Abstract
Objective
The purpose of this study is to compare the dosimetric distribution of ipsilateral proton beam radiation therapy (PBRT) to intensity-modulated radiation therapy (IMRT) in the tooth-bearing region of the mandible in patients with head and neck cancer (HNC).
Patients and Methods
The mandibular dosimetric distribution of HNC patients treated with ≥60 Gy relative biological equivalent (RBE) PBRT were evaluated. The mean radiation doses were calculated in five regions: Ipsilateral molar, ipsilateral premolar, anterior, contralateral premolar and contralateral molar (CM). The CM was used as reference region for comparative analysis. The mandibular dosimetric distribution of patients treated with PBRT was compared to IMRT patients with similar tumor sites and planning target volumes.
Results
The mean radiation dose to the contralateral regions was lower in patients treated with PBRT compared to IMRT. The average mean radiation dose to the reference region (CM) in patients treated with PBRT (RBE) vs. IMRT: oropharynx [2.2 Gy vs. 23.2 Gy, P <0.00002], parotid [0 Gy vs. 11.8 Gy, P = 0.01] and oral cavity [0.4 Gy vs. 15.6 Gy, P = 0.006].
Conclusion
This study demonstrates the effective tissue-sparing capability of PBRT compared to IMRT. Utilization of PBRT could translate to less radiation-related toxicity.
Keywords: Proton therapy, IMRT, head and neck cancer, proton beam radiation therapy
Introduction
Head and neck cancer (HNC) patients frequently undergo radiation therapy for primary tumors as either neoadjuvant or adjuvant therapy, usually in combination with one or more additional modalities such as surgery and chemotherapy1, 2. To limit treatment-related toxicities, radiation oncologists reduce doses to spare adjacent organs and tissues at risk, such as the brain, brainstem, spinal cord, salivary glands, jaw and muscles of mastication.
Intensity-modulated radiation therapy (IMRT) has enabled improved tumor dose conformality and doses are reduced in order to spare the tissues/organs at risk. IMRT has purportedly decreased oral adverse events such as mucositis, xerostomia, trismus and osteoradionecrosis (ORN)3–6. However, some patients still experience these sequelae and complications, which frequently result in decreased quality of life7–13.
Proton beam radiation therapy (PBRT) is a relatively new radiation technique now utilized in the management of HNC that has been shown to have greater dose reduction capability in comparison to IMRT, an advantage that could reduce radiotherapy complications14–19. This advantage owes to a characteristic of proton particles that allows deposition of energy over a discrete range known as the Bragg peak20. The Bragg peak is spread out along the tumor coverage allowing the release of energy within the tumor, thereby eliminating an exit dose2, 18.
In this study, we evaluated the dosimetric distribution to the tooth-bearing region of the mandible in HNC patients treated with ipsilateral PBRT, and compared the tissue-sparing capabilities of PBRT and IMRT in patients with HNC.
Patients and Methods
The study was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center and ProCure Proton Therapy Center. The mandibular dosimetric distributions of 30 HNC patients treated with ipsilateral PBRT receiving ≥60 Gy relative biological equivalent (RBE) between 2014 and 2015 were evaluated. Tumor sites were base of tongue (BOT) (4), tonsil (5), parotid (5), submandibular gland (5), oral cavity (11): [gingiva (5), buccal mucosa (3), retromolar region (1), floor of mouth (1), and palate (1)]. The mandibles were dosimetrically contoured using pre-RT CT planning software (ProCure Proton Therapy Center, New Jersey). The mandible was divided into 5 regions: ipsilateral molar (IM), ipsilateral premolar (IP), anterior (A), contralateral premolar (CP) and contralateral molar (CM). The mean radiation doses were calculated for the 5 regions. The methods for dosimetric contouring were described in our previous study.21.
To compare the tissue-sparing capability of PBRT versus IMRT, the mandibular dosimetric distribution in 16 patients treated with PBRT were compared with 16 patients treated with IMRT based on similar tumor sites, planning target volumes, and radiation dose. The mandibles of patients treated with IMRT were dosimetrically contoured in 5 regions using Memorial Sloan Kettering Cancer Center’s preradiotherapy CT planning software. All patients received ipsilateral radiotherapy. The farthest region from the treated site, CM, was used as reference region for comparative analysis.
Treatment plans were drawn up by the same radiation oncologist. Gross tumor volume (GTV) is defined as gross extent of tumor based on clinical examination and imaging studies (CT, MRI, PET-scans). Patients were treated with a therapeutic intent of 2 Gy per fraction, given 5 fractions per week for 30 – 35 fractions. Clinical target volume (CTV) is defined as GTV + 3–5 mm to cover area of potential microscopic involvement, lymph nodes at high-risk and skin involvement, and planning target volume (PTV) is defined as CTV + 5 mm to account for patient motion and setup error. Radiotherapy technique as earlier described in Romesser et al.17.
Statistical analysis
The patients were group into 3 categories for statistical analysis. Oropharynx (BOT and tonsil) n = 5, parotid n = 5 and oral cavity (submandibular, buccal mucosa, retromolar region, mandibular gingiva) n= 6. Comparisons between cohorts were performed using a 2-tailed Student’s t-test. A P-value of < 0.05 was considered significant.
Results
Table 1 summarizes the dosimetric distribution to the tooth-bearing regions of the mandible following proton beam radiation therapy for 30 HNC patients requiring ipsilateral radiation. The average mean radiation doses to the contralateral region were the highest in patients with BOT tumor and lowest in patients with parotid tumors, in this order: BOT >other oral cavity sites>tonsil > submandibular gland > parotid (Figures 1 and 2).
Table 1.
Dosimetric distribution to the 5 tooth-bearing regions of the mandible following proton beam radiation therapy for head and neck cancer patients requiring ipsilateral radiation (n=30)
| Mean radiation dose to the mandible (Gy) (RBE) | |||||
|---|---|---|---|---|---|
| Tumor site | Ipsilateral molar | Ipsilateral premolar | Anterior | Contralateral premolar | Contralateral molar |
| Base of tongue (n=4) | 32.9 (11.13 – 58.3) | 13.2 (0 – 25.7) | 9.3 (0 – 19.5) | 6.3 (0 – 16.8) | 7.91 (0 – 22.9) |
| Tonsil (n=5) | 31.9 (10.1 – 68.6) | 16.8 (0.1 – 64.8) | 9.5 (0.05 – 38.3) | 2.1 (0 – 5.8) | 0.66 (0 – 2.4) |
| Parotid gland (n=5) | 16.3 (0.4 – 47.5) | 0.4 (0 – 1.9) | 0.002 (0 – 0.01) | 0 | 0 |
| Submandibular gland (n=5) | 64 (61.9 – 66.9) | 28.6 (16.2 – 39.8) | 8.5 (0.01 – 27.3) | 6.8 (0 – 23.9) | 0.45 (0 – 1.3) |
| Other oral cavity sites (n=11) | 45.3 (0 – 68.3) | 39.2 (0 – 69.6) | 27 (0 – 72.4) | 15.3 (0 – 54.9) | 2.6 (0 – 14.5) |
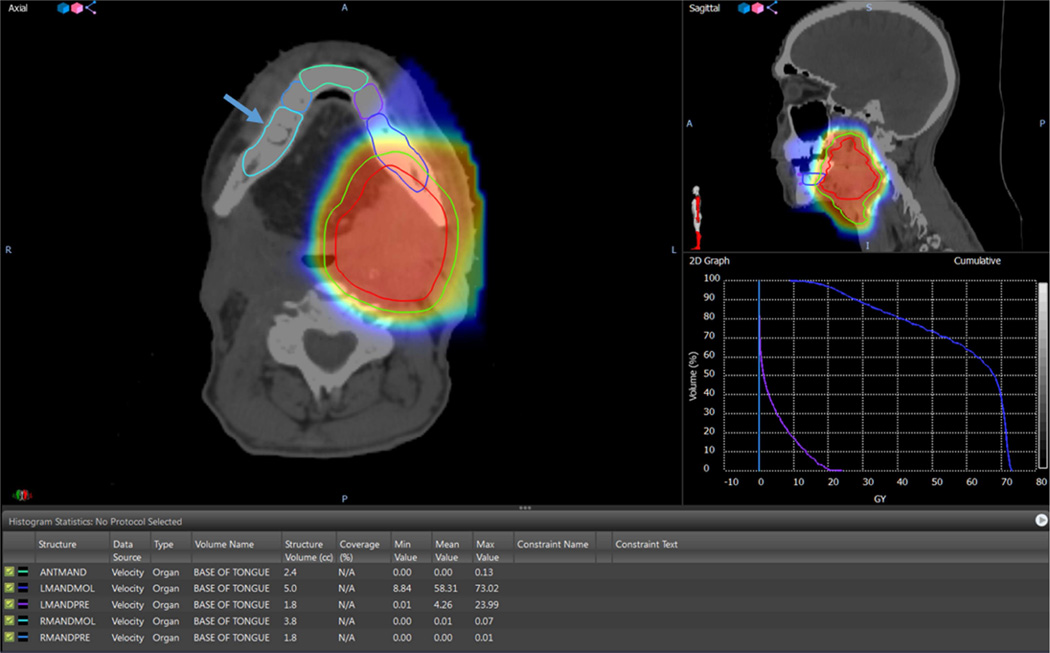
Figure 1.
Shows the dosimetric distribution of the mandible of a base of tongue tumor patient treated with ipsilateral 70 Gy PBRT. The cloud of red, yellow and blue colors represents the radiation isodose color wash in the area of the target volumes. Arrow points to the contralateral molar region (reference region) (RMANDMOL). The mean value of radiation dose to the contralateral molar region was 0.01 Gy. The 2D graph represents the dose volume histogram depicting the isodose lines per volume for each contoured region, the blue isodose line represents the ipsilateral left mandibular molar region (LMANDMOL), the purple isodose line represents the ipsilateral left mandibular premolar region (LMANDPRE) and the cyan isodose line represents the contralateral right mandibular molar region (RMANDMOL). The table shows the minimum, mean and maximum radiation doses to the five contoured regions of the mandible.
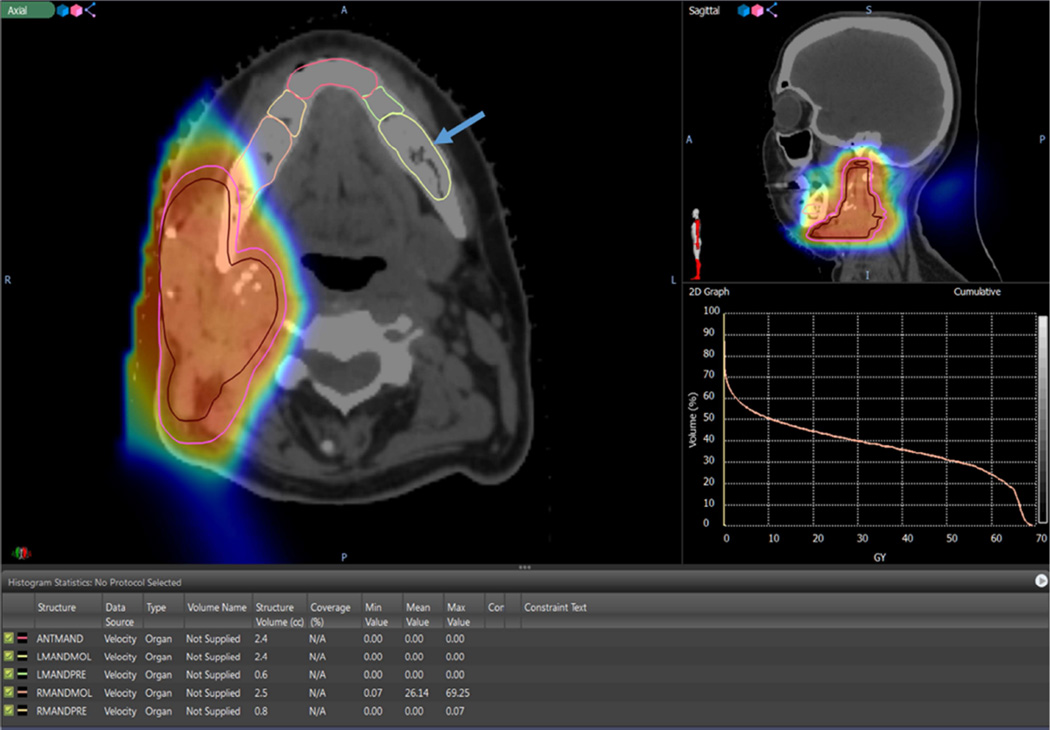
Figure 2.
Shows the dosimetric distribution of the mandible of a parotid gland tumor patient treated with ipsilateral 66 Gy PBRT. The cloud of red, yellow and blue colors represents the radiation isodose color wash in the area of the target volumes. Arrow points to the contralateral molar region (reference region) (RMANDMOL). The mean value of radiation dose to the contralateral molar region was 0 Gy. The 2D graph represents the dose volume histogram depicting the isodose lines per volume for each contoured region, the brown isodose line represents the ipsilateral left mandibular molar region (LMANDMOL) and the yellow isodose line represents the contralateral right mandibular molar region (RMANDMOL). The table shows the minimum, mean and maximum radiation doses to the five contoured regions of the mandible.
Table 2 summarizes the dosimetric distribution to the tooth-bearing regions of the mandible following intensity-modulated radiation therapy for 16 HNC patients requiring ipsilateral radiation.
Table 2.
Dosimetric distribution to the 5 tooth-bearing regions of the mandible following intensity-modulated radiation therapy for head and neck cancer patients requiring ipsilateral radiation (n=16)
| Mean radiation dose to the mandible (Gy) | |||||
|---|---|---|---|---|---|
| Tumor site | Ipsilateral molar | Ipsilateral premolar | Anterior | Contralateral premolar | Contralateral molar |
| Oropharynx (BOT and tonsil) (n=5) | 57.9 (49 – 69.9) | 48.3 (28.8 – 67.1) | 24 (18.4 – 33.9) | 27.9 (20.3 – 35.2) | 23.2 (18.6 – 29.2) |
| Parotid gland (n=5) | 49.8 (35.8 – 62.7) | 38.9 (20.8 – 60.8) | 28.5 (16.6 – 47) | 17.8 (11.4 – 31.3) | 11.8 (4.5 – 21.1) |
| Oral cavity (submandibular, buccal mucosa, retromolar region, mandibular gingiva) (n=6) |
59.6 (37.3 – 73.7) | 58.4 (34.8 – 73.7) | 45.2 (30.3 – 57.9) | 27 (15 – 34.9) | 15.6 (4.1 – 25.9) |
Table 3 summarizes the results of t-tests comparing the mean dose associated with PBRT versus IMRT in 16 patients with HNC treated with ipsilateral PBRT to 16 patients with HNC treated with ipsilateral IMRT. The comparative analysis showed: oropharynx [range: 0 – 8.7 Gy, mean 2.2 Gy (RBE) vs. 18.6 – 29.2 Gy, mean 23.2 Gy, P <0.00002], parotid [range: 0 – 0 Gy, mean 0 Gy (RBE) vs. 4.47 – 21.1 Gy, mean 11.8 Gy, P = 0.01] and oral cavity [range: 0.06 – 1.3 Gy, mean 0.4 Gy (RBE) vs. 4.1 – 25.9 Gy, mean 15.6 Gy, P = 0.006]. The mean radiation dose to the contralateral regions was lower in patients treated with PBRT compared to those treated with IMRT. In addition, the mean radiation dose to the reference region, CM, was statistically lower in patients treated with PBRT.
Table 3.
Comparative analysis of the sparing capability of PBRT versus IMRT
| Tumor sites | PBRT (Gy) (RBE) (n) | IMRT (Gy) (n) | P-value |
|---|---|---|---|
| Oropharynx (BOT and tonsil) | 2.2 (0 – 8.7) (n=5) | 23.2 (18.6 – 29.2) (n=5) | <0.00002 |
| Parotid | 0 (n=5) | 11.8 (4.47 – 21.1) (n=5) | 0.01 |
| Oral cavity (submandibular, buccal mucosa, retromolar region, mandibular gingiva) |
0.4 (0.06 – 1.3) (n=6) | 15.6 (4.1 – 25.9) (n=6) | 0.006 |
Discussion
This is the first study detailing dosimetric distribution of the tooth-bearing region of the mandible in patients treated with PBRT for HNC, as well as the first comparison of PBRT’s mandibular dosimetric distribution with that of IMRT. Our analysis shows that the dosimetric distribution to the tooth-bearing region of the mandible is directly related to the tumor site and location of the tooth on the mandible. BOT tumors and oral cavity tumors in regions such as the gingiva and floor of mouth received the highest radiation doses to the contralateral region while treatment of tumors involving the parotid gland deposited negligible radiation in this region. Also, in all tumor sites the contralateral region of the mandible received the lowest radiation dose with the exception of tumors involving the BOT where the contralateral molar region received a higher dose compared to the contralateral premolar region. Our findings show that PBRT had a far greater sparing capability than IMRT by tumor sites, planning target volume and radiation dose to tumor.
Recent studies of PBRT for the treatment of HNC patients concluded that PBRT lowers radiation dose and improves normal tissue sparing when compared to IMRT without relinquishing target coverage14, 16, 17. This dosimetric benefit translated into lower rates of acute complications such as mucositis, nausea, dysgeusia and fatigue17. Further study of patients treated with PBRT is needed to substantiate the long-term harm reduction associated with this treatment.
Treatment-related toxicity from radiotherapy could be an overwhelming challenge to patients. Osteoradionecrosis (ORN) remains a potentially serious complication following radiotherapy, even in the era of IMRT8, 10, 22. ORN is defined as an area of exposed bone greater than 1 cm in size in an area previously irradiated that failed to heal over a period of 3–6 months, and may require resection of the necrotic segment(s) of the jaw. Reported incidence of ORN ranges from 6.3–6.8% of patients with oral and oropharyngeal cancer treated with IMRT.8, 10. High radiation dose and jaw trauma due to dental extraction remain major risk factors for this complication10, 23–26. Radiation doses >60 Gy to the bone is significantly linked to the risk of developing ORN and mean doses > 40Gy were predictive of increased subsequent dental events25, 27. Our findings show radiation dose to the contralateral premolar and molar ranging from 0 to 15.3 Gy, thus suggesting that the risk for ORN could be avoided in the contralateral region of the mandible in patients who received ipsilateral PBRT. However, areas of the jaw covered by the PTV are at an increased risk of ORN, which also raises the question of the relative biological equivalent (RBE) of a proton to a photon. Report has placed the RBE at 1.1 (i.e 1 Gy of proton beam = 1.1 Gy of photon beam), with some variation18. So areas of the jaw covered by the PTV will be receiving a higher equivalent to a photon beam.
Oral mucositis is an acute complication of head and neck radiotherapy characterized by pain, ulcerations, odynophagia, secondary infections and reduced oral intake28–30. Approximately, one-third of HNC patients receiving radiotherapy suffer from grades 3 and 4 oral mucositis28. Development of oral mucositis has lead to interruptions of patients’ treatment. A recent study by Romesser et al. showed a significant reduction in the development of oral mucositis when comparing patients treated with PBRT to IMRT17.
Xerostomia or dry mouth caused by reduced salivary flow, is the most prevalent complication in patients with HNC treated with radiotherapy31. Xerostomia can lead to poor oral hygiene, dental caries, halitosis, and difficulty with mastication, swallowing and speech. The QUANTEC group suggested that long-term, severe xerostomia may be avoided if at least one parotid gland is spared, to a mean dose of <20 Gy, or if both glands are spared, to a mean dose of <25 Gy to minimize the risk of parotid gland toxicity in patients with HNC treated with conformal radiotherapy31. This radiation doses may be readily achievable with PBRT14, 17.
Trismus is another well-known complication of radiotherapy32, 33. The condition is defined as difficulty with mouth opening secondary to spasm of the muscles of mastication; a maximal interincisal opening measurement of ≤ 35 mm is considered trismus34. Trismus can affect many aspects of daily living, and frequently causes impaired speech and difficulties in eating and chewing, maintaining proper oral hygiene, and receiving dental intervention. Studies have shown a correlation between radiation doses received by the muscles of mastication and post-radiotherapy trismus. High radiation doses to the muscles of mastication predispose patients to trismus35, 36. Oropharyngeal (BOT and tonsil) tumors are in close proximity to the muscles of mastication (pterygoids) making it difficult to spare this normal tissue from radiation dose. However, by attempting to eliminate an exist dose, PBRT may be able to spare the muscles of mastication.
Limitations of this study are the small patient numbers. In addition, we could not account for the subtle differences in target volume delineation that may have occurred as the treating radiation oncologist evolved in practice.
In conclusion, this study demonstrates the potential superior tissue-sparing capability of PBRT compared to IMRT. Clinically, this advantage could lead to fewer complications in patients treated with proton therapy by reducing doses to adjacent organs at risk, such as the jaw thereby minimizes the risk for ORN.
Statement of Clinical Relevance.
In this study, proton beam radiation therapy demonstrated a superior tissue-sparing capability compared to intensity-modulated radiation therapy. Clinically, this advantage could translate to less radiation-related toxicity.
Acknowledgments
This study was supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: All authors declare that there are no financial conflicts associated with this study and that the funding source has no role in conceiving and performing the study.
Abstract of this study was presented at the Annual meeting of the American Academy of Oral Medicine (AAOM), 2016.
References
- 1.Chen AM, Zahra T, Daly ME, et al. Definitive radiation therapy without chemotherapy for human papillomavirus-positive head and neck cancer. Head Neck. 2013;35:1652–1656. doi: 10.1002/hed.23209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holliday EB, Frank SJ. Proton radiation therapy for head and neck cancer: a review of the clinical experience to date. Int J Radiat Oncol Biol Phys. 2014;89:292–302. doi: 10.1016/j.ijrobp.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 3.Vergeer MR, Doornaert PA, Rietveld DH, et al. Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a nonrandomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys. 2009;74:1–8. doi: 10.1016/j.ijrobp.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 4.Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82:291–298. doi: 10.1016/j.ijrobp.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 5.Lohia S, Rajapurkar M, Nguyen SA, et al. A comparison of outcomes using intensity-modulated radiation therapy and 3-dimensional conformal radiation therapy in treatment of oropharyngeal cancer. JAMA Otolaryngol Head Neck Surg. 2014;140:331–337. doi: 10.1001/jamaoto.2013.6777. [DOI] [PubMed] [Google Scholar]
- 6.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770–3776. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 8.Tsai CJ, Hofstede TM, Sturgis EM, et al. Osteoradionecrosis and radiation dose to the mandible in patients with oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2013;85:415–420. doi: 10.1016/j.ijrobp.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh LC, Chen JW, Wang LY, et al. Predicting the severity and prognosis of trismus after intensity-modulated radiation therapy for oral cancer patients by magnetic resonance imaging. PLoS One. 2014;9:e92561. doi: 10.1371/journal.pone.0092561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Studer G, Bredell M, Studer S, Huber G, Glanzmann C. Risk profile for osteoradionecrosis of the mandible in the IMRT era. Strahlenther Onkol. 2016;192:32–39. doi: 10.1007/s00066-015-0875-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao SD, Saleh ZH, Setton J, et al. Dose-volume factors correlating with trismus following chemoradiation for head and neck cancer. Acta Oncol. 2016;55:99–104. doi: 10.3109/0284186X.2015.1037864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen SB, Pedersen AM, Vissink A, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer. 2010;18:1061–1079. doi: 10.1007/s00520-010-0837-6. [DOI] [PubMed] [Google Scholar]
- 13.Beetz I, Steenbakkers RJ, Chouvalova O, et al. The QUANTEC criteria for parotid gland dose and their efficacy to prevent moderate to severe patient-rated xerostomia. Acta Oncol. 2014;53:597–604. doi: 10.3109/0284186X.2013.831186. [DOI] [PubMed] [Google Scholar]
- 14.Kozak KR, Adams J, Krejcarek SJ, Tarbell NJ, Yock TI. A dosimetric comparison of proton and intensity-modulated photon radiotherapy for pediatric parameningeal rhabdomyosarcomas. Int J Radiat Oncol Biol Phys. 2009;74:179–186. doi: 10.1016/j.ijrobp.2008.06.1942. [DOI] [PubMed] [Google Scholar]
- 15.Simone CB, 2nd, Ly D, Dan TD, et al. Comparison of intensity-modulated radiotherapy, adaptive radiotherapy, proton radiotherapy, and adaptive proton radiotherapy for treatment of locally advanced head and neck cancer. Radiother Oncol. 2011;101:376–382. doi: 10.1016/j.radonc.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ladra MM, Edgington SK, Mahajan A, et al. A dosimetric comparison of proton and intensity modulated radiation therapy in pediatric rhabdomyosarcoma patients enrolled on a prospective phase II proton study. Radiother Oncol. 2014;113:77–83. doi: 10.1016/j.radonc.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romesser PB, Cahlon O, Scher E, et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother Oncol. 2016;118:286–292. doi: 10.1016/j.radonc.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukens JN, Lin A, Hahn SM. Proton therapy for head and neck cancer. Curr Opin Oncol. 2015;27:165–171. doi: 10.1097/CCO.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 19.Ramaekers BL, Grutters JP, Pijls-Johannesma M, et al. Protons in head-and-neck cancer: bridging the gap of evidence. Int J Radiat Oncol Biol Phys. 2013;85:1282–1288. doi: 10.1016/j.ijrobp.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 20.van der Laan HP, van de Water TA, van Herpt HE, et al. The potential of intensity-modulated proton radiotherapy to reduce swallowing dysfunction in the treatment of head and neck cancer: A planning comparative study. Acta Oncol. 2013;52:561–569. doi: 10.3109/0284186X.2012.692885. [DOI] [PubMed] [Google Scholar]
- 21.Hansen HJ, Maritim B, Bohle GC, 3rd, et al. Dosimetric distribution to the tooth-bearing regions of the mandible following intensity-modulated radiation therapy for base of tongue cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:e50–e54. doi: 10.1016/j.oooo.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Owosho AA, Kadempour A, Yom SK, et al. Radiographic osteoradionecrosis of the jaw with intact mucosa: Proposal of clinical guidelines for early identification of this condition. Oral Oncol. 2015;51:e93–e96. doi: 10.1016/j.oraloncology.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray CG, Herson J, Daly TE, Zimmerman S. Radiation necrosis of the mandible: a 10 year study. Part I. Factors influencing the onset of necrosis. Int J Radiat Oncol Biol Phys. 1980;6:543–548. doi: 10.1016/0360-3016(80)90380-6. [DOI] [PubMed] [Google Scholar]
- 24.Murray CG, Herson J, Daly TE, Zimmerman S. Radiation necrosis of the mandible: a 10 year study. Part II. Dental factors; onset, duration and management of necrosis. Int J Radiat Oncol Biol Phys. 1980;6:549–553. doi: 10.1016/0360-3016(80)90381-8. [DOI] [PubMed] [Google Scholar]
- 25.Studer G, Gratz KW, Glanzmann C. Osteoradionecrosis of the mandibula in patients treated with different fractionations. Strahlenther Onkol. 2004;180:233–240. doi: 10.1007/s00066-004-1171-z. [DOI] [PubMed] [Google Scholar]
- 26.Thorn JJ, Hansen HS, Specht L, Bastholt L. Osteoradionecrosis of the jaws: clinical characteristics and relation to the field of irradiation. J Oral Maxillofac Surg. 2000;58:1088–1093. doi: 10.1053/joms.2000.9562. discussion 93-5. [DOI] [PubMed] [Google Scholar]
- 27.Gomez DR, Estilo CL, Wolden SL, et al. Correlation of osteoradionecrosis and dental events with dosimetric parameters in intensity-modulated radiation therapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81:e207–e213. doi: 10.1016/j.ijrobp.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253–262. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 29.Saunders DP, Epstein JB, Elad S, et al. Systematic review of antimicrobials, mucosal coating agents, anesthetics, and analgesics for the management of oral mucositis in cancer patients. Support Care Cancer. 2013;21:3191–3207. doi: 10.1007/s00520-013-1871-y. [DOI] [PubMed] [Google Scholar]
- 30.Mallick S, Benson R, Rath GK. Radiation induced oral mucositis: a review of current literature on prevention and management. Eur Arch Otorhinolaryngol. 2015 doi: 10.1007/s00405-015-3694-6. [DOI] [PubMed] [Google Scholar]
- 31.Deasy JO, Moiseenko V, Marks L, et al. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76:S58–S63. doi: 10.1016/j.ijrobp.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao SD, Saleh ZH, Setton J, et al. Dose-volume factors correlating with trismus following chemoradiation for head and neck cancer. Acta Oncol. 2015:1–6. doi: 10.3109/0284186X.2015.1037864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owosho AA, Pedreira Ramalho LM, Rosenberg HI, et al. Objective assessment of trismus in oral and oropharyngeal cancer patients treated with intensity-modulated radiation therapy (IMRT) J Craniomaxillofac Surg. 2016 doi: 10.1016/j.jcms.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dijkstra PU, Huisman PM, Roodenburg JL. Criteria for trismus in head and neck oncology. Int J Oral Maxillofac Surg. 2006;35:337–342. doi: 10.1016/j.ijom.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Teguh DN, Levendag PC, Voet P, et al. Trismus in patients with oropharyngeal cancer: relationship with dose in structures of mastication apparatus. Head Neck. 2008;30:622–630. doi: 10.1002/hed.20760. [DOI] [PubMed] [Google Scholar]
- 36.van der Molen L, Heemsbergen WD, de Jong R, et al. Dysphagia and trismus after concomitant chemo-Intensity-Modulated Radiation Therapy (chemo-IMRT) in advanced head and neck cancer; dose-effect relationships for swallowing and mastication structures. Radiother Oncol. 2013;106:364–369. doi: 10.1016/j.radonc.2013.03.005. [DOI] [PubMed] [Google Scholar]