Abstract
Only scarce data on liver steatosis in children with chronic hepatitis B and C (CHB and CHC) are available. The objective of this study was to evaluate the prevalence, predictors, and impact of hepatic steatosis on children with CHB and CHC. A total of 78 patients aged 11.5 ± 3.4 years were included: 30 (38%) had CHB, and 48 (62%) had CHC. Steatosis was scored on a 5-point scale, as follows: absent; minimal (≤5% hepatocytes affected), mild (6–33%), moderate (34–66%), and severe (>66%). Stepwise logistic regression was used to determine the factors associated with steatosis and moderate-to-severe steatosis. Steatosis was observed in 4/30 (13%) patients with CHB and 13/48 (27%) patients with CHC (P = 0.17). Moderate-to-severe steatosis was observed in 6/78 (8%) patients: 1/30 (3%) had CHB and 5/48 (10%) had CHC (P = 0.40). The body mass index (BMI) z-score was positively associated with the presence of steatosis in children with CHB (odds ratio [OR] = 3.3, 95% confidence interval [CI]: 1.02–10.64). In CHC, steatosis occurred more frequently in patients with hepatitis C virus genotype 3 compared with other genotypes (P = 0.002). In patients with non-3 genotype hepatitis C virus, steatosis was associated with the stage of fibrosis (OR = 3.35, 95% CI: 1.01–11.07) and inversely associated with the duration of infection (OR = 0.74, 95% CI: 0.55–0.97). Moderate-to-severe steatosis was positively associated with the BMI z-score (OR = 3.62, 95% CI: 1.22–10.75) and stage of fibrosis (OR = 3.89, 95% CI: 1.05–14.47). Steatosis is a common finding in children with chronic viral hepatitis. It is associated with metabolic factors in CHB, whereas in patients with CHC, metabolic and viral factors may have a combined effect, leading to more advanced grades of steatosis in children with higher BMI z-scores. Moderate-to-severe steatosis is a predictor of advanced fibrosis in children with CHC.
Keywords: children, chronic hepatitis, hepatitis B virus, hepatitis C virus, steatosis
1. Introduction
Fatty liver is the most common form of chronic liver disease in children and adults worldwide and has a large spectrum of causes including many general or systemic diseases, genetic-metabolic causes, hereditary genetic disorders, and drug hepatotoxicities.[1] Chronic infections with hepatitis B and C viruses (HBV and HCV, respectively) are among the many possible causes of liver steatosis.
The relationship between chronic hepatitis C (CHC) and liver steatosis is well documented.[2–6] Steatosis is a common finding in the course of CHC and occurs in approximately 50% of adults with CHC and up to 74% of patients infected with HCV genotype 3.[7–9] Both host and viral factors may contribute in the formation of steatosis. In adults with CHC, there are 2 different possible pathomechanisms for developing hepatic steatosis, including viral and metabolic, depending on the virus genotype. HCV genotype 3 directly affects the infected hepatocytes, and this cytopathic effect leads to viral-induced steatosis, which is correlated with HCV viral load.[7–10] In this type, the degree of steatosis may improve after antiviral therapy after achieving a sustained viral response.[9] Steatosis associated with non-3 genotype HCV is a marker of metabolic abnormalities, and insulin resistance playing a key role in its pathophysiology.[7,8] Several studies have confirmed that the presence of steatosis in patients with CHC has prognostic and therapeutic implications due to its independent prediction of fibrosis progression and its association with a lower rate of response to the antiviral therapy.[4,7,11–14]
In contrast to CHC, liver steatosis has not been adequately analyzed in chronic hepatitis B (CHB), and available data on its prevalence and significance in patients with HBV infection are much less abundant and consistent.[11,15] The frequency of hepatic steatosis in adults with CHB was estimated to be 22% to 76%.[15–18] Available data suggest that steatosis in HBV infection is more strongly associated with metabolic factors than viral determinants.[11,19] However, the ability of HBV to indirectly facilitate the development of steatosis has also been considered.[20] The implications of liver steatosis on disease progression and response to antiviral treatments in patients with CHB remain unknown.[11]
Only scarce and inconsistent data are available about liver steatosis in children with CHB and CHC with respect to its prevalence, pathogenesis, relation to metabolic and viral determinants, and impact on disease progression or response to the treatment.[3,6,21] However, pediatric patients are considered to be an ideal model to study hepatic steatosis because they are not often affected by other potential confounding risk factors responsible for its development, such as alcohol intake, metabolic syndrome, dyslipidemia, insulin-resistance, or diabetes.[3,21] Thus, the aim of this study was to evaluate the prevalence of liver steatosis in children with CHB and CHC and analyze its relationship with clinical and laboratory factors to assess its predictors and impact on the disease progression.
2. Methods
2.1. Patients
In this retrospective clinicopathological study, we included consecutive treatment-naïve patients chronically infected with HBV and HCV who underwent a liver biopsy in our tertiary health care department between 2002 and 2013. Liver biopsies were performed to qualify for antiviral treatment according to the current recommendations by the European Society of Pediatric Gastroenterology, Hepatology and Nutrition and the European Association for the Study of the Liver.[22,23] All patients and/or their parents/guardians provided written informed consent for the biopsy before the examination.
The diagnosis of chronic viral hepatitis was established in children with a history of hepatitis of at least 6 months and was based on elevated serum levels of alanine aminotransferase and aspartate aminotransferase (ALT and AST, respectively), positive serological testing (HBsAg for HBV infection and anti-HCV for HCV infection), and nucleic acid testing confirmation (positive HBV DNA or HCV RNA determined using a real-time polymerase chain reaction method, Amplicor, Roche Molecular Diagnostics [Pleasanton, CA, USA] and Cobas TaqMan, Roche Molecular Diagnostics [Pleasanton, CA, USA]). The ALT and AST serum levels were analyzed using commercially available laboratory kits (Vitros ECi, Ortho-Clinical Diagnostics, Johnson & Johnson [Raritan, NY, USA]). The serological testing was performed using commercial enzyme-linked immunosorbent assay tests (Vitros ECi, Ortho-Clinical Diagnostics, Johnson & Johnson). All the laboratory tests were performed in the Central Analytical Laboratory and the Molecular Diagnostics Laboratory, Hospital of Infectious Diseases in Warsaw, Poland. The most probable mode and date of infection were established using available medical records. The putative duration of infection was calculated from the beginning of the risk exposure.
Children with HBV/HCV coinfection and patients with hepatitis D virus or human immunodeficiency virus infection were not included in this study. Concomitant liver diseases (autoimmune hepatitis, Wilson's disease, alpha 1-antitripsin deficiency) and diabetes mellitus were exclusion criteria for this study.
Patient's weight and height were obtained on the day of the liver biopsy. Body mass index standard deviation scores (BMI z-scores) were calculated according to the WHO Child Growth Standards and Growth reference data, using the WHO Anthropometric calculator AnthroPlus v.1.0.4. An obesity diagnosis was made when the child's BMI z-score exceeded 2 standard deviations (SD).
2.2. Histological evaluation
Liver tissue samples were obtained using the percutaneous biopsy performed using the Menghini needle (Hepafix kit, B. Braun [Melsungen, Hessen, Germany]). A histopathological evaluation was performed by an experienced pathologist who was unaware of clinical data (Bożena Walewska-Zielecka). Hepatic steatosis was assessed semiquantitatively by scoring the percentage of hepatocytes containing fat droplets and was graded as follows: 0—no steatosis, 1—minimal (≤5% hepatocytes affected), 2—mild (6%–33%), 3—moderate (34%–66%), and severe (>66%). Similar classifications have been used by other authors.[3] Steatosis was described as microvesicular when numerous small fat droplets filled the cytoplasm leaving nucleus in the middle or as macrovesicular when large fat droplets displaced the nucleus to the cell periphery.
To histologically evaluate necroinflammation and fibrosis, we used the numerical scoring system by Knodell et al as modified by international experts.[24–26] The necroinflammatory activity grades were expressed using the histologic activity index, which ranges from 0 to 18 points. Fibrosis was staged using a 5-point scale (0—none fibrosis, 4—cirrhosis).
2.3. Statistical analysis
The Kolmogorov–Smirnov test was used to test whether the data were normally distributed. Continuous variables were expressed as means ± SD or medians with interquartile ranges and were compared using the Student t test or the Mann–Whitney test. Categorical variables were compared with either the chi-square test or Fisher exact test.
Stepwise logistic regression with the accompanying C-statistic was used to determine the factors associated with the presence of steatosis and steatosis of moderate-to-severe grade (3–4 points). The following variables were entered into the model: sex, age at liver biopsy, duration of infection, ALT serum level, AST serum level, HBV or HCV viral load, BMI z-score, mode of infection, genotype, hepatitis B e antigen (HBeAg) positivity, grade of necroinflammation, and stage of fibrosis. To assess model fit, the Hosmer–Lemeshow goodness-of-fit test was used.
All statistical analyses were performed using MedCalc Statistical Software ver. 16.8.4 (MedCalc, Mariakerke, Belgium). A 2-sided P value <0.05 was considered to be significant.
2.4. Ethical statement
The Local Ethics Committee gave an approval for the study and the biopsy procedure. The investigation was concordant with the principles outlined in the Declaration of Helsinki.
3. Results
3.1. Study population
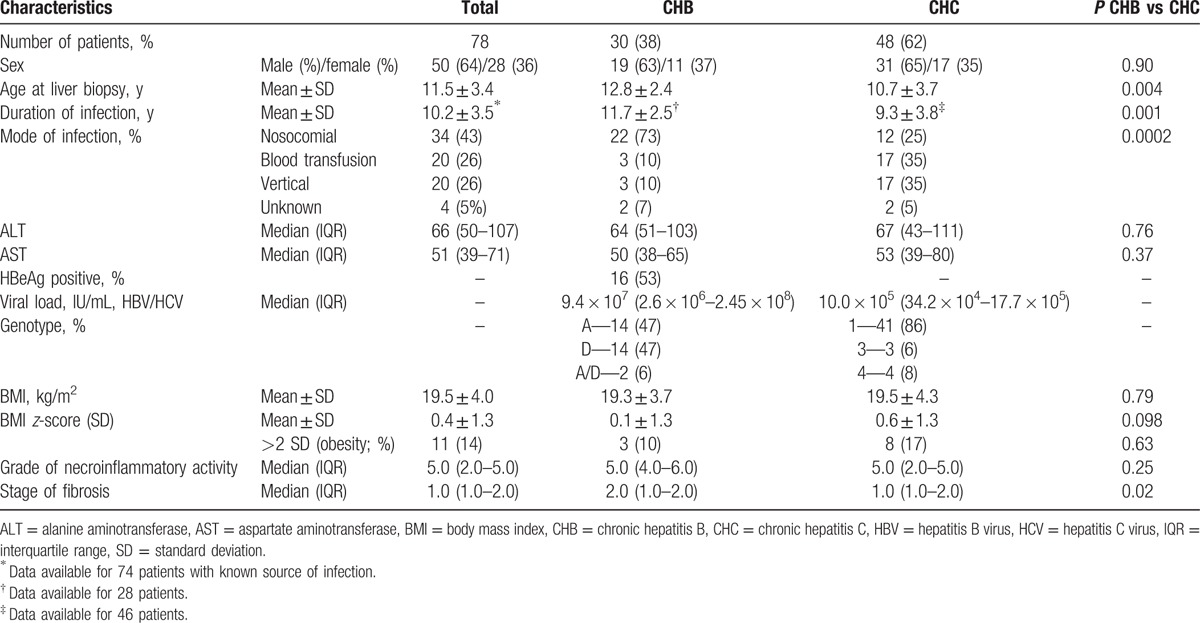
The study group consisted of 78 patients; 30 (38%) had CHB and 48 (62%) had CHC. The baseline characteristics of the study group are presented in Table 1. Children with chronic HBV infection were older compared with HCV-infected patients and had longer duration of infection. Both subgroups differed significantly with respect to the mode of the infection (P = 0.0002), as follows: children with CHB most frequently acquired the infection nosocomially (during hospitalization or surgical intervention, 73% of cases), whereas patients with CHC were infected vertically from an infected mother (35%) or through a transfusion of infected blood products before introduction of routine blood products screening for HCV infection (35%). HBV and HCV-infected patients did not differ in terms of sex, serum ALT and AST levels, and BMI z-scores.
Table 1.
Baseline characteristics of the study group.
3.2. Histological evaluation
Liver steatosis was observed in 17/78 (22%) cases, including 4/30 (13%) patients with CHB and 13/48 (27%) children with CHC (P = 0.17; Fig. 1). Moderate-to-severe steatosis (stage 3 and 4) was observed in 6/78 (8%) of patients overall, 1/30 (3%) infected with HBV and 5/48 (10%) infected with HCV (P = 0.40). Of these HCV-infected children with moderate-to-severe steatosis, there were 4/41 (10%) infected with HCV genotype 1, and 1/3 (33%) with HCV genotype 3 (P = 0.30). Nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH), characterized by the presence of cytologic ballooning, Mallory hyaline scattered inflammation, or predominantly zone 3 pericellular fibrosis, were not observed in any patient.
Figure 1.
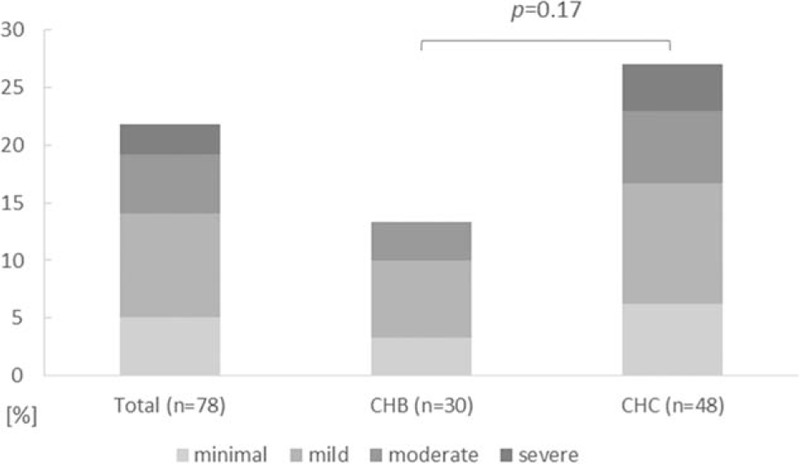
Prevalence of different grades of liver steatosis in the whole study group, in children with chronic hepatitis B and chronic hepatitis C. Minimal steatosis indicates ≤5% of hepatocytes affected; mild: 6–33%, moderate: 34–66%, and severe: >66%.
Histological evaluation revealed mild necroinflammatory activity (median grade 5.0) in the majority of cases. No difference between HBV and HCV-infected children was observed (Table 1). The median stage of fibrosis was 1.0 for the whole group and was higher in children with CHB compared to patients with CHC (P = 0.02; Table 1).
3.3. Predictors of steatosis
Among the analyzed factors (sex, age at liver biopsy, duration of infection, ALT serum level, AST serum level, HBV and HCV viral load, BMI z-score, mode of infection, genotype, HBeAg positivity, grade of necroinflammation, and stage of fibrosis), only the BMI z-score was positively associated with the presence of steatosis in the whole study group (odds ratio [OR] = 1.94, 95% confidence interval [CI]: 1.20–3.14) and in children with CHB (OR = 3.30, 95% CI: 1.02–10.64; Table 2 and Fig. 2). In HCV-infected children, a trend toward an association between steatosis and BMI z-score as well as an association between steatosis and HCV genotype (3 vs non-3) was observed (P = 0.08 and P = 0.002, respectively; Table 2). Steatosis was observed in 9/41 (22%) of patients with the HCV genotype 1, 3/3 (100%) with the genotype 3, and 1/4 (25%) with the genotype 4 (P = 0.013). Only 3 patients had the HCV genotype 3 (all 3 with steatosis), which precluded reliable statistical analysis for this group. In patients with non-3 HCV genotypes, multivariate analysis revealed that steatosis was associated with the stage of fibrosis (OR = 3.35, 95% CI: 1.01–11.07) and inversely associated with the duration of infection (OR = 0.74, 95% CI: 0.55–0.97; Table 2).
Table 2.
Factors associated with steatosis.
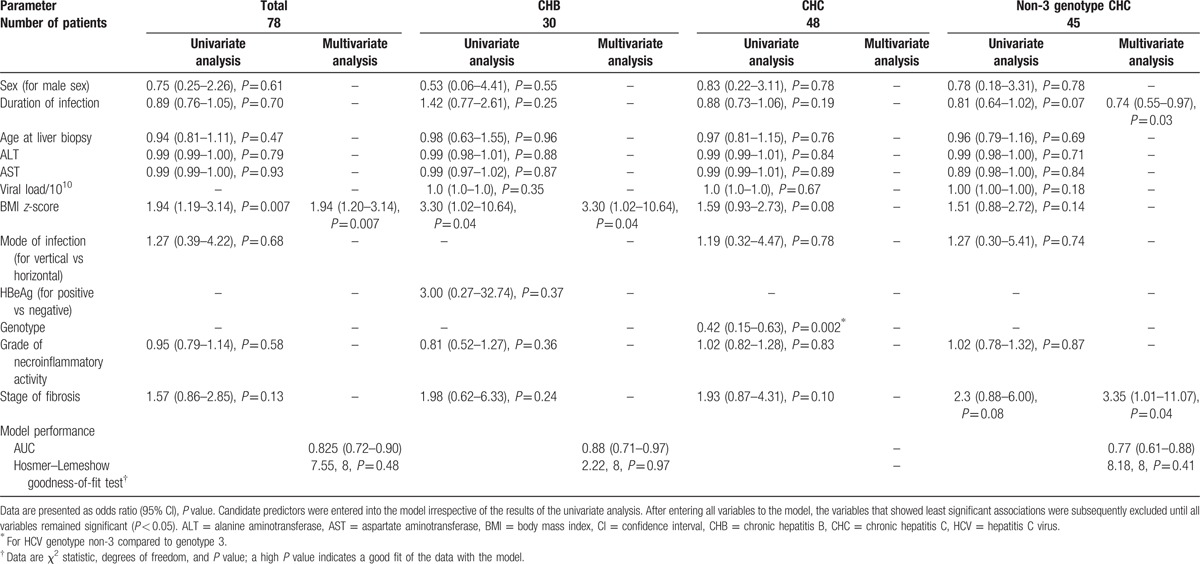
Figure 2.
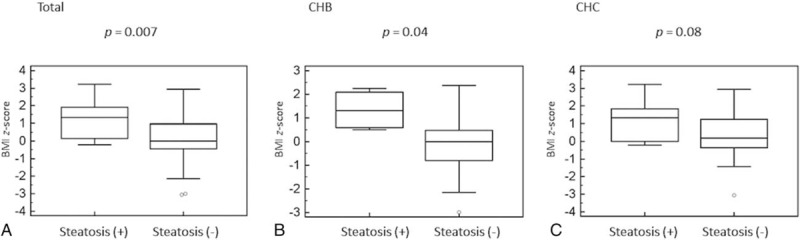
Box-and-whiskers plot for body mass index z-score in children with and without steatosis. The top and bottom of each box are the 25th and 75th percentiles. The line through the box is the median and the error bars are the maximum and minimum. CHB = chronic hepatitis B, CHC = chronic hepatitis C.
3.4. Predictors of moderate-to-severe steatosis
Both univariate and multivariate analysis revealed the positive association between moderate-to-severe steatosis (stages 3 and 4) and BMI z-score in the whole cohort (OR = 3.09, 95% CI: 1.29–7.43) and children with CHC (OR = 3.62, 95% CI: 1.22–10.75; Table 3, Fig. 3). Moreover, univariate analysis revealed that moderate-to-severe steatosis was positively associated with more advanced stage of fibrosis (OR = 3.89, 95% CI: 1.05–14.47) in HCV-infected patients. In addition, there was a trend toward a higher grade of necroinflammatory activity in this group (P = 0.06; Table 3). In patients infected with non-3 genotype HCV, a trend toward higher BMI z-score amongst patients with moderate-to-severe steatosis was observed (P = 0.06; Table 3). Only 1 child with CHB had moderate-to-severe steatosis, precluding reliable statistical analysis in this subgroup.
Table 3.
Factors associated with moderate-to-severe steatosis.
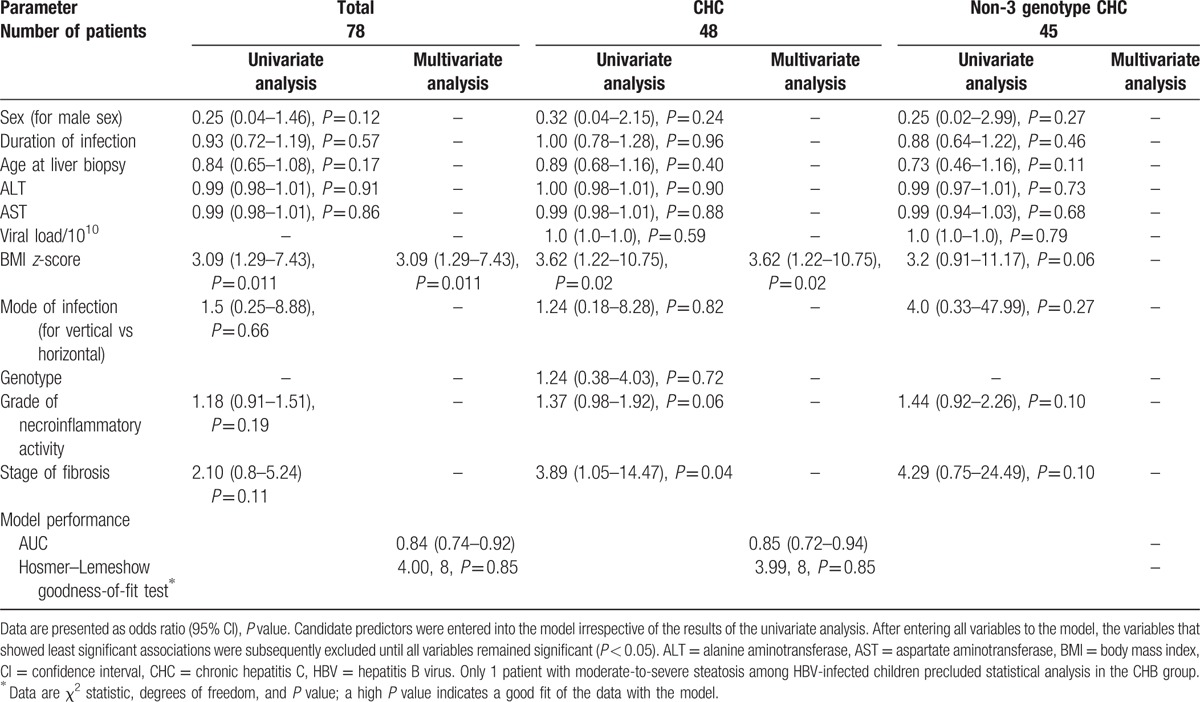
Figure 3.
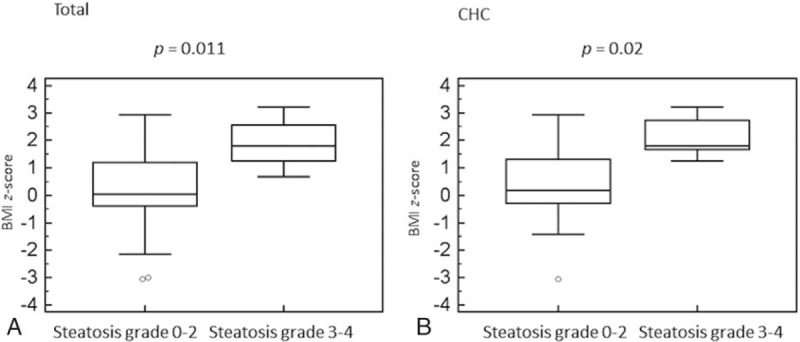
Box-and-whiskers plot for body mass index z-score in children with moderate-to-severe steatosis. The top and bottom of each box are the 25th and 75th percentiles. The line through the box is the median and the error bars are the maximum and minimum. CHC = chronic hepatitis C.
4. Discussion
Histologically proven prevalence of fatty liver in pediatric population in the United States (as revealed at autopsy after accidents) was estimated at 13%, ranging between 9.6% in normal-weight children and 38% in obese individuals.[27] Similar studies in the European children are lacking.[1] Our observations showed that liver steatosis occurs in both HBV and HCV-infected children (with a frequency of 13% and 27%, respectively), with a significant number of patients presenting with moderate-to-severe steatosis (8% in the whole study group). This study confirms results obtained by other authors in that steatosis occurs in about a quarter of children with CHC and is less common than in adults.[3,6,28] Goodman et al[29] demonstrated the presence of steatosis in 41% of their 121 children with CHC in the Peds-C Trial. However, in contrast to our observations, in all these cases the grade of steatosis was minimal to mild. This finding may indicate the influence of geographic trends on the frequency and the extent of steatosis, with European children being less frequently affected than patients from other regions.[29] In contrast to the observations by Giannattasio et al[21] who demonstrated liver steatosis in only 4% of their 56 children with CHB and concluded that hepatic steatosis is uncommon in children with chronic HBV infection, we observed steatosis in a significant percentage (13%) of our patients with CHB. Statistical analyses did not reveal any differences in the prevalence of steatosis between our patients with CHB (4/30, 13%) and CHC (13/48, 27%); however, this finding appears to result from small numbers of patients in both groups.
When evaluating the possible pathomechanism of steatosis in patients with chronic viral hepatitis, it is crucial to determine which of the 2 factors, viral or metabolic, plays a key role in accumulation of fat in the liver. In our study, the presence of steatosis among children with CHB was associated only with the BMI z-score and not with any viral factor, including HBeAg status and viral load. Similar observations were made by other authors who analyzed this issue in both pediatric and adult patients.[15,21] This finding indicates that hepatic steatosis in patients with CHB is mainly associated with host metabolic factors rather than with viral determinants, and in fact its incidence may not be different from the general population.
In children with CHC, the univariate analysis revealed a trend toward higher BMI z-scores and an association between the HCV genotype 3 and steatosis. All children infected with the HCV genotype 3 presented with steatosis, which occurred significantly more frequent compared to patients infected with non-3 genotypes (P = 0.002). The association between steatosis and HCV genotype 3 has been well documented in adult patients but yet not in children.[3,7,16] The HCV genotype 3 is considered to be a modulator of steatosis through directly influencing hepatic lipid homeostasis and can induce liver steatosis without metabolic risk factors.[4,7,30] In adult patients infected with the HCV genotype 3, steatosis correlates with the viral load.[4,7] However, this correlation has not been confirmed in pediatric patients in our study or by other authors.[6] In contrast to genotype 3, in patients infected with non-3 HCV genotypes (mainly the most frequent genotype 1), host metabolic factors (obesity, metabolic syndrome, insulin resistance, hyperglycemia, hypertriglyceridemia, and increased blood pressure) are considered to be crucial in developing steatosis.[4,7,16,31] Data on such correlation in children are inconsistent.[3,6,29] Guido et al[3] found a strong positive association between steatosis and BMI-for-age percentiles and serum triglyceride levels among 66 children who were mainly infected with the HCV genotype 1; they concluded that metabolic rather than viral factors influence the development of steatosis. A similar observation was made by Goodman et al[29], who demonstrated the correlation between steatosis, BMI and BMI z-score as well as with the duration of infection and ALT level. However, in a study by Giannattasio et al[6], there was no difference in the BMI between children with and without steatosis, although most of the children were infected with HCV genotype 1. This observation suggests that there may be a direct viral influence on the development of steatosis in non-3 genotypes in children. To evaluate this possible pathomechanism, we performed a multivariate analysis in children infected with non-3 genotype HCV. This analysis showed that only the duration of infection and the stage of fibrosis were independently associated with the presence of steatosis (P = 0.03 and P = 0.04, respectively). No other factor, including the BMI z-score, was found to be a predictor of steatosis in this group of children. These results confirm observations by Giannattasio et al[6] in that non-3 HCV genotypes in children may be associated with the accumulation of fat in the liver independently from classical metabolic risk factors.
On the other hand, our multivariate analysis showed that moderate-to-severe steatosis is independently associated with BMI z-score in the whole study group and in CHC (P = 0.011 and P = 0.02, respectively). A trend toward a higher BMI z-score was also observed in the univariate analysis in children with moderate-to-severe steatosis infected with non-3 genotype HCV (P = 0.06). This observation suggests that metabolic factors may cooperate with viral determinants responsible for fat accumulation in children with CHC, leading to more advanced stages of steatosis in children with higher BMI z-scores. Weight loss in these patients and a regular physical activity could possibly prevent them from severe steatosis; however, further studies are needed to analyze this issue.
In patients with CHC and steatosis, a differential diagnosis of concomitant NAFLD/NASH should be considered but is difficult to confirm.[1,3,32] The reference method for confirming NAFLD is liver histology.[1] The presence of the following lesions suggests a NAFLD diagnosis: moderate-to-severe macrovesicular steatosis of hepatocytes with displacement of the nucleus, hepatocyte ballooning, Mallory bodies, and zone 3 pericellular fibrosis.[1,11] None of the patients in our study group presented with these lesions. However, the diagnostic criteria for NAFLD in children may differ from those that are typical for adults and steatosis may be associated with mild portal inflammation with or without portal fibrosis and without ballooning degeneration or perisinusoidal fibrosis.[1,3,33] Therefore, in children with CHC, a histological diagnosis of concomitant NAFLD/NASH may be virtually impossible.[3]
To analyze the influence of steatosis on the liver disease progression, we analyzed its association with the necroinflammatory activity and fibrosis. No association was found in children with CHB. By comparison, in patients with CHC, the univariate analysis revealed a significant positive association between moderate-to-severe steatosis and fibrosis (P = 0.04) and trended toward a higher grade of necroinflammation (P = 0.06). In addition, multivariate analysis confirmed that steatosis of any grade was independently associated with more advanced fibrosis in children infected with non-3 genotype HCV. This is a unique finding, because a correlation between steatosis and liver fibrosis has not yet been confirmed in pediatric patients with CHC, despite being well documented in adults.[3,4,6,7,29,31]
Our study provides new data on the role of the viral and metabolic determinants of steatosis in children with chronic viral hepatitis and its implications. However, several limitations should be noted. First, as a retrospective study, this study could not distinguish between causes and effects. In addition, an uninfected control group to compare the prevalence of the steatosis between the study group and the general population is lacking. However, it is ethically unacceptable to perform liver biopsies in healthy children as a control group. Second, low numbers of patients in our group might have influenced the possibility of detecting significant differences and associations. However, a liver biopsy, as an invasive procedure, is currently rarely performed in children with viral hepatitis, and similar studies are therefore lacking in children.[34] Moreover, additional laboratory examinations (e.g., insulin resistance, cholesterol, and triglyceride serum levels) could provide more data on the role of the metabolic factors in the development of steatosis. However, an insulin resistance index assessment was not available, and a lipid serum panel was performed at different durations before liver biopsy, precluding a reliable analysis.
In conclusion, steatosis is a common finding in children with chronic viral hepatitis. It is associated with metabolic factors in CHB, whereas in patients with CHC, metabolic and viral factors may have a combined effect, leading to more advanced grades of steatosis in children with higher BMI z-scores. Moderate-to-severe steatosis is a predictor of advanced fibrosis in children with CHC.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, CHB = chronic hepatitis B, CHC = chronic hepatitis C, CI = confidence interval, HBV = hepatitis B virus, HCV = hepatitis C virus, NAFLD = nonalcoholic fatty liver disease, NASH = nonalcoholic steatohepatitis, OR = odds ratio, SD = standard deviation.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Vajro P, Lenta S, Socha P, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr 2012;54:700–13. [DOI] [PubMed] [Google Scholar]
- [2].Cross TJ, Quaglia A, Hughes S, et al. The impact of hepatic steatosis on the natural history of chronic hepatitis C infection. J Viral Hepat 2009;16:492–9. [DOI] [PubMed] [Google Scholar]
- [3].Guido M, Bortolotti F, Jara P, et al. Liver steatosis in children with chronic hepatitis C. Am J Gastroenterol 2006;101:2611–5. [DOI] [PubMed] [Google Scholar]
- [4].Adinolfi LE, Gambardella M, Andreana A, et al. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology 2001;33:1358–64. [DOI] [PubMed] [Google Scholar]
- [5].Hwang SJ, Luo JC, Chu CW, et al. Hepatic steatosis in chronic hepatitis C virus infection: prevalence and clinical correlation. J Gastroenterol Hepatol 2001;16:190–5. [DOI] [PubMed] [Google Scholar]
- [6].Giannattasio A, Spagnuolo MI, Sepe A, et al. Is HCV infection associated with liver steatosis also in children? J Hepatol 2006;45:350–4. [DOI] [PubMed] [Google Scholar]
- [7].Bjornsson E, Angulo P. Hepatitis C and steatosis. Arch Med Res 2007;38:621–7. [DOI] [PubMed] [Google Scholar]
- [8].Fiel MI. Pathology of chronic hepatitis B and chronic hepatitis C. Clin Liver Dis 2010;14:555–75. [DOI] [PubMed] [Google Scholar]
- [9].Hudacko R, Theise N. Liver biopsies in chronic viral hepatitis: beyond grading and staging. Arch Pathol Lab Med 2011;135:1320–8. [DOI] [PubMed] [Google Scholar]
- [10].Westin J, Nordlinder H, Lagging M, et al. Steatosis accelerates fibrosis development over time in hepatitis C virus genotype 3 infected patients. J Hepatol 2002;37:837–42. [DOI] [PubMed] [Google Scholar]
- [11].Guido M, Mangia A, Faa G. Gruppo Italiano Patologi Apparato D, Societa Italiana di Anatomia Patologica e Citopatologia Diagnostica/International Academy of Pathology Id. Chronic viral hepatitis: the histology report. Dig Liver Dis 2011;43(suppl 4):S331–43. [DOI] [PubMed] [Google Scholar]
- [12].Leandro G, Mangia A, Hui J, et al. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology 2006;130:1636–42. [DOI] [PubMed] [Google Scholar]
- [13].Negro F, Clement S. Impact of obesity, steatosis and insulin resistance on progression and response to therapy of hepatitis C. J Viral Hepat 2009;16:681–8. [DOI] [PubMed] [Google Scholar]
- [14].Westin J, Lagging M, Dhillon AP, et al. Impact of hepatic steatosis on viral kinetics and treatment outcome during antiviral treatment of chronic HCV infection. J Viral Hepat 2007;14:29–35. [DOI] [PubMed] [Google Scholar]
- [15].Tsochatzis E, Papatheodoridis GV, Manesis EK, et al. Hepatic steatosis in chronic hepatitis B develops due to host metabolic factors: a comparative approach with genotype 1 chronic hepatitis C. Dig Liver Dis 2007;39:936–42. [DOI] [PubMed] [Google Scholar]
- [16].Gordon A, McLean CA, Pedersen JS, et al. Hepatic steatosis in chronic hepatitis B and C: predictors, distribution and effect on fibrosis. J Hepatol 2005;43:38–44. [DOI] [PubMed] [Google Scholar]
- [17].Rozario R, Ramakrishna B. Histopathological study of chronic hepatitis B and C: a comparison of two scoring systems. J Hepatol 2003;38:223–9. [DOI] [PubMed] [Google Scholar]
- [18].Papatheodoridis GV, Chrysanthos N, Savvas S, et al. Diabetes mellitus in chronic hepatitis B and C: prevalence and potential association with the extent of liver fibrosis. J Viral Hepat 2006;13:303–10. [DOI] [PubMed] [Google Scholar]
- [19].Peng D, Han Y, Ding H, et al. Hepatic steatosis in chronic hepatitis B patients is associated with metabolic factors more than viral factors. J Gastroenterol Hepatol 2008;23(7 pt 1):1082–8. [DOI] [PubMed] [Google Scholar]
- [20].Mani H, Kleiner DE. Liver biopsy findings in chronic hepatitis B. Hepatology 2009;49(5 suppl):S61–71. [DOI] [PubMed] [Google Scholar]
- [21].Giannattasio A, Cirillo F, Terlizzi V, et al. Hepatic steatosis is uncommon in children with chronic hepatitis B. J Clin Virol 2009;46:360–2. [DOI] [PubMed] [Google Scholar]
- [22].European Association for Study of Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatol 2015;63:199–236. [DOI] [PubMed] [Google Scholar]
- [23].Sokal EM, Paganelli M, Wirth S, et al. Management of chronic hepatitis B in childhood: ESPGHAN clinical practice guidelines: consensus of an expert panel on behalf of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J Hepatol 2013;59:814–29. [DOI] [PubMed] [Google Scholar]
- [24].Brunt EM. Grading and staging the histopathological lesions of chronic hepatitis: the Knodell histology activity index and beyond. Hepatology 2000;31:241–6. [DOI] [PubMed] [Google Scholar]
- [25].Desmet VJ, Gerber M, Hoofnagle JH, et al. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology 1994;19:1513–20. [PubMed] [Google Scholar]
- [26].Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981;1:431–5. [DOI] [PubMed] [Google Scholar]
- [27].Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics 2006;118:1388–93. [DOI] [PubMed] [Google Scholar]
- [28].Mohan P, Barton BA, Narkewicz MR, et al. Evaluating progression of liver disease from repeat liver biopsies in children with chronic hepatitis C—a retrospective study. Hepatology 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Goodman ZD, Makhlouf HR, Liu L, et al. Pathology of chronic hepatitis C in children: liver biopsy findings in the Peds-C Trial. Hepatology 2008;47:836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kumar D, Farrell GC, Fung C, et al. Hepatitis C virus genotype 3 is cytopathic to hepatocytes: Reversal of hepatic steatosis after sustained therapeutic response. Hepatology 2002;36:1266–72. [DOI] [PubMed] [Google Scholar]
- [31].Rubbia-Brandt L, Fabris P, Paganin S, et al. Steatosis affects chronic hepatitis C progression in a genotype specific way. Gut 2004;53:406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ramesh S, Sanyal AJ. Hepatitis C and nonalcoholic fatty liver disease. Semin Liver Dis 2004;24:399–413. [DOI] [PubMed] [Google Scholar]
- [33].Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology 2005;42:641–9. [DOI] [PubMed] [Google Scholar]
- [34].Pokorska-Śpiewak M, Kowalik-Mikołajewska B, Aniszewska M, et al. Is liver biopsy still needed in children with chronic viral hepatitis? World J Gastroenterol 2015;21:12141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


