Abstract
Objective:
To identify the etiology of new-onset seizure in HIV-infected Zambian adults and identify risk factors for seizure recurrence.
Methods:
A prospective cohort study enrolling HIV-infected adults with new-onset seizure within 2 weeks of index seizure obtained clinical, laboratory, and neuroimaging data to determine seizure etiology. Participants were followed to identify risk factors for seizure recurrence. Risk factors for mortality were examined as mortality rates were unexpectedly high.
Results:
Eighty-one patients with CSF for analysis were enrolled and followed for a median of 306 days (interquartile range 61–636). Most (91%) were at WHO stage III/IV and 66 (81%) had a pre-seizure Karnofsky score ≥50. Prolonged or multiple seizures occurred in 46 (57%), including 12 (15%) with status epilepticus. Seizure etiologies included CNS opportunistic infections (OI) in 21 (26%), hyponatremia in 23 (28%), and other infections in 8 (10%). OIs included Cryptococcus (17%), JC virus (7%) and 5% each for tuberculosis, cytomegalovirus, and varicella-zoster virus. No etiology could be identified in 16 (20%). Thirty (37%) patients died during follow-up and 20 (25%) had recurrent seizures with survival being the only identifiable risk factor.
Conclusions:
HIV-infected adults with new-onset seizure in Zambia often have advanced HIV disease with OI being the most frequent seizure etiology. Seizure recurrence is common but no risk factors for recurrence other than survival were identified. These findings suggest an urgent need for immune reconstitution in this population. Initiating treatment for seizure prophylaxis where only enzyme-inducing antiepileptic medications are available could threaten antiretroviral efficacy.
Seizures are common in the HIV-infected population, often occurring in the setting of advanced stages of immunosuppression.1 New-onset seizure has been reported in up to 11% of HIV-infected individuals,2,3 but seizure recurrence risk and risks factors for epilepsy development in HIV-infected individuals with new-onset seizure have not been delineated. Most African regions with high HIV prevalence continue to rely largely on older, enzyme-inducing antiepileptic drugs (AEDs) for epilepsy management. Given the potential for drug interactions between enzyme-inducing AEDs and combined antiretroviral therapy (cART), their co-usage is not recommended,4,5 but other seizure treatment options are often limited or nonexistent.6 Data on the risk of seizure recurrence in HIV-infected people with new-onset seizure are needed to help guide the decision whether or not to initiate AEDs, particularly given the risk of increased drug toxicity and cART failure with the AED–cART combinations available in most resource-limited African settings.
In resource-limited settings, extensive diagnostic investigations are not routinely available and identification of seizure etiology is often challenging. Potential etiologies for new-onset seizure in HIV-infected individuals include metabolic derangements, medication toxicity (e.g., efavirenz or isoniazid), CNS opportunistic infections (OI), immune reconstitution inflammatory syndrome (IRIS), and symptomatic focal lesions from previous injury (e.g., stroke or trauma).
To identify risk factors for seizure recurrence among people with HIV and new-onset seizure, we conducted a prospective cohort study of HIV-infected Zambian adults with new-onset seizure, which included an extensive etiologic assessment.
METHODS
From August 2011 to June 2013, we assessed consecutive HIV-infected Zambian adults presenting with new-onset seizure for enrollment into the Cohort of HIV-Associated Seizure and Epilepsy (CHASE) study. Patients were recruited from the inpatient medical wards and the outpatient HIV clinic at the University Teaching Hospital (UTH) in Lusaka. At the time of the study, UTH was the only tertiary care center in Zambia.
Inclusion criteria were age ≥18 years, HIV infection, new-onset seizure within 2 weeks of enrollment, no seizure history except childhood febrile seizures, and a willingness to receive HIV care through the UTH HIV clinic. Since the primary aim of this study was to evaluate long-term seizure recurrence risks and risk factors for epilepsy development, the original inclusion criteria also required a score ≥50 on the Karnofsky Performance Status scale7 to avoid recruitment of individuals unlikely to survive the acute hospitalization and CSF acquisition to facilitate the diagnosis of etiology for risk factor identification. To optimize population representativeness for outcome, Karnofsky and CSF requirements were dropped on October 17, 2012, until the end of study enrollment, but only CHASE patients who provided CSF were included in this etiologic analysis.
A study nurse made daily rounds through the emergency room and the inpatient wards to screen files for eligible patients. Flyers were also used to solicit staff referrals. For referral of an eligible patient, a hospital or clinic staff member received 10 kwacha (US $2.00) in cell phone talk time to facilitate referral calls. A study investigator (O.K.S., I.S.) then confirmed the eligibility of the patient and obtained informed consent from the patient or health care proxy.
A study physician documented demographic data, presenting symptoms, medical history, and neurologic examination findings. HIV infection was staged using the WHO staging criteria8 with stages III and IV categorized as advanced HIV infection. Whether the patient knew that he or she was HIV-positive prior to the index seizure and any history of cART treatment were also documented. Seizure semiology was based on patient or witness event descriptions and relevant neurologic findings.
Etiologic assessment included serum testing for CD4+ T-cell count, sodium, glucose, malaria infection based upon a Plasmodium falciparum rapid diagnostic antigen test, syphilis via a screening rapid plasma reagin (RPR) test, and, if needed, confirmatory serum Treponema pallidum hemagglutination assay (TPHA). Toxoplasma antibody testing was performed in patients with neuroimaging suspicious for a toxoplasma lesion. CSF studies included gram stain, cell count, differential, total protein, glucose, venereal disease research laboratory test for neurosyphilis, and cryptococcal antigen (CrAg) testing. Serum CrAg testing was performed on those patients who were unable to have CSF CrAg as this is almost uniformly positive in patients with active cryptococcal meningitis.9 CSF DNA PCR testing was conducted for Mycobacterium tuberculosis, Epstein-Barr virus (EBV), JC virus, varicella-zoster virus, cytomegalovirus, herpes simplex virus type 1, herpes simplex virus type 2, and Toxoplasma gondii on a Rotorgene 6000 (Corbett Life Sciences, Sydney, Australia) real-time thermocycler as previously described.10
The presence of a CNS OI was defined as positive CSF or serum CrAg, amplification of DNA from CSF PCR with the exception of EBV, or neuroimaging consistent with a specific CNS OI and confirmed by serum studies or response to treatment. Amplification of CSF EBV was not considered to be a CNS OI as its pathologic role in the CNS is unclear. Patients with bacterial meningitis, positive malaria rapid diagnostic test, or positive serum RPR and TPHA were categorized as having non-OI infections. Hyponatremia was defined as serum sodium <135 mmol/L. CSF pleocytosis was defined as more than 5 leukocytes/µL in the CSF not associated with blood contamination from a traumatic tap using the adjustment of 1 leukocyte for every 500–1,000 erythrocytes.
Neuroimaging studies were offered to patients who did not have imaging as part of routine clinical care and who did not have a seizure etiology established after initial clinical and laboratory evaluation. These were performed on a Siemens (Malvern, PA) CT2007YS CT scanner or a Magnetom Essenza (Siemens) 1.5T MRI scanner. CT protocols included 1.5 mm contiguous axial imaging from foramen magnum through vertex for 3D reconstruction with 4 mm oblique axial imaging precontrast and postcontrast. MRI protocols included sagittal T1, axial T2, fluid-attenuated inversion recovery, diffusion-weighted imaging with apparent diffusion coefficients, T1 precontrast and postcontrast, and coronal T2 images. Magnevist MRI contrast was administered by hand injection. Structural brain abnormalities were further characterized as the presence of one or more of the following: cortical, white matter, deep structural, or posterior fossa abnormalities; masses or mass effect; fluid collections; intracranial bleeds; calcifications; abnormal ventricular size; abnormal brain volume; or contrast enhancement. An experienced neuroradiologist (M.J.P.) used an early version of the NeuroInterp software program to characterize imaging findings.11
All relevant laboratory and neuroimaging findings were shared with treating health care providers. Clinical characteristics were dichotomized as follows: age <40 years (yes/no), sex (male/female), WHO stage (early/advanced), CD4 <200 (yes/no), Glasgow Coma Scale score at presentation <11 (yes/no), and engagement in HIV care (yes/no) with engagement defined as either being on cART or being able to identify the clinic where one was receiving HIV care and treatment. Patients were classified as having focality (yes/no) if they had one or more of the following: focal neurologic examination, focal seizure semiology, focal EEG findings, or a focal lesion on neuroimaging. Seizure etiology in the primary analysis was categorized as follows: any CNS OI (yes/no), non-OI infection (yes/no), hyponatremia (yes/no), structural brain abnormality (yes/no), and etiology unknown (yes/no). Two study clinicians independently assigned patients to etiologic categories. When there was disagreement, a third study clinician provided arbitration. Hyponatremia was considered a secondary and not primary cause of seizure if a patient had hyponatremia in the setting of another evident seizure etiology. Baseline clinical characteristics were evaluated for any association with seizure etiology using χ2 tests or t tests, as appropriate.
After enrollment, patients were followed throughout their hospitalization. After discharge, patients were followed via their clinical care visits at the Adult Infectious Disease Center for Excellence (AIDC), which encompasses the UTH HIV clinic. Reminder phone calls and transport reimbursements of 20 kwacha (∼US $4.00) were provided to all patients to support their attendance at follow-up appointments at the AIDC. Patients were followed until death or study closure on December 18, 2013.
A primary outcome of interest was risk factors for seizure recurrence. Seizure recurrence was defined as a convulsive seizure that occurred after the patient was stabilized and discharged home from the acute hospitalization. To assess seizure etiology and other clinical characteristics as potential risk factors, the associations between these characteristics and seizure recurrence were evaluated via χ2 or Fisher exact tests using SAS 9.4 (SAS Institute, Cary, NC). Despite inclusion criteria aimed at excluding individuals with high early mortality risks (i.e., a Karnofsky score ≥50), 37% of the cohort died during follow-up, so the risk factor analysis was repeated, controlling for death. Clinical characteristics associated with mortality were examined in a post hoc analysis. A p value of <0.05 was considered significant.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the University of Zambia Biomedical Research Ethics Committee and Michigan State University's Biomedical Institutional Review Board. Written informed consent was obtained from all participants or their proxies, if applicable.
RESULTS
Overall, 81/95 CHASE participants had CSF obtained and were included in this etiologic analysis. All but 2 of these patients were recruited from the inpatient setting. There was no difference in baseline characteristics between those who had CSF taken and those who did not. Sixty-one (75%) of the 81 patients with CSF samples had CSF PCR studies in addition to routine testing and 35/81 (43%) underwent neuroimaging. PCR testing was not available in some patients due to the lack of a sufficient sample volume for additional testing.
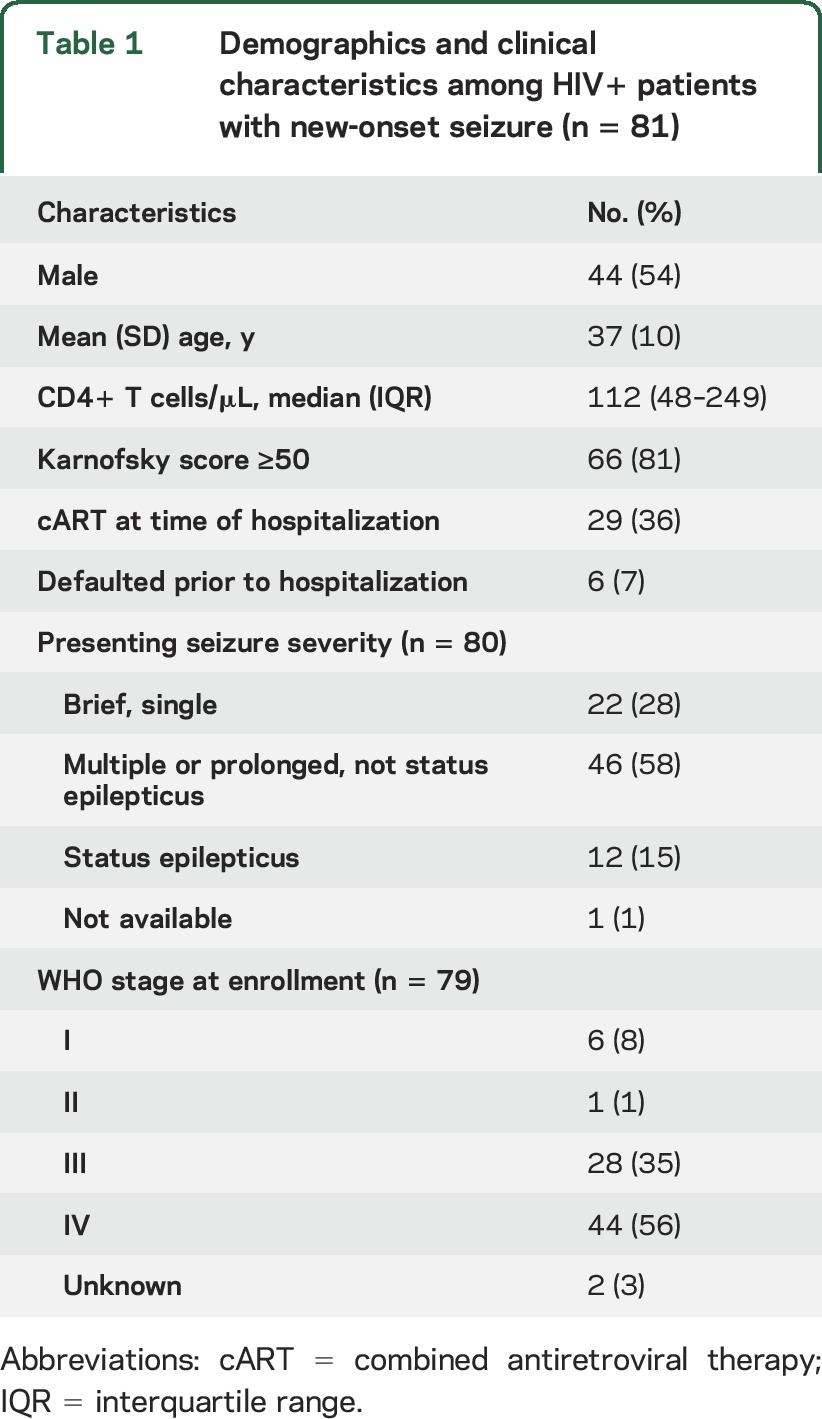
Two patients had MRI scans and the remainder had CT scans. The demographic characteristics of the patients are shown in table 1. The sex distribution slightly favored male participants. The majority of participants were severely immunosuppressed, with a median CD4 count of 112 cells/μL (interquartile range [IQR] 48–249), and 72 (88%) had WHO stage III or IV disease. Of those patients with a known HIV diagnosis prior to the index seizure, 29 (36%) were on cART.
Table 1.
Demographics and clinical characteristics among HIV+ patients with new-onset seizure (n = 81)
Laboratory data are shown in table 2. Comprehensive testing could not be completed on every patient due to a variety of factors that included small sample volume, loss to follow-up, and death prior to completion of testing. The appropriate denominator is indicated in table 2. Pathogens were detected in 32 (40%) CSF samples. EBV was the most common virus, detected in 17/61 of samples tested. Among patients with EBV+ CSF who also had neuroimaging (n = 8), none had imaging indicative of primary CNS lymphoma. Ninety-five percent of patients had sufficient evaluation for Cryptococcus when including serum CrAg testing. Cryptococcus was the most common CNS OI (17%). Fourteen (17%) patients had multiple pathogens in the CSF or serum. Etiologies for new-onset seizure were as follows: CNS OI in 21 (26%), hyponatremia in 23 (28%), and non-OI infection in 8 (10%). Sixteen (20%) patients had secondary hyponatremia. A structural brain abnormality was found in 22/35 patients imaged. Fifty-five (68%) patients had evidence of focality. Nineteen (23%) patients had more than one potential seizure etiology identified. For 16 (20%) patients, no etiology could be identified.
Table 2.
Pathogens detected during etiologic evaluation in HIV+ patients with new-onset seizure (n = 81)

In terms of the relationship between baseline characteristics and seizure etiology, a CD4 <200 was associated with CNS OI (p = 0.027). No other presenting characteristics were associated with seizure etiology.
Of the 81 patients, 20 (25%) had recurrent seizures and 30 (37%) died over a median follow-up period of 306 days (IQR 61–636). The median time to death was 296 days (IQR 55–566). There was no difference in recurrent seizures or death when stratifying by Karnofsky score <50. Table 3 demonstrates the relationship between clinical characteristics including seizure etiology and seizure recurrence. Survival was the only identifiable risk factor for seizure recurrence. After controlling for the risk of death, no clinical characteristics or seizure etiology was associated with seizure recurrence. Table 4 demonstrates the relationship between the same clinical characteristics and mortality. Patients had a higher mortality if their seizure etiology was unknown.
Table 3.
Clinical risk factors associated with seizure recurrence

Table 4.
Clinical risk factors associated with death

DISCUSSION
Despite the wide availability of cART, a substantial proportion of Zambian adults with new-onset seizure were unaware of their HIV status prior to their first seizure12 and had advanced HIV disease, with only about a third of the study population on cART prior to their first seizure. The majority of participants were recruited from the inpatient setting, indicating that HIV-infected patients with new-onset seizure most often present acutely.
Testing for infectious diseases showed a heterogeneous combination of pathogens. We detected a high rate of EBV in the CSF both in isolation and during coinfection. The pathologic significance of EBV in the CSF of HIV-infected patients remains an open question.10,13–15 Cryptococcal meningitis (17%) was the most frequently detected CNS OI. The mechanism whereby cryptococcal meningitis may cause seizures is not fully understood. Neuroimaging in cryptococcal meningitis may be normal or can exhibit dilated Virchow-Robin spaces, enlarged ventricles, or the presence of cryptococcoma. Possible mechanisms include cortical irritation from fungal burden in the CSF, cortical microabscess formation, or elevated intracranial pressure. Therapeutic lumbar puncture is standard of care in cryptococcal meningitis, but the effect of lumbar puncture on seizures in this situation is unknown.16 Twenty percent of patients had no identifiable etiology for their seizure. HIV alone is known to cause inflammation within the brain,17,18 which may further predispose to or precipitate seizure activity.19,20 In a study of 37 South African HIV-infected patients with new-onset seizure, 27% had no identifiable cause,21 and demonstrated detectable CSF HIV viral loads. SPECT studies further support the possibility that HIV itself can precipitate seizures.22
Even though only 17% of CHASE participants had functional impairment prior to the index seizure, the mortality rate was high, with more than one third of patients dying during the relatively brief (<1 year) follow-up period. Deaths almost exclusively occurred outside of UTH and often at home so we have limited insights into the proximate cause of death in the study participants. Diagnostic limitations (i.e., no viral load data and no serial data on CD4 counts) also limited our ability to confidently identify IRIS. Interestingly, patients with no seizure etiology identified were at greater risk of death. Among HIV-infected individuals with new-onset seizure of unknown etiology, both the development of epilepsy and subsequent death may be related to an unrecognized CNS OI or CNS reservoir of HIV. This clearly warrants further study.
There are several limitations related to this study. The representativeness of the CHASE study was limited by identification of etiology only among patients with CSF obtained. Public apprehension in Zambia about the safety of lumbar puncture limits its acceptance16 and patients with a higher level of disability were more likely to provide CSF.12 The PCR assays do not have perfect sensitivity so a negative assay does not guarantee the absence of infection. Although the commonest OIs expected in an African population with new-onset seizures were evaluated,21 we did not perform exhaustive tests to detect all the possible viral, parasitic, fungal, and bacterial infections that could affect this population. In addition, the presence of a pathogen in CSF or blood does not indicate causality. Without obtaining neuroimaging on each participant, we may have missed seizure etiologies such as neurocysticercosis. Some patients with no seizure etiology identified died before neuroimaging could be obtained. Only 2 out of 16 (13%) who had no etiology identified had the full battery of CSF tests and neuroimaging. The etiology may have been identified in the other 14 patients if they had comprehensive testing. The most problematic limitation was the unexpectedly high and early mortality rate in this cohort. Death, a competing outcome, was in fact more common than seizure recurrence.
HIV-infected individuals with new-onset seizure in Zambia were largely people with advanced, untreated HIV who experienced high rates of early mortality during a relatively brief follow-up period. These findings suggest that the top priority for care in this population should be urgent immune reconstitution. Combined antiretroviral therapy is widely available throughout Zambia as well as most other African regions affected by HIV. AED options remain largely limited to older enzyme-inducing agents that could negatively affect the efficacy of cART. Deciding whether and when to initiate AEDs in HIV-infected adults with new-onset seizure remains a major challenge in neurologic care in resource-limited settings.
ACKNOWLEDGMENT
The authors thank the UTH staff of the Adult Infectious Diseases Clinic and the Neurology Research Office for their support and assistance and the patients and families who participated in this study.
GLOSSARY
- AED
antiepileptic drug
- AIDC
Adult Infectious Disease Center for Excellence
- cART
combined antiretroviral therapy
- CHASE
Cohort of HIV-Associated Seizure and Epilepsy
- CrAg
cryptococcal antigen
- EBV
Epstein-Barr virus
- IQR
interquartile range
- IRIS
immune reconstitution inflammatory syndrome
- OI
opportunistic infections
- RPR
rapid plasma reagin
- TPHA
Treponema pallidum hemagglutination assay
- UTH
University Teaching Hospital
AUTHOR CONTRIBUTIONS
Omar K. Siddiqi: study design, data acquisition, study supervision, drafted manuscript. Melissa A. Elafros: data acquisition, study design, analysis, study supervision. Christopher M. Bositis: study design, interpretation, critical review of manuscript for intellectual content. Igor J. Koralnik: study design, interpretation, critical review of manuscript for intellectual content. William H. Theodore: study design, data acquisition and interpretation. Jason F. Okulicz: study design, interpretation, critical review of manuscript for intellectual content. Lisa Kalungwana: study design, data acquisition, interpretation. Michael J. Potchen: study design, data acquisition, interpretation, study supervision. Izukanji Sikazwe: study concept and design, data acquisition, interpretation, study supervision. Gretchen L. Birbeck: study concept and design, interpretation, study supervision.
STUDY FUNDING
The Fogarty International Center and the National Institute of Neurological Disorders and Stroke (NINDS) supported this work under award 1R21NS073509. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Department of Defense, or the Departments of the Army, Navy, or Air Force. M.A.E. was supported in part by an MD/PhD Fellowship from Spectrum Health, the AMA Foundation, and the Lois C. Walker Endowed Fund for Student Research in the Michigan State University College of Human Medicine. O.K.S. was supported by an American Academy of Neurology Clinical Research Fellowship. I.J.K. is supported in part by NINDS R01 047029 and 074995. The International League against Epilepsy supported work by W.H.T.
DISCLOSURE
O. Siddiqi received grant funding from the NIH and American Academy of Neurology. M. Elafros and C. Bositis report no disclosures relevant to the manuscript. I. Koralnik received grant funding through the NIH. W. Theodore, J. Okulicz, and L. Kalungwana report no disclosures relevant to the manuscript. M. Potchen received grant funding through the NIH and Dana Foundation. I. Sikazwe reports no disclosures relevant to the manuscript. G. Birbeck received grant funding through the NIH. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Garg RK. HIV infection and seizures. Postgrad Med J 1999;75:387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kellinghaus C, Engbring C, Kovac S, et al. Frequency of seizures and epilepsy in neurological HIV-infected patients. Seizure 2008;17:27–33. [DOI] [PubMed] [Google Scholar]
- 3.Wong MC, Suite ND, Labar DR. Seizures in human immunodeficiency virus infection. Arch Neurol 1990;47:640–642. [DOI] [PubMed] [Google Scholar]
- 4.Birbeck GL, French JA, Perucca E, et al. Evidence-based guideline: antiepileptic drug selection for people with HIV/AIDS: report of the Quality Standards Subcommittee of the American Academy of Neurology and the ad hoc task force of the Commission on Therapeutic Strategies of the International League Against Epilepsy. Neurology 2012;78:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birbeck GL, French JA, Perucca E, et al. Antiepileptic drug selection for people with HIV/AIDS: evidence-based guidelines from the ILAE and AAN. Epilepsia 2012;53:207–214. [DOI] [PubMed] [Google Scholar]
- 6.Chomba EN, Haworth A, Mbewe E, et al. The current availability of antiepileptic drugs in Zambia: implications for the ILAE/WHO “out of the shadows” campaign. Am J Trop Med Hyg 2010;83:571–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schag CC, Heinrich RL, Ganz PA. Karnofsky Performance Status revisited: reliability, validity, and guidelines. J Clin Oncology 1984;2:187–193. [DOI] [PubMed] [Google Scholar]
- 8.Kalungwana L, Elafros M, Siddiqi O, et al. Cognitive impairment and psychiatric morbidity in HIV+ Zambians with new-onset seizure. Am J Trop Med Hyg 2014;91:1254–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perfect JR, Bicanic T. Cryptococcosis diagnosis and treatment: what do we know now. Fungal Genet Biol 2015;78:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqi OK, Ghebremichael M, Dang X, et al. Molecular diagnosis of central nervous system opportunistic infections in HIV-infected Zambian adults. Clin Infect Dis 2014;58:1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potchen MJ, Kampondeni SD, Ibrahim K, et al. NeuroInterp: a method for facilitating neuroimaging research on cerebral malaria. Neurology 2013;81:585–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sikazwe I, Elafros MA, Bositis CM, et al. HIV and new onset seizures: slipping through the cracks in HIV care and treatment. HIV Med 2016;17:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajasingham R, Rhein J, Klammer K, et al. Epidemiology of meningitis in an HIV-infected Ugandan cohort. Am J Trop Med Hyg 2015;92:274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly MJ, Benjamin LA, Cartwright K, et al. Epstein-Barr virus coinfection in cerebrospinal fluid is associated with increased mortality in Malawian adults with bacterial meningitis. J Infect Dis 2012;205:106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinberg A, Bloch KC, Li S, Tang YW, Palmer M, Tyler KL. Dual infections of the central nervous system with Epstein-Barr virus. J Infect Dis 2005;191:234–237. [DOI] [PubMed] [Google Scholar]
- 16.Thakur KT, Mateyo K, Hachaambwa L, et al. Lumbar puncture refusal in sub-Saharan Africa: a call for further understanding and intervention. Neurology 2015;84:1988–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Portegies P, Berger JR. HIV/AIDS and the nervous system. In: Aminoff MJ, Boller F, Swaab DF, eds. Handbook of Clinical Neurology. Edinburgh: Elsevier; 2007:1–2. [Google Scholar]
- 18.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol 2005;64:529–536. [DOI] [PubMed] [Google Scholar]
- 19.Ravizza T, Balosso S, Vezzani A. Inflammation and prevention of epileptogenesis. Neurosci Lett 2011;497:223–230. [DOI] [PubMed] [Google Scholar]
- 20.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol 2006;147(suppl 1):S232–S240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Modi M, Mochan A, Modi G. New onset seizures in HIV: seizure semiology, CD4 counts, and viral loads. Epilepsia 2009;50:1266–1269. [DOI] [PubMed] [Google Scholar]
- 22.Modi G, Modi M, Martinus I, Vangu M. New onset seizures in HIV-infected patients without intracranial mass lesions or meningitis: a clinical, radiological and SPECT scan study. J Neurol Sci 2002;202:29–34. [DOI] [PubMed] [Google Scholar]


