Abstract
Background
Prostate cancer has become a serious threat to the life of patients. microRNAs are small non-coding RNA molecules that regulate the growth and apoptosis of cells. We aimed to investigate the regulation and mechanism of microRNA (miR-143) in the proliferation and apoptosis of prostate cancer LNCap cells.
Material/Methods
miR-143 and control scramble miRNA were synthesized and respectively transfected into LNCap cells. The proliferation and apoptosis were detected by MTT assay, flow cytometry, and caspase-3 activity assay. The intracellular expression of Bcl-2 was determined by Western blot. Further, LNCap cells were transfected with small interfering RNA (siRNA) targeting Bcl-2 (siBcl-2) or plasmid expressing Bcl-2, followed by transfection of miR-143 or control miRNA. Bcl-2 expression was detected by Western blot, and cell apoptosis was measured by caspase-3 activity assay.
Results
Transfection of miR-143 significantly inhibited the proliferation of LNCap cells (P=0.0073), increased the percentage of externalized phosphatidylserine (P=0.0042), activated the caspase-3 (P=0.0012), and decreased the expression of Bcl-2 (P=0.012) when compared with the control miRNA group. The expression of Bcl-2 was significantly reduced after siBcl-2 transfection. The apoptosis in the siBcl-2+miR-143 group was significantly increased compared with that in the miR-143 group (P=0.036), whereas there was no significant difference in the apoptosis between the siBcl-2+miRNA and miRNA groups. The expression of Bcl-2 was obviously higher after the transfection of Bcl-2-expressing plasmid. The apoptosis in Bcl-2+miR-143 group was significantly reduced compared with the miR-143 group (P=0.031), whereas no significant difference in the apoptosis was detected between the miRNA and Bcl-2+miRNA groups.
Conclusions
Transfection of miR-143 induces the apoptosis of prostate cancer LNCap cells by down-regulating Bcl-2 expression, suggesting that Bcl-2 might be a potential therapeutic target for prostate cancer.
MeSH Keywords: Amlodipine; Apoptosis; Genes, bcl-2
Background
Prostate cancer is a malignant cancer of the male reproductive system with an incident rate of 3/10,000 in men [1]. The pathogenesis of prostate cancer is a complex process involving several factors, among which the genetic factor is known as the main determining factor [2]. Diet, ethnic, and geographic factors may also increase the incidence rate of prostate cancer. The disease has become a serious threat to the health and life of patients [3,4]. Currently, chemotherapy, radiotherapy, and surgery remain the primary treatment methods for prostate cancer, although there are several drawbacks such as the side effects during chemotherapy, potential bleeding in surgery, etc. [5–7]. Therefore, current medical science has been focused on how to improve the accuracy and success rate of the treatment for prostate cancer.
In recent years, molecular targeted therapy has become a hotspot in cancer research, including prostate cancer [8–11]. Nevertheless, the efficacy of the known gene targets for prostate cancer (such as the anti-apoptotic proteins survivin and apollon) is barely satisfactory [12]. It is therefore urgent to identify more effective molecular targets for the malignant disease [12,13]. microRNAs (miRNAs) are a class of small non-coding RNA molecules involved in the regulation of a wide range of biological processes such as cell cycle, apoptosis, organ development, tissue regeneration, aging, and even the pathogenesis of several diseases including prostate cancer. For instance, it is known that microRNA-218 can inhibit the growth of prostate cancer, and microRNA-34a is associated with the metastasis of prostate tumor [14,15], suggesting that miRNAs are involved in the occurrence and progression of prostate cancer [14–16]. In our previous analyses on the expression of miRNAs, we have found that miRNA (miR-143) expression in prostate cancer tissue is significantly higher compared with expression in adjacent non-cancerous tissue [17,18], indicating an association between the molecule and the development of the disease. In this study, we further investigated the regulatory role of miR-143 in the prostate cancer cell line LNCap.
Ideally, an anti-tumor treatment should efficiently kill tumor cells without affecting normal cells. Cell apoptosis is coordinately regulated by multiple anti-apoptotic and pro-apoptotic proteins [19,20]. One of the anti-tumor strategies is to induce the expression of pro-apoptotic proteins while suppressing the expression of anti-apoptotic proteins with anti-tumor drugs. Bcl-2 protein is an extensively studied anti-apoptotic molecule [21,22]. Although there are numerous reagents targeting Bcl-2 protein, none of them can effectively reduce the intracellular level of the protein [23]. In this study, we also investigate the association between miR-143 and Bcl-2 in LNCap cells to elucidate the regulatory role and possible mechanism of the miRNA in prostate cancer. This study provides a theoretical basis for the selection of an effective therapeutic target for the treatment of prostate cancer.
Material and Methods
Cells and reagents
The prostate cancer LNCap cells were purchased from American Type Culture Collection (ATCC, Rockville, Maryland, USA). Dulbecco’s Modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Hualan Biotech (Beijing, China). miR-143, negative scramble miRNA, and siBcl-2 were synthesized by Genepharma Biotech (Suzhou, China). Bcl-2–expressing plasmid was previously constructed in our lab.
Lipofectamine 2000 transfection reagent was purchased from Invitrogen (Camarillo, California, USA). The MTT cell viability assay kit was purchased from Dingguo Changsheng Biotech (Beijing, China). The Annexin V-FITC apoptosis assay kit, caspase-3 activity assay kit, protein extraction kit, and BCA kit were purchased from Beyotime Institute of Biotechnology (Shanghai, China). Mouse anti-human Bcl-2 monoclonal antibody and actin monoclonal antibody, and horseradish peroxidase (HRP)-labeled rabbit anti-mouse IgG were purchased from Santa Cruz Biotech (Santa Cruz, California, USA).
Cell culture
LNCap cells were cultured in DMEM containing 10% FBS, penicillin 100 U/mL, and streptomycin 0.1 mg/mL at 37°C and 5% CO2 in an incubator. Cells in the logistic growth phase were used for subsequent experiments.
Transfection
The miR-143 and control miRNA were transfected into LNCap cells using the Lipofectamine 2000 transfection kit following the manufacturer’s instruction. Briefly, LNCap cells were inoculated into each well on 6-well plates and cultured at 37°C in a 5% CO2 incubator until cells reached 50% confluence. miR-143 or control miRNA (2 μL of 0.5 μg/μL) was mixed with 5 μL of liposomal transfection reagent. The mixture was added to the cell culture, and cells were incubated for 12 h. The DMEM was replaced, and cells were incubated for another 24 h.
MTT assay
The viability of LNCap cells was detected in all groups by MTT assay as previously described [10]. Briefly, LNCap cells were inoculated into each well of 96-well plates at a density of 1×104 cells/well. Cells at 70% confluence were transfected with miR-143 and control miRNA as described above. After 24 h, cells were rinsed 3 times with phosphate-buffered saline (PBS), and 5 μL of 0.2M MTT was added to each well. Medium was discarded after 2 h, and 150 μL of dimethyl sulfoxide (DMSO) was added to each well. The optical density of dissolved MTT crystals was measured by a plate reader at 490 nm. Each sample was measured independently three times, and the percentage of viable cells was calculated.
Annexin VFITC staining
The apoptosis of LNCap cells in all groups was detected by Annexin V-FITC staining. Briefly, LNCap cells were transfected with miR-143 and control miRNA as described above. After 24 h, cells were collected and prepared into suspension. Cell suspension (250 μL) was incubated with 1 μL of Annexin V-FITC solution and 50 μL of binding buffer at room temperature in the dark for 40 min. Cell apoptosis was detected by flow cytometry at an emission wavelength of 448 nm and an absorbance wavelength of 570 nm. The experiment was repeated three times, and the percentage of cells with externalized phosphatidylserine was calculated.
Caspase-3 activity assay
The apoptosis of LNCap cells in all groups was also detected by caspase-3 activity assay. Briefly, LNCap cells in 96-well plates were transfected with miR-143 and control miRNA as described above. After 24 h, 20 μL of lysis buffer was added to each well. Cell lysate was incubated with 5 μL of chromogenic substrate at room temperature in the dark for 20 min. The optical density of each well was measured by a plate reader under 560 nm. The relative caspase-3 activity of each sample was calculated as the absorbance of the well subtracted by that of the control miRNA.
Western blot
The expression of Bcl-2 in LNCap cells in all groups was compared by Western blot analysis. Briefly, cells transfected with miR-143 or control miRNA were collected at 24 h after transfection. Total protein was extracted using protein extraction kits and quantified using a BCA kit according to the manufacturer’s instruction. Equal amounts of total protein (10 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes. The membrane was blocked in phosphate-buffered saline with Tween® 20 (PBST) buffer with 5% skim milk at room temperature for 1 h, and incubated with mouse anti-human Bcl-2 monoclonal antibody or actin monoclonal antibody (1: 200 dilution) overnight at 4°C with gentle shaking. The membrane was washed three times with PBST buffer and incubated with HRP-labeled rabbit anti-mouse IgG (1:200) at 37°C for 2 h. The membranes were washed twice with Tris Buffered Saline with Tween® 20 (TBST) and subjected to ECL detection. The intensity of bands was detected by a Molecular Imager® ChemiDocTM XRS System (Bio-Rad Laboratories). The gray value of bands was analyzed by Image J 6.0 software (Bio-Rad Laboratories). The relative expression of Bcl-2 was calculated as the ratio of the gray value of Bcl-2 to that of actin.
Detection of the effect of Bcl-2 interference or overexpression on miR-143-transfected LNCap cells
In order to assess the interference or overexpression of Bcl-2 on miR-143-transfected LNCap cells, cells were transfected with siBcl-2 or Bcl-2-expressing plasmid, followed by transfection of miR-143 or control miRNA. Briefly, cells were inoculated into 6-well plates. Cells at 50% confluence were transfected with 1 μL of 1 μg/μL siBcl-2 or Bcl-2 plasmid as described above. Cells were incubated and transfected with 2 μL of 0.5 μg/μL miR-143 or control miRNA. After 24 h, cells were collected and subjected to caspase-3 activity assay and Western blot analysis as described above.
Statistical analyses
All data were expressed as mean ± standard deviation. Statistical analyses were performed using SPSS 14.0 (SPSS Inc., Chicago, Illinois, USA). Differences between groups were analyzed by t tests. P values smaller than 0.05 were considered statistically significant.
Results
miR-143 inhibited the proliferation of LNCap cells
The proliferation of LNCap cells in all groups was detected by MTT assay. As shown in Figure 1, the percentage of viable cells in the miR-143 group was significantly lower than that in the miRNA group (P=0.0073). There was no significant difference in the cell viability between the miRNA group and cells without transfection (P>0.05). Therefore, cells in the miRNA group were used as controls in subsequent experiments.
Figure 1.
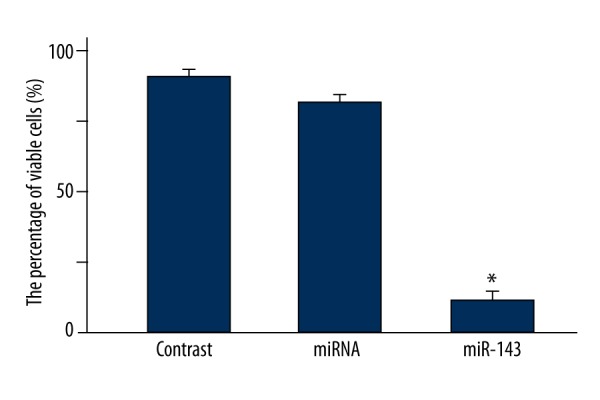
Comparison of cell viability in the miR-143, miRNA, and non-transfected groups by MTT assay. * P<0.01 compared with miRNA group.
miR-143 induced the apoptosis of LNCap cells
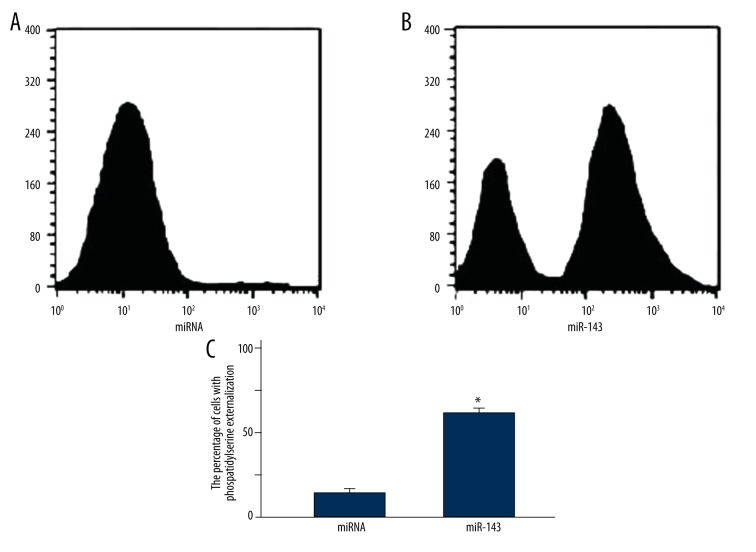
The apoptosis of LNCap cells in the miR-143 and miRNA groups was compared by Annexin VFITC staining. As shown in Figure 2, the percentage of phosphatidylserine externalization in the miR-143 group was significantly increased compared with that in the miRNA group (P=0.0042), suggesting that miR-143 substantially induced the apoptosis of LNCap cells.
Figure 2.
(A, B) Comparison of LNCap cell apoptosis in the miR-143 and miRNA groups by Annexin VFITC staining. * P<0.01 compared with miRNA group.
miR-143 induced caspase-3 activation
The apoptosis of LNCap cells in the miR-143 and miRNA groups was also compared by caspase-3 activity assay. As shown in Figure 3, the relative caspase-3 activity in miR-143 group was significantly higher than that in the miRNA group (P=0.012), indicating that miR-143 transfection greatly induced the caspase-3 activation in LNCap cells.
Figure 3.
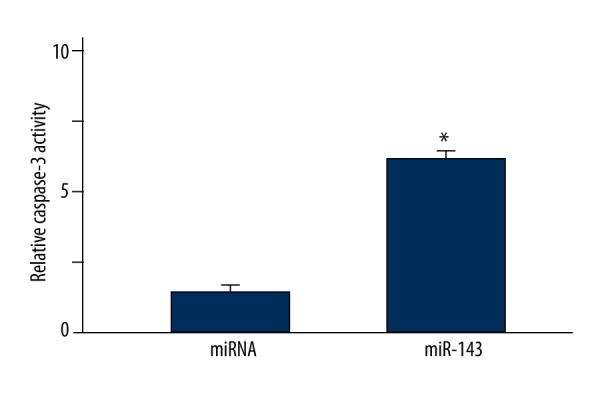
Comparison of LNCap cell apoptosis in the miR-143 and miRNA groups by caspase-3 activity assay. * P<0.05 compared with miRNA group.
miR-143 reduced intracellular Bcl-2 expression
The expression of Bcl-2 protein in LNCap cells in the miR-143 and miRNA groups was compared by Western blot. As shown in Figure 4, Bcl-2 expression in the miR-143 group was significantly lower compared with that in the miRNA group (P=0.012), suggesting that miR-143 reduced intracellular Bcl-2 expression.
Figure 4.
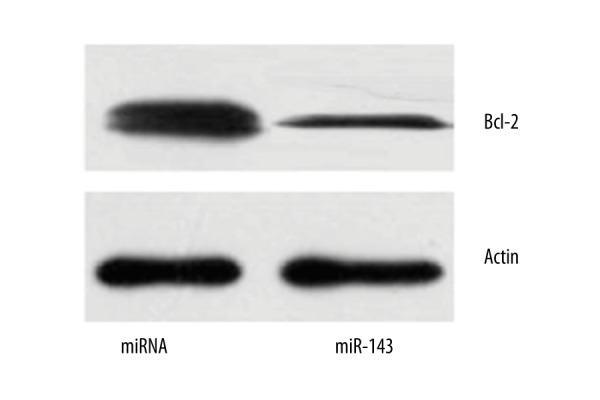
Western blot analyses of Bcl-2 expression in LNCap cells in the miR-143 and miRNA groups.
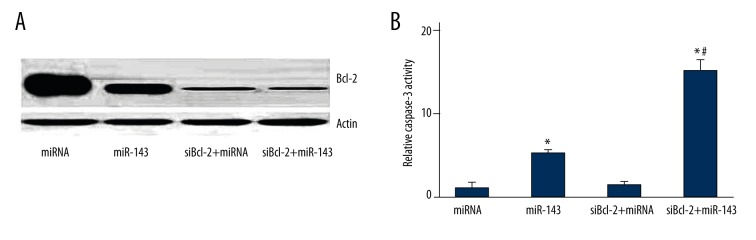
Bcl-2 interference enhanced the miR-143-induced apoptosis of LNCap cells
In order to detect the effect of Bcl-2 interference on the miR-143–induced apoptosis of LNCap cells, cells were transfected with siBcl-2, followed by miR-143 transfection. Western blot showed a markedly reduced Bcl-2 expression in the siBcl-2+miR-143 and siBcl-2+miRNA groups compared with the miR-143 and miRNA groups (Figure 5A), suggesting effective Bcl-2 interference by siBcl-2. The caspase-3 activity in the siBcl-2+miR-143 group was significantly increased compared with that in the miR-143 group (P=0.036), whereas there was no significant difference in the caspase-3 activity between the miRNA and siBcl-2+miRNA groups (Figure 5B).
Figure 5.
Bcl-2 interference enhanced the miR-143-induced apoptosis of LNCap cells. (A) Western blot analyses of Bcl-2 expression in different groups. (B) Caspase-3 activity assay in different groups. * P<0.05 compared with miRNA group. # P<0.05 compared with miR-143 group.
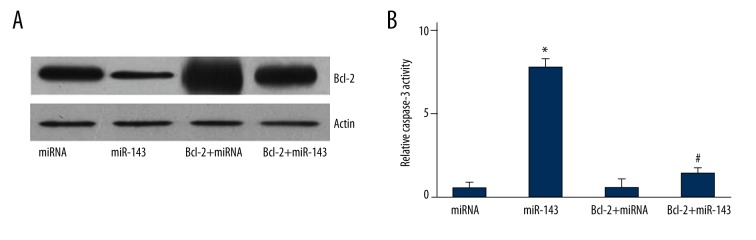
Bcl-2 overexpression inhibited the miR-143-induced apoptosis of LNCap cells
LNCap cells were also transfected with siBcl-2 plasmid, followed by miR-143 transfection. The Bcl-2 expression in the Bcl-2+miRNA and Bcl-2+miR-143 groups was obviously increased compared with that in the miRNA and miR-143 groups, as suggested by Western blot (Figure 6A). The caspase-3 activity in the Bcl-2+miR-143 group was significantly reduced compared with that in the miR-143 group (P=0.031), whereas there was no significant difference in the caspase-3 activity between the miRNA and Bcl-2+miRNA groups (Figure 6B).
Figure 6.
Bcl-2 overexpression inhibited the miR-143-induced apoptosis of LNCap cells. (A) Western blot analyses of Bcl-2 expression in different groups. (B) Caspase-3 activity assay in different groups. * P<0.05 compared with miRNA group. # P<0.05 compared with miR-143 group.
Discussion
To date, there are only a few studies on the regulatory role of miRNAs in prostate cancer [24,25]. MicroRNA-218 can inhibit the growth of prostate cancer, and microRNA-34a is associated with the metastasis of prostate tumor [14,15], suggesting that miRNAs may be involved in the occurrence and development of prostate cancer. In this study, the effect and molecular mechanism of miR-143 in prostate cancer were investigated using the LNCap cell model. It was found that miR-143 transfection reduced the cell viability and induced the apoptosis of LNCap cells, which was consistent with the widely recognized regulatory effect of miRNAs on cell growth and viability.
Bcl-2 protein has been well known as an anti-apoptotic protein [26]. Nevertheless, whether Bcl-2 is regulated by miR-143 in prostate cancer has not been previously investigated. In this study, Western blot analysis showed that Bcl-2 expression in LNCap cells was significantly reduced after miR-143 transfection. Moreover, siBcl-2-mediated Bcl-2 interference enhanced the miR-143-induced apoptosis of LNCap cells, whereas Bcl-2 overexpression inhibited the miR-143-induced cell apoptosis. These results demonstrated that Bcl-2 was involved in the miR-143-induced apoptosis of LNCap cells, indicating that Bcl-2 might be a target for prostate cancer gene therapy [27]. To our best knowledge, the current study is the first report on the association between miR-143 and Bcl-2 in prostate cancer, although the anti-apoptosis effect of Bcl-2 has been previously detected in other types of cancers [23,25].
The current study has several limitations such as the lack of clinical data on the Bcl-2 expression in prostate cancer tissues and adjacent normal tissues to further support the association between Bcl-2 and prostate cancer. Moreover, no animal experiments were performed to evaluate the feasibility and outcome of miR-143-targeted gene therapy for prostate cancer.
Conclusions
miR-143 inhibited the proliferation and induced the apoptosis of LNCap cells. The inhibitory effect on cell apoptosis was probably achieved by down-regulating the intracellular Bcl-2 expression, indicating that Bcl-2 might be a potential target for prostate cancer treatment. This study provides a theoretical basis for the development of effective molecule targeted therapy of prostate cancer.
Footnotes
Source of support: Departmental sources
References
- 1.Xu B, Huang Y, Niu X, et al. Hsa-miR-146a-5p modulates androgen-independent prostate cancer cells apoptosis by targeting ROCK1. Prostate. 2015;75(16):1896–903. doi: 10.1002/pros.23068. [DOI] [PubMed] [Google Scholar]
- 2.Santovito D, Egea V, Weber C. Small but smart: MicroRNAs orchestrate atherosclerosis development and progression. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbalip.2015.12.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Liu C, Li C, et al. Effects of microRNA-221/222 on cell proliferation and apoptosis in prostate cancer cells. Gene. 2015;572(2):252–58. doi: 10.1016/j.gene.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Wan X, Qiang W, et al. miR-143a suppresses prostate cell proliferation and induces apoptosis via KDM5B protein regulation. Int J Clin Exp Med. 2015;8(4):5329–39. [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J, Song Q, Cai Y, et al. RLIP76-dependent suppression of PI3K/AKT/Bcl-2 pathway by miR-101 induces apoptosis in prostate cancer. Biochem Biophys Res Commun. 2015;463(4):900–6. doi: 10.1016/j.bbrc.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 6.Larne O, Hagman Z, Lilja H, et al. miR-145 suppress the androgen receptor in prostate cancer cells and correlates to prostate cancer prognosis. Carcinogenesis. 2015;36(8):858–66. doi: 10.1093/carcin/bgv063. [DOI] [PubMed] [Google Scholar]
- 7.Ozen M, Karatas OF, Gulluoglu S, et al. Overexpression of miR-145-5p inhibits proliferation of prostate cancer cells and reduces SOX2 expression. Cancer Invest. 2015;33(6):251–58. doi: 10.3109/07357907.2015.1025407. [DOI] [PubMed] [Google Scholar]
- 8.Lu K, Liu C, Tao T, et al. MicroRNA-19a regulates proliferation and apoptosis of castration-resistant prostate cancer cells by targeting BTG1. FEBS Lett. 2015;589(13):1485–90. doi: 10.1016/j.febslet.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 9.Zhang GM, Bao CY, Wan FN, et al. MicroRNA-302a suppresses tumor cell proliferation by inhibiting AKT in prostate cancer. PLoS One. 2015;10(4):e0124410. doi: 10.1371/journal.pone.0124410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xuan H, Xue W, Pan J, et al. Downregulation of miR-221, -30d, and -15a contributes to pathogenesis of prostate cancer by targeting Bmi-1. Biochemistry (Mosc) 2015;80(3):276–83. doi: 10.1134/S0006297915030037. [DOI] [PubMed] [Google Scholar]
- 11.Zhang TW, Xing L, Tang JL, et al. Marchantin M induces apoptosis of prostate cancer cells through endoplasmic reticulum stress. Med Sci Monit. 2015;21:3570–76. doi: 10.12659/MSM.894476. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Liu C, Chen Z, Hu X, et al. MicroRNA-185 downregulates androgen receptor expression in the LNCaP prostate carcinoma cell line. Mol Med Rep. 2015;11(6):4625–32. doi: 10.3892/mmr.2015.3332. [DOI] [PubMed] [Google Scholar]
- 13.Yang CH, Pfeffer SR, Sims M, et al. The oncogenic microRNA-21 inhibits the tumor suppressive activity of FBXO11 to promote tumorigenesis. J Biol Chem. 2015;290(10):6037–46. doi: 10.1074/jbc.M114.632125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han G, Fan M, Zhang X. microRNA-218 inhibits prostate cancer cell growth and promotes apoptosis by repressing TPD52 expression. Biochem Biophys Res Commun. 2015;456(3):804–9. doi: 10.1016/j.bbrc.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 15.Chen WY, Liu SY, Chang YS, et al. MicroRNA-34a regulates WNT/TCF7 signaling and inhibits bone metastasis in Ras-activated prostate cancer. Oncotarget. 2015;6(1):441–57. doi: 10.18632/oncotarget.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang FQ, Liu M, Li W, et al. Combination of quercetin and hyperoside inhibits prostate cancer cell growth and metastasis via regulation of microRNA-21. Mol Med Rep. 2015;11(2):1085–92. doi: 10.3892/mmr.2014.2813. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Padi SK, Tindall DJ, Guo B. Polycomb protein EZH2 suppresses apoptosis by silencing the proapoptotic miR-31. Cell Death Dis. 2014;5:e1486. doi: 10.1038/cddis.2014.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai ZK, Chen Q, Chen YB, et al. microRNA-155 promotes the proliferation of prostate cancer cells by targeting annexin 7. Mol Med Rep. 2015;11(1):533–38. doi: 10.3892/mmr.2014.2744. [DOI] [PubMed] [Google Scholar]
- 19.Bian XJ, Zhang GM, Gu CY, et al. Down-regulation of Dicer and Ago2 is associated with cell proliferation and apoptosis in prostate cancer. Tumour Biol. 2014;35(11):11571–78. doi: 10.1007/s13277-014-2462-3. [DOI] [PubMed] [Google Scholar]
- 20.Shen KH, Liao AC, Hung JH, et al. α-Solanine inhibits invasion of human prostate cancer cell by suppressing epithelial-mesenchymal transition and MMPs expression. Molecules. 2014;19(8):11896–914. doi: 10.3390/molecules190811896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lázaro-Ibáñez E, Sanz-Garcia A, Visakorpi T, et al. Different gDNA content in the subpopulations of prostate cancer extracellular vesicles: Apoptotic bodies, microvesicles, and exosomes. Prostate. 2014;74(14):1379–90. doi: 10.1002/pros.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin EA, Sohn EJ, Won G, et al. Upregulation of microRNA135a-3p and death receptor 5 plays a critical role in Tanshinone I sensitized prostate cancer cells to TRAIL induced apoptosis. Oncotarget. 2014;5(14):5624–36. doi: 10.18632/oncotarget.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakurai MA, Ozaki Y, Okuzaki D, et al. Gefitinib and luteolin cause growth arrest of human prostate cancer PC-3 cells via inhibition of cyclin G-associated kinase and induction of miR-630. PLoS One. 2014;9(6):e100124. doi: 10.1371/journal.pone.0100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Yang Y, Gan R, et al. Down-regulation of mir-221 and mir-222 restrain prostate cancer cell proliferation and migration that is partly mediated by activation of SIRT1. PLoS One. 2014;9(6):e98833. doi: 10.1371/journal.pone.0098833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Gao H, Ren L, et al. Demethylation of the miR-146a promoter by 5-Aza-2′-deoxycytidine correlates with delayed progression of castration-resistant prostate cancer. BMC Cancer. 2014;14:308. doi: 10.1186/1471-2407-14-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afshar AS, Xu J, Goutsias J. Integrative identification of deregulated miRNA/TF-mediated gene regulatory loops and networks in prostate cancer. PLoS One. 2014;9(6):e100806. doi: 10.1371/journal.pone.0100806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saini S, Majid S, Shahryari V, et al. Regulation of SRC kinases by microRNA-3607 located in a frequently deleted locus in prostate cancer. Mol Cancer Ther. 2014;13(7):1952–63. doi: 10.1158/1535-7163.MCT-14-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]