Abstract
Purpose
The aim of this study was to estimate the incidence of trismus in oral and oropharyngeal cancer patients (OOPC) treated with intensity-modulated radiation therapy (IMRT) and to identify the role of risk factors in patients who developed trismus.
Materials and Methods
A retrospective cohort study of OOPC treated with IMRT in our institution from 2009 through 2014 was performed. Patients eligible for this study had pre-RT and post-RT maximal inter-incisal opening (MIO) measurements at 6 – 48 months post-RT, treated with high-dose radiation (≥60 Gy) and pre-RT MIO ≥ 36 mm. A descriptive analysis to identify the incidence of trismus, with trismus stated as MIO ≤ 35 mm at or after 6 months post-RT measurement was performed. The role of risk factors such as age, gender, tumor site, tumor size (T), tumor stage, pre-RT MIO measurements and radiation dose to the tumor were assessed using Fisher exact test and the radiation doses to the ipsilateral muscles of mastication in patients who developed trismus were assessed by matching with control (non-trismus) patients using Wilcoxon Signed Rank test.
Results
The study consisted of 54 patients with a median age of 55 years and 81% were males. The median follow-up time was 10 months. The incidence of trismus was 14.8%. Patients with pre-RT MIO measurements ≤40 mm were at risk of developing trismus (P<0.001). In trismus patients, the average mean radiation dose to the masseter and medial pterygoid muscles was numerically higher but not significantly different (P=0.08; P=0.22, respectively) to matched control patients. Age, gender, radiation dose to the tumor, tumor site, size (T) and stage were also found to be not significant.
Conclusion
Pre-RT MIO measurement was a significant risk factor for the development of trismus. However, this is a non-modifiable factor. Limiting radiation dose to the muscles of mastication could prevent this complication.
Keywords: Trismus, Intensity-Modulated Radiation Therapy, Oral Cancer, Oropharyngeal Cancer
Graphical abstract
1. Introduction
Oral and oropharyngeal cancers represent a heterogeneous group of tumors. These tumors are united by a common anatomy and are difficult to manage due to their proximity to vital structures. This group of malignancies, which includes the increasingly prevalent human papilloma virus–positive squamous cell carcinoma, is sensitive to radiation therapy (RT) (Chen et al., 2013).
The last two decades have seen advances in radiation therapy from the conventional/2-dimensional radiotherapy to three-dimensional conformal radiation therapy (3D-CRT) to intensity-modulated radiation therapy (IMRT), a specialized form of 3D-CRT. RT is known to cause complications such as dermatitis, fibrosis, soft tissue atrophy, osteoradionecrosis, mucositis, dysphagia, trismus, and loss of salivary function. The advent of IMRT allows dose distribution that is more conformal to tumor, involved neck, and high-risk areas than that achieved by its predecessor while reducing radiation doses to normal structures (Mendenhall et al., 2006). Therefore, IMRT-associated toxicity and locoregional control rates have been promising and have led to lower percentages and severity of radiation-associated complications (Kraaijenga et al., 2015; Lohia et al., 2014; Vergeer et al., 2009; Setton et al., 2012). The past few years have seen the widespread implementation of IMRT as the standard of care for oral and oropharyngeal cancer radiation therapy.
Trismus is a well-known complication defined as an inability to open the mouth or difficulty with mouth opening secondary to spasm of the muscles of mastication (Bensadoun et al., 2010; Melchers et al., 2009). Maximal inter-incisal opening (MIO) in the healthy population ranges from 36–55 mm; measurements ≤ 35 mm are considered to be trismus (Dijkstra et al., 2006). It can be caused by tumor infiltrating the muscle of mastication, their nerves, and the temporomandibular joint or as a complication of surgery or radiation therapy (Ichimura and Tanaka, 1993; Stubblefield et al., 2010). Trismus may cause difficulty in many aspects of daily living, such as impaired speech, difficulty eating, difficulty in maintaining proper oral hygiene and receiving dental intervention, thereby affecting the patient’s quality of life (Louise Kent et al., 2008; Lee et al., 2015; Weber et al., 2010; Pauli et al., 2013).
Reported incidences of trismus in oral and oropharyngeal cancer patients treated with IMRT can range from 4% to 77.3% (Hsieh et al., 2014; Rao et al., 2015; Ingle et al., 2010; Chao et al., 2004; Gomez et al., 2009). This wide range is likely due to different inclusion criteria; subjective or objective assessments of trismus, cut-off values for trismus, and follow-up periods. The risk of developing trismus can be attributed to a multitude of factors, namely age and gender of the patient, tumor location, pre-RT MIO measurements radiation dose to the primary tumor and radiation doses received by the muscles of mastication (Rao et al., 2015; Kamstra et al., 2015; van der Molen et al., 2013; Lindblom et al., 2014; Teguh et al., 2008).
The objectives of this study were: 1. To identify the incidence of trismus in OOPC treated with IMRT. 2. To assess the role of risk factors such as age, gender, tumor site, tumor size (T), tumor stage, pre-RT MIO measurements, radiation dose to the primary tumor and radiation dose to the ipsilateral muscles of mastication in patients who developed trismus after IMRT. We hypothesized that patients most likely to develop trismus were those with lower pre-RT MIO measurements and higher radiation doses to the muscles of mastication.
2. Materials and Methods
2.1. Study design
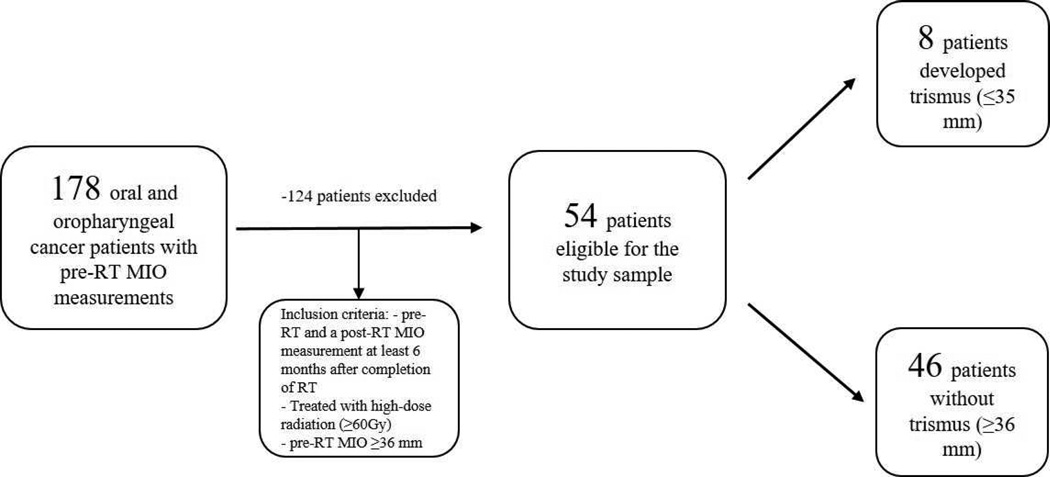
The study was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center (MSKCC). To address our research questions, we designed a retrospective cohort study focusing on patients treated with IMRT for oral/oropharyngeal cancer at MSKCC. The study population consisted of patients who presented for pre- and post-RT evaluation to the MSKCC-Dental Service between 2009 and 2014. Inclusion criteria for the study sample were patients who had pre-RT and a post-RT MIO measurements at least 6 months following RT, were treated with high-dose radiation (≥60 Gy) and had pre-RT MIO ≥ 36 mm (Figure 1). Previously published studies have defined trismus as mouth opening of 35 mm or less and the highest incidence of trismus was identified 6 months post-RT (Dijkstra et al., 2006; Pauli et al., 2013). Risk factors such as gender, tumor site, tumor size (T), overall tumor stage, pre-RT MIO measurements and the radiation doses to the ipsilateral muscles of mastication in patients who developed trismus were reviewed and analyzed. The mean radiation dose to the ipsilateral muscles of mastication (medial pterygoid and masseter) in patients who developed trismus were compared to matched control (non-trismus) patients with similar tumor site, tumor size and radiation dose to the primary tumor.
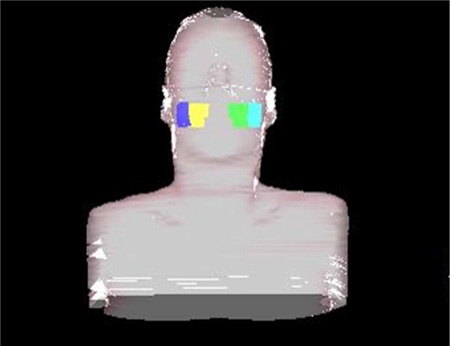
Figure 1.
Example of contoured muscles of mastication (masseter muscles: purple/cyan colors and medial pterygoid muscles: yellow/green colors.
2.2. Variables
The predictor variables assessed in this study were: age, gender, tumor site, tumor size (T), overall tumor stage, pre-RT MIO measurements, radiation dose to the primary tumor and the radiation doses to the ipsilateral muscles of mastication. The patient cohort was divided into 2 groups: patients with pre-RT MIO >40 mm and patients with pre-RT MIO ≤40 mm. The outcome variables were the presence or absence of trismus. Patients with post-RT MIO measurements ≤ 35 mm were considered to have trismus. Trismus was graded as mild/grade I (35–26 mm), moderate/grade II (25–16 mm), and severe/grade III (15-0 mm).
2.3. Data collection methods
The mouth opening assessments were recorded as the maximal distance between the tips of upper and lower incisors in patients with complete frontal dentition; the denture teeth were used for as landmarks in patients without their natural teeth. The pre-RT measurements were recorded. Post-RT measurements were made 6 months or later following completion of RT. The mean radiation dose to the ipsilateral muscles of mastication (masseter and medial pterygoid) was calculated using the MSKCC treatment-planning software (Figure 2).
Figure 2.
Flow chart of oral and oropharyngeal cancer patients evaluated for trismus.
2.4. Statistical analysis
A descriptive analysis was performed to identify the incidence of trismus and patient/tumor characteristics. The role of risk factors such as age, gender, tumor site, tumor size (T), overall tumor stage, pre-RT MIO measurements and radiation dose to the primary tumor were assessed using Fisher exact test and the mean radiation doses to the ipsilateral muscles of mastication in patients who developed trismus were assessed by matching with control (non-trismus) patients using Wilcoxon Signed Rank test. A P-value of < 0.05 was considered significant.
3. Results
A total of 54 patients met the inclusion criteria at a median follow-up time of 10 months (range: 6 – 48 months). There were 44 males and 10 females with a median age of 55 years (range 32–75 years). Patients were pathologically diagnosed with squamous cell carcinoma (51), adenoid cystic carcinoma (2) and mucoepidermoid carcinoma (1). The tumor sites were base of tongue (22), tonsil (20), oral tongue (8), floor of mouth (2) and palate (2). Forty patients had tumor Stage IV, 10 patients had Stage III, 2 Stage II, 1 patient had Stage I and 1 patient had TxN2c. Total radiation dose to the primary tumor ranged from 60 – 73.3 Gy delivered at 2Gy per fraction and 5 fractions per week with a mean of 68.9 Gy, 50 of 54 patients received ≥ 66 Gy. Pre-RT MIO measurements ranged from 36 – 58 mm, mean of 45.14 mm (14 patients ≤40 mm and 40 patients >40mm). Table 1 summarizes the characteristics of the patients.
Table 1.
Patient Characteristics (n=54)
| Characteristic | Total (%) |
|---|---|
| Age | |
| Median (range) | 55 (32–75) |
| ≤50 | 15 (28%) |
| >50 | 39 (72%) |
| Gender | |
| Female | 10 (19%) |
| Male | 44 (81%) |
| Tumor site | |
| Oral Tongue | 8 (15%) |
| Tonsil | 20 (37%) |
| Base of Tongue | 22 (41%) |
| Floor of mouth | 2 (3.7%) |
| Palate | 2 (3.7%) |
| Pathologic diagnosis | |
| Squamous cell carcinoma | 51 (94%) |
| Adenoid cystic carcinoma | 2 (4%) |
| Mucoepidermoid carcinoma | 1 (2%) |
| T classification (n=53) | |
| T1 | 17 (32%) |
| T2 | 20 (38%) |
| T3 | 10 (19%) |
| T4 | 6 (11%) |
| N classification | |
| N0 | 4 (8%) |
| N1 | 11 (20%) |
| N2 | 38 (70%) |
| N3 | 1 (2%) |
| Tumor stage (n=53) | |
| I | 1 (2%) |
| II | 2 (4%) |
| III | 10 (19%) |
| IV | 40 (75%) |
| Radiation to the primary tumor site | |
| ≥70 Gy | 43 (80%) |
| ≥66 Gy and < 70 Gy | 7 (13%) |
| ≥60 Gy and < 66 Gy | 4 (7%) |
| Trismus status | |
| Trismus | 8 (14.8%) |
| No trismus | 46 (85.2%) |
| Pre-RT incisal measurement | |
| Median (range) | 45 (36–58) |
| ≤40 | 14 (26%) |
| >40 | 40 (74%) |
Eight (14.8%, 6 males and 2 females; P = 0.63) patients with ages ranging 46 – 71 years (6 patients ≤50 years and 2 patients >50 years; P = 1.00) developed trismus after IMRT with MIO measurements ≤ 35 mm at a median time of 10 months. Their MIO measurements at a minimum of 6 months post-RT ranged from 10–35 mm, mean of 27 mm; radiation doses to the primary tumor in these patients ranged from 63 – 70 Gy, mean of 69Gy (7 patients ≥70 Gy and 1 patient 63 Gy; P = 1.00). Five patients with trismus were grade I/mild, 2 patients were grade II/moderate, and 1 patient grade III/severe. The primary tumor sites of patients with trismus were base of tongue (4), oral tongue (2), tonsil (1) and floor of mouth (1) (P = 0.32) and tumor stages were Stage IV (7) and Stage II (1) (P = 0.66). The tumor sizes (T) were T1 and T2 (5), T3 and T4 (3) (P = 0.69). The pre-RT MIO measurements in trismus patients ranged from 36 – 44 mm, mean of 38.25 mm (7 patients ≤40 mm and 1 patient >40mm; P <0.001). Table 2 shows patient characteristics by trismus status.
Table 2.
Patient Characteristics by Trismus Status
| Number (%) | ||||
|---|---|---|---|---|
| Characteristic | Totals (%) | No Trismus (n=46) | Trismus (n=8) | P |
| Age | 1.00 | |||
| ≤50 | 15 (28%) | 13 (87%) | 2 (13%) | |
| >50 | 39 (72%) | 33 (85%) | 6 (15%) | |
| Gender | 0.63 | |||
| Female | 10 (19%) | 8 (80%) | 2 (20%) | |
| Male | 44 (81%) | 38 (86%) | 6 (14%) | |
| Tumor site | 0.32 | |||
| Oral Tongue | 8 (15%) | 6 (75%) | 2 (25%) | |
| Tonsil | 20 (37%) | 19 (95%) | 1 (5%) | |
| Base of Tongue | 22 (41%) | 18 (82%) | 4 (18%) | |
| Other | 4 (7%) | 3 (75%) | 1 (25%) | |
| T classification (n=53) | 0.69 | |||
| T1/T2 | 37 (70%) | 32 (86%) | 5 (14%) | |
| T3/T4 | 16 (30%) | 13(81%) | 3 (19%) | |
| Tumor stage (n=53) | 0.66 | |||
| I – III | 13 (25%) | 12 (92%) | 1 (8%) | |
| IV | 40 (75%) | 33 (83%) | 7 (17%) | |
| Radiation to the primary tumor site | 1.00 | |||
| ≥70 Gy | 43 (80%) | 36 (84%) | 7 (16%) | |
| < 70 Gy | 11 (20%) | 10 (91%) | 1 (9%) | |
| Pre-RT incisal measurement | <0.001 | |||
| ≤40 | 14 (26%) | 7 (50%) | 7 (50%) | |
| >40 | 40 (74%) | 39 (98%) | 1 (2%) | |
P-values calculated with Fisher exact test.
Using the Gothenburg trismus questionnaire, 5 of 8 patients with trismus reported significant changes in their quality of life. Patients with significant changes in their quality of life all reported moderate to very severe difficulty with feeding and jaw opening. Trismus patients not reporting any significant change in quality of life had post-RT MIO measurements of 33 – 35 mm.
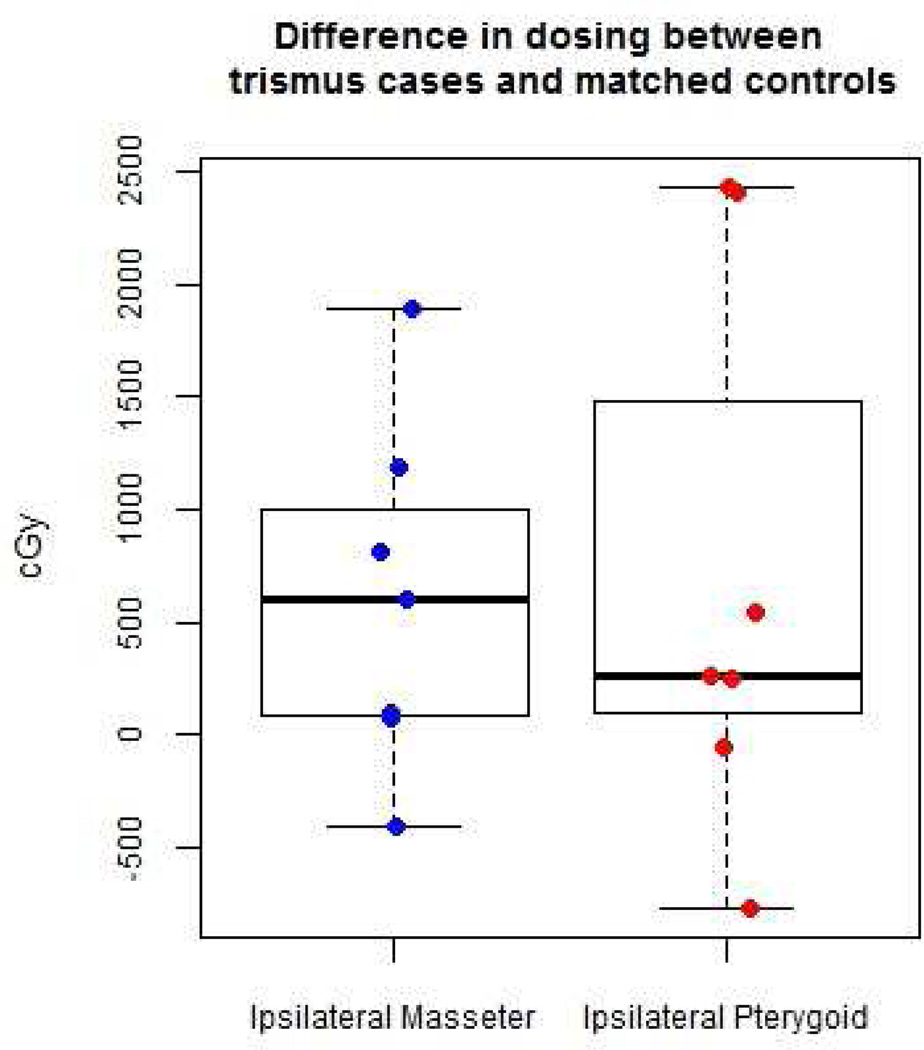
To identify the role of radiation dose to the muscles of mastication, the mean radiation dose to the muscles of mastication (masseter and medial pterygoid) of 7 patients who developed trismus were matched and compared to 7 control (non-trismus) patients with similar tumor site, tumor size (T) and radiation dose to the primary tumor. The dosimetric data for 1 trismus patient was not retrievable. The mean radiation doses to the medial pterygoid were consistently higher than to the masseter in all patients dosimetrically contoured. In patients with trismus, the average mean radiation dose received by the masseter and medial pterygoid muscles was numerically higher but not statistically significant (masseter: P = 0.08, medial pterygoid: P = 0.22) when compared to control (non-trismus) patients. Table 3 compares the radiation dose to the ipsilateral muscle of mastication in patients with trismus vs. matched controls.
Table 3.
Radiation dose to the ipsilateral muscle of mastication in patients with trismus and matched controls (N=7)*
| Dose difference between trismus cases and controls |
Median (range) | P |
|---|---|---|
| Ipsilateral Masseter (cGy) | 598.9 (−412.0, 1897.0) | 0.08 |
| Ipsilateral Medial Pterygoid (cGy) | 261.0 (−769.9, 2431.0) | 0.22 |
Contour dosing not available for one trismus case.
P-values calculated with paired Wilcoxon Signed Rank test.
4. Discussion
Trismus is a well known complication in patients receiving radiation therapy either as a neoadjuvant or as an adjuvant therapy in combination with surgery or chemotherapy. The purpose of this study was to identify the incidence of trismus in OOPC treated with IMRT and the role of risk factors such as age, gender, tumor site, tumor size (T), tumor stage, pre-RT measurements, radiation dose to the primary tumor and radiation dose to the ipsilateral muscles of mastication in patients who developed trismus. We hypothesized that patients at high risk for trismus were those with lower pre-RT MIO measurements and higher radiation doses to the muscles of mastication.
The patients were followed up for a median of 10 months. We found the incidence of trismus to be 14.8%. Patients with pre-RT MIO measurements ≤40 mm were at significant risk of developing trismus. The average mean radiation dose received by the ipsilateral muscles of mastication (masseter and medial pterygoid) was numerically higher in matched patients who developed trismus compared to control (non-trismus) patients but not significantly different. Age, gender, radiation dose to the primary tumor, tumor site, tumor size (T), and tumor stage were also not found to be statistically significant.
Recent studies on IMRT have reported better treatment outcomes and far fewer complications in the treatment of patients with head and neck cancer (Kraaijenga et al., 2015; Lohia et al., 2014; Vergeer et al., 2009; Setton et al., 2012). A study by Chen et al. reported an incidence of 5.7% in nasopharyngeal carcinoma patients treated by IMRT (Chen et al., 2011). Rao et al. reported 14.1% of patients with oropharyngeal cancer treated with IMRT developed chronic trismus (Rao et al., 2015). In another study of head and neck cancer patients treated with 3D-CRT or IMRT, the incidence of trismus was 28.3% (Steiner et al., 2015). Hsieh et al. reported that 77.3% of the patients with oral cancer treated with IMRT experienced trismus over a course of 2 years (Hsieh et al., 2014). The result of the study was probably overestimated as there was a limited sample size of 22 patients; furthermore, the study failed to indicate the cut-off value for trismus and the approximate time interval between radiation and the measurement of MIO. Most patients typically present with trismus secondary to the immediate complication of RT (mucositis) and not due to the late complication of radiation. In this study trismus was assessed at a minimum of 6 months post-RT, at which time the immediate complication of radiotherapy (mucositis) should have subsided and the highest incidence of trismus could be captured (Pauli et al., 2013).
Kamstra et al. showed that patients with smaller pre-RT MIO measurements were at risk of developing trismus (Kamstra et al., 2015). Previous studies have also shown significant correlation between radiation doses received by the muscles of mastication and post-RT trismus (van der Molen et al., 2013; Lindblom et al., 2014; Teguh et al., 2008). One study showed that trismus in patients treated with IMRT was associated with radiation doses received by both the masseter and pterygoid muscles (van der Molen et al., 2013). Another study reported that it was associated with radiation dose received by the masseter muscle (Lindblom et al., 2014), while in another study it was reported that for every additional 10 Gy of radiation dose received by the medial pterygoid muscle there was a 24% probability of developing trismus (Teguh et al., 2008). In our study, the mean radiation dose received by the ipsilateral masseter muscle related to trismus showed a tendency toward significance (P =0.08). The reasons why the mean radiation dose to the muscles of mastication in our study did not show significance might be related to the low number of patients who developed trismus and our patient cohort were treated for oral and oropharyngeal cancer compared to the general head and neck sites.
IMRT allows conformal distribution of radiation thereby reducing toxicity. However, in a subset of patients with tumors adjacent to the muscles of mastication or TMJ, sparing of these structures from the risk of radiation exposure without compromising locoregional cancer control can be challenging. The use of a more modern radiation technique, intensity-modulated proton therapy, which allows for superior conformal distribution, minimizing radiation to adjacent organs at risk in the treatment of oral and oropharyngeal cancer may be more beneficial.
The relatively small sample size of our study precludes us from making any definitive conclusions regarding the incidence of trismus and its risk factors. Retrospective analyses as well as prospective studies with large sample sizes examining radiation dose to the muscles of mastication, baseline MIO measurements, and the benefit of jaw opening exercises during and after radiation therapy would add to our current knowledge.
Since management of trismus could be challenging, focus should be placed on its prevention. Prophylactic and immediate conservative management of trismus has been shown to be effective in some patients (Loorents et al., 2014; Cohen et al., 2005). The use of exercise therapy and jaw opening devices such as stacked tongue depressors, corkscrews, TheraBite and Dynasplint devices are routinely employed. Pre-RT MIO measurement is a non-modifiable factor. Therefore, limiting radiation dose to the muscles of mastication and temporomandibular joint structure may help minimize trismus in oral and oropharyngeal cancer patients treated with IMRT.
5. Conclusion
This study demonstrates that trismus remains a complication of IMRT in the management of oral and oropharyngeal cancer patients. Patients with natural small mouth opening or limited mouth opening secondary to surgery or with tumor infiltrating the muscle of mastication, their nerves, or temporomandibular joint are at an increased risk of developing trismus. These patients should be monitored closely and have jaw exercise therapy initiated early at the beginning of radiotherapy.
Acknowledgments
Disclosure: All authors declare that there are no financial conflicts associated with this study and that the funding source has no role in conceiving and performing the study. This study was supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bensadoun RJ, Riesenbeck D, Lockhart PB, Elting LS, Spijkervet FK, Brennan MT, et al. A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Support Care Cancer. 2010;18(8):1033–1038. doi: 10.1007/s00520-010-0847-4. [DOI] [PubMed] [Google Scholar]
- Chao KS, Ozyigit G, Blanco AI, Thorstad WL, Deasy JO, Haughey BH, et al. Intensity-modulated radiation therapy for oropharyngeal carcinoma: impact of tumor volume. Int J Radiat Oncol Biol Phys. 2004;59(1):43–50. doi: 10.1016/j.ijrobp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Chen AM, Zahra T, Daly ME, Farwell DG, Luu Q, Gandour-Edwards R, et al. Definitive radiation therapy without chemotherapy for human papillomavirus-positive head and neck cancer. Head Neck. 2013;35(11):1652–1656. doi: 10.1002/hed.23209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YY, Zhao C, Wang J, Ma HL, Lai SZ, Liu Y, et al. Intensity-modulated radiation therapy reduces radiation-induced trismus in patients with nasopharyngeal carcinoma: a prospective study with >5 years of follow-up. Cancer. 2011;117(13):2910–2916. doi: 10.1002/cncr.25773. [DOI] [PubMed] [Google Scholar]
- Cohen EG, Deschler DG, Walsh K, Hayden RE. Early use of a mechanical stretching device to improve mandibular mobility after composite resection: a pilot study. Arch Phys Med Rehabil. 2005;86(7):1416–1419. doi: 10.1016/j.apmr.2004.10.035. [DOI] [PubMed] [Google Scholar]
- Dijkstra PU, Huisman PM, Roodenburg JL. Criteria for trismus in head and neck oncology. Int J Oral Maxillofac Surg. 2006;35(4):337–342. doi: 10.1016/j.ijom.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Gomez DR, Zhung JE, Gomez J, Chan K, Wu AJ, Wolden SL, et al. Intensity-modulated radiotherapy in postoperative treatment of oral cavity cancers. Int J Radiat Oncol Biol Phys. 2009;73(4):1096–1103. doi: 10.1016/j.ijrobp.2008.05.024. [DOI] [PubMed] [Google Scholar]
- Hsieh LC, Chen JW, Wang LY, Tsang YM, Shueng PW, Liao LJ, et al. Predicting the severity and prognosis of trismus after intensity-modulated radiation therapy for oral cancer patients by magnetic resonance imaging. PLoS One. 2014;9(3):e92561. doi: 10.1371/journal.pone.0092561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura K, Tanaka T. Trismus in patients with malignant tumours in the head and neck. J Laryngol Otol. 1993;107(11):1017–1020. doi: 10.1017/s0022215100125149. [DOI] [PubMed] [Google Scholar]
- Ingle CJ, Yip K, Caskie V, Dyson C, Ford A, Scrase CD. Intensity modulated radiotherapy (IMRT) in the management of locally advanced oropharyngeal squamous cell carcinomata (SCC): disease control and functional outcome using the therapy outcome measure (TOM) score--report from a single U.K. institution. Head Neck Oncol. 2010;2:28. doi: 10.1186/1758-3284-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamstra JI, Dijkstra PU, van Leeuwen M, Roodenburg JL, Langendijk JA. Mouth opening in patients irradiated for head and neck cancer: a prospective repeated measures study. Oral Oncol. 2015;51(5):548–555. doi: 10.1016/j.oraloncology.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Kraaijenga SA, Oskam IM, van der Molen L, Hamming-Vrieze O, Hilgers FJ, van den Brekel MW. Evaluation of long term (10-years+) dysphagia and trismus in patients treated with concurrent chemo-radiotherapy for advanced head and neck cancer. Oral Oncol. 2015;51(8):787–794. doi: 10.1016/j.oraloncology.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Lee LY, Chen SC, Chen WC, Huang BS, Lin CY. Postradiation trismus and its impact on quality of life in patients with head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(2):187–195. doi: 10.1016/j.oooo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Lindblom U, Garskog O, Kjellen E, Laurell G, Levring Jaghagen E, Wahlberg P, et al. Radiation-induced trismus in the ARTSCAN head and neck trial. Acta Oncol. 2014;53(5):620–627. doi: 10.3109/0284186X.2014.892209. [DOI] [PubMed] [Google Scholar]
- Lohia S, Rajapurkar M, Nguyen SA, Sharma AK, Gillespie MB, Day TA. A comparison of outcomes using intensity-modulated radiation therapy and 3-dimensional conformal radiation therapy in treatment of oropharyngeal cancer. JAMA Otolaryngol Head Neck Surg. 2014;140(4):331–337. doi: 10.1001/jamaoto.2013.6777. [DOI] [PubMed] [Google Scholar]
- Loorents V, Rosell J, Karlsson C, Lidback M, Hultman K, Borjeson S. Prophylactic training for the prevention of radiotherapy-induced trismus - a randomised study. Acta Oncol. 2014;53(4):530–538. doi: 10.3109/0284186X.2014.892211. [DOI] [PubMed] [Google Scholar]
- Louise Kent M, Brennan MT, Noll JL, Fox PC, Burri SH, Hunter JC, et al. Radiation-induced trismus in head and neck cancer patients. Support Care Cancer. 2008;16(3):305–309. doi: 10.1007/s00520-007-0345-5. [DOI] [PubMed] [Google Scholar]
- Melchers LJ, Van Weert E, Beurskens CH, Reintsema H, Slagter AP, Roodenburg JL, et al. Exercise adherence in patients with trismus due to head and neck oncology: a qualitative study into the use of the Therabite. Int J Oral Maxillofac Surg. 2009;38(9):947–954. doi: 10.1016/j.ijom.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Mendenhall WM, Amdur RJ, Palta JR. Intensity-modulated radiotherapy in the standard management of head and neck cancer: promises and pitfalls. J Clin Oncol. 2006;24(17):2618–2623. doi: 10.1200/JCO.2005.04.7225. [DOI] [PubMed] [Google Scholar]
- Pauli N, Johnson J, Finizia C, Andrell P. The incidence of trismus and long-term impact on health-related quality of life in patients with head and neck cancer. Acta Oncol. 2013;52(6):1137–1145. doi: 10.3109/0284186X.2012.744466. [DOI] [PubMed] [Google Scholar]
- Rao SD, Saleh ZH, Setton J, Tam M, McBride SM, Riaz N, et al. Dose-volume factors correlating with trismus following chemoradiation for head and neck cancer. Acta Oncol. 2015:1–6. doi: 10.3109/0284186X.2015.1037864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setton J, Caria N, Romanyshyn J, Koutcher L, Wolden SL, Zelefsky MJ, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82(1):291–298. doi: 10.1016/j.ijrobp.2010.10.041. [DOI] [PubMed] [Google Scholar]
- Steiner F, Evans J, Marsh R, Rigby P, James S, Sutherland K, et al. Mouth opening and trismus in patients undergoing curative treatment for head and neck cancer. Int J Oral Maxillofac Surg. 2015;44(3):292–296. doi: 10.1016/j.ijom.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Stubblefield MD, Manfield L, Riedel ER. A preliminary report on the efficacy of a dynamic jaw opening device (dynasplint trismus system) as part of the multimodal treatment of trismus in patients with head and neck cancer. Arch Phys Med Rehabil. 2010;91(8):1278–1282. doi: 10.1016/j.apmr.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Teguh DN, Levendag PC, Voet P, van der Est H, Noever I, de Kruijf W, et al. Trismus in patients with oropharyngeal cancer: relationship with dose in structures of mastication apparatus. Head Neck. 2008;30(5):622–630. doi: 10.1002/hed.20760. [DOI] [PubMed] [Google Scholar]
- van der Molen L, Heemsbergen WD, de Jong R, van Rossum MA, Smeele LE, Rasch CR, et al. Dysphagia and trismus after concomitant chemo-Intensity-Modulated Radiation Therapy (chemo-IMRT) in advanced head and neck cancer; dose-effect relationships for swallowing and mastication structures. Radiother Oncol. 2013;106(3):364–369. doi: 10.1016/j.radonc.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Vergeer MR, Doornaert PA, Rietveld DH, Leemans CR, Slotman BJ, Langendijk JA. Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a nonrandomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys. 2009;74(1):1–8. doi: 10.1016/j.ijrobp.2008.07.059. [DOI] [PubMed] [Google Scholar]
- Weber C, Dommerich S, Pau HW, Kramp B. Limited mouth opening after primary therapy of head and neck cancer. Oral Maxillofac Surg. 2010;14(3):169–173. doi: 10.1007/s10006-010-0220-2. [DOI] [PubMed] [Google Scholar]