocytes in a FimD/VLA-5 integrin-dependent manner (15,44). The specific FimD adhesin receptor on membrane surfaces of gobblet cells is not known and the FimD protein region directly involved in binding has not been identified. We believe that HBE cells may be used to explore the molecular interaction between B. pertussis fimbriae and membrane receptors. The cell model system may also be implemented to study other B. pertussis adherence factors such as pertactin, tracheal colonization factor, and FHA.
Quantitative adherence assays, microscopy, and fimbrial expression studies show that fimbriae are involved in adherence of B. pertussis to HBE cells. Adherence to HeLa cells was significantly lower, suggesting that this undifferentiated cell line may not express specific B. pertussis fimbrial receptors. Adherence to PHAE cell was high yet it was not dependent on fimbriae suggesting that other B. pertussis factors may be involved in adherence to these cells such as pertactin or FHA. We hypothesize that HBE and PHAE cells may express specific B. pertussis colonization factor receptors that may be identical to those expressed by human airway epithelial cells in vivo. This study may have implications not only on the role of fimbriae in B. pertussis adherence, colonization, and pathogenesis, but also in the characterization of known and novel B. pertussis molecules involved in cell adherence. We believe that this model may also be important to determine the potential role of fimbriae and other B. pertussis adhesins as vaccine candidates, as current acellular B. pertussis vaccines may not contain the most efficient antigens for protection. Read-outs using signaling molecules from infected and uninfected cells may provide important clues on innate immunity. Cytokine levels measurements in response to infection with wild type and specific mutants may also provide clues on the type of immune responses that maybe induced in response to different antigens.
In summary, the B. pertussis PHAE and HEB cell infection assays are an excellent model to study B. pertussis infection in vitro; to evaluate the role of B. pertussis-associated surface molecules in adherence, invasion, and cytoskeletal effects; and, more importantly, to explore the potential role that these molecules may have in vaccine development. The relevance of this model lies on the striking physiologic and morphologic similarity between the PHAE and HBE cells and the human airway cilial and goblet cells in vivo, respectively. These similarities include the ability of these cells to proliferate, differentiate, and to express the same genetic profile as human respiratory cells in vivo.
Acknowledgments
We are grateful with Dr. Joseph Zabner at the Department of Medicine, University of Iowa, for critical discussions and helpful scientific advice. We are thankful to Central Microscopy Research Facility, In Vitro Models and Cell Culture Core and Cystic Fibrosis Research Center staff at the University of Iowa Carver College of Medicine for technical assistance. We are grateful with Dr. Janice Williams at the Electron Microscopy Core Laboratory, Vanderbilt University for technical assistance and helpful discussions.
Declaration of interest:Research support was provided to O.G.G-D by Sanofi Pasteur; Department of Pediatrics, University of Iowa Carver College of Medicine; and Department of Pediatrics, Vanderbilt University School of Medicine. Research support was provided to JAG from the Department of Veterans Affairs CDA-2 1IK2BX001701. Imaging experiments were performed in part through the use of the VUMC Cell Imaging Shared Resource supported by NIH Grants CA68485, DK20593, DK58404, DK59637 and EY08126.
References
- 1.Melvin JA, Scheller EV, Miller JF, Cotter PA. Bordetella pertussis pathogenesis: current and future challenges. Nat Rev Microbiol. 2014;12(4):274–288. doi: 10.1038/nrmicro3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18(2):326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surridge J, Segedin ER, Grant CC. Pertussis requiring intensive care. Arch Dis Child. 2007;92(11):970–975. doi: 10.1136/adc.2006.114082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns DL, Meade BD, Messionnier NE. Pertussis resurgence: perspectives from the Working Group Meeting on pertussis on the causes, possible paths forward, and gaps in our knowledge. J Infect Dis. 2014;209(Suppl 1):S32–S35. doi: 10.1093/infdis/jit491. [DOI] [PubMed] [Google Scholar]
- 5.Crowcroft NS, Stein C, Duclos P, Birmingham M. How best to estimate the global burden of pertussis? Lancet Infect Dis. 2003;3(7):413–418. doi: 10.1016/s1473-3099(03)00669-8. [DOI] [PubMed] [Google Scholar]
- 6.Cherry JD. Adult pertussis in the pre- and post-vaccine eras: lifelong vaccine-induced immunity? Expert Rev Vaccines. 2014;13(9):1073–1080. doi: 10.1586/14760584.2014.935765. [DOI] [PubMed] [Google Scholar]
- 7.Sala-Farré M-R, Arias-Varela C, Recasens-Recasens A, Simó-Sanahuja M, Muñoz-Almagro C, Pérez-Jové J. Pertussis epidemic despite high levels of vaccination coverage with acellular pertussis vaccine. Enfermedades Infecc Microbiol Clínica. 2015;33(1):27–31. doi: 10.1016/j.eimc.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Sheridan SL, Ware RS, Grimwood K, Lambert SB. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA J Am Med Assoc. 2012;308(5):454–456. doi: 10.1001/jama.2012.6364. [DOI] [PubMed] [Google Scholar]
- 9.Smits K, Pottier G, Smet J, Dirix V, Vermeulen F, Schutter I De, et al. Different T cell memory in preadolescents after whole-cell or acellular pertussis vaccination. Vaccine. 2013;32(1):111–118. doi: 10.1016/j.vaccine.2013.10.056. [DOI] [PubMed] [Google Scholar]
- 10.Mooi FR, Maas NATVANDER, Melker HE De. Pertussis resurgence: waning immunity and pathogen adaptation - two sides of the same coin. Epidemiol Infect. 2013:1–10. doi: 10.1017/S0950268813000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willems R, Paul A, Heide HG van der, Avest AR ter, Mooi FR. Fimbrial phase variation in Bordetella pertussis: a novel mechanism for transcriptional regulation. EMBO J. 1990;9(9):2803–2809. doi: 10.1002/j.1460-2075.1990.tb07468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willems RJ, Geuijen C, Heide HG van der, Renauld G, Bertin P, Akker WM van den, et al. Mutational analysis of the Bordetella pertussis fim/fha gene cluster: identification of a gene with sequence similarities to haemolysin accessory genes involved in export of FHA. Mol Microbiol. 1994;11(2):337–347. doi: 10.1111/j.1365-2958.1994.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 13.Heck DV, Trus BL, Steven AC. Three-dimensional structure of Bordetella pertussis fimbriae. J Struct Biol. 1996;116(2):264–269. doi: 10.1006/jsbi.1996.0041. [DOI] [PubMed] [Google Scholar]
- 14.Hallander H, Advani A, Alexander F, Gustafsson L, Ljungman M, Pratt C, et al. Antibody responses to Bordetella pertussis Fim2 or Fim3 following immunization with a whole-cell, two-component, or five-component acellular pertussis vaccine and following pertussis disease in children in Sweden in 1997 and 2007. Clin Vaccine Immunol CVI. 2014;21(2):165–173. doi: 10.1128/CVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hazenbos WL, Geuijen CA, Berg BM van den, Mooi FR, Furth R van. Bordetella pertussis fimbriae bind to human monocytes via the minor fimbrial subunit FimD. J Infect Dis. 1995;171(4):924–929. doi: 10.1093/infdis/171.4.924. [DOI] [PubMed] [Google Scholar]
- 16.Willems RJ, Geuijen C, Heide HG van der, Matheson M, Robinson A, Versluis LF, et al. Isolation of a putative fimbrial adhesin from Bordetella pertussis and the identification of its gene. Mol Microbiol. 1993;9(3):623–634. doi: 10.1111/j.1365-2958.1993.tb01722.x. [DOI] [PubMed] [Google Scholar]
- 17.Berg BM van den, Beekhuizen H, Willems RJ, Mooi FR, Furth R van. Role of Bordetella pertussis virulence factors in adherence to epithelial cell lines derived from the human respiratory tract. Infect Immun. 1999;67(3):1056–1062. doi: 10.1128/iai.67.3.1056-1062.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds HY. Modulating airway defenses against microbes. Curr Opin Pulm Med. 2002;8(3):154–165. doi: 10.1097/00063198-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Geuijen CA, Willems RJ, Bongaerts M, Top J, Gielen H, Mooi FR. Role of the Bordetella pertussis minor fimbrial subunit, FimD, in colonization of the mouse respiratory tract. Infect Immun. 1997;65(10):4222–4228. doi: 10.1128/iai.65.10.4222-4228.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, et al. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol Clifton NJ. 2002;188:115–137. doi: 10.1385/1-59259-185-X:115. [DOI] [PubMed] [Google Scholar]
- 21.Pezzulo AA, Starner TD, Scheetz TE, Traver GL, Tilley AE, Harvey B-G, et al. The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2011;300(1):L25–L31. doi: 10.1152/ajplung.00256.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mooi FR, Jansen WH, Brunings H, Gielen H, Heide HG van der, Walvoort HC, et al. Construction and analysis of Bordetella pertussis mutants defective in the production of fimbriae. Microb Pathog. 1992;12(2):127–135. doi: 10.1016/0882-4010(92)90115-5. [DOI] [PubMed] [Google Scholar]
- 23.Aldallal N, McNaughton EE, Manzel LJ, Richards AM, Zabner J, Ferkol TW, et al. Inflammatory response in airway epithelial cells isolated from patients with cystic fibrosis. Am J Respir Crit Care Med. 2002;166(9):1248–1256. doi: 10.1164/rccm.200206-627OC. [DOI] [PubMed] [Google Scholar]
- 24.Granio O, Ashbourne Excoffon KJD, Henning P, Melin P, Norez C, Gonzalez G, et al. Adenovirus 5-fiber 35 chimeric vector mediates efficient apical correction of the cystic fibrosis transmembrane conductance regulator defect in cystic fibrosis primary airway epithelia. Hum Gene Ther. 2010;21(3):251–269. doi: 10.1089/hum.2009.056. [DOI] [PubMed] [Google Scholar]
- 25.Comolli JC, Waite LL, Mostov KE, Engel JN. Pili binding to asialo-GM1 on epithelial cells can mediate cytotoxicity or bacterial internalization by Pseudomonas aeruginosa. Infect Immun. 1999;67(7):3207–3214. doi: 10.1128/iai.67.7.3207-3214.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruenert DC, Basbaum CB, Welsh MJ, Li M, Finkbeiner WE, Nadel JA. Characterization of human tracheal epithelial cells transformed by an origin-defective simian virus 40. Proc Natl Acad Sci U S A. 1988;85(16):5951–5955. doi: 10.1073/pnas.85.16.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swords WE, Buscher BA, Steeg Ii K Ver, Preston A, Nichols WA, Weiser JN, et al. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol Microbiol. 2000;37(1):13–27. doi: 10.1046/j.1365-2958.2000.01952.x. [DOI] [PubMed] [Google Scholar]
- 28.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 29.Gómez-Duarte OG, Dehio M, Guzmán CA, Chhatwal GS, Dehio C, Meyer TF. Binding of vitronectin to opa-expressing Neisseria gonorrhoeae mediates invasion of HeLa cells. Infect Immun. 1997;65(9):3857–3866. doi: 10.1128/iai.65.9.3857-3866.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clavijo AP, Bai J, Gómez-Duarte OG. The Longus type IV pilus of enterotoxigenic Escherichia coli (ETEC) mediates bacterial self-aggregation and protection from antimicrobial agents. Microb Pathog. 2010;48(6):230–238. doi: 10.1016/j.micpath.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radin JN, Gaddy JA, González-Rivera C, Loh JT, Algood HMS, Cover TL. Flagellar localization of a Helicobacter pylori autotransporter protein. mBio. 2013;4(2):e00613–e00612. doi: 10.1128/mBio.00613-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerr JR, Matthews RC. Bordetella pertussis infection: pathogenesis, diagnosis, management, and the role of protective immunity. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2000;19(2):77–88. doi: 10.1007/s100960050435. [DOI] [PubMed] [Google Scholar]
- 33.Leininger E, Ewanowich CA, Bhargava A, Peppler MS, Kenimer JG, Brennan MJ. Comparative roles of the Arg-Gly-Asp sequence present in the Bordetella pertussis adhesins pertactin and filamentous hemagglutinin. Infect Immun. 1992;60(6):2380–2385. doi: 10.1128/iai.60.6.2380-2385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Locht C, Antoine R, Jacob-Dubuisson F. Bordetella pertussis, molecular pathogenesis under multiple aspects. Curr Opin Microbiol. 2001;4(1):82–89. doi: 10.1016/s1369-5274(00)00169-7. [DOI] [PubMed] [Google Scholar]
- 35.Paddock CD, Sanden GN, Cherry JD, Gal AA, Langston C, Tatti KM, et al. Pathology and pathogenesis of fatal Bordetella pertussis infection in infants. Clin Infect Dis Off Publ Infect Dis Soc Am. 2008;47(3):328–338. doi: 10.1086/589753. [DOI] [PubMed] [Google Scholar]
- 36.Soane MC, Jackson A, Maskell D, Allen A, Keig P, Dewar A, et al. Interaction of Bordetella pertussis with human respiratory mucosa in vitro. Respir Med. 2000;94(8):791–799. doi: 10.1053/rmed.2000.0823. [DOI] [PubMed] [Google Scholar]
- 37.Wilson R, Read R, Thomas M, Rutman A, Harrison K, Lund V, et al. Effects of Bordetella pertussis infection on human respiratory epithelium in vivo and in vitro. Infect Immun. 1991;59(1):337–345. doi: 10.1128/iai.59.1.337-345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Funnell SG, Robinson A. A novel adherence assay for Bordetella pertussis using tracheal organ cultures. FEMS Microbiol Lett. 1993;110(2):197–203. doi: 10.1111/j.1574-6968.1993.tb06320.x. [DOI] [PubMed] [Google Scholar]
- 39.Mills KHG, Gerdts V. Mouse and pig models for studies of natural and vaccine-induced immunity to Bordetella pertussis. J Infect Dis. 2014;209(Suppl 1):S16–S19. doi: 10.1093/infdis/jit488. [DOI] [PubMed] [Google Scholar]
- 40.Ark AA van der, Hozbor DF, Boog CJP, Metz B, Dobbelsteen GP van den, Els CACM van. Resurgence of pertussis calls for re-evaluation of pertussis animal models. Expert Rev Vaccines. 2012;11(9):1121–1137. doi: 10.1586/erv.12.83. [DOI] [PubMed] [Google Scholar]
- 41.Vandebriel RJ, Hellwig SMM, Vermeulen JP, Hoekman JHG, Dormans JAMA, Roholl PJM, et al. Association of Bordetella pertussis with host immune cells in the mouse lung. Microb Pathog. 2003;35(1):19–29. doi: 10.1016/s0882-4010(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 42.Tuomanen EI, Hendley JO. Adherence of Bordetella pertussis to human respiratory epithelial cells. J Infectn Dis. 1983;148(1):125–130. doi: 10.1093/infdis/148.1.125. [DOI] [PubMed] [Google Scholar]
- 43.Tuomanen E, Weiss A. Characterization of two adhesins of Bordetella pertussis for human ciliated respiratory-epithelial cells. J Infect Dis. 1985;152(1):118–125. doi: 10.1093/infdis/152.1.118. [DOI] [PubMed] [Google Scholar]
- 44.Hazenbos WL, Berg BM van den, Geuijen CW, Mooi FR, Furth R van. Binding of FimD on Bordetella pertussis to very late antigen-5 on monocytes activates complement receptor type 3 via protein tyrosine kinases. J Immunol Baltim Md 1950. 1995;155(8):3972–3978. [PubMed] [Google Scholar]