Abstract
To examine and quantify the potential relation between diabetic retinopathy (DR) and risk of all-cause mortality, stroke and heart failure (HF).
The resources of meta-analysis of epidemiological observational studies were from Pub-med, EMBASE, CINAHL, Cochrane Library, conference, and proceedings.
Random/fixed effects models were used to calculate pooled subgroup analysis stratified by different grades of DR was performed to explore the potential source of heterogeneity. Statistical manipulations were undertaken using program STATA.
Of the included 25 studies, comprising 142,625 participants, 19 studies were concluded to find the relation of DR to all-cause mortality, 5 for stroke, and 3 for HF. Risk ratio (RR) for all-cause mortality with the presence of DR was 2.33 (95% CI 1.92–2.81) compared with diabetic individuals without DR. Evidences showed a higher risk of all-cause mortality associated with DR in patients with T2D or T1D (RR 2.25, 95% CI 1.91–2.65. RR 2.68, 95% CI 1.34–5.36). According to different grades of DR in patients with T2D, RR for all-cause mortality varied, the risk of nonproliferative diabetic retinopathy (NPDR) was 1.38 (1.11–1.70), while the risk of proliferative diabetic retinopathy (PDR) was 2.32 (1.75–3.06). There was no evidence of significant heterogeneity (Cochran Q test P = 0.29 vs 0.26, I2 = 19.6% vs 22.6%, respectively). Data from 5 studies in relation to DR and the risk of stroke showed that DR was significantly associated with increased risk of stroke (RR = 1.74, 95%CI: 1.35–2.24), compared with patients without DR. Furthermore, DR (as compared with individuals without DR) was associated with a marginal increased risk of HF in patients with diabetes mellitus (DM) (n = 3 studies; RR 2.24, 95% CI 0.98–5.14, P = 0.056).
Our results showed that DR increased the risk of all-cause mortality, regardless of the different stages, compared with the diabetic individuals without DR. DR predicted increased risk of stroke and HF. Although only 3 studies about HF were available, the association between DR and HF should be careful.
Keywords: all-cause mortality, diabetic retinopathy, heart failure, stroke
1. Introduction
It has been proposed that the impact of diabetic retinopathy (DR) on vision is well known. There are more than 93 million patients with DR out of which 17 million have proliferative diabetic retinopathy (PDR).[1] In China, an estimated 40% (8% for vision-threatening retinopathy) of people with type 2 diabetes (T2D) and 86% (42%) with type 1 diabetes (T1D) have DR.[2] DR has been associated with an increased cardiovascular(CV) events risk in both T2D and T1D.[3,4]
Evidence from cohort studies of DR and risk of stroke and heart failure (HF) are controversial.[5–10] The mechanism by which DR might play a role in the physiology of stroke and HF in diabetes remains to be elucidated. Additionally, the association between DR and all-cause mortality in most studies has been examined by categorizing DR into dichotomous variable. There exists much uncertainty about relationship between different stages of DR and the risk of all-cause mortality.
Therefore, our study addresses an important gap in the published data. The aim of our study is to evaluate the risk of different stages of DR to the all-cause mortality, and to assess the association between DR and risk of all-cause mortality, stroke, and HF, by conducting an accumulated evidence of cohort studies.
2. Methods
Our research was performed in accordance with the recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) statements.[11,12] And the ethical approval was not necessary because our meta-analysis was based on data from previously published studies.
2.1. Search strategy and selection criteria
PubMed, EMBASE, and the Cochrane Library were searched for researches to April, 2016, using the terms: “diabetic retinopathy” in combination with the following terms – “survival, mortality, heart failure, stroke, and cereo-vascular events.” Further, complementary searches in the reference lists of selected articles were added. The supplementary strategy was implemented in addition to a manual search of proceedings of relevant conferences.
Studies were eligible to be included in the meta-analyses if they met the following criteria: a cohort study on the association between diabetic retinopathy or DR and risk of all-cause mortality, HF, and stroke. To be included, study results were presented as a risk ratio (RR) or hazard risk (HR) together with 95% confidence interval (CI), or enough data to perform their calculations, language of articles was limited to English.
2.2. Data synthesis and analysis
We used the results of the original studies from multivariable models with the most complete adjustment for potential confounders. We used the inverse variance weighted method to obtain overall hazard ratios and 95% CIs for an increase in risk of DR. A significant Q-statistic (P < 0.10) indicated heterogeneity across studies. Heterogeneity was quantified with the I2 metric, which is independent of the number of studies in the systematic review.[13] The pooled RR was estimated using fixed effects (FEs, Mantel and Haenszel) and random effects (REs, DerSimonian and Laird) models. When there is heterogeneity between studies, the pooled RR was estimated using the random effects model.[14] Statistical manipulations were undertaken using program STATA (version 13.0, StataCorp LP, TX).
3. Results
3.1. Characteristics of the included studies
Of the 25 studies included (Fig. 1), comprising 142,625 participants, 19 studies were concluded to find the relation between DR to all-cause mortality (n = 19,813, Table 1), and 5 studies about stroke (n = 7727, Table 2), 3 studies about HF (n = 117,451, Table 3).
Figure 1.
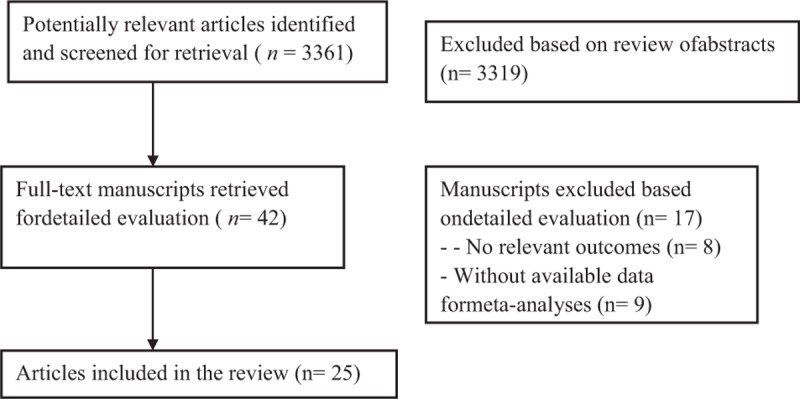
Flow chart demonstrated those studies that were processed for inclusion in our meta-analysis.
Table 1.
Characteristics of studies included in meta-analysis of associations of DR with risk of all-cause mortality.
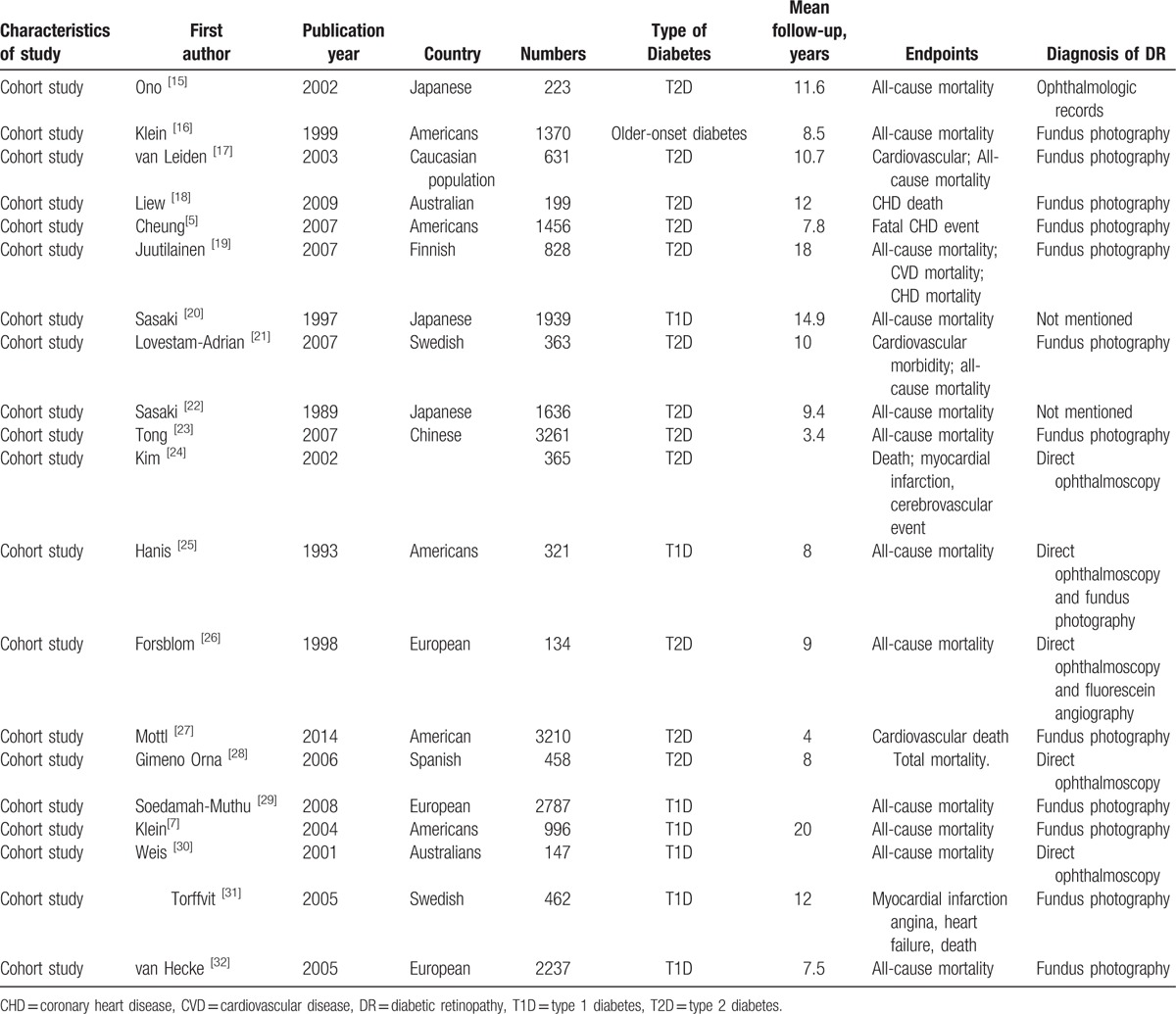
Table 2.
Characteristics of studies included in meta-analysis of associations of DR with risk of stroke.
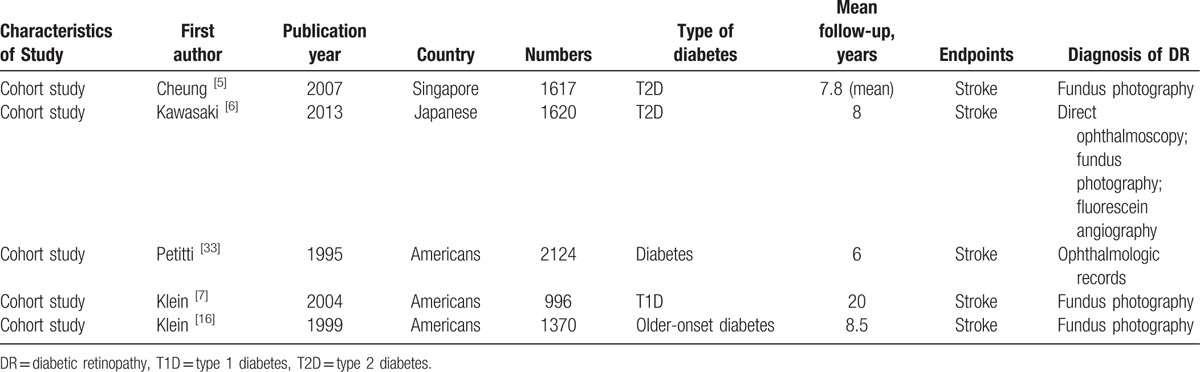
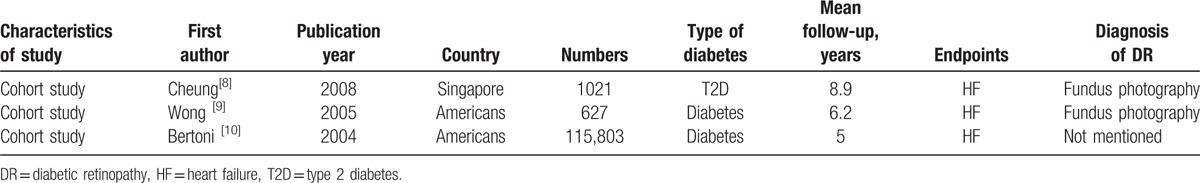
Table 3.
Characteristics of studies included in meta-analysis of associations of DR with risk of HF.
3.2. DR and the risk of all-cause mortality
Analysis of 19 studies showed that the RR for all-cause mortality with the presence of DR was 2.33 (95% CI 1.92–2.81) compared with patients without DR in patients of diabetes mellitus (DM) (Fig. 2). This analysis was associated with significant heterogeneity (Cochran Q test P = 0.001, I2 = 69%). To explore the contribution of age, Hbalc at baseline to relation between DR and overall mortality, meta-regression showed a nonsignificance of age and Hbalc to DR (P = 0.395, P = 0.907).
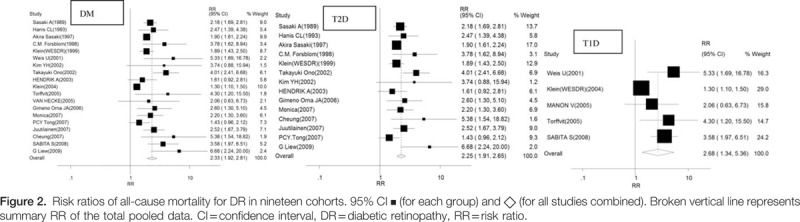
Evidences of 14 observational studies were pooled to evaluate the risk of all-cause mortality associated with DR in patients with T2D, which demonstrated a higher risk of all-cause mortality (RR 2.25, 95% CI 1.91–2.65). There was marginal significance of heterogeneity (Cochran Q test P = 0.041, I2 = 43.7%).
DR was associated with a significant increase in the RR of all-cause mortality in patients with T1D based on meta-analysis of observational studies (n = 5 studies; RR 2.68, 95% CI 1.34–5.36). The results were inconsistent across studies (Cochran Q test P = 0.001, I2 = 78.7%). In T1D studies, because of lack in data of the different stages of DR, DR is not categorized as nonproliferative diabetic retinopathy (NPDR) and PDR to assess risk for all-cause mortality.
According to different grades of DR in patients with T2D, RR for all-cause mortality varied as well, the risk of NPDR was 1.38 (1.11–1.70), PDR was 2.32 (1.75–3.06). There was no evidence of significant heterogeneity (Cochran Q test P = 0.29 vs 0.26, I2 = 19.6% vs 22.6%, respectively).
3.3. DR and the risk of stroke
Data from 5 studies on DR and the risk of stroke were pooled. DR was associated with a significantly increased risk of stroke (RR = 1.74, 95%CI 1.35–2.24), compared with patients without DR. The results were consistent across studies (Cochran Q test P = 0.78, I2 = 0%, Table 4).
Table 4.
Meta-analysis of DR and risk of all-cause mortality, stroke, and HF.
3.4. DR and the risk of HF
DR (as compared with patients without DR) was marginally associated with a significant increase in the risk of HF in patients with DM (n = 3 studies; RR 2.24, 95% CI 0.98–5.14, P = 0.056) (Fig. 3). There was a significant heterogeneity (Cochran Q test P = 0.01, I2 = 85.6%, Table 4).

3.5. Publication bias
Funnel plots and the Egger regression test suggested a borderline significant asymmetry in the analysis of T2D (P = 0.10). However, the trim-and-fill computation revealed that there were no missing trials, indicating that the publication bias did not interfere with the interpretation of the results. There were no publication biases in analysis of T1D in both tests (P = 0.21).
The potential presence of publication bias was evaluated by a funnel plot of the estimate of log-RR. The Begger funnel plots appeared symmetric. And there was no evidence of bias using the Egger method (τ = 1.94, P = 0.15), as well as using the Begg test (z = 1.85, P = 0.18), suggesting that no publication bias was observed in this meta-analysis.
4. Discussions
There are limited prospective data on the relationship between DR and all-cause mortality, HF, or stroke. We conducted meta-analysis to evaluate the risk of DR in association with these events. Our results show that the different stages of DR were associated with the increase of risk of all-cause mortality in patients with T2D. DR was significantly associated with the rising risk of stroke in individuals with diabetes.
The novelty of our results indicated a graded relation between severity of DR and the risk of all-cause mortality in patients with T2D, which is indicative that PDR individuals have more risk factors than NPDR. Due to scarce data on T1D, analysis could not be performed. The association between DR and all-cause mortality has been noted to be of similar magnitude, regardless of diabetes type,[27,34] which is in accordance to our results in this paper (RR 2.68, 95% CI 1.34–5.36, in T1D; RR 2.25, 95% CI 1.91–2.65, in T2D).
The association between DR and HF was significant in this meta-analysis, although the significance was marginal (P = 0.056). The mechanism by which DR might play a role in the pathophysiology of HF in DM remains to be elucidated. The key evidence is reduced coronary microcirculation leading to chronic myocardial ischemia, which is induced by lack of compensatory angiogenesis in response to myocardial remodeling.[35,36] Evidence indicated that individuals with DR were more likely to have left ventricular concentric remodeling, a precursor for HF (OR 1.72, 95% CI: 1.20–2.47).[37] Reports manifest that DR reflects diastolic cardiac dysfunction, which is indicative of HF.[38–40] Therefore, another explanation was that retinopathy simply lies along a continuum of disease that eventually leads to macrovascular damage and HF.[41]
Up to one-third of symptomatic strokes are major contributor of morbidity and mortality in diabetic individuals, they can be attributed to disease of the small cerebral arteries,[42] especially in folks with diabetes.[43] Due to the paucity of noninvasive tools to explore the cerebral microcirculation, relatively little is known about these arterioles pathologies.[44] Our findings showed that DR was significantly associated with the rising risk of stroke in characters with diabetes (pooled RR = 1.74, 95%CI 1.35–2.24). The feasible mechanism between DR and stroke might be that embryological origin, anatomical features, and physiological properties were shared.[45,46]
Vascular lesions in eyes with DR may mirror similar pathological disease processes in the cerebral microcirculation. Therefore, DR might demonstrate microvascular dysfunction not only in the retina but also in other organs such as heart and brain.[47] Our findings are in accordance with the concept that DR, stroke, and HF may have shared certain pathophysiological mechanisms.[48] Clearly, future studies are needed to verify this hypothesis and to perhaps uncover other noncirculatory backgrounds that could explain the risk of DR.
5. Limitation
The strengths of our meta-analysis are related to our extensive literature search and no evidence of publication bias. The quality of included studies was checked according to the Newcastle scale statement, and most of the included studies fulfilled all criteria. Nevertheless, some limitations should also be noted. First, possible limitation is that our adjusted meta-analyses undertaken were not ideal because the authors of the original studies used different statistical models. Second, we did not conduct different stages of DR for stroke and HF because of scarcity of data to classify DR. Furthermore, we did not perform sensitivity analyses or meta-regression to examine the contribution of participants’ baseline characteristics (such as age, HbA1c, and duration of T2D) in assessing relationship between DR and stroke and HF. Finally, none of the included studies were designed to explore association between DR and “target organ damage.” Hence, any conclusions regarding hard outcomes, such as all-cause mortality, stroke, or HF, should be considered with caution.
6. Conclusions
The meta-analysis showed that DR was associated with increased risk for all-cause mortality, regardless of the different stages of DR, compared with the diabetic individuals without DR. DR increased the risk of stroke and HF. Only 3 studies about HF were available, the exact association between DR and HF needs further studies to clarify.
Acknowledgement
The authors thank Shoaib Ahmed from UK, studying in Capital Medical University, for his assistance with revision of this manuscript; and all the participants and staff in this study. The authors also thank the National Science Foundation Council of China (No. 81471014, 81300650, 81300726, 81670738) for the support.
Footnotes
Abbreviations: CI = confidence interval, DM = diabetes mellitus, DR = diabetic retinopathy, HF = heart failure, NPDR = nonproliferative diabetic retinopathy, PDR = proliferative diabetic retinopathy, RR = risk ratio.
Authorship: JBZ and JKY have contributed to the design of the study, analysis and interpretation of data, and prepared all figures and tables. JBZ, JKY, XRZ, YPZ, LB and XLZ drafted a part of manuscript. XRZ and LB took part in analyzing data, and drafting a part of manuscript. All authors reviewed the manuscript.
Funding/support: This work was supported by the National Science Foundation Council of China (No. 81471014, 81300650, 81300726, 81670738).
The authors have no conflicts of interest to disclose.
References
- [1].Morehouse KA, Hobley L, Capeness M, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA 2010;304:649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang FH, Liang YB, Zhang F, et al. Prevalence of diabetic retinopathy in rural China: the Handan Eye Study. Ophthalmology 2009;116:461–7. [DOI] [PubMed] [Google Scholar]
- [3].Guo VY, Cao B, Wu X, et al. Prospective association between diabetic retinopathy and cardiovascular disease – a systematic review and meta-analysis of cohort studies. J Stroke Cerebrovasc Dis 2016;25:1688–95. [DOI] [PubMed] [Google Scholar]
- [4].Brownrigg JR, Hughes CO, Burleigh D, et al. Microvascular disease and risk of cardiovascular events among individuals with type 2 diabetes: a population-level cohort study. Lancet Diabetes Endocrinol 2016;47:588–97. [DOI] [PubMed] [Google Scholar]
- [5].Cheung N, Rogers S, Couper DJ, et al. Is diabetic retinopathy an independent risk factor for ischemic stroke? Stroke 2007;38:398–401. [DOI] [PubMed] [Google Scholar]
- [6].Kawasaki R, Tanaka S, Tanaka S, et al. Risk of cardiovascular diseases is increased even with mild diabetic retinopathy: the Japan Diabetes Complications Study. Ophthalmology 2013;120:574–82. [DOI] [PubMed] [Google Scholar]
- [7].Klein BE, Klein R, McBride PE, et al. Cardiovascular disease, mortality, and retinal microvascular characteristics in type 1 diabetes: Wisconsin epidemiologic study of diabetic retinopathy. Arch Intern Med 2004;164:1917–24. [DOI] [PubMed] [Google Scholar]
- [8].Cheung N, Wong TY. Diabetic retinopathy and systemic vascular complications. Prog Retin Eye Res 2008;27:161–76. [DOI] [PubMed] [Google Scholar]
- [9].Wong TY, Rosamond W, Chang PP, et al. Retinopathy and risk of congestive heart failure. JAMA 2005;293:63–9. [DOI] [PubMed] [Google Scholar]
- [10].Bertoni AG, Hundley WG, Massing MW, et al. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 2004;27:699–703. [DOI] [PubMed] [Google Scholar]
- [11].Stroup DF1, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [12].von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [14].Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird's methodology to perform multivariate random effects meta-analyses. Stat Med 2010;29:1282–97. [DOI] [PubMed] [Google Scholar]
- [15].Ono T, Kobayashi J, Sasako Y, et al. The impact of diabetic retinopathy on long-term outcome following coronary artery bypass graft surgery. J Am Coll Cardiol 2002;40:428–36. [DOI] [PubMed] [Google Scholar]
- [16].Klein R, Klein BE, Moss SE, et al. Association of ocular disease and mortality in a diabetic population. Arch Ophthalmol 1999;117:1487–95. [DOI] [PubMed] [Google Scholar]
- [17].van Leiden HA, Dekker JM, Moll AC, et al. Blood pressure, lipids, and obesity are associated with retinopathy: the Hoorn study. Diabetes Care 2002;25:1320–5. [DOI] [PubMed] [Google Scholar]
- [18].Liew G, Wong TY, Mitchell P, et al. Retinopathy predicts coronary heart disease mortality. Heart 2009;95:391–4. [DOI] [PubMed] [Google Scholar]
- [19].Juutilainen A, Lehto S, Ronnemaa T, et al. Retinopathy predicts cardiovascular mortality in type 2 diabetic men and women. Diabetes Care 2007;30:292–9. [DOI] [PubMed] [Google Scholar]
- [20].Sasaki A, Uehara M, Horiuchi N, et al. A 15-year follow-up study of patients with non-insulin-dependent diabetes mellitus (NIDDM) in Osaka, Japan. Factors predictive of the prognosis of diabetic patients. Diabetes Res Clin Pract 1997;36:41–7. [DOI] [PubMed] [Google Scholar]
- [21].Lovestam-Adrian M, Hansson-Lundblad C, Torffvit O. Sight-threatening retinopathy is associated with lower mortality in type 2 diabetic subjects: a 10-year observation study. Diabetes Res Clin Pract 2007;77:141–7. [DOI] [PubMed] [Google Scholar]
- [22].Sasaki A, Horiuchi N, Hasegawa K, et al. Mortality and causes of death in type 2 diabetic patients. A long-term follow-up study in Osaka District, Japan. Diabetes Res Clin Pract 1989;7:33–40. [DOI] [PubMed] [Google Scholar]
- [23].Tong PC, Kong AP, So WY, et al. Interactive effect of retinopathy and macroalbuminuria on all-cause mortality, cardiovascular and renal end points in Chinese patients with Type 2 diabetes mellitus. Diabet Med 2007;24:741–6. [DOI] [PubMed] [Google Scholar]
- [24].Kim YH, Hong MK, Song JM, et al. Diabetic retinopathy as a predictor of late clinical events following percutaneous coronary intervention. J Invasive Cardiol 2002;14:599–602. [PubMed] [Google Scholar]
- [25].Hanis CL, Chu HH, Lawson K, et al. Mortality of Mexican Americans with NIDDM. Retinopathy and other predictors in Starr County, Texas. Diabetes Care 1993;16:82–9. [DOI] [PubMed] [Google Scholar]
- [26].Forsblom CM, Sane T, Groop PH, et al. Risk factors for mortality in Type II (non-insulin-dependent) diabetes: evidence of a role for neuropathy and a protective effect of HLA-DR4. Diabetologia 1998;41:1253–62. [DOI] [PubMed] [Google Scholar]
- [27].Mottl AK, Pajewski N, Fonseca V, et al. The degree of retinopathy is equally predictive for renal and macrovascular outcomes in the ACCORD Trial. J Diabetes Complications 2014;28:874–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gimeno Orna JA, Castro Alonso FJ, Sánchez Vañó R, et al. Diabetic retinopathy and mortality in type 2 diabetic patients. Med Clin (Barc) 2006;126:686–9. [DOI] [PubMed] [Google Scholar]
- [29].Soedamah-Muthu SS, Chaturvedi N, Witte DR, et al. Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care 2008;31:1360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Weis U, Turner B, Gibney J, et al. Long-term predictors of coronary artery disease and mortality in type 1 diabetes. QJM 2001;94:623–30. [DOI] [PubMed] [Google Scholar]
- [31].Torffvit O, Lovestam-Adrian M, Agardh E, et al. Nephropathy, but not retinopathy, is associated with the development of heart disease in Type 1 diabetes: a 12-year observation study of 462 patients. Diabet Med 2005;22:723–9. [DOI] [PubMed] [Google Scholar]
- [32].van Hecke MV, Dekker JM, Stehouwer CD, et al. Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB prospective complications study. Diabetes Care 2005;28:1383–9. [DOI] [PubMed] [Google Scholar]
- [33].Petitti DB, Bhatt H. Retinopathy as a risk factor for nonembolic stroke in diabetic subjects. Stroke 1995;26:593–6. [DOI] [PubMed] [Google Scholar]
- [34].Kramer CK, Rodrigues TC, Canani LH, et al. Diabetic retinopathy predicts all-cause mortality and cardiovascular events in both type 1 and 2 diabetes: meta-analysis of observational studies. Diabetes Care 2011;34:1238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev 2006;20:3347–65. [DOI] [PubMed] [Google Scholar]
- [36].Shigeta T, Aoyama M, Bando YK, et al. Dipeptidyl peptidase-4 modulates left ventricular dysfunction in chronic heart failure via angiogenesis-dependent and -independent actions. Circulation 2012;126:1838–51. [DOI] [PubMed] [Google Scholar]
- [37].Cheung N, Bluemke DA, Klein R, et al. Retinal arteriolar narrowing and left ventricular remodeling: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol 2007;50:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cheung N, Wang JJ, Rogers SL, et al. Diabetic retinopathy and risk of heart failure. J Am Coll Cardiol 2008;51:1573–8. [DOI] [PubMed] [Google Scholar]
- [39].Kurioka S, Ose H, Fukuma K, et al. Severity of diabetic retinopathy is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Res Clin Pract 2013;99:287–91. [DOI] [PubMed] [Google Scholar]
- [40].Takeda Y, Sakata Y, Mano T, et al. Diabetic retinopathy is associated with impaired left ventricular relaxation. J Card Fail 2011;17:556–60. [DOI] [PubMed] [Google Scholar]
- [41].Ventura HO, Reddy M. The eye as an indicator of heart failure in diabetic patients. J Am Coll Cardiol 2008;51:1579–80. [DOI] [PubMed] [Google Scholar]
- [42].Greenberg SM. Small vessels, big problems. N Engl J Med 2006;354:1451–3. [DOI] [PubMed] [Google Scholar]
- [43].Alex M, Baron EK, Goldenberg S, et al. An autopsy study of cerebrovascular accident in diabetes mellitus. Circulation 1962;25:663–73. [DOI] [PubMed] [Google Scholar]
- [44].Wardlaw JM, Dennis MS, Warlow CP, et al. Imaging appearance of the symptomatic perforating artery in patients with lacunar infarction: occlusion or other vascular pathology? Ann Neurol 2001;50:208–15. [DOI] [PubMed] [Google Scholar]
- [45].Patton N1, Aslam T, Macgillivray T, et al. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat 2005;206:319–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wong TY. Is retinal photography useful in the measurement of stroke risk? Lancet Neurol 2004;3:179–83. [DOI] [PubMed] [Google Scholar]
- [47].Hiller R, Sperduto RD, Podgor MJ, et al. Diabetic retinopathy and cardiovascular disease in type II diabetics. The Framingham Heart Study and the Framingham Eye Study. Am J Epidemiol 1988;128:402–9. [DOI] [PubMed] [Google Scholar]
- [48].van Hecke MV, Dekker JM, Nijpels G, et al. Inflammation and endothelial dysfunction are associated with retinopathy: the Hoorn Study. Diabetologia 2005;48:1300–6. [DOI] [PubMed] [Google Scholar]


