Abstract
Objective:
To explore rural–urban differences and trends in tissue plasminogen activator (tPA) utilization among acute ischemic stroke (AIS) patients and examine the association between primary stroke center (PSC) growth and geographic disparity in tPA use.
Methods:
We used hospital discharge data from the National Inpatient Sample (NIS) from 2000 to 2010 and indicators of tPA utilization and describe temporal trends in geographic disparities in AIS care during PSC growth. The Gini coefficient was used to quantify rural–urban inequity in tPA use at the state level (from 0% to 100% of maximum potential rural–urban inequity) in tPA use.
Results:
Of 914,500 cases of AIS between 2001 and 2010, 2.3% (n = 21, 190) received tPA. The rural–urban disparity in tPA worsened: tPA use in urban hospitals quadrupled (1.17%–4.87%) compared to rural hospitals (0.87%–1.59%). Of 33 states with NIS data, 15 reached at least 75% of the maximum rural–urban inequality from 2004 to 2010.
Conclusions:
Geographic disparities in tPA use for AIS are increasing. Greater understanding of the effectors of tPA utilization is necessary to ensure that access to tPA treatment is equitable for all communities in the United States.
Thrombolysis using recombinant tissue plasminogen activator (tPA) is a highly effective treatment for acute ischemic stroke (AIS). tPA reduces stroke symptom burden, decreases poststroke dependency, and is cost-effective.1–3 In spite of these benefits, tPA utilization rates in the United States are less than 5%.4,5
To improve stroke care quality and outcomes and reduce disparities in stroke care in the United States, the Joint Commission on Accreditation of Health Care Organizations (JCAHO) began certifying hospitals as primary stroke centers (PSCs) in 2004. Several studies suggest that AIS care subsequently improved. The tPA utilization rate in PSCs is nearly 3 times the national rate.6 However, the PSC certification program currently does not include a specific strategy for the 20% of the US population that lives in rural areas. Rural Americans are at greater risk of cerebrovascular disease due to higher rates of tobacco use, alcohol abuse, and hypertension.7,8 Yet no studies have examined whether the geographic distribution of tPA has trended toward equity or inequity since PSC certification began.
To address this knowledge gap and guide future programmatic efforts to further improve disparities in stroke care, we used data from the Nationwide Inpatient Sample (NIS) to measure rural–urban differences in tPA utilization for AIS and describe temporal trends in geographic disparities in tPA utilization, particularly since PSC designation began in 2004. A greater understanding of the drivers of tPA utilization is necessary to guide the design of additional measures to ensure that access to tPA treatment is equitable for all communities in the United States.
METHODS
This study was approved by the Human Research Protection Office at the University of Pennsylvania, Philadelphia.
Study design and dataset.
We performed a serial cross-sectional analysis of hospitalizations in the NIS from 2001 to 2010. The NIS is one of the databases developed for the Healthcare Cost and Utilization Project (HCUP),9 sponsored by the Agency for Healthcare Research and Quality. The NIS contains clinical (diagnoses, procedures) and nonclinical (demographic and insurance information, charges, length of stay, disposition) weighted data on over 36 million community (nonfederal, general, short-term, and specialty) hospitalizations nationally. The NIS has been widely used to examine disparities in acute stroke care.6,10–13
Study population.
Our study population consisted of all discharges with a diagnosis of AIS using the ICD-9 codes 433.x1, 434.x1, or 436. Previous research supports the use of these ICD-9 codes for AIS identification in administrative datasets.14 We excluded discharges of patients younger than 18 years.
Individual characteristics.
The following patient data were extracted: sex, age (categorized as <60 years, 60–69 years, 70–79 years, 80+ years, and also analyzed as a continuous variable), type of admission (emergent, urgent, elective, other), insurance (Medicare, Medicaid, private insurance, self-pay, no charge, other), and discharge status (routine, died, transferred). Race (black, white, Asian/Pacific Islander, and Hispanic) is not reported by all states that participate in the NIS, but was extracted when available. Comorbid disease burden was quantified using the Charlson Comorbidity Index score calculated by HCUP comorbidity software.15
Hospital and geographic characteristics.
Hospitals in the NIS are categorized according to the following characteristics: (1) geographic: state, Census region; (2) location: urban, rural; (3) bed size: small, medium, large; and (4) teaching status: teaching, nonteaching. In the NIS, a hospital location was identified as urban if it was located in a metropolitan statistical area (MSA) and a non-MSA was classified as rural, based on 1990 US Census data. Beginning with 2004 NIS data, the classification of urban or rural hospital location used core-based statistical area (CBSA) codes, based on the 2000 US Census data. Hospitals residing in metropolitan CBSA counties were considered urban, while hospitals with a CBSA micropolitan or non-core designation were classified as rural. To account for PSC growth, we calculated the number of PSCs per US county for each year of data using accreditation records from the JCAHO. For example, the data from 2005 would include county-level data on the number of PSCs that had active accreditation in 2005.16
Primary outcome.
Our primary outcome was IV tPA (ICD-9 procedure code 99.10) use for AIS from 2001 to 2010.
Statistical analysis.
tPA utilization.
We calculated annual thrombolysis rates for AIS in the NIS dataset from 2001 to 2010 stratified by individual and hospital characteristics. Unconditional logistic regression models were built to examine the odds of tPA receipt predicted by hospital location (urban vs rural) controlling for individual characteristics (race, sex, age, insurance type, comorbidity burden, length of hospitalization), hospital characteristics (bed size, teaching status), and year.
Trends in rural–urban tPA disparities.
We examined the geographic trends in tPA usage by directly comparing national weighted tPA rates in urban vs rural hospitals from 2001 to 2010. State-level data were used to determine the extent to which geographic disparities in tPA have changed between 2004 and 2010. These years were chosen because JCAHO certification of hospitals as PSCs began in 2004 and the most recent NIS dataset available at the time of this study was 2010. State-level disparities in tPA were quantified by calculating the rural–urban hospital tPA rate ratio and also by calculating the rural–urban Gini index for 2004 and 2010. Rural–urban rate ratios were calculated using the formula (Riu/Rir), where R is the unadjusted rate of tPA utilization (i = year, u = urban, r = rural). The Gini index is widely used to measure geographic inequity in income, health care access, treatment utilization, and outcomes.17–19 Defined mathematically as half of the absolute difference between any 2 values of an outcome (e.g., income, vaccination, or adverse event) normalized on the mean, the Gini index can be calculated using the following formula:
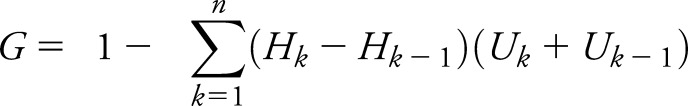 |
where Hk is a cumulated portion of the hospitals by geography (i.e., urban and rural) and Uk is a cumulated portion of tPA utilized by the respective geographic portions. The calculated Gini index can be expressed as a percent ranging from 0% (perfect equality, rural hospital tPA rates are equal to urban hospital tPA) and 100% (perfect inequality, no rural hospital AIS patients receive tPA, all urban hospital AIS patients do). In practice, the Gini coefficient can be categorized as low (<20%), moderate (20%–30%), high (30%–40%), or extreme (>40%) inequality.20,21 We calculated a Gini index (expressed as a percent) to quantify the inequality in tPA between urban and rural hospitals.
We performed several sensitivity analyses focused on stroke patients who may have presented to a rural hospital with stroke symptoms, but were transferred to urban or PSC hospitals for care, or who may have received tPA and were transferred immediately thereafter. The latter are commonly referred to as drip and ship cases. The ICD-9 code for drip and ship treatment, V45.88, did not become available until October 1, 2008, limiting our ability to specifically identify these cases prior to that date. However, the NIS includes a variable that indicates a patient was transferred from another hospital. We repeated our primary analyses, first excluding individuals who were transfer patients (according to NIS data), and then excluding patients with ICD-9 code V45.88 (2009 and 2010 data). A third sensitivity analysis was performed using only individuals with acute stroke recorded as the primary diagnosis. Statistical analyses were performed using Statistical Analysis System 9.3 (SAS; SAS Institute Inc., Cary, NC). Maps to display state-level differences in tPA were prepared with ArcMap 10.1 (Environmental Systems Research Institute, Redlands, CA).
RESULTS
We identified 914,500 discharges for AIS between 2001 and 2010. Of these cases, 2.3% (n = 21,190) received thrombolysis. The distributions of patient, hospital, and geographic characteristics of AIS patients stratified by tPA treatment status are displayed in table 1. Although the NIS data do not contain research measures of stroke severity (such as the NIH Stroke Scale score) or assessments of tPA eligibility, AIS patients cared for at rural hospitals did not have a higher prevalence of diagnoses that are viewed as a contraindication to tPA receipt (table e-1 at Neurology.org).
Table 1.
Thrombolysis for acute ischemic stroke in the Nationwide Inpatient Sample, 2001–2010
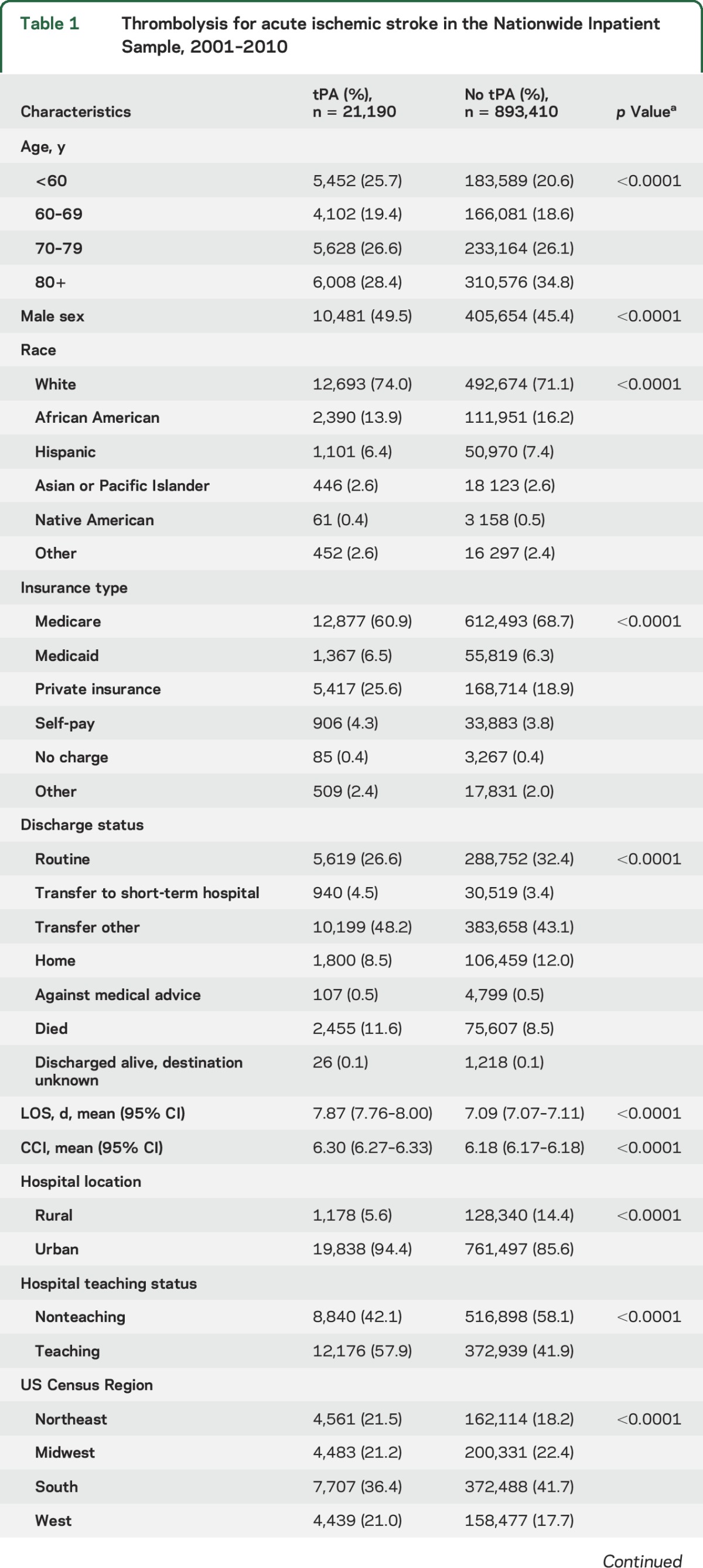
Rural–urban disparity in acute stroke care has increased.
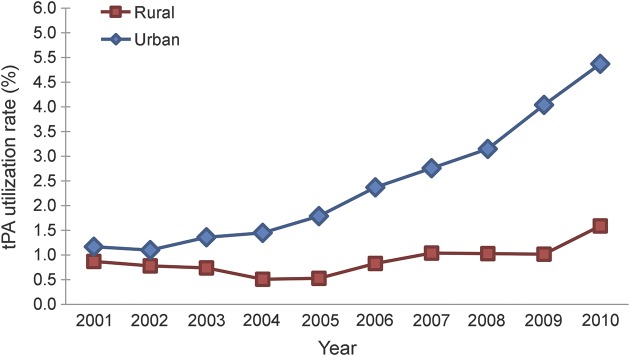
Since 2001, tPA utilization in urban hospitals quadrupled (from 1.17% to 4.87%). tPA utilization in rural hospitals has increased at a much slower pace; the utilization rate in 2010 was less than twice the rate of 2001 (0.87%–1.59%). In addition, as figure 1 illustrates, while tPA utilization rates in urban hospitals have increased yearly since 2002, tPA in rural hospitals decreased initially, and only appreciably increased twice between in 2006 and 2007 (+0.30%) and between 2009 and 2010 (+0.48%). Adjusted logistic regression demonstrated that tPA thrombolysis for AIS was twice as likely to occur in an urban hospital (adjusted odds ratio [AOR] 2.11; 1.97–2.27).
Figure 1. Trends in tissue plasminogen activator (tPA) utilization rates by hospital location, Nationwide Inpatient Sample, 2001–2010.
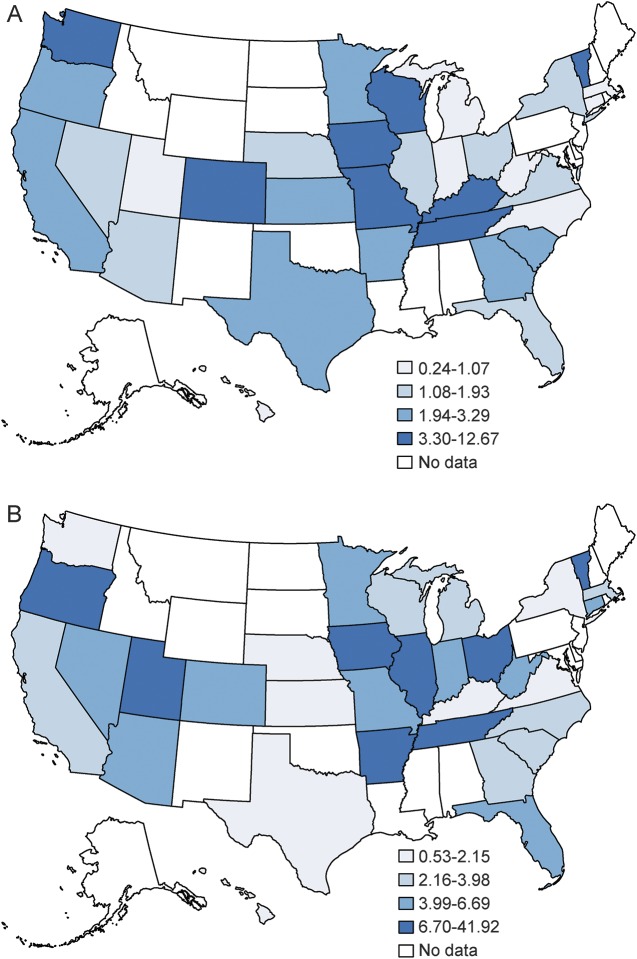
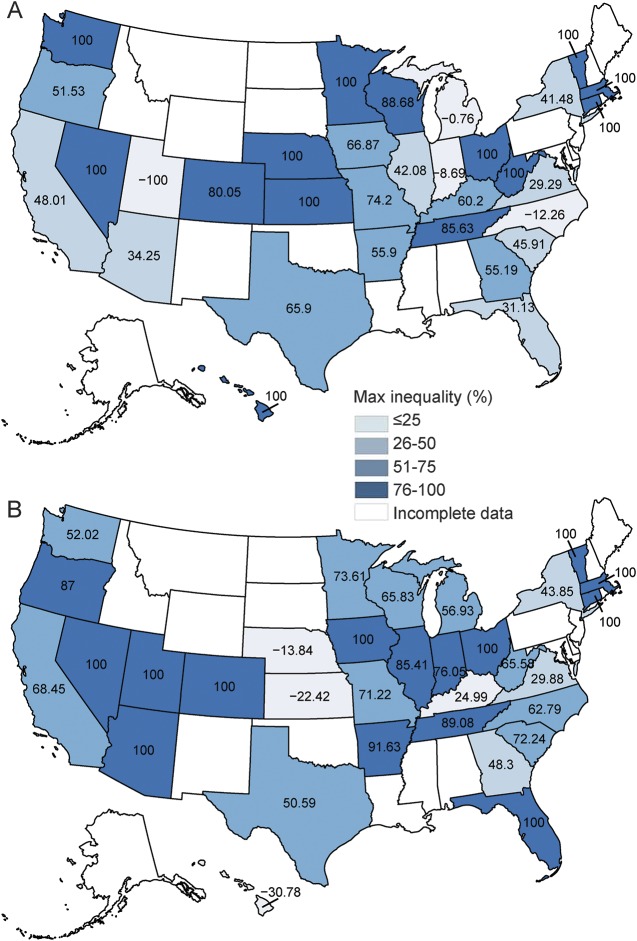
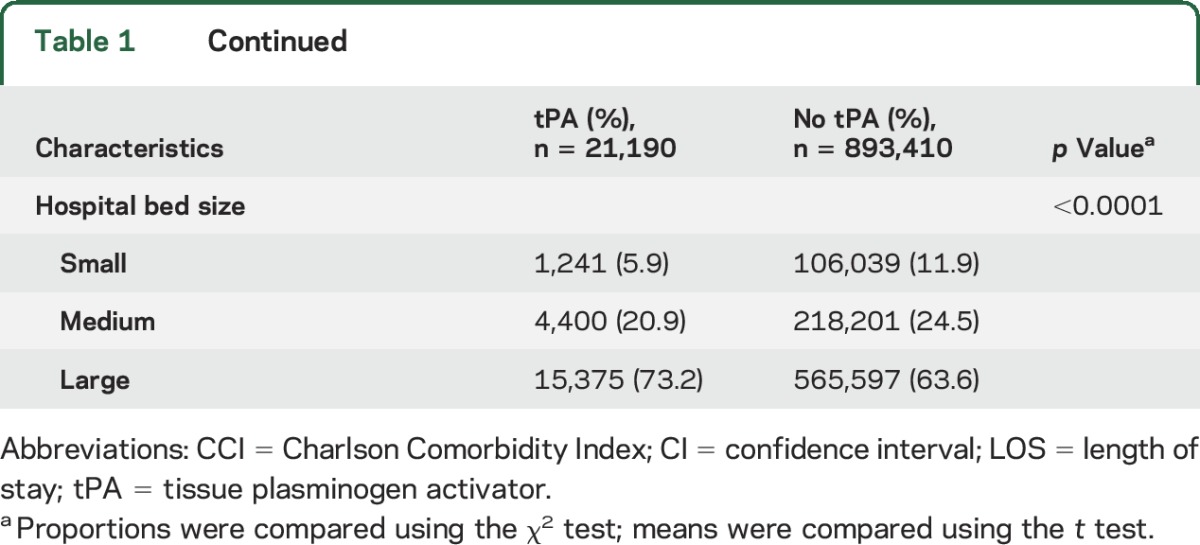
Figure 2 illustrates rural–urban tPA rate ratios in 2004 and 2010 for states that had NIS data for both years. Of the 30 states with available data in 2010, the greatest disparity was found in Ohio (41.9, 95% confidence interval [CI] 38.51–45.62). An rural–urban hospital tPA rate ratio equal to 1, indicating no disparity, was found for Maryland (0.99, 95% CI 0.91–1.08). A ratio of <1, indicating a disparity favoring rural hospitals, was present in Hawaii, Kansas, and Maine. Individual state rural–urban ratios for 2004 and 2010 are available in table 2. Figure 3 illustrates the percentage of maximum observable inequality calculated using the Gini index expressed as a percentage. Per conventional nomenclature, a Gini index of 100% indicates complete inequity, an index of >40% indicates severe inequity, and an index of 0% indicates no inequity. Simply stated, in this study, the higher the Gini index, the greater the inequity in rural vs urban areas for the outcome variable. Of the 33 states with available data, 15 were found have a Gini index of 75% or greater for both 2004 and 2010. Furthermore, the number of states with a Gini inequity index between 50% and 75% grew from 7 to 10 during the time of PSC growth.
Figure 2. Geographic disparity in rural–urban tissue plasminogen activator rate ratio in the United States: 2004 (A) and 2010 (B).
Table 2.
Predictors of tissue plasminogen activator utilization for acute ischemic stroke, Nationwide Inpatient Sample 2001–2010
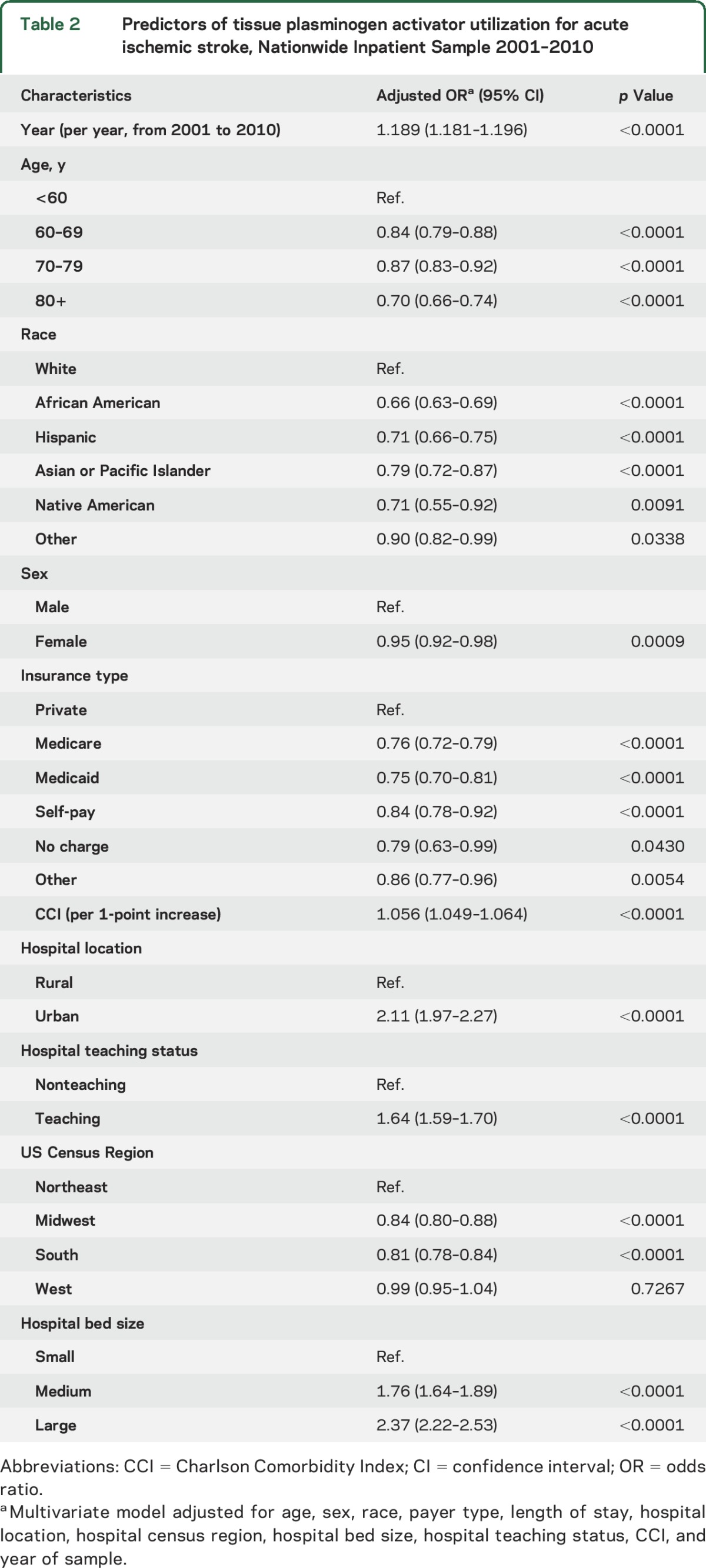
Figure 3. Rural–urban inequity in tissue plasminogen activator use in the United States: 2004 (A) and 2010 (B).
In our sensitivity analyses, we found no use of the ICD-9 code for drip and ship until 2008, as expected. A total of 17% (n = 764) and 20.5% (n = 1785) of tPA-treated stroke patients were drip and ship cases in 2009 and 2010. After excluding these, AIS patients presenting to urban hospitals in 2009 or 2010 were still over 2.5 times more likely to receive tPA (AOR 2.56, 95% CI 2.26–2.90) (table e-2). Excluding transferred patients (n = 32, 490) from our analytic dataset did not meaningfully affect the magnitude or direction our primary findings (table e-3). Finally, excluding individuals who had acute stroke as a secondary discharge diagnosis (n = 200,658) also produced the same findings as our primary analyses (table e-4).
DISCUSSION
In 2005, the Centers for Disease Control and Prevention partnered with government, academic, and private stakeholders to produce a public action plan to reduce the burden of stroke in the United States. A fundamental tenet of that plan was to provide access to standard care without disparity.22 Stroke incidence is greatest in the rural southeast and coastal plains regions of the United States23; however, there are limited data on geographic inequities in stroke care or outcomes. Using data from the largest publicly available all-payer database, we found that fewer patients with AIS receive tPA at rural hospitals compared to urban hospitals and this disparity has increased. Further, urban–rural disparities in acute stroke treatment are increasing in spite of massive public and private investment in multilevel (community, hospital, region) and multifaceted (public awareness campaigns, provider incentives, increased reimbursement) interventions to improve stroke outcomes for all Americans. There are several potential contributors to the growing rural urban disparities in acute stroke care, which can be grouped as structure (provider, facility, and organizational characteristics, ability to access care), process (diagnosis, treatment). and patient (tPA eligibility, disease severity) barriers.
Utilization of a particular health service is contingent upon that service being available where and when the patient needs it. Few PSCs are located in rural areas, and PSC growth in rural areas has been minimal. In the NIS dataset, 2.4% of rural hospitals had PSC status in 2010, compared to 18.7% of urban hospitals, increased from 1.2% and 14.9% in 2004 when the PSC program began. Overcoming this imbalance in infrastructure is unlikely, in part because of the prerequisite certifications for a PSC application.24 The JCAHO is the only organization that offers stroke program certification.25 More than 95% of urban hospitals are JCAHO accredited, compared with 35% of rural critical access hospitals.26 Many rural hospitals cannot afford the JCAHO accreditation process, or have a patient case mix or bed size that would nullify the usefulness of JCAHO accreditation from a payer perspective. At the state level, lack of state stroke legislation and limited state economic output may also contribute to fewer PSCs and less stroke care infrastructure in predominantly rural states.27 Finally, neurologists, the physicians who are best trained to evaluate AIS patients, may be geographically segregated, with fewer practicing in rural areas. Such supply-side disparities are likely to persist in the absence of centrally supported programmatic efforts to improve access to neurologic care.
Geographic differences in preventative health care effectiveness may manifest as a greater percentage of rural stroke patients having appropriate tPA contraindications. The lack of PCP availability in rural areas has been demonstrated in many studies.28–31 We did not find a disproportionate number of inpatient diagnoses that would absolutely prohibit tPA use; however, comorbid disease severity, prestroke disability, life expectancy, or other factors that result in appropriate tPA contraindication may vary in rural vs urban populations, even after accounting for baseline sociodemographic differences and standard comorbidity scoring in our study. Optimal primary care in rural areas would prevent some, but not all, strokes that may benefit from tPA therapy, reinforcing the need to address access barriers.
As currently delivered, tPA requires that patients present for emergent care within a certain time period. There are several reasons other than a nocturnal onset or unwitnessed stroke that may lead rural AIS patients to have longer prehospital delay. A multiyear cross-sectional analysis of the Behavioral Risk Factor Surveillance System survey of 13 states and the District of Columbia revealed that adults who had low heart attack and stroke knowledge scores were more likely to be rural (AOR 1.218, 95% CI 1.216–1.219) area residents, using the same definitions for rural and urban as the NIS.32 Rural African American (AOR 1.76, 1.758, 1.768) and rural Hispanic respondents (AOR 2.787, 2.772, 2.802) were especially vulnerable to having minimal stroke and heart knowledge.31 Similar studies suggest that stroke awareness in urban populations approaches 60%,33,34 highlighting a potential need for increased stroke awareness campaigns in rural areas. Even if stroke awareness in rural areas responded to community or media-based campaigns, rural AIS patients may still face a transportation barrier, with fewer and less reliable means to travel to the hospital (e.g., public transportation, private vehicle, or ambulance) and greater distances to travel to reach care.
This is the largest study to date of geographic disparities in AIS care, and provides a benchmark for the development and testing of structural, process, and patient-level interventions that have the potential to reduce the disparities reported here. In spite of these strengths, there are limitations to the use of observational data (e.g., prospective cohorts, disease registries, medical claims data) that relate to incorrectly measured variables (coding errors), unmeasurable variables (participation bias), or incomplete data (stroke severity). ICD-9 data may underestimate tPA use,35 but there is no evidence that undercoding or coding errors are biased to rural hospitals. We were unable to assess behavioral factors such as community awareness of stroke symptoms and whether this affects individual-level response to stroke symptoms. Other local-level characteristics such as hospital density, urban adjacency, and ratio of PSC to non-PSC hospitals may also contribute to bias in our measurements. We did not have clinical trial or stroke registry level data on stroke severity or tPA eligibility. We did not find striking differences in tPA contraindications between rural and urban stroke patients, but more refined analyses are needed. NIS data for the time period during which other AIS treatments, such as mechanical thrombectomy, have emerged are not yet available. Future iterations of the NIS dataset may be used to examine changes in the approach to AIS with endovascular treatment, which may affect IV tPA use. In spite of these limitations, and need for much further study, we show a striking, growing disparity in tPA utilization among 59,492,276 people, or 19.3% of Americans living in rural areas. Greater understanding of the effectors of tPA utilization is necessary to ensure that access to tPA treatment is equitable for all communities in the United States.
Supplementary Material
GLOSSARY
- AIS
acute ischemic stroke
- AOR
adjusted odds ratio
- CBSA
core-based statistical area
- CI
confidence interval
- HCUP
Healthcare Cost and Utilization Project
- ICD-9
International Classification of Diseases–9
- JCAHO
Joint Commission on Accreditation of Health Care Organizations
- MSA
metropolitan statistical area
- NIS
Nationwide Inpatient Sample
- PSC
primary stroke center
- tPA
tissue plasminogen activator
Footnotes
Supplemental data at Neurology.org
Editorial, page 422
AUTHOR CONTRIBUTIONS
Sergio Gonzales: carried out the secondary analyses, performed primary writing of the manuscript, and approved the final manuscript as submitted. Lesli Skolarus: reviewed and revised the manuscript and approved the final manuscript as submitted. Dylan Thibault: carried out the primary analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted. Uduak Udoeyo: reviewed and revised the manuscript and approved the final manuscript as submitted. Michael Mullen: reviewed and revised the manuscript and approved the final manuscript as submitted. Allison Willis: conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology 2000;55:1649–1655. [DOI] [PubMed] [Google Scholar]
- 2.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014;384:1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tung CE, Win SS, Lansberg MG. Cost-effectiveness of tissue-type plasminogen activator in the 3- to 4.5-hour time window for acute ischemic stroke. Stroke 2011;42:2257–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skolarus LE, Meurer WJ, Shanmugasundaram K, Adelman EE, Scott PA, Burke JF. Marked regional variation in acute stroke treatment among Medicare beneficiaries. Stroke 2015;46:1890–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke 2011;42:1952–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullen MT, Kasner SE, Kallan MJ, Kleindorfer DO, Albright KC, Carr BG. Joint Commission primary stroke centers utilize more rt-PA in the nationwide inpatient sample. J Am Heart Assoc 2013;2:e000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldwin LM, Chan L, Andrilla CH, Huff ED, Hart LG. Quality of care for myocardial infarction in rural and urban hospitals. J Rural Health 2010;26:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellamy GR, Bolin JN, Gamm LD. Rural Healthy People 2010, 2020, and beyond: the need goes on. Fam Community Health 2011;34:182–188. [DOI] [PubMed] [Google Scholar]
- 9.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. Rockville: Agency for Healthcare Research and Quality; 2014. [Google Scholar]
- 10.Brinjikji W, Rabinstein AA, Cloft HJ. Socioeconomic disparities in the utilization of mechanical thrombectomy for acute ischemic stroke. J Stroke Cerebrovasc Dis 2014;23:979–984. [DOI] [PubMed] [Google Scholar]
- 11.Fargen KM, Neal D, Blackburn SL, Hoh BL, Rahman M. Health disparities and stroke: the influence of insurance status on the prevalence of patient safety indicators and hospital-acquired conditions. J Neurosurg 2015;122:870–875. [DOI] [PubMed] [Google Scholar]
- 12.Kimball MM, Neal D, Waters MF, Hoh BL. Race and income disparity in ischemic stroke care: nationwide inpatient sample database, 2002 to 2008. J Stroke Cerebrovasc Dis 2014;23:17–24. [DOI] [PubMed] [Google Scholar]
- 13.Rahman M, Neal D, Fargen KM, Hoh BL. Establishing standard performance measures for adult stroke patients: a nationwide inpatient sample database study. World Neurosurg 2013;80:699–708. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein LB. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke 1998;29:1602–1604. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 16.US Joint Commission Standards. Stroke Center Certification data currently available at: https://www.qualitycheck.org/data-download/certification-data-download/. Accessed August 2013.
- 17.Green C, Yu BN, Marrie RA. Exploring the implications of small-area variation in the incidence of multiple sclerosis. Am J Epidemiol 2013;178:1059–1066. [DOI] [PubMed] [Google Scholar]
- 18.Massie AB, Gentry SE, Montgomery RA, Bingaman AA, Segev DL. Center-level utilization of kidney paired donation. Am J Transpl 2013;13:1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothenbuhler M, O'Sullivan CJ, Stortecky S, et al. Active surveillance for rheumatic heart disease in endemic regions: a systematic review and meta-analysis of prevalence among children and adolescents. Lancet Glob Health 2014;2:e717–e726. [DOI] [PubMed] [Google Scholar]
- 20.Ameryoun A, Meskarpour-Amiri M, Dezfuli-Nejad ML, Khoddami-Vishteh H, Tofighi S. The assessment of inequality on geographical distribution of non-cardiac intensive care beds in Iran. Iran J Public Health 2011;40:25–33. [PMC free article] [PubMed] [Google Scholar]
- 21.Horev T, Pesis-Katz I, Mukamel DB. Trends in geographic disparities in allocation of health care resources in the US. Health Policy 2004;68:223–232. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. A Public Health Action Plan to Prevent Heart Disease and Stroke. Atlanta: Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 23.Howard G, Evans GW, Pearce K, et al. Is the stroke belt disappearing? An analysis of racial, temporal, and age effects. Stroke 1995;26:1153–1158. [DOI] [PubMed] [Google Scholar]
- 24.Mullen MT, Wiebe DJ, Bowman A, et al. Disparities in accessibility of certified primary stroke centers. Stroke 2014;45:3381–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brasure M, Stensland J, Wellever A. Quality oversight: why are rural hospitals less likely to be JCAHO accredited? J Rural Health 2000;16:324–336. [DOI] [PubMed] [Google Scholar]
- 26.Lutfiyya MN, Sikka A, Mehta S, Lipsky MS. Comparison of US accredited and non-accredited rural critical access hospitals. Int J Qual Health Care 2009;21:112–118. [DOI] [PubMed] [Google Scholar]
- 27.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014;129:399–410. [DOI] [PubMed] [Google Scholar]
- 28.American Heart Association. State stroke legislation increases U.S. primary stroke centers. Science Daily 2015. Available at: sciencedaily.com/releases/2015/06/150618174157.htm. Accessed February 2015. [Google Scholar]
- 29.Rabinowitz HK. Evaluation of a selective medical school admissions policy to increase the number of family physicians in rural and underserved areas. N Engl J Med 1988;319:480–486. [DOI] [PubMed] [Google Scholar]
- 30.Salsberg E, Grover A. Physician workforce shortages: implications and issues for academic health centers and policymakers. Acad Med 2006;81:782–787. [DOI] [PubMed] [Google Scholar]
- 31.Colwill JM, Cultice JM. The future supply of family physicians: implications for rural America. Health Aff 2003;22:190–198. [DOI] [PubMed] [Google Scholar]
- 32.Swanoski MT, Lutfiyya MN, Amaro ML, Akers MF, Huot KL. Knowledge of heart attack and stroke symptomology: a cross-sectional comparison of rural and non-rural US adults. BMC Public Health 2012;12:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kothari R, Sauerbeck L, Jauch E, et al. Patients' awareness of stroke signs, symptoms, and risk factors. Stroke 1997;28:1871–1875. [DOI] [PubMed] [Google Scholar]
- 34.Schneider AT, Pancioli AM, Khoury JC. Trends in community knowledge of the warning signs and risk factors for stroke. JAMA 2003;289:343–346. [DOI] [PubMed] [Google Scholar]
- 35.Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National US estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke 2008;39:924–928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.