Abstract
Each month, subscribers to The Formulary Monograph Service receive 5 to 6 well-documented monographs on drugs that are newly released or are in late phase 3 trials. The monographs are targeted to Pharmacy & Therapeutics Committees. Subscribers also receive monthly 1-page summary monographs on agents that are useful for agendas and pharmacy/nursing in-services. A comprehensive target drug utilization evaluation/medication use evaluation (DUE/MUE) is also provided each month. With a subscription, the monographs are are available online to subscribers. Monographs can be customized to meet the needs of a facility. Through the cooperation of The Formulary, Hospital Pharmacy publishes selected reviews in this column. For more information about The Formulary Monograph Service, contact Wolters Kluwer customer service at 866-397-3433. The January 2017 monograph topics are bezlotoxumab, buprenorphine buccal, deflazacort, dupilumab, and olaratumab. The DUE is on buprenorphine buccal.
INDICATIONS
Lixisenatide, a glucagonlike peptide 1 (GLP-1) receptor agonist, is approved for use as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.1,2 Lixisenatide has been studied as monotherapy and in combination with other antidiabetic medications or with basal insulin.1,3–12
The other US Food and Drug Administration (FDA)–approved GLP-1 receptor agonists (albiglutide, dulaglutide, exenatide immediate-release injection, exenatide extended-release injection, and liraglutide) are also indicated as adjunctive therapies to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Each agent has been studied for use as monotherapy and in combination with other antidiabetic medications; however, the current labeling for each advises that these agents are not recommended as first-line therapy for patients inadequately controlled on diet and exercise.13–17
Similar to other GLP-1 receptor agonists, lixisenatide has not been studied in patients with chronic pancreatitis or a history of unexplained pancreatitis, is not a substitute for insulin, has not been studied and is not recommended in concurrent use with short-acting insulin, and has not been studied and is not recommended in patients with gastroparesis.1,13–17
CLINICAL PHARMACOLOGY
Lixisenatide is a synthetic GLP-1 receptor agonist. 18,19 Similar to other GLP-1 receptor agonists, lixisenatide improves glycemic control through its effects on glucose-dependent insulin secretion, glucagon secretion, gastric emptying, and food intake.18–27
PHARMACOKINETICS
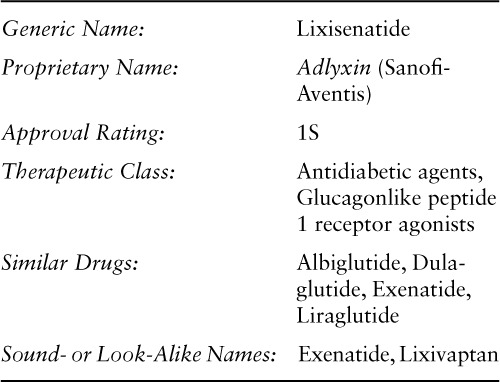
Peak plasma concentrations (Cmax) of lixisenatide occur 1.25 to 3.5 hours after subcutaneous injection. 1,21 The rate of absorption did not change when administered subcutaneously in the abdomen, thigh, or arm.1 Cmax was dose dependent: With once-daily subcutaneous administration, Cmax was 37.3 pg/mL after lixisenatide 5 mcg, 82.7 pg/mL after lixisenatide 10 mcg, and 187.2 pg/mL after lixisenatide 20 mcg; with twice-daily subcutaneous administration, Cmax was 50.9 pg/mL after lixisenatide 5 mcg, 108.6 pg/mL after lixisenatide 10 mcg, and 234.4 pg/mL after lixisenatide 20 mcg.21
The apparent volume of distribution of lixisenatide after subcutaneous administration is 100 L. Lixisenatide is thought to be eliminated by glomerular filtration and proteolytic degradation, with a mean half-life of about 3 hours and mean apparent clearance of about 35 L/h. Age, body weight, gender, and race had no apparent effect on pharmacokinetic parameters.1
Significant changes in renal function may influence the elimination of lixisenatide. Patients with mild to moderate renal impairment had similar area under the curve (AUC) and Cmax as those with normal renal function, but patients with severe renal impairment (creatinine clearance [CrCl] < 30 mL/min but not requiring dialysis) had increased drug exposure. 1,21 No pharmacokinetic studies were conducted in patients with acute or chronic hepatic impairment.1
Elderly patients (65 years or older) had no change in Cmax or time to maximal concentration (Tmax) following a 20 mcg subcutaneous injection compared with younger healthy subjects (24–44 years of age). However, mean exposure to lixisenatide was increased by 30%, and terminal half-life was increased approximately 1.6 times.28
Table 1 compares the pharmacokinetic parameters of FDA-approved GLP-1 receptor agonists.1,13–17
Table 1.
COMPARATIVE EFFICACY
The phase 3 studies sponsored by Sanofi (the GetGoal program) evaluated lixisenatide as monotherapy and in combination with various oral antidiabetic agents or in combination with basal insulin. Patients enrolled in these studies had type 2 diabetes that had been inadequately controlled with diet and exercise and/or metformin therapy. Their baseline glycated hemoglobin (HbA1c) was at least 7% and less than 10%.3,5,6–9,21,29,30
Indication: Type 2 Diabetes Mellitus—Monotherapy
Guidelines
Guideline: Standards of medical care in diabetes—2016
Reference: American Diabetes Association, 201631
Comments: Metformin, unless contraindicated at the time of diagnosis, is recommended as first-line therapy in patients with type 2 diabetes mellitus. In patients with metformin intolerance or contraindications, initial therapy should be selected from one of the following classes: sulfonylureas, thiazolidinediones, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium glucose cotransporter 2 (SGLT2) inhibitors, GLP-1 receptor agonists, or basal insulin. Selection of an agent should be based on patient- and disease-specific factors.
Guideline: Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2016 executive summary
Reference: Garber, 201632
Comments: According to the consensus statement, monotherapy is preferred in patients with recent-onset type 2 diabetes mellitus or HbA1c less than 7.5%. Metformin is recommended as first-line monotherapy, unless contraindicated. Acceptable alternative initial therapies in a suggested hierarchy of use include GLP-1 receptor agonists, SGLT2 inhibitors, DPP-4 inhibitors, or thiazolidinediones. Alpha-glucosidase inhibitors, sulfonylureas, and glinides may be appropriate as monotherapy for select patients. Selection of an agent should take into consideration patient- and medication-specific factors.
Studies
Drug: Lixisenatide vs Placebo
Reference: Fonseca VA, et al, 2012 (GetGoal-Mono trial)5
Study Design: Randomized, double-blind, multicenter, international, noninferiority study
Study Funding: Sanofi
Patients: 361 adults with type 2 diabetes not currently receiving glucose-lowering therapy and with HbA1C of 7% to 10%. Exclusion criteria were treatment with a glucose-lowering agent in the previous 3 months; fasting plasma glucose (FPG) greater than 250 mg/dL at screening; amylase and/or lipase greater than 3 times the upper limit of normal (ULN); history of gastrointestinal (GI) disease with prolonged nausea and vomiting during the previous 6 months, chronic pancreatitis and/or stomach/gastric surgery; history of myocardial infarction, stroke, or heart failure requiring hospitalization in the previous 6 months; or hepatic disease, end-stage renal disease (ESRD), and/or dialysis at screening. Of the 795 patients screened, 361 were randomized to lixisenatide 2-step treatment (n = 120), lixisenatide 1-step treatment (n = 119), or placebo (n = 122). Mean patient age was about 54 years, median duration of diabetes was about 1.4 years, and mean baseline HbA1c was about 8%.
Intervention: Lixisenatide or placebo was administered subcutaneously once daily for 12 weeks using a dose-increase regimen. The lixisenatide 2-step group received 10 mcg for 1 week, 15 mcg for 1 week, and then 20 mcg for the remaining 10 weeks. The lixisenatide 1-step group received 10 mcg for 2 weeks and then 20 mcg for the remaining 10 weeks. The 2 placebo groups (2-step [n = 61] and 1-step [n = 61]) followed the same schedule as the lixisenatide groups, but the results from the 2 groups were combined for analysis.
Results
Primary Endpoint(s)
Least squares (LS) mean change in HbA1c from baseline to week 12 was −0.73% with 2-step lixisenatide, −0.85% with 1-step lixisenatide, and −0.19% with placebo. The LS mean difference from placebo was −0.54% with 2-step lixisenatide (p < .001) and −0.66% with 1-step lixisenatide (p < .001).
Secondary Endpoint(s)
HbA1c less than 7% was achieved in 52% of patients in the 2-step lixisenatide group (p < .01 vs placebo) and in 47% of patients in the 1-step lixisenatide group (p < .01 vs placebo), compared with 27% of patients in the placebo group. HbA1c 6.5% or less was achieved in 32% of patients in the 2-step lixisenatide group (p < .01 vs placebo) and in 25% of patients in the 1-step lixisenatide group (p < .01 vs placebo), compared with 13% of patients in the placebo group.
Body weight decreased by about 2 kg in all groups, with no significant differences between lixisenatide and placebo.
Comments: Randomization of subjects and drug allocation were performed using an interactive voice-response system. Lixisenatide as monotherapy administered once daily provided significant improvement in glycemic control with a pronounced postprandial effect. Lixisenatide was well tolerated, with no relevant difference in safety and tolerability between the 1-step and 2-step dose increase regimens, which supports a role for once-daily lixisenatide monotherapy using a 1-step dose increase regimen.
Limitations: This study did not include a study arm with another GLP-1 receptor agonist to determine whether differences exist among drugs in the same class.
Indication: Type 2 Diabetes Mellitus—Add-On Therapy With Metformin
Guidelines
Guideline: Standards of medical care in diabetes—2016
Reference: American Diabetes Association, 201631
Comments: If metformin at maximal tolerated doses does not achieve or maintain the target HbA1c after 3 months, a sulfonylurea, thiazolidinedione, DPP-4 inhibitor, SGLT2 inhibitor, GLP-1 receptor agonist, or basal insulin should be added. A dual combination may also be considered as initial therapy when HbA1c is 9% or greater. Considerations when selecting therapy for an individual patient should include efficacy, cost, potential adverse effects, weight, comorbidities, hypoglycemia risk, and patient preferences.
Guideline: Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2016 executive summary
Reference: Garber, 201632
Comments: If glycemic targets are not achieved within 3 months with metformin or an alternative first-line agent, another agent should be added in the following suggested hierarchy of use: GLP-1 receptor agonist, SGLT2 inhibitor, DPP-4 inhibitor, thiazolidinedione, basal insulin, colesevelam, bromocriptine, alpha-glucosidase inhibitor, or sulfonylurea/glinide. Initial dual combination therapy is recommended for patients presenting with HbA1c greater than 7.5%.
Studies
Drug: Lixisenatide vs Placebo
Reference: Bolli GB, et al, 2014 (GetGoal-F1 trial)3
Study Design: Randomized, double-blind, multicenter study
Study Funding: Sanofi
Patients: 482 adults with type 2 diabetes inadequately controlled with metformin. Patients were receiving at least 1.5 g/day of metformin as monotherapy and had HbA1C of 7% to 10%. Main exclusion criteria were use of injectable or oral glucose-lowering agents (other than metformin) within 3 months prior to screening; FPG greater than 250 mg/dL at screening; or history of unexplained pancreatitis, chronic pancreatitis, pancreatectomy, stomach/gastric surgery, or inflammatory bowel disease. Of 884 patients screened, 484 were randomized, with 482 included for analysis (lixisenatide 2-step, n = 161; lixisenatide 1-step, n = 161; placebo, n = 160). Mean baseline characteristics were as follows: patient age was 56.1 years, duration of disease was 6 years, HbA1c was 8%, and body mass index (BMI) was 32.5 kg/m2.
Intervention: Lixisenatide or placebo was administered subcutaneously once daily for 24 weeks using a dose-increase regimen. The lixisenatide 2-step group received 10 mcg for 1 week, then 15 mcg for 1 week, and then 20 mcg for the remaining 22 weeks; the lixisenatide 1-step group received 10 mcg for 2 weeks and then 20 mcg for the remaining 22 weeks. The 2 placebo groups (2-step [n = 80] and 1-step [n = 82]) followed the same schedule as the lixisenatide groups, but results from the 2 groups were combined for analysis. Established metformin doses were continued. Patients were stratified according to HbA1C values and BMI.
Results
Primary Endpoint(s)
LS mean improvement in HbA1c from baseline to week 24 was −0.8% with lixisenatide 2-step, −0.9% with lixisenatide 1-step, and −0.4% with placebo. The LS mean difference from placebo was −0.5% (95% CI, −0.7% to −0.3%; p < .001) with lixisenatide 1-step and −0.4% (95% CI, −0.6% to −0.2%; p < .001) with lixisenatide 2-step.
Secondary Endpoint(s)
HbA1c less than 7% was achieved in 42.1% of patients with lixisenatide 2-step (p < .001 vs placebo) and 47.4% of patients with lixisenatide 1-step (p < .001 vs placebo), compared with 24.1% of patients with placebo. HbA1c 6.5% or less was achieved in 20.4% of patients with lixisenatide 2-step (p < .001 vs placebo) and 25.6% of patients with lixisenatide 1-step (p < .001 vs placebo), compared with 7.6% of patients with placebo.
Improvements in FPG and body weight were observed in both the 1- and 2-step lixisenatide groups compared with placebo.
Comments: Rescue therapy was required by 3.1% of the lixisenatide 2-step group, 1.3% of the lixisenatide 1-step group, and 4.4% of the combined placebo group. Both dosing schedules of lixisenatide produced better glycemic control than placebo. The 24-week study was followed by a 52-week variable, double-blind extension period, during which efficacy was maintained. In general, the 2 lixisenatide regimens were similar in efficacy and tolerability, which supports the 1-step dosage increase recommended in the lixisenatide prescribing information.
Limitations: This study did not include a study arm with another GLP-1 receptor agonist to determine if differences exist among drugs in the same class.
Drug: Lixisenatide vs Exenatide
Reference: Rosenstock J, et al, 2013 (GetGoal-X trial)9
Study Design: Randomized, open-label, parallel-group, multicenter, noninferiority study
Study Funding: Sanofi
Patients: 634 adults with type 2 diabetes inadequately controlled with metformin. Patients were receiving a metformin dose of at least 1.5 g/day and had HbA1C 7% to 10%. Exclusion criteria were use of oral or injectable glucose-lowering agents other than metformin within 3 months prior to screening; FPG greater than 250 mg/dL at screening; history of unexplained pancreatitis, chronic pancreatitis, pancreatectomy, stomach/gastric surgery, or inflammatory bowel disease; history of metabolic acidosis, including diabetic ketoacidosis, within 1 year before screening; history of myocardial infarction, stroke, or heart failure requiring hospitalization in the previous 6 months; and clinically relevant history of GI disease with prolonged nausea and vomiting during the previous 6 months. Of 1,243 patients screened, 639 were randomized, with 634 patients included for analysis (lixisenatide, n = 318; exenatide, n = 316). Mean baseline characteristics were as follows: patient age was 57.4 years, duration of diabetes was 6.8 years, HbA1c was 8%, and BMI was 33.6 kg/m2; 92.7% of patients were White.
Intervention: Patients were randomized (1:1) to the lixisenatide 2-step group or exenatide 1-step group, with lixisenatide administered subcutaneously once daily and exenatide administered subcutaneously twice daily for 24 weeks. Lixisenatide was given 1 hour before the morning meal, and exenatide was given before the morning and evening meals. Both drugs were started at lower doses and increased in a stepwise manner to the target dose: The lixisenatide group received 10 mcg once daily for 1 week, then 15 mcg once daily for 1 week, and then 20 mcg once daily for the remaining 22 weeks; and the exenatide group received 5 mcg twice daily for 4 weeks and then 10 mcg twice daily for the remaining 20 weeks.
Results
Primary Endpoint(s)
LS mean change in HbA1c from baseline to week 24 was −0.79% with lixisenatide once daily and −0.96% with exenatide twice daily. The LS mean change difference between groups was 0.17% (95% CI, 0.033% to 0.297%); therefore, noninferiority (upper CI margin of 0.4%) was demonstrated.
Secondary Endpoint(s)
No differences were found in the impact on FPG (LS mean change difference between the 2 groups was 0.23 mmol/L [95% CI, −0.052 to 0.522]) or in the proportion of patients achieving HbA1c less than 7% (48.5% with lixisenatide and 49.8% with exenatide).
Body weight was decreased by −2.96 kg with lixisenatide and by −3.98 kg with exenatide. The LS mean change difference in body weight between the 2 groups was 1.02 kg (95% CI, 0.456 to 1.58). Weight loss of 5% or greater from baseline to week 24 occurred in 25.1% of patients treated with lixisenatide and 31.4% treated with exenatide.
Endpoint(s)
Nausea occurred more frequently with exenatide (35.1% vs 24.5%; p < .05), whereas there was no difference in the incidence of diarrhea or vomiting.
Symptomatic hypoglycemia occurred less frequently with lixisenatide (2.5% vs 7.9%; p < .05).
Comments: This trial was designed as a noninferiority trial based on the change in HbA1c at week 24. Noninferiority was achieved if the upper limits of the 2-sided 95% CI of the difference in adjusted mean change in HbA1c from baseline to week 24 was less than or equal to 0.4%. Centralized randomization and treatment allocation were performed using an interactive voice-response system. Both GLP-1 receptor agonists were relatively well tolerated; the most common adverse reactions, which were GI in nature, tended to occur more frequently at the start of therapy and subsequently subsided. Both drugs were effective in lowering FPG levels and HbA1c, with the difference in response meeting noninferiority criteria. Incidence of study discontinuation was 12.9% in the lixisenatide group and 14.2% in the exenatide group, with adverse reactions accounting for the majority of discontinuations. The study was conducted at 122 centers in 18 countries.
Limitations: The impact of the different dosing schedules on adherence was not reported.
Drug: Lixisenatide vs Liraglutide
Reference: Novo Nordisk, 2016 (LIRA-LIXI trial)33,34
Study Design: Phase 4 randomized, open-label, multicenter study
Study Funding: Novo Nordisk
Patients: 404 adults with type 2 diabetes inadequately controlled with metformin. Patients had received metformin at a maximum tolerated dose (1,000 to 3,000 mg/day) for 90 days prior to screening, and had HbA1c of 7.5% to 10.5% and BMI of at least 20 kg/m2. Female patients of childbearing potential not using adequate contraception or who were pregnant, breastfeeding, or intending to become pregnant were excluded. Additional exclusion criteria were previous treatment with a GLP-1 receptor agonist, treatment with glucose-lowering agents other than metformin within 90 days of screening, history of chronic pancreatitis or idiopathic acute pancreatitis, screening calcitonin value of 50 ng/L or greater, personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2, impaired liver function (ALT at least 2.5 times the ULN), impaired renal function (estimated glomerular filtration rate <60 mL/min/1.73 m2), or any chronic disorder or severe disease that in the opinion of the investigator might jeopardize patient safety or compliance with the protocol. Of the 619 patients screened, 404 were randomized to treatment with lixisenatide (n = 202) or liraglutide (n = 202). Mean baseline characteristics were as follows: patient age was 56.2 years, duration of diabetes was 6.3 years, HbA1c was 8.4%, and body weight was 101.24 kg; 60% of patients were male.
Intervention: Patients were randomly assigned (1:1) to treatment with lixisenatide or liraglutide subcutaneously once daily for 26 weeks. Liraglutide was started at 0.6 mg/day and increased at weekly intervals of 0.6 mg/day to a maintenance dose of 1.8 mg. Lixisenatide was started at 10 mcg and increased to 20 mcg on day 15. Lixisenatide was given within 1 hour prior to the morning or evening meal, and liraglutide could be given at any time of day, without regard to meals. Patient metformin regimens were continued unchanged.
Results
Primary Endpoint(s)
Estimated change in HbA1c from baseline to week 26 was −1.2% with lixisenatide and −1.8% with liraglutide (estimated difference, 0.6%; 95% CI, −0.8% to −0.4%; p < .001).
Secondary Endpoint(s)
Change in FPG was −1.7 mmol/L with lixisenatide and −2.9 mmol/L with liraglutide (estimated difference, −1.2 mmol/L; 95% CI, −1.5 to −0.8; p < .001).
HbA1c less than 7% was achieved in 45.5% of patients treated with lixisenatide and in 74.2% with liraglutide (p < .001). HbA1c 6.5% or less was achieved in 26.2% of patients treated with lixisenatide and in 54.6% with liraglutide (p < .001).
Change in body weight from baseline to week 26 was −3.7 kg with lixisenatide and −4.3 kg with liraglutide (estimated difference, −0.6; 95% CI, −1.6 to 0.4; p = .2347).
Incidence of adverse reactions was similar for the 2 treatments, with nausea occurring in 21.8% of patients in both groups and diarrhea occurring in 12.4% of liraglutide patients and 9.9% of lixisenatide patients.
Comments: Randomization was performed using an interactive voice-/web-response system. This head-to-head comparison, funded and conducted by the manufacturer of liraglutide (Novo Nordisk), did not enroll patients from the United States. Patients were from the Czech Republic, Finland, France, Germany, Hungary, Italy, Latvia, Lithuania, and the United Kingdom. About 84% of patients (340 of 404) completed the study: 88.1% of liraglutide patients and 80.2% of lixisenatide patients.
Limitations: Potential limitations of this trial included the relatively short 6-month duration, open-label design. In addition, a double-dummy design was not achievable, opening the study to bias due to lack of patient and physician blinding. The effect of liraglutide 1.2 mg versus lixisenatide was not evaluated in this trial.
Drug: Lixisenatide vs Sitagliptin
Reference: Van Gaal L, et al, 201411
Study Design: Randomized, double-blind, double-dummy, multicenter study
Study Funding: Sanofi
Patients: 319 obese (BMI 30 kg/m2 or greater) adults younger than 50 years with type 2 diabetes inadequately controlled with metformin. Patients had to have been diagnosed at least 1 year before screening, be receiving a stable dose of metformin (at least 1.5 g/day) for at least 3 months prior to screening, and have HbA1C 7% to 10%. Exclusion criteria were FPG greater than 250 mg/dL at screening; history of unexplained pancreatitis, chronic pancreatitis, pancreatectomy, stomach/gastric surgery, or inflammatory bowel disease; history of metabolic acidosis, including diabetic ketoacidosis within 1 year prior to screening; history of myocardial infarction, stroke, or heart failure requiring hospitalization within 6 months prior to screening; and amylase and/or lipase values more than 3 times the normal laboratory range. Of 620 patients screened, 319 were randomized to treatment with lixisenatide (n = 158) or sitagliptin (n =161). Mean baseline characteristics were as follows: patient age was 43 years, duration of diabetes was 4.4 years, HbA1c was 8.1%, and BMI was 36.8 kg/m2; 80% of patients were White, and the lixisenatide group had a lower proportion of males (34.8% vs 45.3%).
Intervention: Patients were randomized to lixisenatide 20 mcg subcutaneously once daily initiated in a 2-step regimen or sitagliptin 100 mg orally once daily for 24 weeks. Each group also received a placebo dose of oral or subcutaneous study medication to maintain the double-blind. Patients continued to receive metformin at a stable dose.
Results
Primary Endpoint(s)
A composite endpoint of the proportion of patients achieving HbA1c of less than 7% and body weight loss of at least 5% from baseline at week 24 was achieved by 12% of patients in the lixisenatide group and by 7.5% of the sitagliptin group; weighted average of proportion difference was 4.6% (95% CI, −1.8% to 11%; p = .1696).
Secondary Endpoint(s)
HbA1c less than 7% at week 24 was achieved in 40.7% of patients in the lixisenatide group and in 40% of the sitagliptin group; weighted average of proportion difference was 0.8% (95% CI, −9.7% to 11.3%; p = .884).
LS mean change in HbA1c from baseline was −0.7% for both groups.
Reduction in body weight from baseline to week 24 was −2.5 kg with lixisenatide and −1.2 kg with sitagliptin; mean difference was −1.3 kg (95% CI, −2.1 to −0.6; p < .001).
Comments: This study compared an injectable GLP-1 receptor agonist (lixisenatide) and an oral DPP-4 inhibitor (sitagliptin) using a double-blind, double-dummy design. Patients were censored if a rescue medication had to be added to the drug regimen, and missing values were replaced using last observation carried forward values. Study withdrawal occurred in 10.1% of the lixisenatide group and 6.8% of the sitagliptin group.
Limitations: The study population was younger than in other studies. Whether a difference in response exists between the 2 drugs in older patients remains to be determined.
Indication: Type 2 Diabetes Mellitus—Add-On Therapy With Other Hypoglycemic Drugs
Guidelines
Guideline: Standards of medical care in diabetes—2016
Reference: American Diabetes Association, 201631
Comments: If metformin at maximal tolerated doses does not achieve or maintain the target HbA1c after 3 months, a sulfonylurea, thiazolidinedione, DPP-4 inhibitor, SGLT2 inhibitor, GLP-1 receptor agonist, or basal insulin should be added. A dual combination may also be considered as initial therapy when HbA1c is 9% or greater. If target HbA1c levels are not achieved after 3 months of dual therapy, an additional agent should be added. If adequate control is not achieved after 3 months of triple therapy, patients receiving oral regimens should be switched to injectables, patients receiving a GLP-1 receptor agonist should have basal insulin added, and patients receiving optimally titrated basal insulin should have a GLP-1 receptor agonist or mealtime insulin added. Considerations when selecting therapy for an individual patient should include efficacy, cost, potential adverse effects, weight, comorbidities, hypoglycemia risk, and patient preferences.
Guideline: Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2016 executive summary
Reference: Garber, 201632
Comments: If glycemic targets are not achieved within 3 months with metformin or an alternative first-line agent, another agent should be added in the following suggested hierarchy of use: GLP-1 receptor agonist, SGLT2 inhibitor, DPP-4 inhibitor, thiazolidinedione, basal insulin, colesevelam, bromocriptine, alpha-glucosidase inhibitor, or sulfonylurea/glinide. If glycemic targets are not achieved within 3 months on dual therapy, a third agent should be added in the following suggested hierarchy of usage: GLP-1 receptor agonist, SGLT2 inhibitor, thiazolidinedione, basal insulin, DPP-4 inhibitor, colesevelam, bromocriptine, alpha-glucosidase inhibitor, or sulfonylurea/glinide. Insulin therapy is recommended for patients with HbA1c greater than 9% with symptoms and for those not at glycemic goal after 3 months on triple therapy.
Studies
Drug: Lixisenatide vs Placebo
Reference: Riddle MC, et al, 2013 (GetGoal-L trial)7
Study Design: Randomized, double-blind, multicenter study
Study Funding: Sanofi
Patients: 496 adults with type 2 diabetes insufficiently controlled with basal insulin with or without metformin therapy. Patients were eligible if they had received a basal insulin regimen for at least 3 months and been receiving a stable dose for at least 2 months before screening, and if they had HbA1C 7% to 10%. Patients receiving metformin were required to be on a stable dose (at least 1.5 g/day) for at least 3 months prior to screening. Exclusion criteria were FPG greater than 250 mg/dL; BMI 20 kg/m2 or less; weight change greater than 5 kg over the 3 months before screening; history of unexplained pancreatitis, ESRD, or allergic reaction to any GLP-1RA; or pregnancy. Of 879 patients screened, 496 were randomized, with 495 receiving treatment with lixisenatide (n = 328) or placebo (n = 167). Mean baseline characteristics were as follows: patient age was 57 years, duration of diabetes was 12.5 years, duration of basal insulin use was 3.1 years, and HbA1C was 8.4%; 78% of patients were White.
Intervention: Patients were randomized (2:1) to receive lixisenatide or placebo subcutaneously once daily before the morning meal for 24 weeks in addition to basal insulin with or without metformin therapy. Lixisenatide was administered as a 2-step dose-increase regimen at 10 mcg/day for 1 week, then 15 mcg/day for 1 week, and then 20 mcg/day for the remaining 22 weeks. Prior to starting study drug, the daily dose of basal insulin was reduced by 20% to decrease the risk of hypoglycemia; thereafter, it could be increased.
Results
Primary Endpoint(s)
LS mean change in HbA1c from baseline at week 24 was −0.7% with lixisenatide and −0.4% with placebo. The LS mean difference from placebo was −0.4% (95% CI, −0.6% to −0.2%; p < .001).
Secondary Endpoint(s)
HbA1c less than 7% at week 24 was achieved in 28.3% of patients treated with lixisenatide and in 2% with placebo (p < .001). HbA1c of 6.5% or less at week 24 was achieved in 14.5% of patients treated with lixisenatide and in 3.8% with placebo (p < .001).
Body weight decreased from baseline to week 24 by 1.8 kg with lixisenatide and by 0.5 kg with placebo. The LS mean difference from placebo was −1.3 kg (p < .001).
No difference was observed between treatments in FPG, but 7-point self-measurement of plasma-referenced glucose showed reductions throughout the day with lixisenatide, and postprandial glucose concentrations were lower with lixisenatide.
Comments: Treatment was allocated using an interactive voice-response system. Rescue therapy with rapid-acting insulin or with an increase in basal insulin of more than 20% occurred in 6% of lixisenatide patients and in 7% of placebo patients. GetGoal-L-Asia was a similar study, but it used basal insulin with or without sulfonylurea therapy instead of metformin and only included patients from Japan, the Republic of Korea, Taiwan, and the Philippines.10 Similar to the GetGoal-L trial, these patients had improved glycemic control compared to the placebo group.
Limitations: This study did not include a study arm with another GLP-1 receptor agonist to determine whether differences exist among drugs in the same class.
Reference: Riddle MC, et al, 2013 (GetGoal-Duo 1 trial)8
Study Design: Randomized, double-blind, multicenter study
Study Funding: Sanofi
Patients: 446 adults with type 2 diabetes inadequately controlled on metformin alone or with a sulfonylurea, glinide, or thiazolidinedione. Patients were eligible if they had been diagnosed at least 1 year prior to screening, were receiving metformin at a stable dose (at least 1.5 g/day) as monotherapy or as part of combination therapy for at least 3 months, had HbA1c 7% to 10%, and had BMI greater than 20 kg/m2. Exclusion criteria were use of oral or injectable antihyperglycemic agents other than metformin, sulfonylureas, glinides, and thiazolidinediones within 3 months; use of weight-loss drugs if not at a stable dose for at least 3 months; history of hypoglycemia unawareness, GI disease associated with prolonged nausea, and vomiting; and hypersensitivity to insulin glargine or allergic reaction to any GLP-1 receptor agonists. Of 1,470 patients screened, 825 patients completed the run-in phase; of these 825 patients, 446 met requirements for randomization to treatment with lixisenatide (n = 223) or placebo (n = 223). Mean baseline characteristics were as follows: patient age was 56 years, duration of diabetes was 9.2 years, and BMI was 31.8 kg/m2.
Intervention: Sulfonylurea or glinide use was discontinued at the beginning of the study. Previous thiazolidinedione (12%) and metformin (100%) therapy was continued unchanged throughout the study. Insulin glargine was initiated and titrated during the 12-week run-in phase to achieve FPB of 80 to 100 mg/dL in all patients. Patients with an HbA1c of 7% to 9% after the insulin glargine run-in phase were randomized (1:1) to receive lixisenatide or placebo subcutaneously once daily for 24 weeks. The lixisenatide dose was titrated in 2 steps at 10 mcg/day for 1 week, then 15 mcg/day for 1 week, and then 20 mcg/day as a maintenance dosage for the remaining 22 weeks.
Results
Primary Endpoint(s)
Mean HbA1c decreased from 8.6% to 7.6% during the insulin glargine run-in phase. The LS mean change from baseline (7.6%) was −0.7% with lixisenatide and −0.4% with placebo. The LS mean difference from placebo was −0.3% (95% CI, −0.5% to −0.2%; p < .001).
Secondary Endpoint(s)
HbA1c less than 7% was achieved in 56% of patients treated with lixisenatide and in 39% with placebo (p = .001). HbA1c 6.5% or less was achieved in 32% of patients treated with lixisenatide and in 16% with placebo (p < .001).
The 2-hour postbreakfast plasma glucose LS mean was decreased from baseline by 3.1 mmol/L with lixisenatide and increased by 0.1 mmol/L with placebo. The LS mean difference from placebo was −3.2 mmol/L (95% CI, −4 to −2.4; p < .001).
FPG was increased from baseline by 0.3 mmol/L with lixisenatide and by 0.5 mmol/L with placebo. The LS mean difference from placebo was −0.1 mmol/L (95% CI, −0.5 to 0.2; p = .5142).
Comments: Randomization was performed using a centralized interactive voice-response system. Meaningful changes in HbA1c and glucose control were seen with the addition of lixisenatide to metformin and basal insulin glargine. The impact of postmeal insulin administration was not assessed. The proportion of patients with treatment-emergent adverse events (primarily GI events) was higher with lixisenatide than placebo (79.8% vs 68.2%); serious adverse events were reported in 7.6% of patients in the lixisenatide group compared with 4.5% in the placebo group.
Limitations: Although the run-in period could be considered short, 3 months is enough time for a meaningful change in HbA1c. Approximately 40% of patients randomized to lixisenatide did not attain the HbA1c goal of 7% or lower, and what further measures might improve their success remains unknown. This study did not include a study arm with another GLP-1 receptor agonist to determine if differences exist among drugs in the same class.
Reference: Pinget M, et al, 2013 (GetGoal-P trial)6
Study Design: Randomized, double-blind, multicenter study
Study Funding: Sanofi
Patients: 484 adults with type 2 diabetes inadequately controlled with at least 30 mg/day of pioglitazone with or without metformin (1.5 g/day) for at least 3 months, with an HbA1c of 7% to 10%. Exclusion criteria were use of oral or injectable glucose-lowering agents other than pioglitazone and metformin within 3 months prior to screening; FPG greater than 250 mg/dL at screening; history of unexplained pancreatitis, chronic pancreatitis, pancreatectomy, stomach/gastric surgery, or inflammatory bowel disease; ESRD and/or dialysis, or creatinine greater than 1.4 mg/dL in women or greater than 1.5 mg/dL in men; history of allergic reaction to any GLP-1 receptor agonist; and clinically relevant history of GI disease with prolonged nausea and vomiting during the previous 6 months. Of 906 patients screened, 484 were eligible for randomization to treatment with lixisenatide (n = 323) or placebo (n = 161). Mean baseline characteristics were as follows: patient age was 55.8 years, duration of diabetes was 8.1 years, HbA1c was 8.1%, and BMI was 33.9 kg/m2.
Intervention: Patients were randomized (2:1) to receive lixisenatide or placebo subcutaneously once daily for 24 weeks. Lixisenatide was administered in a 2-step dose-increase regimen at 10 mcg/day for 1 week, then 15 mcg/day for 1 week, and then 20 mcg/day for the remaining 22 weeks. Previous pioglitazone and/or metformin therapy was continued unchanged throughout the study. Randomization was stratified by HbA1c and metformin use.
Results
Primary Endpoint(s)
LS mean change in HbA1c from baseline to week 24 was −0.9% with lixisenatide and −0.34% with placebo. The LS mean difference from placebo was −0.56% (95% CI, −0.73% to −0.39%; p < .001).
Secondary Endpoint(s)
HbA1c less than 7% was achieved in 52.3% of patients treated with lixisenatide and in 26.4% with placebo (p < .001). HbA1c 6.5% or less was achieved in 28.9% of patients treated with lixisenatide and in 10.1% with placebo (p < .001).
FPG was decreased from baseline by 1.1 mmol/L with lixisenatide and by 0.3 mmol/L with placebo. The LS mean difference from placebo was −0.84 mmol/L (95% CI, −1.21 to −0.47; p < .001).
Body weight decreased by 0.2 kg with lixisenatide and increased by 0.2 kg with placebo. The LS mean difference from placebo was −0.41 kg (95% CI, −1.03 to 0.2; p = .1864). Patients using metformin at baseline had an LS mean difference in body weight of −0.54 kg (95% CI, −1.23 to 0.14) and those not using metformin had a difference of 0.13 kg (95% CI, −1.27 to 1.53).
Comments: This was a multicenter, international study; approximately half of the study patients were from North America. Fewer patients required rescue therapy at week 24 with lixisenatide (3.8% vs 11.3%). The nausea associated with lixisenatide was more frequent during the first week of therapy or the week following an increase in dose. The incidence of injection-site reactions was similar in both groups. The beneficial effects of lixisenatide were maintained during the variable extension phase (52 weeks or more). At week 76, HbA1c was reduced from baseline by 1.1 in the lixisenatide group and by 0.6 in the placebo group, and mean HbA1c was 6.9% in the lixisenatide group and 7.3% in the placebo group. Maintenance of HbA1c less than 7% occurred in 58.5% of patients in the lixisenatide group and in 38% in the placebo group, and HbA1c of 6.5% or less was achieved by 35.6% and 21.1%, respectively. The mean change in body weight from baseline to week 76 was 0.88 kg in the lixisenatide group and 0.86 kg in the placebo group.
Reference: Rosenstock J, et al, 2014 (GetGoal-S trial)30
Study Design: Randomized, double-blind, multicenter study
Study Funding: Sanofi
Patients: 859 adults with type 2 diabetes inadequately controlled with sulfonylurea therapy with or without metformin, and with HbA1C 7% to 10%. Exclusion criteria were use of oral or injectable glucose-lowering agents other than a sulfonylurea or metformin within 3 months prior to screening; FPG greater than 250 mg/dL; history of unexplained pancreatitis, chronic pancreatitis, pancreatectomy, stomach/gastric surgery, or inflammatory bowel disease; history of GI disease with prolonged nausea and vomiting in the 6 months prior to study initiation, metabolic acidosis (including diabetic ketoacidosis) within 1 year prior to screening, or myocardial infarction, stroke, or heart failure requiring hospitalization within the previous 6 months; uncontrolled/inadequately controlled hypertension at the time of screening, with a resting systolic blood pressure greater than 180 mm Hg or diastolic blood pressure greater than 95 mm Hg; amylase and/or lipase greater than 3 times or AST, ALT, or alkaline phosphatase greater than 2 times the ULN; and ESRD (defined as serum creatinine clearance <15 mL/min) and/or dialysis. In the case of treatment with metformin, patients with renal impairment (defined as creatinine >1.4 mg/dL in women and >1.5 mg/dL in men) were excluded. Of 1,438 patients screened, 850 were randomized to treatment with lixisenatide (n = 573) or placebo (n = 286). Monotherapy with a sulfonylurea was used by 16% of patients at baseline, and combination therapy with sulfonylurea and metformin was used by 84% of patients. Mean baseline characteristics were as follows: patient age was 57.4 years, duration of diabetes was 9.5 years, HbA1c was 8.3%, and BMI was 30.3 kg/m2; 52.2% of patients were White and 44.8% were Asian.
Intervention: Patients were randomized (2:1) to receive treatment with lixisenatide or placebo subcutaneously once daily within 1 hour before the morning meal for 24 weeks. Using a 2-step doseincrease regimen, lixisenatide was administered as 10 mcg/day for 1 week, then 15 mcg/day for 1 week, and then 20 mcg/day for the remaining 22 weeks. Previous sulfonylurea and metformin regimens were continued unchanged. Randomization was stratified by HbA1c and metformin use.
Results
Primary Endpoint(s)
HbA1c decreased from baseline to week 24 by 0.85% with lixisenatide and by 0.1% with placebo (p < .001); LS mean difference between the 2 groups was −0.74% (95% CI, −0.867% to −0.621%; p < .001).
Secondary Endpoint(s)
HbA1c less than 7% was achieved in 36.4% of patients treated with lixisenatide and in 13.5% with placebo (p < .001). HbA1c 6.5% or less was achieved in 19.3% of patients treated with lixisenatide and in 4.7% with placebo (p < .001).
FPG decreased more with lixisenatide; LS mean difference was −0.6 mmol/L (95% CI, −0.9 to −0.3; p < .001).
Body weight decreased by 1.76 kg with lixisenatide and by 0.93 kg with placebo; LS mean difference was −0.84 kg (95% CI, −1.25 to −0.42; p < .001). Weight loss of 5% or greater from baseline to week 24 was achieved in 14.4% of patients treated with lixisenatide and in 7.2% with placebo.
Comments: Rescue medication was required by 4% of patients in the lixisenatide group and by 12.6% of in the placebo group during the 24-week treatment period. These patients were censored for efficacy assessment at the time rescue medication was started. The 24-week study was completed by 87% of the lixisenatide group and 89% of the placebo group. The discontinuation rate due to adverse reactions was 8.4% with lixisenatide and 3.8% with placebo. The majority of adverse reactions were mild or moderate cases of nausea and vomiting that subsided over the first 4 weeks. A similar study conducted in Asian patients with type 2 diabetes inadequately controlled on metformin with or without a sulfonylurea (GetGoal-M-Asia) found that lixisenatide improved glycemic control in this patient population and was well tolerated throughout the 24-week study.12
Limitations: GetGoal-S did not include a study arm with another GLP-1 receptor agonist to determine whether differences exist among drugs in the same class.
CONTRAINDICATIONS, WARNINGS, AND PRECAUTIONS
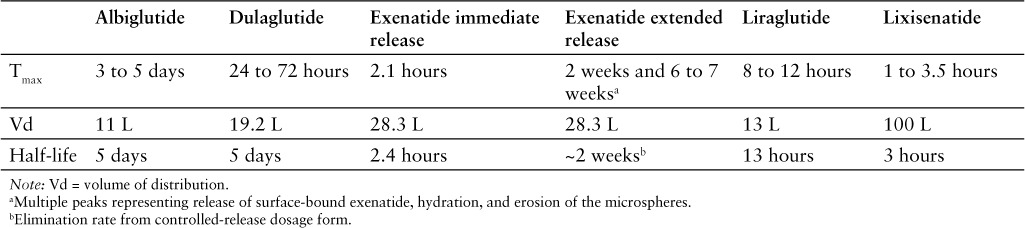
The contraindications, warnings, and precautions for lixisenatide are similar to those included in the product labeling of albiglutide, dulaglutide, exenatide immediate-release injection, exenatide extended-release injection, and liraglutide (Table 2).1,13–17
Table 2.
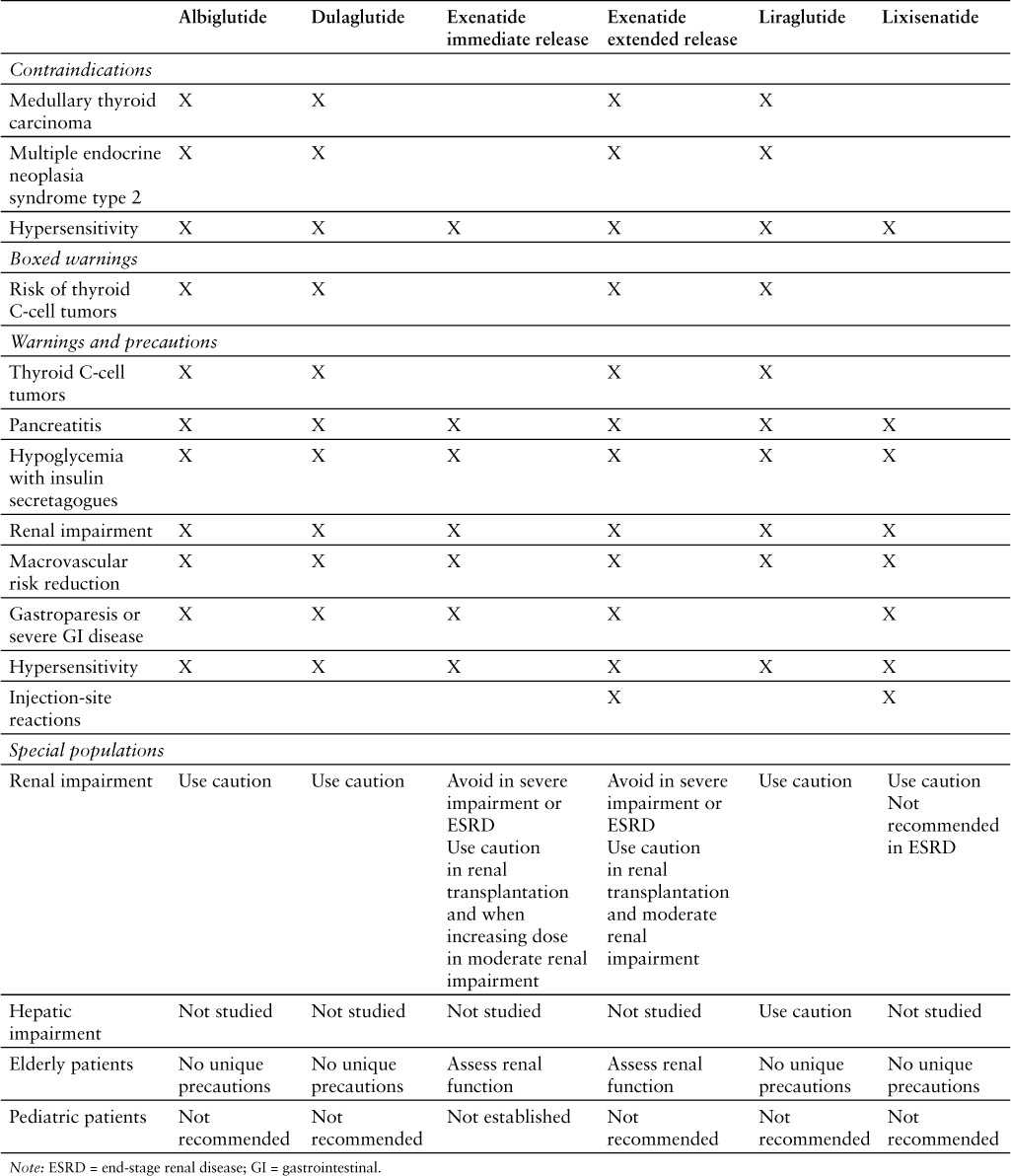
Some GLP-1 receptor agonists (ie, albiglutide, dulaglutide, exenatide, liraglutide) are contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia syndrome type 2.13–17 Boxed warnings regarding the risk of thyroid C-cell tumors are required for some GLP-1 receptor agonists (ie, albiglutide, dulaglutide, exenatide, liraglutide).13–17
Contraindications
Lixisenatide is contraindicated in patients with known hypersensitivity to lixisenatide or to any component of the product (ie, glycerol, sodium acetate trihydrate, methionine, metacresol, water for injection).1
Warnings and Precautions
Anaphylaxis and serious hypersensitivity reactions (eg, angioedema) have been reported. The risk of cross-sensitivity between GLP-1 receptor agonists is unknown.1
Acute pancreatitis has been reported, including cases of fatal and nonfatal hemorrhagic or necrotizing pancreatitis. This risk may be increased in patients with a history of cholelithiasis or alcohol abuse. Patients should be monitored for signs and symptoms of pancreatitis; if pancreatitis is suspected, the drug should be discontinued and appropriate management started. If pancreatitis is confirmed, lixisenatide should not be restarted.1
Concomitant use with a sulfonylurea or basal insulin is associated with an increased frequency of hypoglycemia. Reduction in the dose of sulfonylurea or basal insulin may be necessary.1
Acute kidney injury and worsening chronic renal failure have been reported with GLP-1 receptor agonists. Monitor renal function in patients with preexisting renal impairment or in patients reporting severe GI reactions. Lixisenatide is not recommended for patients with ESRD.1
Development of antibodies to lixisenatide may occur during treatment. Allergic reactions and injection-site reactions may occur in patients who develop these antibodies. Patients experiencing worsening glycemic control or failure to achieve targeted glycemic control, allergic reactions, or significant injection-site reactions may require treatment with an alternative antidiabetic therapy.1
The impact of lixisenatide on the reduction of risk of developing negative macrovascular outcomes is unknown.1
The safety and effectiveness of lixisenatide in patients with gastroparesis is unknown. Gastroparesis was an exclusion criterion in clinical trials. Because lixisenatide may slow gastric emptying, its use in patients with gastroparesis is not recommended.1
Information regarding the safety of lixisenatide during pregnancy is not sufficient to determine the risk of major birth defects and miscarriage. Lixisenatide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.1
No information is available regarding the safety of lixisenatide during breastfeeding. In a study in rats, lixisenatide and its metabolites were excreted in small amounts.1
The safety and effectiveness of lixisenatide have not been established in patients younger than 18 years.1
ADVERSE REACTIONS
In clinical trials, the most common adverse reactions associated with lixisenatide therapy included nausea (25%), vomiting (10%), headache (9%), diarrhea (8%), and dizziness (7%).1
Infrequent adverse reactions included allergic reactions (eg, pruritus, urticaria) and injection-site reactions.1
The incidence of hypoglycemia is influenced by concurrent use with other antidiabetic agents; in clinical studies, the incidence of hypoglycemia during monotherapy with lixisenatide was the same as with placebo (2%). The incidence of hypoglycemia with lixisenatide when administered with metformin or pioglitazone with or without metformin was 1% with placebo and 3% with lixisenatide. In patients treated with lixisenatide and a sulfonylurea with or without metformin, the incidence of hypoglycemia was approximately 15%. In those treated with lixisenatide and basal insulin with or without metformin, the incidence of hypoglycemia was 28%. In those treated with lixisenatide, basal insulin, and a sulfonylurea, the incidence of hypoglycemia was 47%.1
In clinical trials, there were 21 cases of pancreatitis with lixisenatide and 14 cases in comparator-treated patients (incidence rate of 21 vs 17 per 10,000 patient-years).1 In one clinical study, administration of lixisenatide to patients with type 2 diabetes and a history of acute coronary syndrome (eg, myocardial infarction, hospitalization for unstable angina) within the past 6 months did not alter the rate of major cardiovascular events (eg, rate of hospitalization for heart failure, rate of death) or other serious adverse events compared to placebo.35 This study met noninferiority criteria for lixisenatide but did not meet superiority criteria.
In a dose-ranging study that enrolled 542 patients, anti-lixisenatide antibody–positive frequency ranged from 43.1% in patients receiving 10 mcg once daily to 71.2% in patients receiving 20 mcg twice daily.29 In another study that enrolled 361 patients and used a stepwise dosing increase to the target dosage of 20 mcg/day, 56% of those treated using the 2-step dosing schedule and 60% treated with the 1-step dosing schedule developed anti-lixisenatide antibodies.5 The presence of antibodies in these 2 studies did not appear to affect the safety or efficacy of lixisenatide.5,29
DRUG INTERACTIONS
Any changes in warfarin pharmacokinetics with concurrent lixisenatide therapy are believed to be clinically insignificant.36
Absorption of some orally administered drugs may be delayed, especially if taken within 4 hours of lixisenatide administration, due to the impact of lixisenatide on gastric emptying time and GI motility. However, absorption of acetaminophen and oral contraceptives taken 1 hour before administration of lixisenatide were unaffected.1
RECOMMENDED MONITORING
As in all patients with type 2 diabetes, blood glucose and periodic HbA1c monitoring is advised.
DOSING
Lixisenatide should be started at 10 mcg subcutaneously once daily for 14 days. The injection should be given in the abdomen, thigh, or upper arm 1 hour before the first meal of the day, and the injection site should be rotated with each dose.1 The dose should be increased to 20 mcg once daily starting on day 15.1 Recommended dosages for the GLP-1 receptor agonists are compared in Table 3.1,13–17
Table 3.
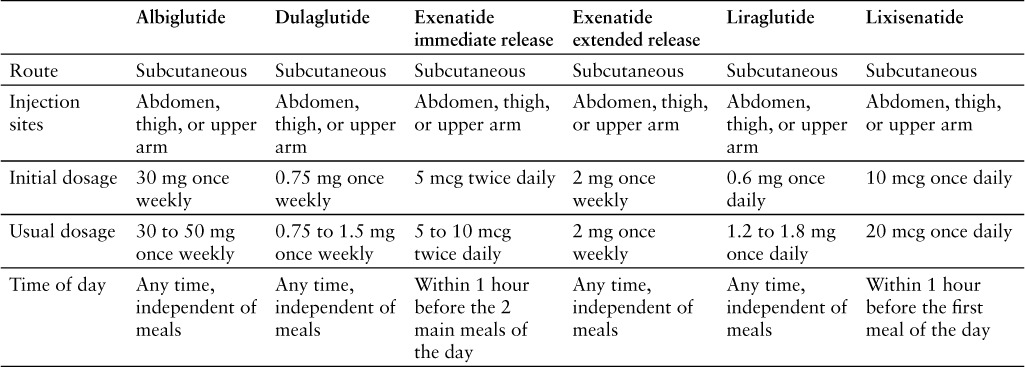
The prescribing information states that lixisenatide should be given 1 hour before the first meal of the day; however, subcutaneous administration of lixisenatide 20 mcg within 1 hour before the main meal of the day was shown to be noninferior to lixisenatide 20 mcg before the morning meal in patients insufficiently controlled with metformin therapy.1,37 In another study that evaluated morning versus evening before-meal dosing in patients insufficiently controlled with metformin therapy, both times of day were associated with improvement in glycemic control and were well tolerated.38
No dosage adjustments are needed for patients with mild to moderate renal impairment; however, monitoring for hypoglycemia and increased frequency of nausea and vomiting in these patients is recommended. Experience in patients with severe renal impairment or ESRD is limited. Lixisenatide should be used with caution in patients with severe renal impairment and is not recommended in patients with ESRD.1
PRODUCT AVAILABILITY
Lixisenatide was approved by the FDA on July 27, 2016.2 It is available in a prefilled pen for subcutaneous administration. The starter pen contains 50 mcg/mL of lixisenatide in 3 mL solution and delivers 14 doses of 10 mcg/dose. The maintenance pen contains 100 mcg/mL of lixisenatide in 3 mL solution and delivers 14 doses of 20 mcg/dose. Both pens are intended for single-patient use.1 Table 4 compares the availability, storage, and handling of GLP-1 receptor agonists.1,13–17
Table 4.
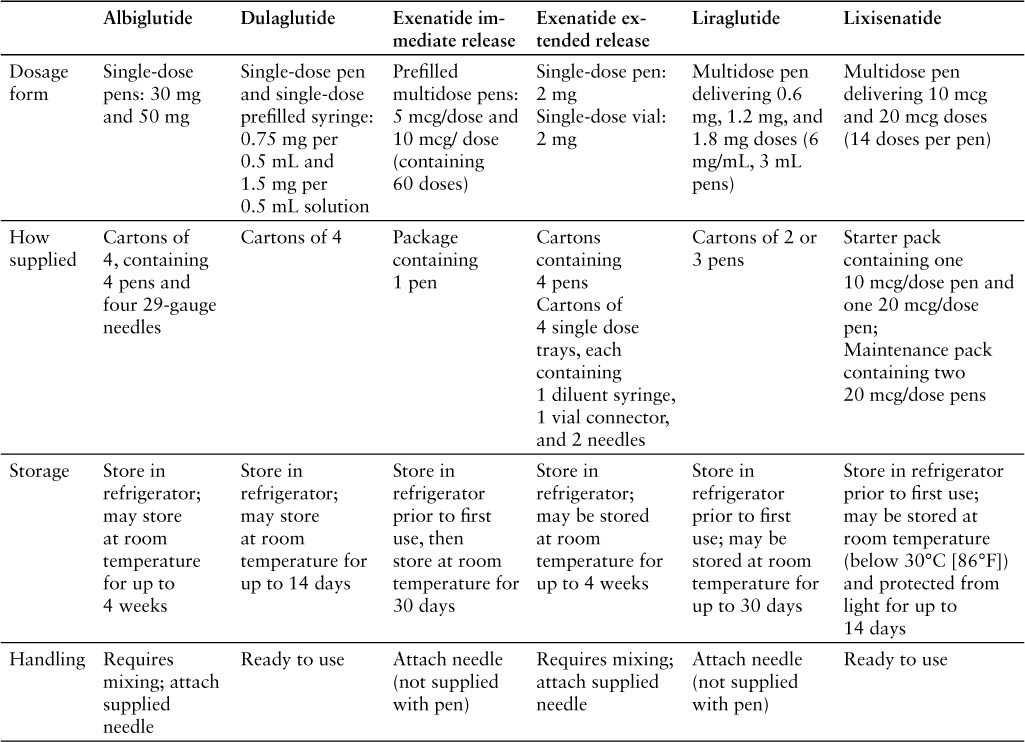
Unused pens should be stored in the refrigerator at 2°C to 8°C (36°F to 46°F) in the original packaging to protect them from light. After the first use, a pen can be stored below 30°C (86°F) and protected from light. Pens should not be frozen. Pens should be discarded 14 days after first use.1
DRUG SAFETY/RISK EVALUATION AND MITIGATION STRATEGY (REMS)
No REMS is required for lixisenatide.2
CONCLUSION
Lixisenatide, approved as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus, offers an alternative to other GLP-1 receptor agonists, especially in patients with inadequate response to monotherapy with sulfonylureas or metformin, or to a combination of the 2. Lixisenatide once daily demonstrated noninferiority to exenatide twice daily, was less effective than liraglutide once daily, and had a similar therapeutic effect to sitagliptin, a DPP-4 inhibitor. Differences in dosing schedules, cardiovascular outcome studies, and cost will influence lixisenatide's place in therapy. The lack of cardiovascular adverse effects with lixisenatide is similar to that observed with other GLP-1 receptor agonists; however, studies addressing the role of lixisenatide in decreasing the risk of heart attack, stroke, and cardiovascular death in high-risk type 2 diabetes patients would be beneficial.
REFERENCES
- 1. Adlyxin (lixisenatide) [prescribing information]. Bridgewater, NJ: Sanofi-Aventis; July 2016. [Google Scholar]
- 2. Parks MH. NDA approval letter: Adlyxin (lixisenatide NDA 208471). US Food and Drug Administration website. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2016/208471Orig1s000ltr.pdf. Published July 27, 2016. Accessed August 1, 2016.
- 3. Bolli GB, Munteanu M, Dotsenko S, . et al. Efficacy and safety of lixisenatide once daily vs placebo in people with type 2 diabetes insufficiently controlled on metformin (GetGoal-F1). Diabet Med. 2014; 31( 2): 176– 184. [DOI] [PubMed] [Google Scholar]
- 4. Charbonnel B, Bertolini M, Tinahones FJ, Domingo MP, Davies M.. Lixisenatide plus basal insulin in patients with type 2 diabetes mellitus: A meta-analysis. J Diabetes Complications. 2014; 28( 6): 880– 886. [DOI] [PubMed] [Google Scholar]
- 5. Fonseca VA, Alvarado-Ruiz R, Raccah D, Boka G, Miossec P, Gerich JE.. Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: A randomized, doubleblind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care. 2012; 35( 6): 1225– 1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pinget M, Goldenberg R, Niemoeller E, Meuhlen-Bartmer I, Guo H, Aronson R.. Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P). Diabetes Obes Metabol. 2013; 15( 11): 1000– 1007. [DOI] [PubMed] [Google Scholar]
- 7. Riddle MC, Aronson R, Home P, . et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: A 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care. 2013; 36( 9): 2489– 2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riddle MC, Forst T, Aronson R, . et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: A 24-week, randomized, placebo-controlled comparison (GetGoal-Duo 1). Diabetes Care. 2013; 36( 9): 2497– 2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenstock J, Raccah D, Korányi L, . et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: A 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care. 2013; 36( 10): 2945– 2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seino Y, Min KW, Niemoeller E, Takami A; EFC10887 GETGOAL-L Asia Study Investigators. . Randomized, doubleblind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metabol. 2012; 14( 10): 910– 917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Gaal L, Souhami E, Zhou T, Aronson R.. Efficacy and safety of glucagon-like peptide-1 receptor agonist lixisenatide versus the dipeptidyl peptidase-4 inhibitor sitagliptin in young (<50 years) obese patients with type 2 diabetes mellitus. J Clin Trans Endocrinol. 2014; 1( 2): 31– 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu Pan C, Han P, Liu X, . et al. Lixisenatide treatment improves glycaemic control in Asian patients with type 2 diabetes mellitus inadequately controlled on metformin with or without sulfonylurea: A randomized, double-blind, placebo-controlled, 24-week trial (GetGoal-M-Asia). Diabetes Metabol Res Rev. 2014; 30( 8): 726– 735. [DOI] [PubMed] [Google Scholar]
- 13. Bydureon (exenatide) [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals; September 2015. [Google Scholar]
- 14. Byetta (exenatide) [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals; February 2015. [Google Scholar]
- 15. Tanzeum (albiglutide) [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; July 2015. [Google Scholar]
- 16. Trulicity (dulaglutide) [prescribing information]. Indianapolis, IN: Eli Lilly and Company; July 2015. [Google Scholar]
- 17. Victoza (liraglutide) [prescribing information]. Princeton, NJ: Novo Nordisk Inc; April 2016. [Google Scholar]
- 18. Werner U, Haschke G, Herling AW, Kramer W.. Pharmacological profile of lixisenatide: A new GLP-1 receptor agonist for the treatment of type 2 diabetes. Regul Pept. 2010; 164 ( 2–3): 58– 64. [DOI] [PubMed] [Google Scholar]
- 19. Wilkins JJ, Dubar M, Sébastien B, Laveille C.. A drug and disease model for lixisenatide, a GLP-1 receptor agonist in type 2 diabetes. J Clin Pharmacol. 2014; 54( 3): 267– 278. [DOI] [PubMed] [Google Scholar]
- 20. Aroda VR, Ratner R.. The safety and tolerability of GLP-1 receptor agonists in the treatment of type 2 diabetes: A review. Diabetes Metabol Res Rev. 2011; 27( 6): 528– 542. [DOI] [PubMed] [Google Scholar]
- 21. Barnett AH. Lixisenatide: Evidence for its potential use in the treatment of type 2 diabetes. Core Evid. 2011; 6: 67– 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Becker RH, Stechl J, Msihid J, Kapitza C.. Lixisenatide resensitizes the insulin-secretory response to intravenous glucose challenge in people with type 2 diabetes--a study in both people with type 2 diabetes and healthy subjects. Diabetes Obes Metabol. 2014; 16( 9): 793– 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Becker RH, Stechl J, Steinstraesser A, Golor G, Pellissier F.. Lixisenatide reduces postprandial hyperglycaemia via gastrostatic and insulinotropic effects. Diabetes Metabol Res Rev. 2015; 31( 6): 610– 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lorenz M, Pfeiffer C, Steinstraber A, Ruus P.. Effects of lixisenatide once daily on gastric emptying and relationship to postprandial glycemia in type 2 diabetes mellitus [abstract]. 72nd Annual Scientific Sessions of the American Diabetes Association; June 8–12, 2012; Philadelphia, PA Abstract 1085-P. [Google Scholar]
- 25. Nock V, Ruppel D, Kloft C.. Comparison of three PK/PD models for glycated haemoglobin in diabetes type 2 patients treated with lixisenatide [abstract]. 2012 Annual Meeting of Population Approach Group in Europe; June 5–8, 2012; Venice, Italy Abstract 2335. [Google Scholar]
- 26. Werner U, Kuhlmann-Gottke J, Schfer HL, Herling AW.. Effect of the once-daily GLP-1R agonist lixisenatide on gastric emptying and prandial carbohydrate utilization in animal models: A comparison with liraglutide [abstract]. 71st Annual Scientific Sessions of the American Diabetes Association; June 24–28, 2011; San Diego, CA Abstract 2267-PO. [Google Scholar]
- 27. Werner U, Gerlach M, Hofmann M, Herling AW.. Combination of lixisenatide and insulin glargine demonstrates complementary pharmacological activity on glycemic control in animal models of diabetes [abstract]. 72nd Annual Scientific Sessions of the American Diabetes Association; June 8–12, 2012; Philadelphia, PA Abstract 1051-P. [Google Scholar]
- 28. Raccah E, Miossec P, Esposito V, Niemoeller E, Cho M, Gerich JE.. Efficacy and safety of lixisenatide in elderly (> 65 yr) patients with type 2 diabetes: An analysis from the Get-Goal Phase 3 Program [abstract]. 72nd Annual Scientific Sessions of the American Diabetes Association; June 8–12, 2012; Philadelphia, PA Abstract 972-P. [Google Scholar]
- 29. Ratner RE, Rosenstock J, Boka G; DRI6012 Study Investigators. . Dose-dependent effects of the once-daily GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes inadequately controlled with metformin: A randomized, double-blind, placebo-controlled trial. Diabet Med. 2010; 27( 9): 1024– 1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosenstock J, Hanefeld M, Shamanna P, . et al. Beneficial effects of once-daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal-S). J Diabetes Complications. 2014; 28( 3): 386– 392. [DOI] [PubMed] [Google Scholar]
- 31. American Diabetes Association. . Standards of medical care in diabetes—2016. Diabetes Care. 2016; 39( suppl 1): S1– S112. http://care.diabetesjournals.org/content/suppl/2015/12/21/39.Supplement_1.DC2/2016-Standards-of-Care.pdf. Accessed September 7, 2016. [DOI] [PubMed] [Google Scholar]
- 32. Garber AJ, Abrahamson MJ, Barzilay JI, . et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE) Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2016 executive summary. Endocr Pract. 2016; 22( 1): 84– 113. [DOI] [PubMed] [Google Scholar]
- 33. Novo Nordisk. . Efficacy and safety of liraglutide versus lixisenatide as add-on to metformin in subjects with type 2 diabetes (LIRA-LIXI). ClinicalTrials.gov website. https://clinicaltrials.gov/ct2/show/NCT01973231?term=nct01973231&rank=1. Updated December 21, 2015. Accessed August 9, 2016. NLM Identifier: NCT01973231.
- 34. Nauck M, Rizzo M, Johnson A, Bosch-Traberg H, Madsen J, Cariou B.. Once-daily liraglutide versus lixisenatide as add-on to metformin in type 2 diabetes: A 26-week randomized controlled clinical trial. Diabetes Care. 2016; 39( 9): 1501– 1509. [DOI] [PubMed] [Google Scholar]
- 35. Pfeffer MA, Claggett B, Diaz R, . et al; ELIXA Investigators Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015; 373( 23): 2247– 2257. [DOI] [PubMed] [Google Scholar]
- 36. Liu YH, Russ P, Steinstraesser A, Teichert L.. Effect of the GLP-1 agonist lixisenatide on the pharmacokinetics of warfarin [abstract]. 70th Annual Scientific Sessions of the American Diabetes Association; June 25–29, 2010; Orlando, FL Abstract 2128-PO. [Google Scholar]
- 37. Ahrén B, Vorokhobina N, Souhami E, Demil N, Ye J, Aronson R.. Equal improvement in glycaemia with lixisenatide given before breakfast or the main meal of the day. J Diabetes Complications. 2014; 28( 5): 735– 741. [DOI] [PubMed] [Google Scholar]
- 38. Ahrén B, Leguizamo Dimas A, Miossec P, Saubadu S, Aronson R.. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M). Diabetes Care. 2013; 36( 9): 2543– 2550. [DOI] [PMC free article] [PubMed] [Google Scholar]


